Abstract
Context
Androgen deprivation therapy (ADT) is increasingly used for the treatment of prostate cancer (PCa), even in clinical settings in which there is no evidence-based proof of prolonged overall survival (OS). ADT, however, may be associated with numerous side effects, including an increased therapy-related cardiovascular mortality.
Objective
To discuss different clinical settings in which ADT is currently used and to critically weigh the benefits of ADT against its possible side effects.
Evidence acquisition
A MEDLINE search was conducted to identify original articles and review articles addressing the efficacy and side effects of ADT for the treatment of PCa. Keywords consisted of prostate cancer, hormonal therapy, adverse effects, radical prostatectomy, and radiotherapy. The articles with the highest level of evidence for the various examined end points were identified with the consensus of all authors and were reviewed.
Evidence synthesis
Even short-term use of ADT may lead to numerous side effects, such as osteoporosis, obesity, sarcopenia, lipid alterations, insulin resistance, and increased risk for diabetes and cardiovascular morbidity. Despite these side effects, ADT is commonly used in various clinical settings in which a clear effect on improved OS has not been shown.
Conclusions
ADT is associated with an increased risk of multiple side effects that may reduce quality of life and/or OS. Consequently, these issues should be discussed in detail with patients and their families before initiation of ADT. ADT should be used with knowledge of its potential long-term side effects and with possible lifestyle interventions, especially in settings with the highest risk–benefit ratio, to alleviate comorbidities.
Keywords: Prostate cancer, Hormonal therapy, Adverse effects, Radical prostatectomy, Radiotherapy
1. Introduction
Since the pivotal studies of Huggins and Hodges [1–3], which demonstrated that the development and growth of prostate cancer (PCa) cells are dependent on androgens, androgen deprivation therapy (ADT) has been increasingly utilized for the treatment of PCa. Although relatively unknown, Paul Niehans from Switzerland published his 13-yr experience with the prevention and treatment of PCa with ADT in 1940 [4], 1 yr prior to the publications of Huggins and Hodges. ADT has historically been considered the treatment of choice for advanced or metastatic PCa, but there are numerous other settings in which ADT is used. These include administration in the neoadjuvant or adjuvant setting combined with surgery or radiation therapy (RT) or when there is biochemical recurrence (BCR), defined as a new rise of the prostate-specific antigen (PSA) serum level, after therapy with curative intent. Additionally, hormonal therapy as the treatment of choice for localized disease has gained in popularity. A 1999–2001 survey showed that primary ADT had become the second most common treatment approach, after surgery, for localized PCa [5,6].
Smith noted in a recent publication that of 2 million men diagnosed with PCa in the United States, approximately 600 000 (30%) receive ADT [7]. Because androgens are essential for the physiologic activity of various body functions, the multitude of possible ADT side effects include loss of libido, erectile dysfunction, fatigue, osteoporosis and induced skeletal complications, hot flushes, altered body composition, arterial stiffness, new-onset diabetes mellitus, and cognitive decline [8–14]. Even more striking are the findings of recent publications highlighting that ADT may be associated with increased cardiovascular morbidity and mortality [15–17]. This finding is of extreme importance because the use of ADT for prolonging overall survival (OS) is unclear in several clinical scenarios [18]; however, another important aspect of ADT is palliation of symptoms related to advanced or metastatic PCa [19]. Therefore, various end points beyond survival, such as quality of life (QoL), must also be seriously considered.
The aim of this paper is to review the existing literature concerning ADT for PCa and to understand the circumstances in which objective evidence exists that use of ADT is beneficial for prolonging OS and/or improving QoL. The forms of hormonal therapy that are most frequently used in daily practice, including orchiectomy, antiandrogens, and luteinizing hormone-releasing hormone (LHRH) agonists (LHRHa), are considered. The benefits and side effects of these therapeutic hormonal manipulations are presented. Although LHRH antagonists are also used for the treatment of PCa [20], they are not considered in this article because no long-term data are available.
2. Evidence acquisition
A MEDLINE search was conducted to identify original articles and review articles addressing the efficacy and the side effects of ADT for the treatment of PCa. Keywords consisted of prostate cancer, hormonal therapy, adverse effects, radical prostatectomy, and radiotherapy. All of the keywords are within the Medical Subject Headings (MeSH) database, which represents the controlled vocabulary used for indexing articles for MEDLINE and PubMed. The articles with the highest level of evidence for the various examined end points were identified with the consensus of all of the collaborative authors and were reviewed.
3. Evidence synthesis
3.1. Critical evaluation of the efficacy of androgen deprivation therapy in different clinical scenarios
The different scenarios in which ADT is predominantly used are introduced, and the efficacy of ADT is discussed. Table 1 summarizes different clinical settings in which hormonal therapy has been evaluated and highlights the positive and negative results.
Table 1.
