Abstract
Vaccination for hepatitis B virus (HBV) in the setting of hepatitis C virus (HCV) infection is recommended, but responses to vaccination are blunted when compared to uninfected populations. The mechanism for this failure of immune response in HCV-infected subjects remains unknown but is thought to be a result of lymphocyte dysfunction during chronic viral infection. We have recently demonstrated that PD-1, a novel negative immunomodulator for T cell receptor (TCR) signaling, is involved in T and B lymphocyte dysregulation during chronic HCV infection. In this report, we further investigated the role of the PD-1 pathway in regulation of CD4+ T cell responses to HBV vaccination in HCV-infected individuals. In a prospective HCV infected cohort, a poor response rate to HBV vaccination as assayed by seroconversion was observed in HCV-infected subjects (53%), while a high response rate was observed in healthy or spontaneously HCV-resolved individuals (94%). CD4+ T cell responses to ex vivo stimulations of anti-CD3/CD28 antibodies or hepatitis B surface antigen (HBsAg) were found to be lower in HBV vaccine non-responders compared to those responders in HCV-infected individuals who had received a series of HBV immunizations. PD-1 expression on CD4+ T cells were detected at relatively higher levels in these HBV vaccine non-responders than those who responded, and this was inversely associated with the cell activation status. Importantly, blocking the PD-1 pathway improved T cell activation and proliferation in response to ex vivo HBsAg or anti-CD3/CD28 stimulation in HBV vaccine non-responders. These results suggest that PD-1 signaling may be involved in impairing CD4+ T cell responses to HBV vaccination in subjects with HCV infection, and raise the possibility that blocking this negative signaling pathway might improve success rates of immunization in the setting of chronic viral infection.
Keywords: vaccine, hepatitis C, hepatitis B, PD-1, T cell
Introduction
Hepatitis C virus (HCV) infection is a common cause of chronic liver disease and is the leading indication for liver transplantation in the United States. Given the shared risk factors for transmission, co-infection of hepatitis B virus (HBV) with HCV is quite common, and may lead to more significant liver disease, including higher rates and more rapid progression to liver cirrhosis and liver cancer1-5. As such, HBV vaccine is recommended as the primary means to prevent HBV super-infection and its associated increase in morbidity and mortality in HCV-infected subjects. However, vaccine response (seroconversion with a hepatitis B surface antibody titer >10 IU/L) in this setting is often blunted, with poor response rates to a standard course of HBV vaccinations in chronically HCV-infected individuals when compared to the healthy populations (40∼60% versus 90∼95%); this is especially noted in the setting of advanced fibrosis and liver cirrhosis6-8. To improve seroconversion rates following HBV immunization, approaches have included different administration routes (intradermal), higher doses of HBV vaccine (40 μg), or adding adjuvants (CPG 7909, levamisole, GM-CSF), but success rates remain disappointing in the setting of chronic HCV infection. Additionally, a poor immune response in HCV-infected subjects is also observed in the clinical setting following other adult immunizations--including hepatitis A, influenza and pneumococcal vaccines---a phenomenon that observed in the setting of human immunodeficiency virus (HIV) infection9,10. This suggests the possibility of a more global immune dysregulation and a shared mechanism in individuals with chronic viral infections.
The mechanism for vaccine-induced immune response is activation and expansion of antigen-specific memory T and B lymphocytes upon encountering antigen. Activation and proliferation of lymphocytes requires two signals: an antigen specific signal and a co-stimulatory signal. Co-stimulatory signaling is pivotal to determining whether recognition of antigen by lymphocytes leads to full cell activation/proliferation or to cell exhaustion/apoptosis. Recently, it has been shown that a virus-induced negative co-stimulatory cell modulator, programmed death-1 (PD-1), contributes significantly to lymphocyte dysregulation11, 12. PD-1 is an inhibitory immune-receptor predominantly expressed on activated T and B lymphocytes. PD-1 ligand, PDL-1, is expressed on both haematopoetic and parenchymal cells. PD-1/PDL-1 engagement induces immune-receptor tyrosine phosphorylation and delivers a negative signal to T/B cell receptor (TCR/BCR) signaling pathways. We have recently demonstrated that HCV core-mediated PD-1 expression is involved in T and B cell dysregulation during chronic HCV infection13-15. However, the role of PD-1 in regulating T cell responses to HBV vaccine in HCV-infected individuals has yet to be determined. In this report, we found that patients with chronic HCV infection have poor responses to HBV immunizations, and that PD-1 is involved in regulating CD4+ T cell responses to HBV vaccines in chronically HCV-infected individuals.
Materials and Methods
Subjects
The study protocol was approved by an institutional review board at East Tennessee State University and James H. Quillen VA Medical Center (ETSU/VA IRB, Johnson City, TN), which has contributed to a database for the storage of blood samples from HCV-infected individuals for the purpose of viral immunology studies. A total of 61 HCV-infected subjects and 16 controls without serologic evidence of prior exposure to HBV or HAV were recruited into this study to receive either Engerix® HBV (if HAV antibody negative), or Twinrix® HAV/HBV combination vaccines as appropriate, and peripheral blood was collected at 1∼6 months post immunizations to determine seroconversion, with an HBsAb titer >10 IU/L deemed as HBV vaccine response. All subjects gave written informed consent for this study. The subjects were divided into four groups of populations: 1) HBV vaccine non-responders with HCV infection (HBV-NR, n=29); 2) HBV vaccine responders with HCV infection (HBV-R, n=32); 3) subjects with HCV who spontaneously resolved their infection and were HCV RNA-negative (HCV-resolved individuals, n=6); and 4) healthy subjects who were negative for HBV, HCV, and HIV (n=10). The demographic features of each group, including HCV genotype and viral load (performed by Lexington VAMC) are shown in Table 1. The majority of the study subjects were male. The mean age of the HCV-infected individuals was higher but comparable to healthy subjects.
