Abstract
Background
Individual effects of hyperglycemia and obesity to impair vascular health are recognized. However, the relative contributions of dysglycemia versus other obesity-related traits to vascular dysfunction have not been systematically evaluated.
Methods
We undertook a cross-sectional evaluation of factors contributing to vascular function in 271 consecutive subjects, categorized as non-obese normal glucose tolerant (n=115), non-obese dysglycemic (n=32), obese normal glucose tolerant (n=57), obese dysglycemic (n=38), or type 2 diabetic (n=29). Vascular function was measured invasively as leg blood flow responses to methacholine chloride, an endothelium-dependent vasodilator. Categorical and continuous analyses were used to assess the contributions of hyperglycemia to vascular dysfunction.
Results
Even among normoglycemic subjects, obese subjects had impaired vascular function compared to non-obese subjects (p=0.004). Vascular function was also impaired in non-obese dysglycemic subjects (p=0.04 versus non-obese normoglycemic subjects), to a level comparable to normoglycemic obese subjects. Within obese subject groups, gradations of dysglycemia including the presence of diabetes were not associated with further worsening of these vascular responses beyond the effect of obesity alone (p=NS comparing all obese groups, p<0.001 versus lean normoglycemic subjects). In univariate and multivariable modeling analyses we found that effects of glycemia were less powerful than effects of insulin resistance and obesity on vascular dysfunction.
Conclusions
Dysglycemia contributes to impaired vascular function in non-obese subjects, but obesity and insulin resistance are more important determinants of vascular function in obese and diabetic subjects.
Keywords: Dysglycemia, Endothelium, Obesity, Diabetes
Introduction
In vivo vascular function measured in peripheral vessels is currently used as a surrogate of cardiovascular disease status. Detrimental effects of short-term hyperglycemia on peripheral vascular function have been demonstrated in some but not all studies of healthy normoglycemic humans [1–4]. Chronic hyperglycemia in the setting of diabetes mellitus is reliably associated with impaired vascular function [5], but effects of chronic exposure to pre-diabetic levels of hyperglycemia have not been systematically evaluated. Further, there is very little data comparing the relative contributions of glucose versus other concurrent determinants of vascular dysfunction in diabetes and pre-diabetes. In particular, insulin resistance and obesity are recognized as important contributors to vascular dysfunction [6], but the importance of an added effect of dysglycemia has not been systematically evaluated. We hypothesized that glycemic status contributes to impaired endothelium-dependent vasodilation (EDV) beyond the effects of obesity.
Methods
Participants
All consecutive subjects who participated in ongoing studies in our laboratory from July 1997 to May 2009 were included in the current analyses. For subjects who participated in more than one such study, only the first study for that participant was included in the dataset. These studies varied in the particular physiologic or pharmacologic manipulations of vascular physiology being evaluated, but in all cases a shared set of baseline (untreated) data were collected representing the natural state of the participants. These baseline data were aggregated for the present analyses. Subjects were classified for the current analyses according to their body mass index (non-obese: BMI <29.9 kg/m2, obese: >30 kg/m2). By virtue of the design of the original studies, all diabetic subjects were also obese. Participants abstained from cigarette smoking for 3 days in advance of the studies. Antihypertensive medications, lipid-lowering medications and ASA were withheld for 3 days. In order to evaluate the diabetic state without confounding effects of antidiabetic medications, subjects with diabetes were studied after 3 days’ withdrawal from current treatment; subjects taking PPARgamma agonists were excluded from participation, and no insulin-treated subjects were studied. All studies were performed according to the guidelines of the Helsinki declaration, approved by the local Institutional Review Board, and all subjects gave written informed consent.
Metabolic phenotyping
Subjects were classified as dysglycemic if fasting glucose was >100 mg/dL or 2 hour glucose following a 75g oral glucose load was between 140 and 200 mg/dL. Subjects with diabetes mellitus were recruited by prior diagnosis, confirmed with fasting glucose >126 mg/dL or 2 hour 75g OGTT >200 mg/dL. Subjects could also be newly diagnosed as diabetic using these criteria on screening glucose tolerance testing. Glucose concentrations were measured at the bedside using a Yellow Springs Instrument YSI StatPlus 3000 (Yellow Springs, OH). Blood for determination of plasma insulin was collected in heparinized tubes, processed immediately, and frozen at −80°C. Insulin concentrations were measured in batches by study at a later date, using a dual-site radioimmune assay specific for human insulin and with cross-reactivity with proinsulin <0.2% (R&D Systems Inc, Minneapolis MN). The lower detection limit is 0.56 pmol/L, and in our laboratory the inter- and intra-assay coefficients of variation (CV) are 4.1% and 2.6% respectively. Fasting lipid profiles were measured using standard methodologies for cholesterol and triglyceride determinations performed through our local hospital’s clinical laboratory.