Clinical settings for the use of androgen deprivation therapy (ADT) in current clinical practice
| Clinical setting | Effect |
|---|---|
| Combination with external-beam radiation therapy | • Improves overall survival of patients with locally advanced or high-risk prostate cancer |
| Neoadjuvant before low-dose and high-dose brachytherapy | • Downsizing of the prostate can efficiently be achieved |
| • No proof of survival benefit | |
| Neoadjuvant before radical prostatectomy | • Lowers adverse histopathologic features in the prostatectomy specimen but does not prolong overall survival |
| Adjuvant after radical prostatectomy | • Improves progression-free survival and cancer-specific survival but does not prolong overall survival in N0 patients |
| • Immediate ADT vs deferred ADT may improve overall survival in N+ patients | |
| Organ-confined prostate cancer | • No proof that ADT vs no therapy at all improves overall survival |
| • Usage of bicalutamide may reduce overall survival | |
| Locally advanced prostate cancer | • Immediate ADT vs deferred ADT significantly reduces cancer-related complications and may show a small overall survival benefit, especially in high-risk patients |
| • Optimal timing of ADT remains unclear | |
| Biochemical failure after therapy with curative intent | • No proof that immediate ADT vs deferred ADT prolongs overall survival; timing of ADT remains unclear |
| Metastatic prostate cancer | • Immediate ADT may improve quality of life in symptomatic disease but does not prolong overall survival |
3.1.1. Androgen deprivation therapy combined with radiation therapy
The beneficial role of ADT with external-beam RT (EBRT) has been repeatedly reported. Several well-conducted randomized trials uniformly demonstrated a statistically significant improvement in OS when EBRT was combined with ADT [21–23]. Consequently, the combination of RT and ADT is widely accepted for patients with high-grade or locally advanced PCa, which is also stated in the guidelines of the European Association of Urology (EAU) [18] and of the American Urological Association (AUA) [24]. The duration of ADT to achieve an optimal benefit is dependent on both the radiation dose and the risk profile of the patient. If the local radiation dose is relatively low (60–70 Gy, as used in earlier trials), ADT improves the local tumor control. This may be partially achieved by the direct effect of ADT on the PCa cells. It has been demonstrated, however, that ADT makes androgen-receptor expressing PCa cells more vulnerable to radiation in this setting [25]. This is supported by prospective randomized trials in which the outcome was significantly better in patients who were treated with RT and ADT, even if the patients received only 4 mo or 6 mo of ADT at the time of RT [21]. In Radiation Therapy Oncology Group (RTOG) protocol 92-02 [26], patients with a Gleason score of 8–10 had a significant survival benefit when treated with long-term ADT (28 mo) compared with patients treated with short-term ADT (4 mo). Based on the available literature, patients with low- and intermediate-risk PCa may be sufficiently treated with short-term ADT combined with RT, at least if the local radiation dose is <80 Gy. Conversely, poor-risk patients with locally advanced disease and high-risk PCa may also profit from long-term ADT.
3.1.2. Androgen deprivation therapy combined with brachytherapy
ADT is frequently used for prostate volume reduction before initiation of brachytherapy [27,28]; however, neoadjuvant ADT did not show an improvement in OS [29–31]. On the contrary, a retrospective analysis suggested a significantly reduced OS rate when brachytherapy was combined with neoadjuvant ADT [32]. Due to the retrospective nature of the study and other methodologic limitations, these findings must be interpreted with caution. Men receiving ADT, for example, had significantly more adverse PCa baseline features. Nevertheless, the EAU and AUA guidelines state that there is no benefit from adding ADT to brachytherapy [18,24].
3.1.3. Androgen deprivation therapy combined with radical prostatectomy
In a recent Cochrane meta-analysis, the role of neoadjuvant ADT with radical prostatectomy (RP) was addressed. The conclusion of this analysis was that neoadjuvant ADT does significantly improve adverse histopathologic parameters like pathologic tumor (pT) stage or surgical margin stage but does not improve OS [33]. Accordingly, neoadjuvant ADT before RP is not recommended in the EAU or AUA guidelines [18,24].
In the adjuvant setting after RP, an increase in biochemical-free survival and progression-free survival (PFS) has been reported in favor of the adjuvant ADT treatment [34,35]; however, the effect on OS is unclear and seems to be highly dependent on the risk profile of the patient. For example, patients receiving bicalutamide 150 mg/day immediately after therapy with curative intent for early, nonmetastatic PCa had a significantly worse OS compared with their counterparts receiving placebo [36]. In trials comparing immediate versus deferred ADT for patients with low-risk disease, a significant difference in PFS has been reported in favor of the intermediate arm [34,35]. When these patients progress, however, they may have hormone-refractory or less hormone-sensitive disease while patients on the deferred-hormone arm still have hormone-sensitive disease. Studies on PCa cell lines demonstrated that early administration of hormonal therapy after failure of first-line treatment is associated with a clonal selection of aggressive, androgen-independent PCa cells [37]. Consequently, despite the significant differences in PFS, the cancer-specific survival did not differ in these patients.
For patients undergoing RP and having lymph node invasion (LNI), an advantage in OS has been reported for immediate ADT [38]. In this series, which was recently updated, 98 patients with nodal metastases were randomly assigned to receive immediate ADT versus ADT at the time of distant metastases or of symptomatic recurrences. After a median follow-up of 11.9 yr, patients in the immediate-ADT arm had a significantly improved OS (hazard ratio [HR]: 1.84; p = 0.04) [39]. Delayed ADT was not given in case of PSA recurrence, which many clinicians would recommend today, but at a much more advanced stage of the disease. Moreover, two-thirds of the patients also had seminal vesicle invasion and/or positive margins; as such, these patients were at a very high risk of recurrence, regardless of the lymph node status. Consequently, the findings of the trial cannot necessarily be transposed to any existing patients with a single microscopic metastasis in one or more of 20 lymph nodes because the prognosis for these patients may not be as poor as for those studied [40]. Recently, Boorjian et al retrospectively analyzed the data of 507 patients with +N disease after RP in the PSA era [41]. Patients who received adjuvant ADT had a statistically significantly decreased risk of BCR and local recurrence. There was no statistically significant difference, however, in the rate of systemic progression or of cancer-specific survival between the two groups.
In summary, no proof currently exists that adjuvant ADT for pN0 PCa after RP prolongs OS. Although immediate ADT is superior to delayed ADT at the time of clinical recurrence in +N disease, it is not known whether a similar advantage could be seen in those who are treated at the time of subclinical recurrence or on the basis of PSA parameters. Immediate ADT in case of +N disease is considered to be an indication in the EAU guidelines, but it is not explicitly recommended [18]. The AUA guidelines do not address this clinical setting [24].