Table 1. Demographic features of the study subjects.
| Groups | No. of Subjests | Mean age (Range) | % of Males | % of Genotype 1 | HCV RNA (IU/ml) |
|---|---|---|---|---|---|
| HBV-NR | 29 | 56 (31-69) | 93 | 72 | 4,890∼50,000,000 |
| HBV-R | 32 | 54 (29-71) | 96 | 69 | 45,800∼50,000,000 |
| HCV-resolved | 6 | 51 (37-62) | 100 | NA | NA |
| Healthy subjects | 10 | 45 (28-58) | 80 | NA | NA |
NA = not applicable.
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of study subjects by Ficoll-density centrifugation with lympho-H (Atlanta biological, Lawrenceville, GA), which were then viably cryopreserved in freezing medium in liquid nitrogen15. If indicated, CD4+ T cells were further purified from isolated PBMC by incubation with a magnetic beads-conjugated with anti-CD4 antibody, followed by positive selection per the manufacturer's instruction (Miltenyi Biotec., Auburn, CA). Purified cells were cultured as described14.
Flow cytometry
To determine the role of PD-1 in T cell activation, 1 × 106 purified PBMC were stimulated with either anti-CD3/CD28 (1 μg/ml each; BD Pharmingen) or HBsAg (2.5 μg/ml, Biospcific, Emeryville, CA) for 24 hours, followed by double or triple immune-staining with the following antibody conjugates: PE-anti-CD69, APC-anti-CD25, PE-anti-PD-1, PE-anti-PDL-1, and FITC-anti-CD4 (BD Pharmingen or eBioscience). The primary isotype controls were used to determine the level of background staining. 20,000 events were acquired after gating on lymphocyte populations using a BD FACS caliber instrument. Percentages of CD69/CD25 or PD-1/PDL-1 expressions in the CD4+ T cells were analyzed using FACS express software.
PD-1/PDL-1 blockade
PBMC isolated from HBV-NR in HCV-infected subjects were incubated with 10 μg/ml anti-PDL-1 (eBioscience, San diego, CA, anti-human CD274 or B7-H1 clone: M1H1) or control IgG antibodies overnight, followed by stimulation with anti-CD3/CD28 or HBsAg for 5 days. T cell activation and proliferation were evaluated by detecting CD69 expression and CFSE dilution as described previously13.
Statistical analysis
Results are expressed as means ± SD with additional confidence intervals of the means included where appropriate. Linear regression models were run predicting percentages of CD69 expression among different groups and beta-estimates of predictor variables generated using the NPAR1WAY procedure in SAS software, version 9.1. The difference of CD69/CD25 or PD-1/PDL-1 expression levels between each group was determined using independent samples T test program by SPSS 18 software (IBM, Somers, NY). Correlation between T cell activation markers and PD-1/PDL-1 expression was analyzed using a Pearson Correlation program. Values of *P < 0.05 were considered significant, and **P< 0.01 was considered very significant.
Results
Subjects with chronic HCV infection have a poor response to HBV vaccines
Individuals with chronic HCV infection are at increased risk of severe outcomes upon co-infection with HBV. In this prospective study to evaluate the efficacy of HBV vaccinations in subjects with HCV infection, those without serologic evidence of prior exposure to HBV were vaccinated with either Engerix® HBV, or Twinrix® HAV/HBV combination vaccine if hepatitis A virus (HAV) nonimmune, and response was defined as the development of anti-HBV surface antibody (HBsAb). A total of 61 HCV-infected subjects received vaccination for HBV: 28% (17/61) received Engerix, and 72% (44/61) Twinrix. Of the 61 Subjects vaccinated, 53% (32/61) of HCV-infected subjects exhibited seroconversion with an HBsAb titer >10 IU/L. The response to HBV vaccination was 47% in subjects receiving Engerix and 55% in those vaccinated with Twinrix. Although the administration of the combination vaccine appeared somewhat superior to HBV vaccine alone, this difference was not statistically significant. In contrast, 94% (15/16) controls (10 healthy subjects and 6 spontaneously HCV-resolved individuals) achieved seroconversion after receiving either Engerix or Twinrix vaccinations, consistent with a large body of data showing excellent rates of HBV vaccine response in healthy individuals6,8. This was higher than HCV subjects who received HBV vaccines (p=0.002), and in keeping with data suggesting that subjects with chronic HCV infection may have poorer responses to HBV vaccination7.
Specific and non-specific CD4+ T cell responses are impaired in HBV vaccine non-responders with HCV infection
CD4+ T cells play a critical role in mounting an effective immune response to immunogens, including the provision of help to B cells to facilitate antibody production. To determine whether the poor HBV vaccine responses we observed were associated with poor CD4+ T cell responses during HCV infection, we first compared the activation status of CD4+ T cells in HCV-infected subjects and healthy subjects who had received a standard series of HBV vaccinations. To this end, PBMC isolated from HCV-infected and healthy subjects were ex vivo stimulated with anti-CD3/CD28 or HBsAg for 24 hours, and expression of CD69 (an early T cell activation marker) on the surface of CD4+ T cells was examined by flow cytometry. Antigen-specific or non-specific stimuli were employed as these are required to observe CD69 expression, which is quite low in unstimulated cells. No differences in absolute CD4+ T cell number were found amongst the groups (data not shown). As shown in Fig. 1 (left panel), CD69 expression on CD4+ T cells in response to anti-CD3/CD28 stimulation was detected at significantly lower levels in the grouped subjects with chronic HCV infection (HBV-R + HBV-NR, 47.38% ± 17.52%, n=61) versus the control subjects (HCV-resolved + healthy subjects, 76.01% ± 7.81%, n=16, p<0.01). Additionally, the group of HBV-NR exhibited significantly lower CD69 expression on their CD4+ T cells than those HBV-R (37.79% ± 18.52%, n=29 versus 56.06% ± 10.98%, n=32, p<0.01); while no significant differences in CD69 expressions were observed between HCV-resolved individuals and healthy subjects (71.93% ± 10.83% versus 78.47% ± 4.31%, p>0.05).