Vascular function measurements
All studies were performed in a quiet temperature-controlled room following an overnight fast. The methodology used has been previously published [5, 6]. Briefly, a 6F sheath (Cordis Corp, Miami, FL) was placed into the right femoral vein to allow the insertion of a custom-designed thermodilution catheter (Baxter Scientific, Edwards Division, Irvine, CA) to measure leg blood flow (LBF). The right femoral artery was cannulated with a 5.5F double-lumen catheter to allow simultaneous infusion of vasoactive agents and intra-arterial blood pressure monitoring. Basal LBF and mean arterial pressure measurements were obtained following ≥30 minutes of rest after the insertion of the catheters. Basal unstimulated readings were obtained by averaging 24 sets of measurements obtained at ~30-second intervals. Methacholine chloride was then infused at 3 successive infusion rates (10 replicates each) to measure endothelium-dependent vasodilation; the mean of the 10 readings of the vasodilator response to the highest rate (15 mcg/min) is used in the current analyses, expressed as percent increase in leg blood flow from each individual’s baseline readings, and subsequently root transformed as described below.
Statistics
Variables were compared across groups using one-way analysis of variance, with post-hoc Tukey-Kramer pairwise comparisons. A goodness-of-fit test (Kolmogorov-Smirnov and Anderson Darling) along with quantile-quantile (Q-Q) plot was used to assess whether continuous variables were normally distributed. For variables that were not normally distributed, transformations were applied to achieve normalization in order to be able to apply parametric testing. In most cases logarithmic transformations achieved this result, except for the measurements of vascular function which required square root transformation. The analyses and results presented below use these transformed variables directly, without back-transformation. The extremely skewed distribution of fasting blood glucose was not satisfactorily normalized by any transformation, but area under the curve for glucose across the first 2 hours of a 75 gram oral glucose tolerance test was normalized after logarithmic transformation.
The pre-specified dependent variable was percent increase in leg blood flow at maximal infusion rate of methacholine chloride (endothelium-dependent vasodilation). The principal analysis was a comparison of this measure of vascular function across groups using one-way ANOVA with pairwise post-hoc comparisons to distinguish differences attributable to obesity versus glycemia. Partial correlation analysis was undertaken to evaluate adjusted univariate relationships of the principal variables of interest (measures of insulin resistance, obesity, glycemia) with vascular function. Multivariable linear modeling was then applied to assess simultaneous relationships between these variables, first without and then with adjustment for other phenotypic factors that differed across groups and were related to the dependent variable. Statistical significance was pre-specified at p<0.05.
Results
In the time frame evaluated, complete vascular function data were available for first studies in 271 subjects. The original studies did not recruit subgroups with particular characteristics that might have resulted in differences between groups, although these studies systematically excluded subjects of any category with extremes of blood pressure (>160.95 mmHg) or cholesterol concentrations (>6.3 mmol/L). Subject characteristics are detailed in Table 1. We divided these into 115 non-obese normoglycemic subjects, 32 non-obese dysglycemic subjects, 57 obese normoglycemic subjects, 38 obese dysglycemic subjects, and 29 obese type 2 diabetic subjects. No subjects with Type 1 diabetes were studied in the time period. There were no statistically significant differences in race/ethnicity distribution across the groups, but sex distribution and prevalence of smokers were different across groups (Table 1). These groups also differed in age, insulin concentrations, insulin sensitivity by homeostasis model index of insulin resistance, blood pressure, and blood lipid concentrations. The basal leg blood flow also differed across groups, as has been noted previously in our data. Because of these findings, multivariate analyses were adjusted for age, sex and smoking status. Because of our prior finding of an interaction effect of race/ethnicity on the relationship between obesity and vascular function [6], this variable was also included in our adjusted multivariable analyses.
Table 1.