3.1.4. Androgen deprivation therapy as the primary therapy for prostate cancer
3.1.4.1. Localized disease
No clear evidence supports the use of ADT for patients with localized disease [19]. Iversen et al demonstrated that patients receiving bicalutamide 150 mg/day for the treatment of early, nonmetastatic PCa had a significantly worse outcome than patients receiving placebo [36]. The authors concluded, “[I]n previously untreated patients there may be a tumor burden below which endocrine therapy provides no benefit or may even decrease survival.” In the above-mentioned EPC program, there was a trend toward reduced OS for patients with localized disease who received 150 mg/day of bicalutamide compared with the placebo-treated patients [35]. Therefore, and in accordance with the EAU guidelines, the usage of bicalutamide as primary therapy for localized PCa is not indicated [18]. In the AUA guidelines, primary ADT for the treatment of localized PCa is not considered as a standard treatment option due to the lack of prospective data [24]. To date, no evidence exists that ADT as primary therapy for localized PCa is in any way superior to no therapy in terms of improving OS, and there are data suggesting reduced OS with ADT.
3.1.4.2. Locally advanced and metastatic disease
The Medical Research Council (MRC) conducted a trial in which 938 patients with locally advanced or metastatic disease were randomly assigned to immediate ADT versus deferred treatment until clinically significant progression occurred [42]. Although an initial survival benefit was documented for the immediate-treatment arm, it disappeared after longer follow-up [43]. Nevertheless, the occurrence of adverse events such as pathologic fractures, spinal cord compression, and ureteral obstruction were twice as frequent in the deferred arm; however, the patients in the deferred arm had not been followed regularly, so part of these differences might be related to inadequate follow-up. The largest randomized trial so far that compared immediate and deferred ADT in patients not suitable for local treatment with curative intent was conducted by the European Organization for Research and Treatment of Cancer (EORTC) [44]. In this trial, there was a small but statistically significant difference in favor of immediate ADT. It seemed, however, that the patients who died from cancer-related complications within 3–6 yr after randomization accounted for this difference. Thus, such patients may benefit from ADT. Subgroup analyses showed that these are mostly the patients who have a PSA doubling time (PSA DT) of <12 mo [45]. PSA DT is defined as the time it takes for the PSA serum level to double. Notably, in the EORTC trial, the cancer-related complications were apparently not much different in patients on the deferred arm compared with those on the immediate arm. Because all the patients were regularly followed, metastases were detected early and ADT was started immediately, explaining the small difference overall between the two arms.
In another trial, patients with localized or locally advanced PCa were randomized to bicalutamide (150 mg/day) or placebo in addition to standard care; so far, there is no difference in OS [46]. Tyrrell et al demonstrated, however, that in patients with metastatic PCa, the OS rate is significantly worse for patients receiving bicalutamide 150 mg/day compared with those who are treated with castration [47]. A study that addressed the tolerability, efficacy, and pharmacokinetics of bicalutamide pointed out that the dosage of bicalutamide would have to be 450 or 600 mg/day if it were to be used as monotherapy in patients with very advanced or metastatic disease to achieve an equal efficacy with castration [48].
Because of the paucity of high-quality data from randomized controlled clinical trials, the EAU guidelines state that definite recommendations are difficult to make as to when to start hormonal therapy for patients with advanced PCa [18]. The AUA guidelines only address the treatment of localized PCa, so no recommendations are made [24]. ADT is the treatment of choice for patients with metastatic disease, although the literature analyzing deferred treatment of asymptomatic M+ patients is sparse. Because median survival in metastatic patients at diagnosis is now approximately 5–6 yr and further cancer progression can cause severe adverse effects, the EAU guidelines suggest that ADT should only be delayed in patients who strongly wish to avoid treatment-related side effects. In symptomatic M+ PCa patients, ADT should be given immediately to palliate symptoms [18].
3.1.5. Androgen deprivation therapy for recurrence after treatment with curative intent
According to the EAU guidelines, ADT may be an option after RP for selected patients in whom systemic progression is expected to prevent symptomatic progression [18]; however, there are no objective data to clearly support this statement. The EAU guidelines further state that local relapses may be treated more efficiently with salvage RT [18]. Because the median time from PSA relapse to PCa-related death is 13 yr [49], factors such as life expectancy, time to PSA relapse after surgery, and PSA DT after relapse should be considered before proposing early ADT [50]. Many patients with a slow PSA relapse may well be considered patients with a low tumor burden; thus, they may not benefit from immediate ADT [36]. Accordingly, the EAU guidelines state that a period of watchful waiting is appropriate, with ADT possible later [18]. As indicated above, this issue is not addressed in the AUA guidelines [24].
3.2. Side effects and sequelae of hormonal therapy
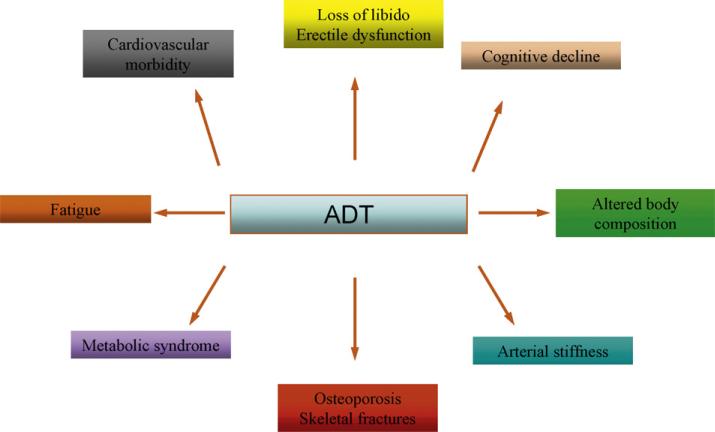
Fig. 1 illustrates which body systems may be impaired by hormonal therapy. The list of castration-induced side effects is long, so emphasis is placed on those that may be directly related to increased morbidity and/or mortality.