Fig. 1. CD4+ T cell activation status in HCV-infected and healthy subjects who received HBV vaccinations.
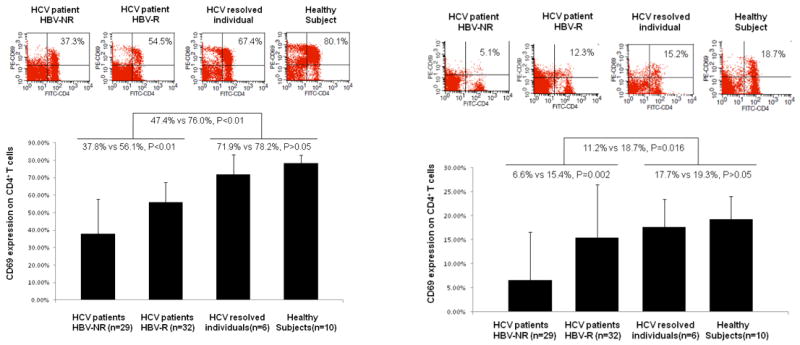
PBMC were isolated from HCV-infected HBV vaccine non-responders (HBV-NR) (n=29), HCV-infected HBV vaccine responders (HBV-R) (n=32), HCV-resolved subjects (n=6), and healthy subjects (n=10), ex vivo stimulated with anti-CD3/CD28 (left panel) or HBsAg (right panel) for 24 hrs, and assayed by flow cytometry for CD69 expression on CD4+ T cells; representative dot plots are shown above and summary data shown below.
To determine the status of antigen-specific T cell activation, we also examined CD4+ T cell responses to HBsAg stimulation ex vivo. Lower CD69 expression was detected on CD4+ T cells in chronically HCV-infected subjects (Fig. 1, right panel), especially in HBV-NR (HBV-NR 6.61% vs HBV-R 15.42%, p=0.002), when compared to those who spontaneously HCV-resolved or healthy subjects (Total HCV-infected subjects 11.23% vs controls 18.66%, p=0.016). To account for potential variance, a summary of these data analyzed employing confidence intervals is shown in Table 2, and further linear regression modeling using Wilcoxon testing for non-parametric data found similar results (data not shown). Because a virus-specific response was being assayed in this CD4+ T cell population, we expected and observed that the percentages of CD4+CD69+ T cells stimulated with HBsAg were much lower than those stimulated more generally with anti-CD3/CD28. We found similar expression patterns for the T cell activation marker, CD25 (IL-2 receptor α chain), on CD4+ T cells in response to anti-CD3/CD28 (HBV-NR 45.27% ± 19.03% n=29 vs HBV-R 65.48% ± 13.65% n=32 p<0.01; total HCV-infected subjects 55.87% ± 19.21% n=61 vs controls 75.49% ± 7.20% n=16, p<0.01) as well as HBsAg stimulation (HBV-NR 16.62% ± 7.73% n=29 vs HBV-R 25.48% ± 11.85% n=32 p=0.001; total HCV-infected subjects 21.27% ± 10.97% n=61 vs control subjects 25.69% ± 3.82% n=16, p=0.011). These results suggest an impaired activation status of helper T cells in chronically HCV-infected subjects, especially in those who failed to respond to HBV vaccination, when compared to HCV-negative subjects.
Table 2. Comparison of CD69 expression amongst different groups after stimulation with anti-CD3/CD28 or HBsAg.
| Comparison groups | N | Percentage of CD69 expression | |||
|---|---|---|---|---|---|
| Anti-CD3/CD28 stimulated | HBsAg stimulated | ||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | ||
| HCV infected | 61 | 47.4 (42.9 – 51.9) | <0.0001 | 11.2 (8.3 – 14.2) | <0.001 |
| Controls | 16 | 76.0 (71.9 – 80.2) | 18.7 (15.5 – 18.7) | ||
| HBV-NR | 29 | 37.8 (30.7 – 44.8) | <0.0001 | 6.6 (2.9 – 10.3) | 0.002 |
| HBV-R | 32 | 56.1 (52.1 – 60.9) | 15.4 (11.2 – 19.6) | ||
| HCV-resolved | 6 | 71.9 (60.6 – 83.3) | 0.207 | 17.7 (10.3 – 25.0) | 0.619 |
| Healthy subjects | 10 | 78.5 (75.4 – 81.5) | 19.3 (15.3 – 23.2) | ||
Abbreviations: CI – confidence interval
PD-1 and PDL-1 expressions are increased in HBV vaccine non-responders with HCV infection
We have previously shown that the PD-1/PDL-1 pathway is involved in T cell dysfunction during HCV infection15. To determine the role of this inhibitory pathway in regulation of CD4+ T cell responses to HBV vaccinations, we further examined PD-1/PDL-1 expressions on CD4+ T cells from chronically HCV-infected and healthy subjects who had received HBV vaccinations, including HBV-NR versus HBV-R. As shown in Figs. 2A and 2B respectively, PD-1 and PDL-1 expressions on CD4+ T cells in response to anti-CD3/CD28 stimulation were detected significantly higher in HCV-infected subjects than controls, in contrast to the expression levels of CD69 and CD25. Importantly, HCV-infected HBV-NR exhibited much higher PD-1 and PDL-1 expressions on their CD4+ T cells than HBV-R, while no significant differences in PD-1/PDL-1 expressions were observed between HCV-resolved individuals and healthy subjects. In the setting of specific antigen stimulation with HBsAg ex vivo, greater expressions of PD-1 (Fig. 2C) and PDL-1 (Fig. 2D) were detected on CD4+ T cells in chronically HCV infected subjects once again, especially in HBV-NR, when compared to controls. As expected, the percentages of PD-1+ or PDL-1+ T cells stimulated in an antigen-specific manner by HBsAg were much lower than following the non-specific TCR stimulation by anti-CD3/CD28.