Subject population
| Parameter | Non-obese NGT |
Non-obese DYS |
Obese NGT | Obese DYS | DM | P value comparing groups |
|---|---|---|---|---|---|---|
| Number | 115 | 32 | 57 | 38 | 29 | |
| Sex (M/F) | 69/46 | 25/7 | 45/12 | 15/23 | 17/12 | 0.0008 |
| Race (Caucasian/AA/Asian) | 56/54/3 | 18/14/0 | 23/24/0 | 21/17/0 | 13/16/0 | 0.59 |
| Smoker (Y/N) | 63/52 | 13/19 | 27/30 | 9/29 | 12/17 | 0.018 |
| Age (yrs) | 36.0±0.8e | 38.2±1.5c | 32.4±1.1b,e | 35.9±1.4e | 43.0±1.6a,c,d | <0.0001 |
| Weight (kg) | 71.4±1.3c,de | 76.4±2.4c,d,e | 102.3±1.8a,b | 106.7±2.2a,b | 105.5±2.5a,b | <0.0001* |
| BMI (kg/m2) | 23.2±0.4b,c,d,e | 25.3±0.7a,c,d,e | 35.2±0.5a,b | 36.8±0.6a,b | 36.7±0.7a,b | <0.0001* |
| Waist (cm) | 98.2±1.7c | 91.4±3.3 | 85.3±2.4a,d,e | 102.0±3.0c | 96.7±3.3c | <0.0001* |
| Fasting Glucose (mmol/L) | 4.7±0.2e | 5.1±0.3e | 4.8±0.2e | 5.5±0.3e | 10.1±0.3a,b,c,d | <0.0001* |
| OGTT 30 min Glucose | 7.9±0.2e | 8.8±0.4c,e | 7.1±0.3b,d,e | 8.6±0.3c,e | 13.2±0.4a,b,c,d | <0.0001* |
| OGTT 120 min Glucose | 5.6±0.2b,d,e | 7.5±0.4a,c,e | 5.6±0.3b,d,e | 8.3±0.3a,c,e | 16.8±0.4a,b,c,d | <0.0001* |
| OGTT Glucose AUC (mmol*L−1* min) | 1310±29b,d,e | 1607±55a,c,e | 1239±43b,d,e | 1664±50a,c,e | 2803±62a,b,c,d | <0.0001* |
| Fasting Insulin (pmol/L) | 52.8±5.6c,d,e | 71.9±10.4 | 81.9±7.4a | 106.6±9.6a | 111.2±11.5a | <0.0001* |
| OGTT 30 min Insulin (pmol/L) | 310±38c,d | 387±72d | 492±50a,e | 697±62a,b,e | 213±85c,d | <0.0001* |
| OGTT Ins AUC (pmol*L−1*min) | 36963±6678d | 50810±12933d | 56638±9051d | 128780±11029a,b,c,e | 41083±14535d | <0.0001* |
| HOMA-IR | 2.3±0.5d,e | 3.1±1.0e | 3.1±0.7d,e | 6.7±0.9a,c | 8.0±1.1a,b,c | <0.0001* |
| Insulinogenic Index (pmol/mmol) | 187±71 | 135±133 | 462±97 | 263±118 | 24±157 | 0.085* |
| Disposition Index | 64.6±41.1d | 88.0±77.5 | 232.1±56.5 | 343.1±68.5a,e | 24.8±91.3d | 0.0027 |
| SBP (mmHg) | 126.5±1.5c,d,e | 129.4±2.8d,e | 133.6±2.1a,e | 140.3±2.5a,b | 144.0±2.9a,b,c | <0.0001 |
| DBP (mmHg) | 73.3±0.9d,e | 74.6±1.6 | 77.1±1.2 | 79.9±1.5a | 80.5±1.7a | <0.0001 |
| Total Cholesterol (mmol/L) | 3.4±0.06e | 3.6±0.12 | 3.6±0.09 | 3.8±0.11 | 3.81±0.12a | 0.0156 |
| Triglycerides (mmol/L) | 1.18±0.08e | 1.15±0.15e | 1.18±0.11e | 1.42±0.14 | 1.89±0.16a,b,c | 0.0008 |
| LDL Cholesterol (mmol/L) | 2.0±0.05d,e | 2.21±0.10 | 2.25±0.07 | 2.36±0.09a | 2.37±0.11a | 0.0014 |
| HDL Cholesterol (mmol/L) | 0.96±0.03d,e | 0.97±0.05e | 0.93±0.03 | 0.82±0.04a | 0.78±0.05a,b | 0.0025 |
| Basal Leg Blood Flow (L/min) | 0.23±0.01c,d,e | 0.25±0.02 | 0.31±0.02a | 0.31±0.02a | 0.30±0.02a | <0.0001 |
Superscript letters indicate pairwise differences, specifying which group is significantly different from the current column (a= Non-obese NGT; b=Non-obese DYS; c=Obese NGT; d=Obese DYS; e=Obese DM).