Fig. 1.
Side effects of androgen deprivation therapy (ADT).
3.2.1. Osteoporosis and fractures
Osteoporosis is an important medical problem in men worldwide [51]. After age 45, approximately one in four men will experience a clinical fracture [52]. Men suffer one-third of all hip fractures and are more likely to die following a hip fracture. Hypogonadism, chronic glucocorticoid therapy, and alcohol abuse are the major causes of acquired osteoporosis in men [53]. Smoking, low dietary calcium intake, vitamin D deficiency, and sedentary lifestyle also contribute to risk for osteoporosis.
ADT is associated with greater risk for clinical fractures. In large population-based studies, for example, use of LHRHa was associated with a 21–45% relative increase in the incidence of clinical fractures [54–56]. Longer treatment duration confers greater fracture risk. Age and comorbidity are also associated with higher fracture incidence in men with PCa.
ADT increases bone turnover [57] and decreases bone mineral density (BMD) [57–60], a surrogate for fracture risk. During initial ADT, BMD on the hip and spine decrease by approximately 3% per year. Most studies have reported that BMD continues to decline steadily during long-term therapy.
Several small randomized controlled trials have demonstrated that bisphosphonates increase BMD in men receiving ADT for PCa. Intravenous pamidronate significantly decreased biochemical markers of bone turnover and increased BMD of the hip and spine in men receiving LHRHa [57,61]. In a 12-mo multicenter placebo-controlled study of 106 men with PCa, intravenous zoledronic acid increased BMD of the hip and spine by 3.9% and 7.3%, respectively [62]. Similar results were reported with annual zoledronic acid [63]. In another study, oral alendronate increased BMD of the hip and spine by 2.3% and 5.1% after 12 mo [64]. It is not known, however, whether bisphosphonate substitution is equivalent to nondemineralized bone.
Two large randomized placebo-controlled trials to prevent fractures during ADT were recently completed. Denosumab is a human monoclonal antibody that binds and neutralizes human receptor activator of nuclear factor-κB ligand (RANKL), a critical regulator of osteoclast activation, differentiation, and survival. Denosumab is in development for the treatment and prevention of postmenopausal osteoporosis, treatment-related osteoporosis in men with PCa and women with breast cancer, and bone metastases. In a recently completed global study, approximately 1500 men who are receiving ADT for PCa were randomly assigned to either denosumab subcutaneously every 6 mo or placebo. The primary study end points are BMD and new fractures. Toremifene is a selective estrogen receptor modulator approved for the treatment of advanced breast cancer. In a recently completed multicenter study, approximately 1400 men in the United States and Mexico who are receiving ADT for PCa were randomly assigned to either toremifene or placebo. The primary study end point is new vertebral fractures. Secondary end points include BMD, serum lipids, vasomotor flushing, and breast symptoms. Complete results from both studies are expected this year.
3.2.2. Obesity and sarcopenia
Androgens are important determinants of body composition in men. In healthy men, serum testosterone levels correlate positively with lean body mass and negatively with fat mass [65]. ADT significantly decreases lean body mass and increases fat mass in men with PCa [9,10,12,66,67]. In two prospective studies of men with nonmetastatic PCa, for example, ADT decreased lean body mass by 2.7–3.8% and increased fat mass by 9.4–11.0% from baseline to 1 yr (p < 0.001 for each comparison) [12,67]. Changes in body composition appear primarily as an early adverse event, with most of the treatment-related changes in fat and lean body mass apparent within the first year of therapy [68]. ADT increases subcutaneous rather than visceral fat [12].
Sarcopenia is associated with frailty and increased risk of falls in older men [69]. Accordingly, ADT may increase fracture risk by decreasing BMD and muscle mass. Treatment-related sarcopenia may also contribute to fatigue and decrease QoL.
3.2.3. Lipid alterations and insulin resistance
ADT increases serum cholesterol and triglyceride levels [67,70]. In a prospective 12-mo study of 40 men with PCa, for example, ADT increased serum total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides by 9%, 7%, 11%, and 27%, respectively [67]. Most but not all of the observed long-term adverse effects of serum lipids are apparent within the first 3 mo of treatment [11].
Insulin resistance is a common metabolic abnormality that underlies type 2 diabetes mellitus and is prevalent in about one-quarter of nondiabetic men [71]. Insulin resistance is also an independent risk factor for cardiovascular disease [72,73]. ADT increases fasting plasma insulin levels—a marker of insulin resistance in men with PCa [10,74]. In a 12-wk prospective study of nondiabetic men, ADT significantly decreased insulin sensitivity [11]. The longer term effects of ADT on insulin sensitivity are unknown.
3.2.4. Metabolic syndrome
The metabolic syndrome is a clustering of specific cardiovascular-disease risk factors for which pathophysiology appears to be related to insulin resistance. The National Cholesterol Education Program's Adult Treatment Panel (NCEP ATP III) defines the metabolic syndrome in men as any three or more of the following factors: waist circumference >102 cm, serum triglycerides ≥1.7 mmol/l, blood pressure ≥130/80 mmHg, HDL cholesterol <1.0 mmol/l, or serum glucose ≥6.1 mmol/l. The World Health Organization (WHO) defines the metabolic syndrome using different but related criteria [75].