Fig. 2. PD-1 / PDL-1 expressions on CD4+ T cells in HCV-infected and healthy subjects who received HBV vaccination.
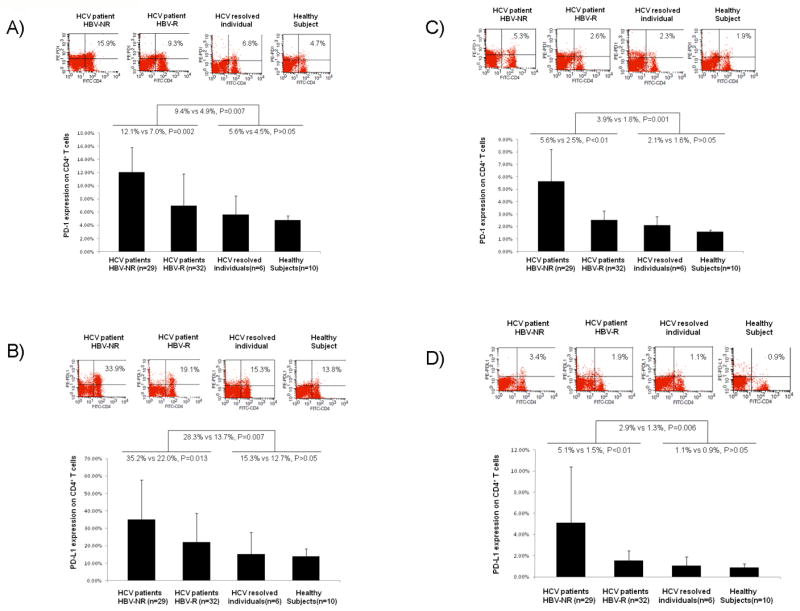
PBMC were isolated from HCV-infected HBV vaccine non-responders (HBV-NR) (n=29), HCV-infected HBV vaccine responders (HBV-R) (n=32), HCV-resolved subjects (n=6), and healthy subjects (n=10), ex vivo stimulated with anti-CD3/CD28 or HBsAg for 24 hrs, and assayed by flow cytometric analysis for PD-1 or PDL-1 expressions on CD4+ T cells; representative dot plots are shown above and summary data shown below. A) PD-1 expression on CD4+ T cells stimulated with anti-CD3/CD28. B) PDL-1 expression on CD4+ T cells stimulated with anti-CD3/CD28. C) PD-1 expression on CD4+ T cells stimulated with HBsAg. D) PDL-1 expression on CD4+ T cells stimulated with HBsAg. Statistical findings are shown between the comparison groups.
Because we have previously shown that the intracellular inhibitory molecule, SOCS-1, is up-regulated in T cells from chronically HCV-infected individuals16, we also compared the level of SOCS-1 expression in CD4+ T cells isolated from HCV-infected HBV-NR and HBV-R, ex vivo stimulated with HBsAg and assayed by RT-PCR and immunoblotting for SOCS-1 gene and protein expressions (Fig. 3). SOCS-1 was found to be increased by both methods in CD4+ T cells from HBV-NR subjects versus HBV-R. The differences were also found using bulk PBMC from HBV-NR and HBV-R when the cells were stimulated by anti-CD3/CD28 antibodies ex vivo (data not shown). Data were reproducible in independent experiments using cells isolated from three different HCV-infected subjects per condition.
Fig. 3. Increase in SOCS-1 expression in CD4+ T cells isolated in HCV-infected HBV vaccine non-responders.
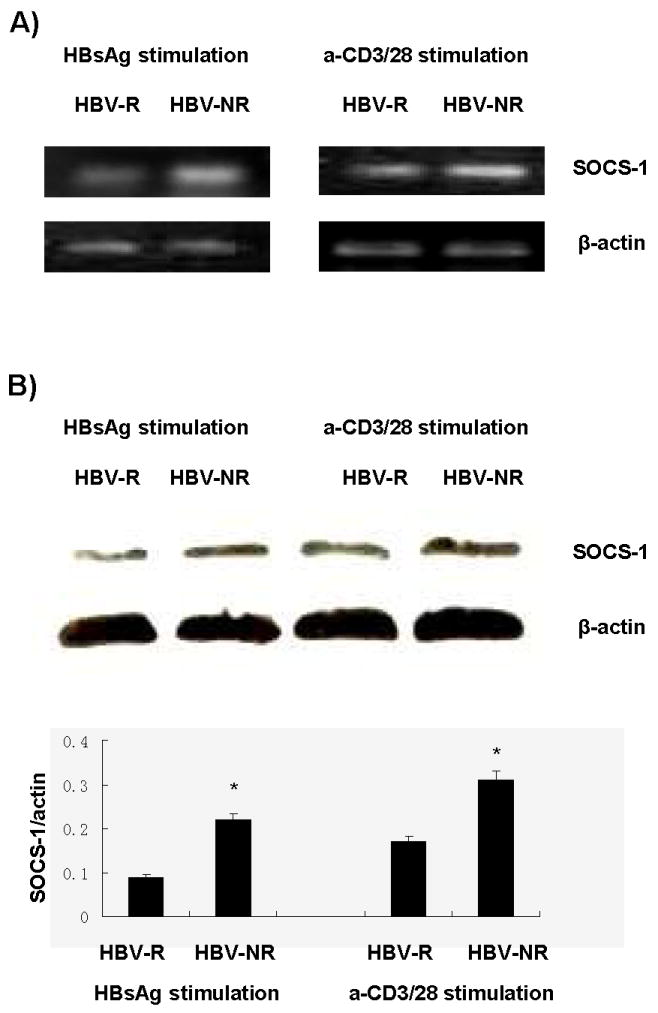
Purified CD4+ T cells were isolated by incubation of PBMCs with magnetic beads-conjugated with anti-CD4 antibody, followed by positive selection per the manufacturer's instruction, from three HBV-R and three HBV-NR. Following stimulation with either HBsAg or anti-CD3/CD28 for 24 h as described above, cells were either lysed for immunoblotting (A) or RNA isolation for RT PCR (B) as we have described13,16,32. SOCS-1 gene expression was detected by quantitative RT-PCR, and β2M gene served as an internal control. Densitometric analysis of immunoblots normalized to actin are shown with error bars below. Data were reproducible in independent experiments using cells isolated from three different HCV-infected subjects per condition.