indicates normalized values were used, see text. Data reported as mean ± SEM
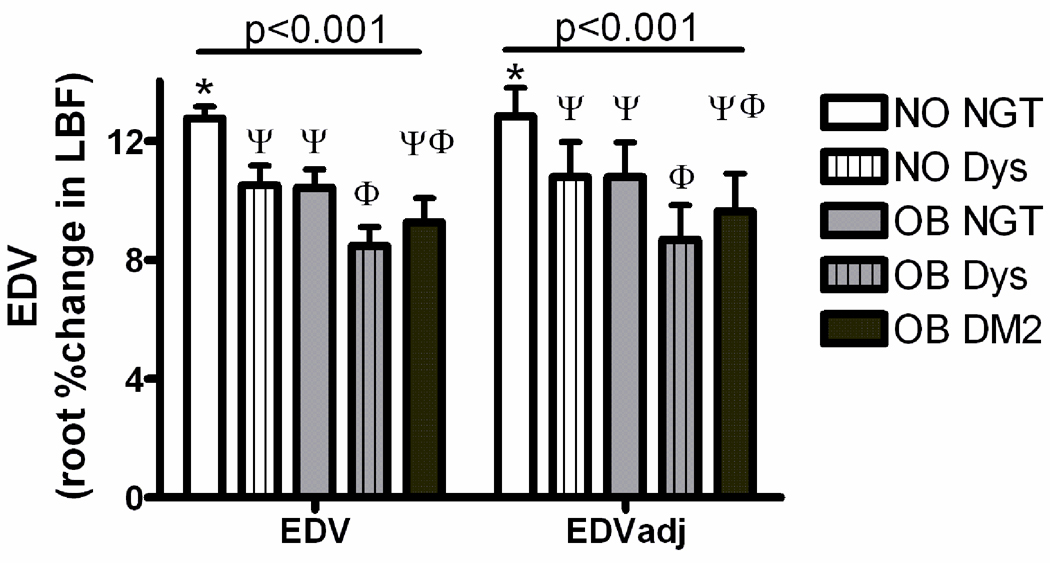
The groups differed in endothelium-dependent vasodilation (EDV) responses in both unadjusted and age/sex/race/smoking status- adjusted analyses (p<0.001 by ANOVA; Figure 1). Pairwise comparisons revealed that non-obese dysglycemic subjects had impaired responses compared to non-obese normoglycemic subjects (p=0.04). All obese subject groups exhibited lower responses than non-obese normoglycemic subjects (p<0.001). Within the obese subjects, the mean EDV response was nominally reduced across groups with increasing degrees of dysglycemia. Pairwise comparisons revealed a greater impairment in obese dysglycemic subjects than normoglycemic subjects, but the more profoundly dysglycemic state of Type 2 diabetes did not confer a further worsening of endothelium-dependent vasodilation than was seen with obesity plus non-diabetic levels of dysglycemia.
Figure 1.
Endothelium-dependent vasodilation (EDV) and insulin resistance by weight and glycemia categories. EDV is plotted on the Y axis as the square root transformed percent change of leg blood flow with application of methacholine chloride. Clusters represent unadjusted group means (left), and estimated means following adjustment for age, sex and race (right). For both analyses the group means were statistically different by ANOVA; groups sharing the same symbol within each cluster are not different from each other on posthoc pairwise analyses. DM2 = type 2 diabetes; Dys = dysglycemic; NGT = normal glucose tolerant; NO = non-obese; OB = obese.
In univariate analyses adjusted for the confounding effects of age, sex, race/ethnicity, and smoking status, significant relationships were evident between endothelium-dependent vasodilation and measures of obesity, measures of insulin sensitivity, and measures of glycemic response to an oral glucose load (Table 2). (Due to incomplete data we were unable to calculate glucose AUC in 13/271 (4.8%) subjects (1 non-obese dysglycemic, 6 obese normoglycemic, 1 obese dysglycemic, and 5 obese type 2 diabetic subjects). The EDV responses in this subgroup did not differ statistically from those of the entire group (p=0.09)). The relationship of EDV with fasting glucose (i.e. ambient glucose at the time of vascular function testing) did not achieve significance (p=0.07; Table 2) but warranted inclusion in subsequent multivariable modeling. Other phenotypic variables were also individually related to EDV including blood pressure (Table 2). No parameters of beta cell function were significantly related to EDV. The Disposition Index (a quantitative measure that describes the relationship between β-cell sensitivity and insulin sensitivity) achieved a statistical significant p value (<0.011), but this reflected a highly significant effect of insulin resistance alone. Notably, in this population blood lipids were not significantly related to vascular function after adjustment as above.
Table 2.