A cross-sectional study reported a higher prevalence of the metabolic syndrome (as defined by NCEP ATP III) in 18 men receiving ADT compared with age-matched control groups of untreated men with PCa and men without PCa [76]. Men receiving ADT were more likely to have increased abdominal girth, elevated triglycerides, and elevated fasting plasma glucose. These findings were consistent with results of published prospective studies. In contrast to the metabolic syndrome, however, prospective studies have shown that ADT preferentially increases subcutaneous rather than visceral fat, increases rather than decreases HDL cholesterol, and does not alter blood pressure or waist–hip ratio [67]. Additionally, the metabolic syndrome is characterized by low levels of adiponectin and elevated markers of inflammation. Conversely, ADT significantly increases serum adiponectin levels and does not alter levels of C-reactive protein or other markers of inflammation [77,78]. Taken together, these observations suggest that ADT causes a pattern of metabolic changes that is distinct from the classically defined metabolic syndrome.
There may be practical implications of distinguishing the phenotype of men receiving ADT from the classic metabolic syndrome. In the general population, the label of the metabolic syndrome is applied to indicate risk for cardiovascular disease. The metabolic syndrome, however, is not precisely defined and has little or no independent value as a marker of cardiovascular disease [79,80]. In other words, the composite definition of the metabolic syndrome is no greater than the sum of its parts. Given this limitation, the management of men with PCa should focus on evaluation and treatment of specific risk factors without concern for whether an individual meets diagnostic criteria for the metabolic syndrome.
3.2.5. Diabetes and cardiovascular disease
A landmark study evaluated the relationship between ADT and incident diabetes mellitus and cardiovascular disease using the linked Surveillance, Epidemiology, and End Results (SEER) and Medicare database [15]. The study included the records of 73 196 men diagnosed with local or local-regional PCa between 1992 and 1999. The primary outcomes were new diagnosis of diabetes mellitus, new cardiovascular disease, and admission or myocardial infarction. Cox proportional hazards models with time-varying treatment variables and time-varying covariates were used to assess the relationship between LHRHa or orchiectomy and primary study outcomes. About one-third of the men received treatment with LHRHa. The unadjusted rates of incident diabetes, coronary heart disease, and myocardial infarction were higher for men receiving LHRHa than for untreated men. After controlling for other variables, current use of LHRHa was associated with a significantly increased risk of incident diabetes (adjusted HR: 1.42; p < 0.001), coronary heart disease (adjusted HR: 1.16; p < 0.001), and admission for myocardial infarction (adjusted HR: 1.11; p = 0.03) compared with men who did not receive ADT. Similar results were obtained using the propensity score methods to match treated patients with similar untreated patients, suggesting that potential differences in baseline characteristics between the groups are unlikely to explain the observed significant associations.
A subsequent study using the SEER–Medicare database reached similar conclusions [16]. The study evaluated 22 816 men diagnosed with PCa between 1992 and 1996. Multivariate models were used to assess the risk of incident cardiovascular morbidity. Men who received ADT for at least 1 yr were found to have a 20% greater risk for new cardiovascular disease compared with their respective counterparts who were not exposed to any form of ADT. Consistent with the observations of Keating et al [15], greater risk for cardiovascular morbidity was apparent in men with both short-term and long-term exposure to ADT.
Whether the observed association between ADT and incident cardiovascular mortality is accompanied by greater cardiovascular mortality is not entirely clear. Several recent studies provide insights into the possible relationship between ADT and cardiovascular mortality in men with PCa.
Recently updated results of a RTOG study (protocol 8610) show no statistically significant association between short-term ADT and cardiovascular mortality [81]. Overall, 456 men with locally advanced PCa were randomly assigned to RT with or without neoadjuvant and concomitant ADT. Combined treatment with RT and ADT significantly improved disease-free survival (HR: 1.52; p = 0.009) but not OS. There were a total of 348 deaths; 57 were classified as cardiovascular deaths. Combined modality treatment was associated with a slightly higher rate of cardiovascular mortality (11% vs 14%) without reaching statistical significance (p = 0.4).
The EORTC trial 30891 randomized 985 men with newly diagnosed PCa T0-4 N0-2 M0 to either immediate ADT (n = 493) or ADT at symptomatic disease progression or occurrence of serious complications [44]. Baseline characteristics were similar between the groups. At a median follow-up of 7.8 yr, 541 of 985 patients had died, mostly of PCa (n = 193) or of cardiovascular disease (n = 185). The overall survival HR was 1.25 (95% CI: 1.05–1.48; p < 0.01), favoring immediate treatment and seemingly due to fewer deaths from non-PCa causes (p = 0.06). Rates of cardiovascular mortality were not statistically significantly different between the immediate and the deferred ADT groups (17.9% vs 19.7%).
In contrast, analyses of the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database suggested that neoadjuvant or adjuvant ADT was associated with higher rates of cardiovascular death [17]. The analyses included a total of 4890 men (1015 received ADT) and 131 fatal cardiovascular events. Greater risk of cardiovascular death was observed in the subset of men who underwent RP (adjusted HR: 2.6; 95% CI: 1.4–4.7; p = 0.002) but not in the overall study population. The authors suggested that this inconsistency might be explained by the fewer patients and the fewer events in the non-RP group, which limited the power of the analysis to detect a statistically significant increase in the incidence of cardiovascular deaths related to ADT use. A pooled analysis of three randomized controlled trials (n = 1372) of RT with or without ADT for immediate- and high-risk PCa also showed that ADT was statistically significantly associated with shorter time to fatal myocardial infarction [82]; however, the overall rate of cardiovascular death was not statistically significantly different between the different treatment arms. Moreover, the analyses included only 51 primary events, and the association was observed only in a subset of men >65 yr.