PD-1 and PDL-1 expressions are inversely associated with the CD4+ T cell responses to HBsAg in HCV-infected individuals
We next analyzed the association between the expressions of PD-1/PDL-1 and CD69 on CD4+ T cells from chronically HCV-infected as well as healthy subjects who had received HBV vaccination. To this end, PBMC isolated from the subjects who had received HBV immunizations were ex vivo stimulated with HBsAg or anti-CD3/CD28 antibodies, followed by flow cytometric analysis as described above. A Pearson correlation program in SPSS 18 software was used to statistically analyze the association between the markers of PD-1 and T cell activation. As shown in Figs. 4A, PD-1 expression was found to be inversely associated with CD69 expression on CD4+ T cells stimulated with HBsAg (left panel) or CD3/CD28 (right panel), respectively. Additionally, PDL-1 expression was closely and inversely associated with CD69 expression on CD4+ T cells stimulated with HBsAg (Fig. 4B, left panel) but a statistically significant correlation was not found to occur between CD69 and PDL-1 following non-specific stimulation (Fig. 4B, right panel).
Fig. 4. PD-1 and PDL-1 expressions are inversely associated with CD4+ T cell responses to HBV vaccine in HCV-infected individuals.
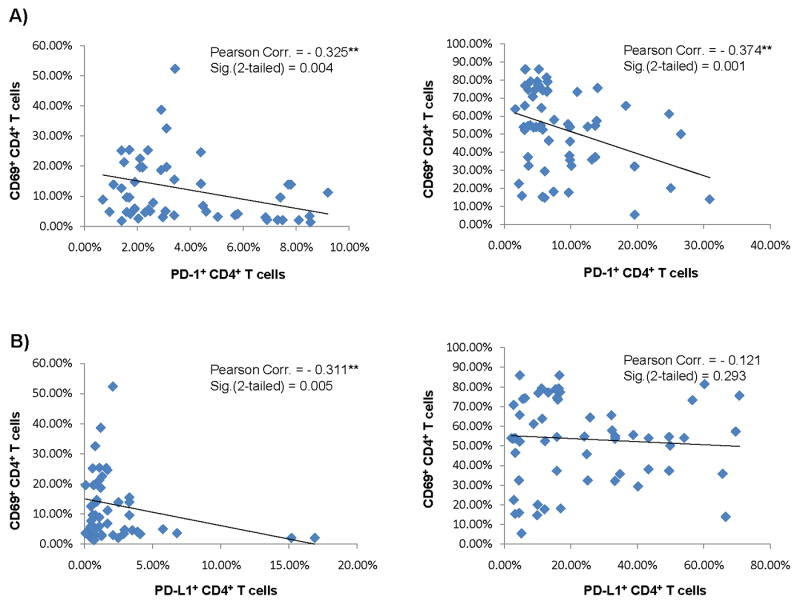
Each symbol represents an individual expressing cell surface marker for PD-1 inhibitory pathway as well as T cell activation in subjects who had received HBV vaccines and ex vivo stimulated with HBsAg (left panels) or anti-CD3/CD28 (right panels). The inverse association between PD-1 and CD69 (A) and PDL-1 and CD69 (B) expressions on CD4+ T cells was evaluated using Pearson Correlation analysis. Pearson correlation and 2-tailed significance are shown in the upper right corner of each analysis.
Blocking the PD-1 pathway can improve CD4+ T cell responses in HBV vaccine non-responders infected with HCV
We have previously shown that blocking the PD-1 pathway can abrogate T cell inhibition and apoptosis in healthy cells exposed to HCV core protein, a viral antigen with immunomodulatory properties15. To determine whether blocking PD-1 signaling can improve the impaired T cell function in chronically HCV-infected HBV-NR, we examined T cell activation and proliferation by flow cytometric analysis of CD69 expression and CFSE dilution, respectively. We incubated PBMC isolated from HCV-infected subjects who had failed to respond to HBV vaccination with anti-PDL-1 or control antibody overnight, followed by stimulation of the cells with either HBsAg or anti-CD3/CD28 antibodies for 5 days. As shown in Fig.5A, CD69 expressions on CD4+ T cells were found to be significantly improved by pre-incubating the cells with anti-PDL-1 antibody followed by stimulating with HBsAg or anti-CD3/CD28 antibodies. Similarly, T cell proliferation, as represented by CFSE dilutional analysis (left shifting) on gated T cell populations (Fig. 5B), was observed more significantly upon PD-1 pathway blockade versus control treatment. The improvement in proliferative ability varied under the two-stimulating conditions: TCR activation by anti-CD3/CD28 stimulation elicited a more general and significant T cell proliferation as compared to HBsAg specific stimulation, in agreement with our observations that CD69/CD25 and PD-1/PDL-1 expressions on T cells differ following these different stimulations. These data are reproducible in repeated experiments using cells isolated from three different individuals with HBV-NR in the setting of chronic HCV infection.