Individual Determinants of Endothelium-Dependent Vasodilation (partial correlations adjusted for age, sex, race/ethnicity, and smoking status).
| Parameter | R value | P value |
|---|---|---|
| BMI* | −0.31 | <0.0001 |
| Waist* | −0.21 | 0.0007 |
| Systolic Blood Pressure | −0.19 | 0.002 |
| Diastolic Blood Pressure | −0.28 | <0.0001 |
| Total Cholesterol | −0.007 | 0.92 |
| Triglycerides | 0.06 | 0.39 |
| LDL Cholesterol | −0.07 | 0.28 |
| HDL Cholesterol | 0.03 | 0.60 |
| Fasting Glucose | 0.11 | 0.07 |
| Glucose AUC* | −0.20 | 0.001 |
| Insulinogenic Index* | −0.06 | 0.36 |
| Disposition Index* | −0.18 | 0.011 |
| HOMA-IR* | −0.26 | <0.0001 |
| Insulin AUC* | −0.30 | <0.0001 |
indicates variables were transformed to normalize their distributions, see text. AUC, area under the curve for a 2 hour oral glucose tolerance test; BMI, body mass index; HDL, high density lipoprotein; HOMA-IR, homeostasis model of insulin resistance; LDL, low density lipoprotein.
In multivariable modeling we assessed the concurrent contributions of insulin resistance, obesity and glycemia to EDV after adjusting confounding variables (Table 3). Under Model 1 we applied metabolic parameters of glycemia and insulin resistance derived from fasting measures. Under Model 2 we applied parameters derived from oral glucose tolerance testing. In model 3 both sets of parameters were entered; entering these parameter simultaneously was valid despite the physiologic relationships between these variables because no multicollinearity was evident, with variance inflation statistics less than 1.85 in all cases. All models were performed without and then with adjustment as above. In all cases the relationship of body mass index with EDV was seen. Under Model 1 HOMA-IR was significantly related to EDV in unadjusted analysis, but not with the application of adjustment. Under Model 2 insulin AUC as a measure of insulin resistance achieved significance with and without adjustment. Under Model 3 this was again seen, with retained significance of insulin AUC despite the adjustments and despite the concurrent inclusion of HOMA-IR. Measures of glycemic status were not significant contributors to vascular function in any version of the multivariable models.
Table 3.
Multivariable Modeling for Determinants of Endothelium-Dependent Vasodilation.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Model 1 | N=217 | Model r2(adj) = 0.102 | Model p <0.001 | N=216 | Model r2(adj) = 0.15 | Model p <0.001 |
| Parameter | Beta | SE (beta) | P value | Beta | SE (beta) | P value |
| BMI | −0.14 | 0.05 | 0.002 | −0.16 | 0.05 | 0.001 |
| HOMA-IR* | −2.17 | 1.03 | 0.036 | −1.62 | 1.02 | 0.11 |
| Fasting Glucose* | −0.12 | 0.96 | 0.90 | 0.001 | 0.97 | 0.99 |
| Model 2 | N=201 | Model r2(adj) = 0.14 | Model p<0.001 | N=200 | Model r2(adj) = 0.18 | Model p <0.001 |
| Parameter | Beta | SE (beta) | P value | Beta | SE (beta) | P value |
| BMI | −0.11 | 0.05 | 0.015 | −0.13 | 0.05 | 0.009 |
| InsAUC* | −1.33 | 0.39 | 0.001 | −1.06 | 0.49 | 0.007 |
| GlucAUC* | −1.78 | 1.11 | 0.11 | −1.70 | 1.14 | 0.14 |
| Model 3 | N=216 | Model r2(adj) = 0.15 | Model p <0.001 | N=193 | Model r2(adj) = 0.17 | Model p <0.001 |
| Parameter | Beta | SE (beta) | P value | Beta | SE (beta) | P value |
| BMI | −0.10 | 0.05 | 0.038 | −0.13 | 0.05 | 0.015 |
| HOMA-IR* | −0.85 | 1.18 | 0.48 | −0.26 | 1.17 | 0.83 |
| InsAUC* | −1.25 | 0.44 | 0.005 | −1.05 | 0.44 | 0.017 |
| Fasting Glucose* | −0.23 | 1.26 | 0.85 | −0.10 | 1.24 | 0.94 |
| GlucAUC* | −1.35 | 1.40 | 0.34 | −1.58 | 1.41 | 0.27 |
Values were transformed for parametric analyses (see text). Coefficients are presented for the transformed variables. Adjustments for age, sex, race/ethnicity and smoking status were applied for each model. AUC, area under the curve for a 2 hour oral glucose tolerance test; BMI, body mass index; HOMA-IR, homeostasis model of insulin resistance.
