Abstract
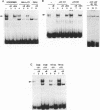
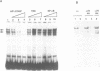
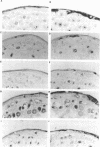
We have previously reported the epidermis-specific expression of the HIV-1 LTR in transgenic mice and its induction by UV-B rays. To dissect the underlying mechanism of the UV induction of the LTR in mice, we developed two approaches. We first demonstrated by gel mobility shift analysis, using mice epidermal extracts, that the NF-kappa B sites of the HIV-1 LTR were one of the targets of the UV induction. The Sp-1 sites and the potential AP-1 sites of the LTR were not involved in this phenomenon. The transient transfection assays of modified LTR in HeLa cells also demonstrated the involvement of the NF-kappa B sites in the UV induction and were consistent with previously published data. Secondly, to study the regulation acting on an integrated gene, we generated transgenic mice carrying the lacZ gene under the control of the partially deleted LTR. All the transgenic lines and unexpectedly those carrying the LTR deleted for the kappa B sites displayed a UV-inducible epidermal expression. This suggests that, in mice, the UV induction might be mediated through other sites than the kappa B sites and may also depend on changes of the chromatin state.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Rupniak H. T., Fusenig N. E. Environmental modulation of the expression of differentiation and malignancy in six human squamous cell carcinoma cell lines. Cancer Res. 1985 Nov;45(11 Pt 2):5582–5592. [PubMed] [Google Scholar]
- Cavard C., Zider A., Vernet M., Bennoun M., Saragosti S., Grimber G., Briand P. In vivo activation by ultraviolet rays of the human immunodeficiency virus type 1 long terminal repeat. J Clin Invest. 1990 Oct;86(4):1369–1374. doi: 10.1172/JCI114849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Picot I., Nicole A., Briand P., Grimber G., Delacourte A., Defossez A., Javoy-Agid F., Lafon M., Blouin J. L., Sinet P. M. Neuronal-specific expression of human copper-zinc superoxide dismutase gene in transgenic mice: animal model of gene dosage effects in Down's syndrome. Brain Res. 1991 Jun 28;552(2):198–214. doi: 10.1016/0006-8993(91)90084-9. [DOI] [PubMed] [Google Scholar]
- Charneau P., Alizon M., Clavel F. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol. 1992 May;66(5):2814–2820. doi: 10.1128/jvi.66.5.2814-2820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991 Jun;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Frucht D. M., Lamperth L., Vicenzi E., Belcher J. H., Martin M. A. Ultraviolet radiation increases HIV-long terminal repeat-directed expression in transgenic mice. AIDS Res Hum Retroviruses. 1991 Sep;7(9):729–733. doi: 10.1089/aid.1991.7.729. [DOI] [PubMed] [Google Scholar]
- Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992 Apr;6(4):347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Giacca M., Gutierrez M. I., Menzo S., d'Adda di Fagagna F., Falaschi A. A human binding site for transcription factor USF/MLTF mimics the negative regulatory element of human immunodeficiency virus type 1. Virology. 1992 Jan;186(1):133–147. doi: 10.1016/0042-6822(92)90067-y. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrich D., Garcia J., Mitsuyasu R., Gaynor R. TAR independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990 Dec;9(13):4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Rabson A. B., Gélinas C. The RxxRxRxxC motif conserved in all Rel/kappa B proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol Cell Biol. 1992 Jul;12(7):3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J., Parrott C., Buckler-White A. J., Turner W., Ross E. K., Martin M. A., Rabson A. B. The NF-kappa B binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989 Nov;63(11):4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey J. D., Bourn S. M., Bunch T. D., Jackson M. K., Sidwell R. W., Barrows L. R., Daynes R. A., Rosen C. A. In vivo activation of human immunodeficiency virus type 1 long terminal repeat by UV type A (UV-A) light plus psoralen and UV-B light in the skin of transgenic mice. J Virol. 1991 Sep;65(9):5045–5051. doi: 10.1128/jvi.65.9.5045-5051.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C., Seeler J. S., Nirula A., Shurland D. L., Gaynor R. B. Regulation of human immunodeficiency virus enhancer function by PRDII-BF1 and c-rel gene products. J Virol. 1992 Jan;66(1):244–250. doi: 10.1128/jvi.66.1.244-250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Baltimore D. The inhibitory ankyrin and activator Rel proteins. Curr Opin Genet Dev. 1992 Apr;2(2):211–220. doi: 10.1016/s0959-437x(05)80276-x. [DOI] [PubMed] [Google Scholar]
- Rattner A., Korner M., Rosen H., Baeuerle P. A., Citri Y. Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol. 1991 Feb;11(2):1017–1022. doi: 10.1128/mcb.11.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Stein B., Krämer M., Rahmsdorf H. J., Ponta H., Herrlich P. UV-induced transcription from the human immunodeficiency virus type 1 (HIV-1) long terminal repeat and UV-induced secretion of an extracellular factor that induces HIV-1 transcription in nonirradiated cells. J Virol. 1989 Nov;63(11):4540–4544. doi: 10.1128/jvi.63.11.4540-4544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K., Rosenberg M. Chromatin structure implicated in activation of HIV-1 gene expression by ultraviolet light. New Biol. 1990 Aug;2(8):712–718. [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagoe S., Kohda T., Oishi M. Poly(ADP-ribose) polymerase inhibitors suppress UV-induced human immunodeficiency virus type 1 gene expression at the posttranscriptional level. Mol Cell Biol. 1991 Jul;11(7):3522–3527. doi: 10.1128/mcb.11.7.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]