Fig. 5. Blocking the PD-1 pathway improves CD4+ T cell responses in HBV vaccine non-responders with HCV infection.
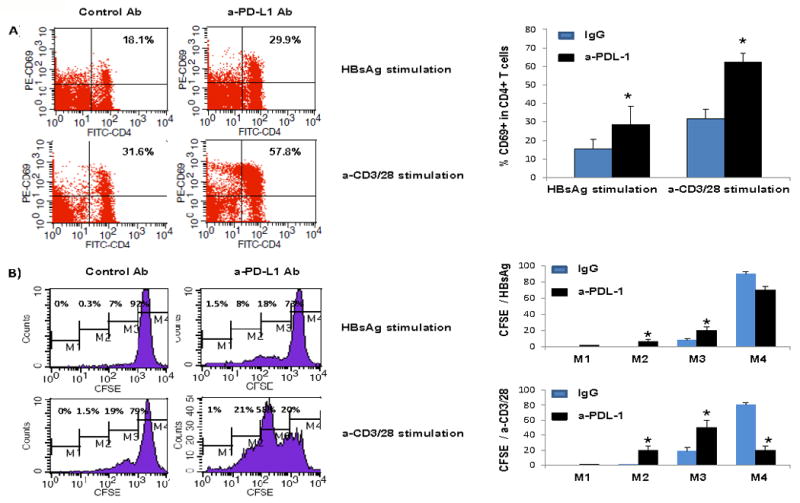
PBMC isolated from HCV-infected subjects who had received HBV vaccinations and failed to seroconvert were incubated with anti-PDL-1 or control antibody overnight, followed by stimulation with either HBsAg or anti-CD3/CD28 antibodies for 5 days. T cell activation and proliferation were evaluated by detection of CD69 expression (A) and CFSE assay (B) in stimulated T cells. Representative percentages of CD69 expression and gating of CFSE in T cells treated with anti-PDL-1 versus control antibody are shown on the left and summary data on the right. Data are reproducible in repeated experiments using cells isolated from three different individuals with HBV-NR in the setting of chronic HCV infection.
Discussion
Vaccine responses in the setting of chronic HCV infection appear to be blunted, with poor response rates to a standard course of HBV vaccination in chronically HCV-infected individuals (and especially in those with advanced fibrosis and liver cirrhosis) when compared to healthy populations (40∼60% versus 90∼95%)6-8. This study confirms this discrepancy and raises the possibility that responses to vaccination may be impaired at least in part by up-regulated expression of the negative immunomodulator, PD-1. Our data consistently demonstrate that HBV vaccine non-responders have up-regulation of PD-1 that correlates with diminished T cell activation in response to both general and virus-specific stimulation, an impairment that can be improved by PDL-1 blockade.
The mechanisms underlying HBV vaccine non-response in 5-10% of healthy subjects and 40-60% of HCV-infected subjects remain poorly understood, although several factors are known to affect this response. These include age, gender, tobacco use, obesity, and certain human leukocyte antigen (HLA) alleles17-20. Given the fact that non-responders also exhibit poor recall responses to tetanus toxoid or Candida, it has been suggested that HBV vaccine non-responsiveness may be due to a defect in HBsAg-reactive T cells21-24, regulatory T cells25, or in APCs26, 27; this has remained controversial22-23. A number of studies have tried to correct non-response by addition of vaccine adjuvants, altered doses, and different vaccine administration routes or strategies28-31. These approaches have led to varying degrees of success in healthy subjects, but have been limited in chronically infected individuals. Notably, similar phenomena are observed in chronic HIV infection as well as in other immunosuppressed settings, such as organ transplantation, cancer chemotherapy, and hemodialysis, following routine adult immunizations that include influenza and pneumococcal vaccination; this suggests a possible shared mechanism of non-response in immunocompromised hosts9,10.
The role of PD-1 in viral clearance during HCV (and HIV) infection has been studied extensively by us and others11, 15, 32-34, but a role in vaccine responses during chronic viral infection is novel in concept. Up-regulation of PD-1 appears to be a marker for T cell anergy, with selective up-regulation observed on “exhausted” T cells; inhibition of its signaling via anti-PD-1 or anti-PDL-1 antibodies has led to improved T cell function and viral clearance in several models34-38. PD-1 is an immunoinhibitory receptor predominantly expressed on activated T cells and function through interaction with its natural ligands including PDL-1 (also known as B7-H1, expressed on both haematopoetic and parenchymal cells) and PDL-2 (B7-DC, primarily expressed on monocytes). Activation of the PD-1 pathway induces immunoreceptor tyrosine phosphorylation and recruitment of tyrosine phosphatases, including src-homology proteins (SHP-1/SHP-2), to deliver a negative signal to TCR activation pathways. Evidence is emerging for the involvement of PD-1 inhibitory pathway in normal immune tolerance, autoimmune responses, antitumor and antiviral immune evasion. Our study adds to compendium by suggesting a role in vaccine responses.