Longer duration of LHRHa therapy was not associated with greater risk for cardiovascular mortality in recent analyses of RTOG 92-02, a randomized trial of 1554 men treated with short-term versus long-term adjuvant goserelin and RT for locally advanced PCa [83]. Cox regression analyses were performed to evaluate the relationship between the treatment arms and cardiovascular mortality. Covariates included age, prevalent cardiovascular disease, hypertension, diabetes, race, PSA level, Gleason score, and stage. Overall, there were 185 cardiovascular-related deaths. There was no increase in cardiovascular mortality for men receiving longer duration of ADT. In multivariate analyses, traditional cardiovascular-disease risk factors (including age, prevalent cardiovascular disease, and diabetes mellitus) but not the duration of ADT were associated with significantly greater cardiovascular mortality.
In summary, the available literature indicates that even short-term usage of ADT (≤6 mo) may be associated with increased cardiovascular morbidity; however, a clear cut-off for the minimum duration of ADT exposure to cause this adverse event has not been identified. Moreover, the results of the studies addressing cardiovascular mortality are inconsistent. Notably, any increase in cardiovascular mortality–associated ADT would likely be smaller than the observed 16–20% excess risk for incident cardiovascular morbidity observed in the SEER–Medicare studies. The retrospective studies described above included between 57 and 185 fatal cardiovascular events. Retrospective analyses of relatively small studies may be inadequate to detect a modest increase in cardiovascular mortality. Similarly, exploratory analyses of small datasets are prone to chance observations in this setting [84]. Both issues should be taken into account when considering these reports.
3.2.6. The effect of the form of hormonal treatment on treatment-related side effects
Because there are currently three different hormonal approaches (LHRHa, orchiectomy, and antiandrogens), it may be possible that a particular approach is less likely to induce severe side effects; however, the most predominantly used form of hormonal therapy is LHRHa. Literature regarding antiandrogens is sparse, and there are virtually no studies evaluating the side effects of orchiectomy-induced hypogonadism alone. Consequently, it is difficult to compare these approaches. Any form of hormonal therapy either lowers the testosterone level or blocks the testosterone receptors; therefore, it is likely that any form of hormonal therapy may be associated with some sort of treatment-related morbidity. Due to a paucity of available data, definite comparisons cannot be made.
3.2.7. The effect of intermittent androgen deprivation therapy on treatment-related side effects
Intermittent ADT is one approach to hormonal therapy for PCa that has been developed with the aim of minimizing the treatment-related side effects while maximizing the clinical outcome and the patient's QoL [85,86]. The potential advantages of intermittent ADT over continuous ADT are 3-fold. First, intermittent ADT may be associated with a reduced incidence of side effects and, consequently, improved QoL. Second, intermittent ADT may be associated with a prolonged period of androgen dependence of the PCa cells. Third, intermittent ADT is more cost efficient than continuous ADT. Studies comparing intermittent versus continuously administered ADT have demonstrated a reduced incidence of side effects in favor of the intermittent approach. Intermittent ADT, for example, was associated with recovery of BMD in the off-treatment phase [87], a beneficial effect on cognitive function and depression [88,89], and an overall increase in general health-related QoL [90]. This is based on the fact that testosterone levels recover during off-treatment phases [91]. Because the majority of these studies are limited by relatively small sample sizes, it is currently unclear whether intermittent ADT may also reduce the incidence of severe side effects such as diabetes and cardiovascular morbidity.
4. Conclusions
Although ADT was initially applied for treatment of metastatic disease, the number of patients receiving hormones for the treatment of clinically organ-confined and locally advanced disease is continually rising. ADT is now the second most common approach, after surgery, for the treatment of clinically organ-confined PCa in the United States [5,6]. As highlighted in Table 1, there are only three clear indications for the use of ADT: (1) to support EBRT in high-risk patients, which prolongs patients’ OS [21–23]; (2) to downsize the prostate gland before starting brachytherapy (although in this setting ADT does not prolong patients’ OS) [27–31]; and (3) to minimize the risk of fatal cancer-related complications and palliation of symptomatic disease in patients with advanced metastatic disease [18,42]. Additionally, ADT may effectively prolong OS in patients with N+ disease after RP, although the optimal time point to initiate therapy is less clear and studies have produced conflicting results [38,39,41]. The monitoring of the PSA DT might be as effective and reasonable as the use of ADT. Moreover, there is a lack of definitive evidence that ADT is beneficial in all other scenarios in which it is currently used.
ADT is associated with an increased prevalence of various adverse events, such as anemia, decreased cognitive function, impotence, and osteoporosis. ADT is also associated with obesity, sarcopenia, metabolic alterations, and greater risk for diabetes and cardiovascular disease [7–17,54,55,59,67,68,76,82,83,92–95]. This constellation of adverse effects may have important consequences for a population of men who are already at risk for a variety of chronic medical conditions. These risks do not exclusively apply to long-term use of ADT; even short-term use of ADT may induce some of these conditions [17,74,82]. Moreover, ADT in the form of medical castration is costly. A recent report indicated that the costs associated with ADT medication use were as high as $1.2 billion in the United States in 2003 [96]. Finally, the early use of ADT after failure of first-line therapy is associated with a clonal selection of aggressive, androgen-independent PCa cells [37], which may lead to an earlier occurrence of an androgen-independent stage of the disease.
In the light of the above-mentioned data, the progressive increase in popularity of hormonal therapy remains intriguing. There are several possible explanations for this phenomenon. Due to the advent of PSA use, most patients in Western countries with a first diagnosis of PCa present with clinically localized disease [97]. Because these tumors usually have a long natural history, interventions such as surgery or RT may be regarded as overtreatment, especially in older patients and patients with comorbidities. Therefore, many urologists may be reluctant to offer what they consider to be aggressive therapy, such as RP, to patients with significant comorbidities and may prefer using ADT [98].