Overall, in the whole population encompassing a range of glycemic status and a range of obesity status, in continuous analyses the effects of BMI and insulin resistance were the dominant determinants of vascular function. The effect of glycemia was evident when evaluating only non-obese subjects, but glycemic status was not a powerful separate determinant of vascular function among obese subjects.
Discussion
We have undertaken an evaluation of the relationships between agonist-mediated endothelium-dependent vasodilation (i.e. vascular function) and phenotypic variables in order to compare relative contributions of obesity, insulin resistance, and dysglycemia. In categorical analyses, the presence of dysglycemia (impaired fasting glucose and/or impaired glucose tolerance) was associated with impaired endothelium-dependent vasodilation in non-obese subjects, such that vascular function in dysglycemic non-obese subjects was reduced comparable to normoglycemic obese subjects. Within the obese subjects, gradations of dysglycemia were not associated with further worsening of these vascular responses beyond the defect associated with obesity alone. In univariate modeling adjusted for relevant confounders, obesity, insulin resistance and dysglycemia were each significantly inversely related to vascular function. Ambient glycemia at the time of vascular function measurement was not significantly related to vascular function. Multivariable modeling analyses revealed that effects of glycemia were not significant when entered in models together with measures of obesity and insulin resistance.
In these analyses progressive increases in dysglycemia within the obese group were not associated with further worsening of vascular function, such that vascular function was not significantly worse in the presence of frank diabetes than among normoglycemic obese subjects. This observation plus the results of the multivariable modeling suggest that obesity-related factors other than glycemia are of prime importance in the pathogenesis of vascular dysfunction in obesity. This observation is consistent with recent clinical trial data where treatments targeting aggressive glycemic control failed to improve vascular disease outcomes. Conversely, these observations suggest that in non-obese patients with type 1 diabetes, glycemia appears to be more viable as a treatment target for improving vascular biology. This flows from our observations comparing normoglycemia and dysglycemic non-obese subjects, and is consistent with recent data suggesting a cardiovascular protective effect of intensive glycemic control in Type 1 diabetic subjects who participated in the Diabetes Control and Complications Trial [7].
Acute hyperglycemia and vascular function
Hyperglycemia has well-documented acute adverse effects on vascular cells. In vitro hyperglycemia induces the production of highly reactive oxidant species [8, 9], and induces the production of vasoconstrictors including endothelin-1 [10].
A number of investigators have evaluated effects of acute hyperglycemia on vascular function in vivo in humans. These studies include evidence demonstrating glucose-induced impairment in vascular function [1, 2], and evidence against such effects [3, 4]. Although there is a temptation to lend more credence to positive studies, the latter reports are carefully performed studies that produced reliable negative results: Natali and colleagues undertook cross-sectional evaluations of vascular responses to acetylcholine in subjects with NGT, IGT and DM, at baseline and following an oral glucose tolerance test. At baseline, DM but not IGT subjects had impaired responses. There was no impairment from acute glucose loading orally or intravenously, in either the forearm resistance or forearm skin circulation [3]. Beneficial countervailing actions of glucose-stimulated insulin production were suggested as a reason for this observation. Reed and colleagues undertook a well-controlled experimental study to separate the effects of glucose excursions, pancreatic hormone responses to changes in glucose, and hyperglycemia per se on vascular function measured as responses to infused acetylcholine [4], and found that under these conditions acute elevations in glucose did not impair vascular function.
Chronic hyperglycemia and vascular function
Vascular dysfunction is a feature of diabetes in animal models. Vascular rings from STZ-treated insulin deficient, chronically hyperglycemic rats exhibited impaired endothelium-dependent responses when studied under normoglycemic conditions [11]. Other groups report impaired acetylcholine-stimulated NO production in STZ diabetic rats [12]. Similarly impaired vascular function has been reported in other animal models of type 2 diabetes [13].
In human studies, type 1 and type 2 diabetes are both associated with impaired vascular function [5, 14–18]. Vascular dysfunction is also a well-recognized feature of obesity and insulin resistance even without concurrent hyperglycemia [6, 19]. As observed in the present report, other groups have found that vascular function is not further impaired by the additional effects of diabetes beyond those of obesity [20].
Among nondiabetic obese and diabetic subjects, the degree and/or duration of dysglycemia may relate to vascular dysfunction [21, 22]. Ceriello and colleagues evaluated vascular function in type 1 diabetic subjects with historically poor glycemic control, and found that acute correction of the impaired vascular function required both reduction of glycemia with insulin and antioxidant treatment with Vitamin C [22]. In those with shorter duration of diabetes, or with better historical control, acute reduction in glycemia was sufficient to improve vascular function. These observations are concordant with the current observation that glycemic status was associated with vascular function, but ambient glycemia did not exert additional effects.