Our findings have several limitations worth noting. For example, CD4+ T cell responses were assayed in the presence of other cells that may be contributing to the dysregulation we observed, and thus these other suppressive cells may be playing a role. Future studies using purified CD4+ T cell populations would clarify this issue. We also do not know the role that costimulatory molecules may be playing in this process, although there are data suggesting that such molecules impact the T cell exhaustion associated with PD-1 up-regulation. In addition, we focused on PD-1 expression in the stimulated state only, in part because we and others have already observed up-regulation of PD-1 in HCV-infected individuals at baseline15. We however do not know if responders differ in baseline PD-1 expression when compared to non-responders prior to stimulation, an area worth exploring. The role of T regulatory cells is also not characterized herein, although their contribution to altering responses is quite possible13. Finally, correlation analyses while yielding associations could have more robust co-efficients with a larger sample size.
The finding of vaccine non-response is perhaps not an absolute phenomenon, but one of a range of low responses to vaccine antigen. Pre-existing cellular responses to HBsAg in PBMCs from HBV non-responders have been reported despite a lack of a humoral response25. A more potent vaccination might circumvent this problem, but our data suggest that augmenting CD4+ T cell function/priming may be feasible through blockade of native, negative cellular immunoreceptors such as PD-1. Clinical studies exploring a role for PD-1 blockade in improving viral clearance will be emerging, and it would seem logical to assess vaccine responsiveness in this setting as well. The potential negative consequences of blocking such a key negative modulator as PD-1 are many, including uncontrolled immune responses, autoimmune phenomenon, and malignancy. Nevertheless, multiple investigators are developing agents that inhibit this pathway and their clinical role will likely be explored in the near future. Additional cellular targets almost assuredly exist, including CD150, expression of which has been shown to be linked to decreased CD4+ T cell proliferative responses in healthy vaccine non-responders39; SOCS-116, that regulates Jak/STAT signaling; and the recently described negative receptor, Tim-340. Further studies focusing on these potent cell regulators in the setting of vaccine responses are ongoing, studies which might provide a novel approach in to immunization.
Acknowledgments
This work was supported by an NIH NIAID grant to ZQY/JPM (1R15A1084057-01). C.L. Zhang, a visiting scholar, holds a grant for viral hepatitis research from Guangzhou Municipal Health Bureau, China. L. Ni, a joint PhD student, was supported in part by the China Scholarship Council (CSC 2008655005). This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- HCV
hepatitis C virus
- PD-1
programmed death-1
- PDL-1
programmed death ligand-1
- HIV
human immunodeficiency virus
- PBMC
peripheral blood mononuclear cells
- FACS
fluorescence-activated cell sorting
- SD
standard deviation
- STAT-1
signal transducer and activator of transcription
- JAK
janus-associated kinase-1
- SOCS-1
suppressor of cytokine signaling-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheruvu S, Marks K, Talal AH. Understanding the pathogenesis and management of hepatitis B/HIV and hepatitis B/hepatitis C virus coinfection. Clinics in liver disease. 2007 Nov;11(4):917–43. ix–x. doi: 10.1016/j.cld.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Duberg AS, Torner A, Davidsdottir L, Aleman S, Blaxhult A, Svensson A, et al. Cause of death in individuals with chronic HBV and/or HCV infection, a nationwide community-based register study. Journal of viral hepatitis. 2008 Jul;15(7):538–50. doi: 10.1111/j.1365-2893.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 3.Filippini P, Coppola N, Pisapia R, Martini S, Marrocco C, Di Martino F, et al. Virological and clinical aspects of HBV-HCV coinfection in HIV positive patients. Journal of medical virology. 2007 Nov;79(11):1679–85. doi: 10.1002/jmv.20992. [DOI] [PubMed] [Google Scholar]
- 4.Torbenson M, Kannangai R, Astemborski J, Strathdee SA, Vlahov D, Thomas DL. High prevalence of occult hepatitis B in Baltimore injection drug users. Hepatology (Baltimore, Md. 2004 Jan;39(1):51–7. doi: 10.1002/hep.20025. [DOI] [PubMed] [Google Scholar]
- 5.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. Journal of hepatology. 1998 Jan;28(1):27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 6.Keeffe EB. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. The American journal of medicine. 2005 Oct;118 10A:21S–7S. doi: 10.1016/j.amjmed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Kramer ES, Hofmann C, Smith PG, Shiffman ML, Sterling RK. Response to hepatitis A and B vaccine alone or in combination in patients with chronic hepatitis C virus and advanced fibrosis. Digestive diseases and sciences. 2009 Sep;54(9):2016–25. doi: 10.1007/s10620-009-0867-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsai IJ, Chang MH, Chen HL, Ni YH, Lee PI, Chiu TY, et al. Immunogenicity and reactogenicity of the combined hepatitis A and B vaccine in young adults. Vaccine. 2000 Oct 15;19(4-5):437–41. doi: 10.1016/s0264-410x(00)00205-x. [DOI] [PubMed] [Google Scholar]