Another explanation could be that many patients are very concerned when they are diagnosed with PCa or with BCR after therapy with curative intent, and it is a difficult task to assure them that not every detected PCa or BCR requires treatment; many such patients may demand therapy. Some form of treatment, such as ADT, may be offered to patients despite lack of evidence-based proof of a positive impact on OS. Although this explanation is speculative, it does not sound unrealistic to say that if surgical castration were still the standard form of ADT, a substantial number of present indications would certainly be reconsidered. From a practical point of view, it seems advisable to consider further clinical parameters, such as the PSA DT, in those clinical settings before recommending ADT, which may help to identify patients at high risk of disease progression [45].
There is clear geographic variability in the use of ADT [99]. Moreover, the treating physician seems to have a non-negligible effect on the particular treatment decision. Patients of nonacademically active urologists, for example, are significantly more likely to receive primary ADT for localized PCa [100]. Financial interests may also be a factor influencing the choice of administered treatment. A study from the United States demonstrated that the use of LHRHa significantly decreased with a decrease in reimbursement [96]. Conversely, the administration of surgical castration, which is regarded as much more cost effective, simultaneously increased.
In summary, it is obvious that a substantial number of patients are exposed to ADT without evidenced-based proof of efficacy. Because ADT may lead to numerous side effects and because medical castration is costly, it may be inappropriate to offer ADT in some clinical settings. Although the majority of the possible side effects are not life threatening, they may substantially affect patients’ QoL. Physicians should discuss the possible benefits and side effects in detail with patients prior to starting ADT and especially before starting ADT for localized PCa or biochemical failure after definite therapy, as these patients have the highest risk–benefit ratio. Physicians should educate patients and other health care providers about the potential harms and consider greater intensity of screening for osteoporosis, diabetes, and cardiovascular disease [101].
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following:
Matthew Smith: Consultant for: Amgen, GTx Incorporated, and Novartis.
Claude Schulman: Lecturer/Adviser/Investigator for: Astellas, Novartis, Pierre Fabre Medicament.
Francesco Montorsi: Paid Consultant for: Pfizer, Bayer-Schering, Pierre Fabre, AMS, Mipharm, Stryker.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostate cancer. II. The effect of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23. [Google Scholar]
- 3.Huggins C. Endocrine-induced regression of cancers. Cancer Res. 1967;27:1925–30. [PubMed] [Google Scholar]
- 4.Niehans P. Prostata-Krebs wie Paraprostata-Hypertrophie sind Folgen hormoneller Störungen, die wir je früher je besser beeinflussen können. 1940.
- 5.Barry MJ, Delorenzo MA, Walker Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: a population-based cohort study. BJU Int. 2006;98:973–8. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247–54. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oefelein MG, Ricchuiti V, Conrad W, et al. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166:1724–8. doi: 10.1016/s0022-5347(05)65661-3. [DOI] [PubMed] [Google Scholar]
- 9.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–7. discussion 2367. [PubMed] [Google Scholar]
- 10.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–7. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–8. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Smith MR. Osteoporosis and obesity in men receiving hormone therapy for prostate cancer. J Urol. 2004;172:S52–6. doi: 10.1097/01.ju.0000141820.17959.2f. discussion S56–7. [DOI] [PubMed] [Google Scholar]
- 14.Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–82. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- 15.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 16.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 17.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Chodak GW, Keane T, Klotz L. Critical evaluation of hormonal therapy for carcinoma of the prostate. Urology. 2002;60:201–8. doi: 10.1016/s0090-4295(02)01677-1. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A. Management of advanced prostate cancer: gonadotropin-releasing hormone blockers might improve prognosis. Eur Urol. 2008;54:726–7. doi: 10.1016/j.eururo.2008.04.101. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 22.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 23.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Pinthus JH, Bryskin I, Trachtenberg J, et al. Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation therapy. Neoplasia. 2007;9:68–80. doi: 10.1593/neo.06739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 27.Merrick GS, Wallner KE, Butler WM. Permanent inter-stitial brachytherapy for the management of carcinoma of the prostate gland. J Urol. 2003;169:1643–52. doi: 10.1097/01.ju.0000035544.25483.61. [DOI] [PubMed] [Google Scholar]
- 28.Lee WR. The role of androgen deprivation therapy combined with prostate brachytherapy. Urology. 2002;60:39–44. doi: 10.1016/s0090-4295(02)01568-6. discussion 44. [DOI] [PubMed] [Google Scholar]
- 29.Martinez A, Galalae R, Gonzalez J, Mitchell C, Gustafson G, Kovacs G. No apparent benefit at 5 years from a course of neoadjuvant/concurrent androgen deprivation for patients with prostate cancer treated with a high total radiation dose. J Urol. 2003;170:2296–301. doi: 10.1097/01.ju.0000096709.05800.48. [DOI] [PubMed] [Google Scholar]
- 30.Machtens S, Baumann R, Hagemann J, et al. Long-term results of interstitial brachytherapy (LDR-Brachytherapy) in the treatment of patients with prostate cancer. World J Urol. 2006;24:289–95. doi: 10.1007/s00345-006-0083-1. [DOI] [PubMed] [Google Scholar]
- 31.Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048–55. doi: 10.1016/j.ijrobp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Beyer DC, McKeough T, Thomas T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;61:1299–305. doi: 10.1016/j.ijrobp.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006:CD006019. doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830–7. doi: 10.1016/j.juro.2008.01.022. discussion 1837. [DOI] [PubMed] [Google Scholar]
- 35.Wirth M, Tyrrell C, Delaere K, et al. Bicalutamide (Casodex) 150 mg plus standard care in early non-metastatic prostate cancer: results from Early Prostate Cancer Trial 24 at a median 7 years’ follow-up. Prostate Cancer Prostatic Dis. 2007;10:87–93. doi: 10.1038/sj.pcan.4500916. [DOI] [PubMed] [Google Scholar]
- 36.Iversen P, Johansson JE, Lodding P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6. J Urol. 2004;172:1871–6. doi: 10.1097/01.ju.0000139719.99825.54. [DOI] [PubMed] [Google Scholar]
- 37.Tso CL, McBride WH, Sun J, et al. Androgen deprivation induces selective outgrowth of aggressive hormone-refractory prostate cancer clones expressing distinct cellular and molecular properties not present in parental androgen-dependent cancer cells. Cancer J. 2000;6:220–33. [PubMed] [Google Scholar]