Improvement of glucose control in diabetes can reverse vascular dysfunction. Type 1 [16] and type 2 [17, 18] diabetic subjects who underwent intensification of diabetic control showed improved vasodilator responses to acetylcholine. Similar beneficial effects have been reported for a variety of diabetes treatments including metformin and pioglitazone [23, 24]. These observations suggest that the effects of chronic exposure to the diabetic state can be reversed with treatments targeting improved glycemic control. However, improvements in glycemia are only one of many concurrent improvements in metabolic status with these treatments so the causal connection between glycemia and vascular function cannot be conclusively argued from these data alone.
Relative contributions of obesity, insulin resistance, and dysglycemia
We observed significant univariate contributions of dysglycemia, insulin resistance and obesity to impairment in endothelium-dependent vasodilation. However, when combined in multivariable modeling the relative contribution of dysglycemia was considerably less than that of obesity. Little published data exist to corroborate this observation, with most authors focusing on relationships of vascular function with one variable of interest. One group undertook analyses similar to ours, and found that flow-mediated vasodilation was inversely related to HbA1c only in non-obese subjects, and that elevated HbA1c or elevated BMI was associated with impaired vascular function but without further impairment seen in subjects with both factors elevated [21].
In the present analysis the contribution of insulin resistance measures was also weaker than, and statistically separate from, that of obesity. This is of interest given the broad acceptance that insulin resistance mediates the effects of obesity on the vasculature [6, 20, 25]. If these effects are not mediated by insulin resistance, what does account for this? With and without insulin resistance, family members of type 2 diabetic subjects exhibit vascular dysfunction [20, 26], perhaps suggesting that such effects are genetically determined. Elevated levels of non-esterified fatty acids (NEFA) are associated with impaired vascular function [27–30] and induce insulin resistance and vascular dysfunction on different time scales [27–31]. Although elevated NEFA have been strongly implicated as a cause of vascular dysfunction in obesity, the available data do not clearly separate this factor from other obesity-associated abnormalities [32]. Acquired factors distinct from the traditionally evaluated metabolic effects of obesity may also be important. For example, the current focus on adipokines such as adiponectin [33], and on activation of innate immunity as a component of obesity [29, 34] may provide some explanation for the residual unexplained effects of obesity to induce vascular dysfunction.
Weaknesses
We did not routinely measure hemoglobin A1c in non-diabetic subjects in the studies that contributed data to the current dataset, and therefore cannot evaluate associations of this measure of durable glycemic exposure with vascular function. The cross-sectional nature of the data collected does not allow us to comment on more detailed mechanistic factors that may contribute to the observed relationships. An insufficient number of the studies that contributed to this dataset included measurement of endothelium-independent vasodilation or measures of insulin-mediated vasodilation, precluding parallel analyses of the relationships of glycemia with these parameters.
Acknowledgements
Dr. Han’s contributions to this work were made possible by a sabbatical supported by Nowon Eulji Hospital, Seoul, Korea. The studies included in this report were supported by the National Institutes of Health (DK 42469) and by the American Diabetes Association (Junior Faculty Award and Career Development Award to Dr Mather). Infrastructure and nursing support was provided by of the Indiana University General Clinical Research Center (NIH M01 RR00750). The expert technical assistance and hyperinsulinemic clamp expertise of Jan VanderLuitgaren RN, Christina Leffler RN and Paula Robinett RN is gratefully acknowledged.