- 9.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. The Journal of infectious diseases. 2005 May 1;191(9):1442–50. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Barradas MC, Alexandraki I, Nazir T, Foltzer M, Musher DM, Brown S, et al. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis. 2003 Aug 1;37(3):438–47. doi: 10.1086/375841. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends in immunology. 2001 May;22(5):265–8. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. International immunology. 2007 Jul;19(7):813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 13.Moorman J, Dong ZP, Ni L, Zhang C, Borthwick T, Yao ZQ. Abnormal B-cell activation associated with TALL-1 over-expression and SOCS-1 suppression during chronic hepatitis C virus infection. Immunology. 2009 Oct;128(2):227–35. doi: 10.1111/j.1365-2567.2009.03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao ZQ, Prayther D, Trabue C, Dong ZP, Moorman J. Differential regulation of SOCS-1 signalling in B and T lymphocytes by hepatitis C virus core protein. Immunology. 2008 Oct;125(2):197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral immunology. 2007 Summer;20(2):276–87. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 16.Moorman JP, Ni L, Ma CJ, Zhang Y, Zhang CL, Dong ZP, Frazier A, Wu XY, Thayer P, Chen XY, Yao ZQ. Differential regulation of T and B lymphocytes by PD-1 and SOCS-1 signaling in hepatitis C virus-associated non-Hodgkin's lymphoma. Immunological Investigations. doi: 10.3109/08820139.2010.534218. In press. [DOI] [PubMed] [Google Scholar]
- 17.De Silvestri A, Pasi A, Martinetti M, Belloni C, Tinelli C, Rondini G, et al. Family study of non-responsiveness to hepatitis B vaccine confirms the importance of HLA class III C4A locus. Genes Immun. 2001 Nov;2(7):367–72. doi: 10.1038/sj.gene.6363792. [DOI] [PubMed] [Google Scholar]
- 18.Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology (Baltimore, Md. 2005 Jun;41(6):1383–90. doi: 10.1002/hep.20716. [DOI] [PubMed] [Google Scholar]
- 19.Kruskall MS, Alper CA, Awdeh Z, Yunis EJ, Marcus-Bagley D. The immune response to hepatitis B vaccine in humans: inheritance patterns in families. The Journal of experimental medicine. 1992 Feb 1;175(2):495–502. doi: 10.1084/jem.175.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lango-Warensjo A, Cardell K, Lindblom B. Haplotypes comprising subtypes of the DQB1*06 allele direct the antibody response after immunisation with hepatitis B surface antigen. Tissue antigens. 1998 Oct;52(4):374–80. doi: 10.1111/j.1399-0039.1998.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 21.Albarran B, Goncalves L, Salmen S, Borges L, Fields H, Soyano A, et al. Profiles of NK, NKT cell activation and cytokine production following vaccination against hepatitis B. Apmis. 2005 Jul-Aug;113(7-8):526–35. doi: 10.1111/j.1600-0463.2005.apm_191.x. [DOI] [PubMed] [Google Scholar]
- 22.Salazar M, Deulofeut H, Granja C, Deulofeut R, Yunis DE, Marcus-Bagley D, et al. Normal HBsAg presentation and T-cell defect in the immune response of nonresponders. Immunogenetics. 1995;41(6):366–74. doi: 10.1007/BF00163994. [DOI] [PubMed] [Google Scholar]
- 23.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006 Jan 30;24(5):572–7. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology. 2004 Aug 15;326(1):20–8. doi: 10.1016/j.virol.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Bauer T, Gunther M, Bienzle U, Neuhaus R, Jilg W. Vaccination against hepatitis B in liver transplant recipients: pilot analysis of cellular immune response shows evidence of HBsAg-specific regulatory T cells. Liver Transpl. 2007 Mar;13(3):434–42. doi: 10.1002/lt.21061. [DOI] [PubMed] [Google Scholar]
- 26.Hohler T, Stradmann-Bellinghausen B, Starke R, Sanger R, Victor A, Rittner C, et al. C4A deficiency and nonresponse to hepatitis B vaccination. Journal of hepatology. 2002 Sep;37(3):387–92. doi: 10.1016/s0168-8278(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 27.Verkade MA, van Druningen CJ, Op de Hoek CT, Weimar W, Betjes MG. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clinical and experimental medicine. 2007 Jun;7(2):65–71. doi: 10.1007/s10238-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacques P, Moens G, Desombere I, Dewijngaert J, Leroux-Roels G, Wettendorff M, et al. The immunogenicity and reactogenicity profile of a candidate hepatitis B vaccine in an adult vaccine non-responder population. Vaccine. 2002 Nov 1;20(31-32):3644–9. doi: 10.1016/s0264-410x(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 29.Rahman F, Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology (Baltimore, Md. 2000 Feb;31(2):521–7. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 30.Kim MJ, Nafziger AN, Harro CD, Keyserling HL, Ramsey KM, Drusano GL, et al. Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine. 2003 Mar 7;21(11-12):1174–9. doi: 10.1016/s0264-410x(02)00626-6. [DOI] [PubMed] [Google Scholar]
- 31.Nystrom J, Cardell K, Bjornsdottir TB, Fryden A, Hultgren C, Sallberg M. Improved cell mediated immune responses after successful re-vaccination of non-responders to the hepatitis B virus surface antigen (HBsAg) vaccine using the combined hepatitis A and B vaccine. Vaccine. 2008 Nov 5;26(47):5967–72. doi: 10.1016/j.vaccine.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Frazier AD, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu XY, et al. Programmed death-1 affects suppressor of cytokine signaling-1 expression in T cells during hepatitis C infection. Viral Immunology. Oct;23(5):487–95. doi: 10.1089/vim.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni L, Ma CJ, Zhang Y, Nandakumar S, Zhang CL, Wu XY, et al. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunology and cell biology. Oct 26; doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006 Feb 9;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 35.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008 Mar 15;180(6):3637–41. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 36.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008 Apr 1;180(7):4875–84. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008 Jun;134(7):1927–37. 37 e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XF, Chen YJ, Wang Q, Ge Y, Dai Q, Yang KF, et al. Distinct expression and inhibitory function of B and T lymphocyte attenuator on human T cells. Tissue antigens. 2007 Feb;69(2):145–53. doi: 10.1111/j.1399-0039.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 39.Pati NT, Sukriti, Hissar S, Agrawal K, Rani R, Sarin SK. Decrease in CD4+ T lymphocyte proliferation responses and enhanced CD150 cell expression in health care workers non-responsive to HBV vaccine. Vaccine. 2007 Feb 26;25(10):1848–55. doi: 10.1016/j.vaccine.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009 Sep;83(18):9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


