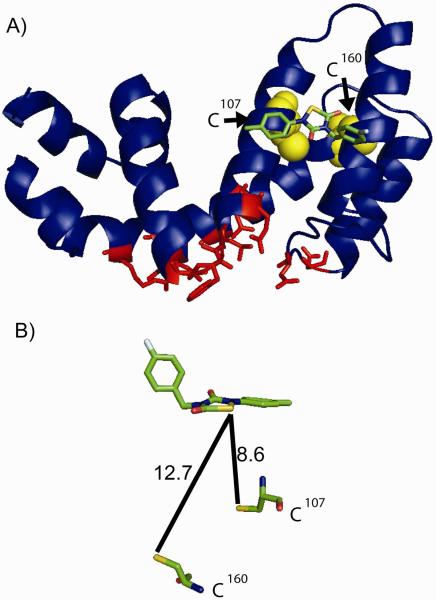
Figure 10. Hypothesized binding site of CCG-50014 on RGS8.
A) CCG-50014 was docked to RSG8 using Autodock software (ver 4.0) as described in Materials and Methods and one docking site showed the greatest predicted affinity (18 μM Ki). This site is far from the Gα binding interaction interface and is near the RGS4 which is important for RGS regulation by calmodulin and acidic phospholipids. Conserved residues between RGS4 and RGS8 that directly contact Gαi in the RGS4-Gαi1 structure (PDB 1AGR (24)) are shown in red. B) Assuming a static protein, this binding site places the compound close to the two cysteine residues in RGS8, but not within a distance compatible with direct covalent reaction. A conformational change must occur in the RGS to allow compound intercalation into the helix bundle. Distances are shown in angstroms. RGS8 structure from 2IHD.