References
- 1.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008 May;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 3.Natali A, Baldi S, Vittone F, Muscelli E, Casolaro A, Morgantini C, et al. Effects of glucose tolerance on the changes provoked by glucose ingestion in microvascular function. Diabetologia. 2008 May;51(5):862–871. doi: 10.1007/s00125-008-0971-6. [DOI] [PubMed] [Google Scholar]
- 4.Reed AS, Charkoudian N, Vella A, Shah P, Rizza RA, Joyner MJ. Forearm vascular control during acute hyperglycemia in healthy humans. Am J Physiol Endocrinol Metab. 2004 Mar;286(3):E472–E480. doi: 10.1152/ajpendo.00348.2003. [DOI] [PubMed] [Google Scholar]
- 5.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002 Dec;51(12):3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 6.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005 Jul 5;112(1):32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. [Review] [82 refs] Free Radical Biology & Medicine. 1994;16(3):383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 9.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000 Aug;85(8):2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 10.Hattori Y, Kasai K, Nakamura T, Emoto T, Shimoda S. Effect of glucose and insulin on immunoreactive endothelin-1 release from cultured porcine aortic endothelial cells. Metabolism. 1991;40(2):165–169. doi: 10.1016/0026-0495(91)90168-v. [DOI] [PubMed] [Google Scholar]
- 11.Hamaty M, Guzman CB, Walsh MF, Bode AM, Levy J, Sowers JR. High glucose-enhanced acetylcholine stimulated CGMP masks impaired vascular reactivity in tail arteries from short-term hyperglycemic rats. Int J Exp Diabetes Res. 2000;1(1):69–79. doi: 10.1155/EDR.2000.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Kamata K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis. 2001 Apr;155(2):313–320. doi: 10.1016/s0021-9150(00)00583-9. [DOI] [PubMed] [Google Scholar]
- 13.Brunner F, Wolkart G, Pfeiffer S, Russell JC, Wascher TC. Vascular dysfunction and myocardial contractility in the JCR:LA-corpulent rat. Cardiovasc Res. 2000 Jul;47(1):150–158. doi: 10.1016/s0008-6363(00)00056-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993 Dec;88(6):2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 15.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(8):771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 16.Franklin VL, Khan F, Kennedy G, Belch JJ, Greene SA. Intensive insulin therapy improves endothelial function and microvascular reactivity in young people with type 1 diabetes. Diabetologia. 2008 Feb;51(2):353–360. doi: 10.1007/s00125-007-0870-2. [DOI] [PubMed] [Google Scholar]
- 17.Vehkavaara S, Makimattila S, Schlenzka A, Vakkilainen J, Westerbacka J, Yki-Jarvinen H. Insulin therapy improves endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000 Feb;20(2):545–550. doi: 10.1161/01.atv.20.2.545. [DOI] [PubMed] [Google Scholar]
- 18.Bagg W, Whalley GA, Gamble G, Drury PL, Sharpe N, Braatvedt GD. Effects of improved glycaemic control on endothelial function in patients with type 2 diabetes. Intern Med J. 2001 Aug;31(6):322–328. doi: 10.1046/j.1445-5994.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 19.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007 Mar;56(3):728–734. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 20.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48(9):1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 21.Voidonikola PT, Stamatelopoulos KS, Alevizaki M, Kollias GE, Zakopoulos NA, Lekakis JP, et al. The association between glycemia and endothelial function in nondiabetic individuals: the importance of body weight. Obesity (Silver Spring) 2008 Dec;16(12):2658–2662. doi: 10.1038/oby.2008.431. [DOI] [PubMed] [Google Scholar]
- 22.Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J Clin Endocrinol Metab. 2009 Aug;94(8):2751–2756. doi: 10.1210/jc.2009-0762. [DOI] [PubMed] [Google Scholar]
- 23.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37(5):1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 24.Martens FM, Visseren FL, de Koning EJ, Rabelink TJ. Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J Cardiovasc Pharmacol. 2005 Dec;46(6):773–778. doi: 10.1097/01.fjc.0000187176.13403.05. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfine AB, Beckman JA, Betensky RA, Devlin H, Hurley S, Varo N, et al. Family history of diabetes is a major determinant of endothelial function. J Am Coll Cardiol. 2006 Jun 20;47(12):2456–2461. doi: 10.1016/j.jacc.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997 Sep 1;100(5):1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind L, Fugmann A, Branth S, Vessby B, Millgard J, Berne C, et al. The impairment in endothelial function induced by non-esterified fatty acids can be reversed by insulin. Clin Sci (Lond) 2000 Sep;99(3):169–174. doi: 10.1042/cs0990169. [DOI] [PubMed] [Google Scholar]
- 29.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003 Dec;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 30.de Kreutzenberg SV, Crepaldi C, Marchetto S, Calo L, Tiengo A, Del Prato S, et al. Plasma free fatty acids and endothelium-dependent vasodilation: effect of chain-length and cyclooxygenase inhibition. J Clin Endocrinol Metab. 2000;85(2):793–798. doi: 10.1210/jcem.85.2.6352. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49(7):1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 32.Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007 Feb;18(1):58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- 33.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003 Jul;88(7):3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 34.Alipour A, Elte JW, van Zaanen HC, Rietveld AP, Cabezas MC. Postprandial inflammation and endothelial dysfuction. Biochem Soc Trans. 2007 Jun;35(Pt 3):466–469. doi: 10.1042/BST0350466. [DOI] [PubMed] [Google Scholar]