- 38.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 39.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 40.Schumacher MC, Burkhard FC, Thalmann GN, Fleischmann A, Studer UE. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol. 2008;54:344–52. doi: 10.1016/j.eururo.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Boorjian SA, Thompson RH, Siddiqui S, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178:864–71. doi: 10.1016/j.juro.2007.05.048. discussion 870–1. [DOI] [PubMed] [Google Scholar]
- 42.Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79:235–46. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 43.Kirk D. Timing and choice of androgen ablation. Prostate Cancer Prostatic Dis. 2004;7:217–22. doi: 10.1038/sj.pcan.4500733. [DOI] [PubMed] [Google Scholar]
- 44.Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24:1868–76. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- 45.Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0–4 N0– 2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891). Eur Urol. 2008;53:941–9. doi: 10.1016/j.eururo.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 46.Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. J Urol. 2004;172:1865–70. doi: 10.1097/01.ju.0000140159.94703.80. [DOI] [PubMed] [Google Scholar]
- 47.Tyrrell CJ, Kaisary AV, Iversen P, et al. A randomised comparison of ‘Casodex’ (bicalutamide) 150 mg mono-therapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33:447–56. doi: 10.1159/000019634. [DOI] [PubMed] [Google Scholar]
- 48.Tyrrell CJ, Iversen P, Tammela T, et al. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU Int. 2006;98:563–72. doi: 10.1111/j.1464-410X.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 49.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 50.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–71. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 51.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474–82. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 52.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–74. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 53.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–4. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 54.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 55.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 56.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–9. doi: 10.1016/S0022-5347(05)00033-9. discussion 139. [DOI] [PubMed] [Google Scholar]
- 57.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 58.Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–6. [PubMed] [Google Scholar]
- 59.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 60.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–6. [PubMed] [Google Scholar]
- 61.Diamond TH, Winters J, Smith A, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–50. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 62.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 63.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 65.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22:110–6. [PubMed] [Google Scholar]
- 66.Tayek JA, Heber D, Byerley LO, Steiner B, Rajfer J, Swerdloff RS. Nutritional and metabolic effects of gonadotropin-releasing hormone agonist treatment for prostate cancer. Metabolism. 1990;39:1314–9. doi: 10.1016/0026-0495(90)90190-n. [DOI] [PubMed] [Google Scholar]
- 67.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 68.Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104:1633–7. doi: 10.1002/cncr.21381. [DOI] [PubMed] [Google Scholar]
- 69.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 70.Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995;154:100–4. [PubMed] [Google Scholar]
- 71.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 72.Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–7. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 73.Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation. 1998;98:398–404. doi: 10.1161/01.cir.98.5.398. [DOI] [PubMed] [Google Scholar]
- 74.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 75.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 76.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–83. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 77.Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–22. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith MR, Lee H, McGovern F, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–94. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 80.Ferrannini E. Metabolic syndrome: a solution in search of a problem. J Clin Endocrinol Metab. 2007;92:396–8. doi: 10.1210/jc.2006-0944. [DOI] [PubMed] [Google Scholar]
- 81.Roach M, III, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG: 8610. J Clin Oncol. 2008;26:585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 82.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–5. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 83.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol. 2008;54:816–24. doi: 10.1016/j.eururo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Roach M., III Regarding the influence of adjuvant suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarction: how real is the risk? J Clin Oncol. 2007;25:5325–6. doi: 10.1200/JCO.2007.13.9931. author reply 5326. [DOI] [PubMed] [Google Scholar]
- 85.Tunn U. The current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlight. BJU Int. 2007;99(Suppl 1):19–22. doi: 10.1111/j.1464-410X.2007.06596.x. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 86.Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U. The role of intermittent androgen deprivation in prostate cancer. BJU Int. 2007;100:738–43. doi: 10.1111/j.1464-410X.2007.07053.x. [DOI] [PubMed] [Google Scholar]
- 87.Higano C, Shields A, Wood N, Brown J, Tangen C. Bone mineral density in patients with prostate cancer without bone metastases treated with intermittent androgen suppression. Urology. 2004;64:1182–6. doi: 10.1016/j.urology.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808–11. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 89.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–81. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer. 2006;42:1083–92. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 91.Mottet N, Lucas C, Sene E, Avances C, Maubach L, Wolff JM. Intermittent androgen castration: a biological reality during intermittent treatment in metastatic prostate cancer? Urol Int. 2005;75:204–8. doi: 10.1159/000087794. [DOI] [PubMed] [Google Scholar]
- 92.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 93.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 94.Smith MR. Osteoclast targeted therapy for prostate cancer: bisphosphonates and beyond. Urol Oncol. 2008;26:420–5. doi: 10.1016/j.urolonc.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 96.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008;112:2195–201. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 97.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–54. [PubMed] [Google Scholar]
- 98.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 99.Akaza H. Trends in primary androgen depletion therapy for patients with localized and locally advanced prostate cancer: Japanese perspective. Cancer Sci. 2006;97:243–7. doi: 10.1111/j.1349-7006.2006.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol. 2007;25:5359–65. doi: 10.1200/JCO.2006.09.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Basaria S. Androgen deprivation therapy, insulin resistance and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]