Abstract
This review critically examines progress in understanding the link between Alzheimer’s disease (AD) molecular pathogenesis and behavior, with an emphasis on the impact of amyloid-β. We present the argument that the AD research field requires more multi-faceted analyses into the impacts of Alzheimer’s pathogenesis which combine simultaneous molecular-, circuit-, and behavior-level approaches. Supporting this argument is a review of particular research utilizing similar, ‘systems-level’ methods in mouse models of AD. Related to this, a critique of common physiological and behavioral models is made – highlighting the likely usefulness of more refined and specific tools in understanding the relationship between candidate molecular pathologies and behavioral dysfunction. Finally, we propose challenges for future research which, if met, may greatly extend our current understanding of how AD molecular pathology impacts neural network function and behavior and possibly may lead to refinements in disease therapeutics.
Keywords: Amyloid-β, APP, cognition, dementia, endocytosis, LTD, LTP, neural connectivity, presenilin, tau, rab5, synapse
Introduction: A preface on systems neuroscience
While a wealth of information on the pathological basis of diseases such as Alzheimer’s disease (AD) has been obtained with classic molecular and genetic methods, linking these findings to known cognitive and behavioral symptoms characteristic of the disease has proved difficult. Mirroring this are issues in establishing replicable methods to modify disease pathology which show functional outcomes. This difficulty is perhaps partly attributable to the unfortunately common practice of ‘chaining experiments’: measuring molecular expression in one group of subjects, then later in another group assessing synaptic function, and finally in a last group assaying behavior. Since our perceptions, thoughts, and behaviors are all linked to the interactive dynamics of neural circuits in the brain, this ‘chaining’ strategy is not ideal for understanding the complex and active orchestration of molecules, cells, and circuits in a functioning system.
Begetting a potential solution for this problem are the theoretical and methodological advances championed by modern systems neuroscientists. The field of systems neuroscience has furthered our understanding of perceptually-and behaviorally-relevant neural circuit function by taking advantage of increasingly popular molecular genetic tools, in combination with physiological and behavioral methods. In this ‘systems-level’ approach, the contributions of specific circuits are simultaneously examined in relation to cognitive and behavioral measures. This approach can yield even more powerful results when identified cell types are probed.
It is the thesis of this review that while only rarely applied within the AD research community, a systems-level approach is a necessary tool for understanding mechanisms of this wide-spread disease. Indeed, it is not the expression of a given molecule which results in someone having AD, but how this candidate molecule alters vulnerable circuit function in a manner resultant in cognitive and behavioral dysfunction. This complexity is further compounded during changes in behavioral state such as transitions in arousal, engagement, and attention – issues which systems neuroscientists are tackling with extraordinary progress (e.g., [1]). Thus, these neurobehavioral assays and technological innovations by systems neuroscientists now allow for more tightly-coupled approaches to understanding why certain circuits are often impacted during AD pathogenesis. Furthermore, these systems approaches may prove uniquely powerful in assessing the efficacy of disease-directed therapeutics.
In this review we summarize basic concepts of AD pathogenesis and what is known about how these factors impact synaptic and neural network activity. Throughout this summary we incorporate ideas regarding the strengths of various approaches, especially electrophysiological and behavioral, to understanding disease-relevant cellular perturbations. Finally, we propose several goals for future research which involve assimilating systems-level theories and methods to improve our understanding of AD mechanisms. It is our intention to not only inform, but also to provoke serious consideration regarding the usefulness and meaning of prevailing AD research methods.
Alzheimer’s Disease: Impact and prevalence
AD, the most prevalent form of dementia, is a progressive and fatal brain disease. Persons who suffer from AD experience sensory and memory loss, which together constitute a loss in intellectual abilities severe enough to impede normal daily functioning. In later stages of the disease, functionally debilitating motor loss becomes evident. Approximately 5 million North American’s over 65 years of age suffer from AD – a number projected to triple by the year 2050 due to the growing population of aging persons [2]. AD seriously impacts those who suffer from it as well as their social network, primary care-givers and the North American health-care system. Indeed, it is estimated that the total annual fiscal impact of AD on the North American economy is approximately 148 billion dollars [2]. These factors combined highlight the major societal problem represented by AD.
AD is most often displayed in an idiopathic, sporadic manner – with disease onset occurring typically >65 years of age. Inheritance of the apolipoprotein E epsilon 4 (ApoE4) allele is a strong risk-factor for sporadic, late-onset AD [3, 4]. However, familial AD (FAD) also accounts for AD occurrences, especially those originating early in life (< 65 years of age). AD is classically characterized by the deposition of amyloid-β (Aβ) and neurofibrillary tangles in the brain [5]. Aβ is a processing product of the amyloid-β precursor protein (APP). In 1990, Levy and colleagues published the discovery of a mutation in the APP gene on chromosome 21, resulting is a single amino-acid substitution (E693Q) at position 22 of Aβ in Dutch patients with severe Aβ deposits within their cerebrovascular walls [6]. Since this initial finding, numerous other mutations in the APP gene have been discovered among families with inherited AD (for review see [7]). Genetic testing among FAD populations with similar FAD patterns has provided evidence for the role of genetic loci in chromosomes 1 and 14 in AD [8], leading to the identification of mutations in the genes coding the presenilins, which are part of the γ-secretase complex that generates Aβ. In particular, mutations in the PS-1 (Presenilin-1; [9]) and while less prevalent, PS-2 (Presenilin-2; [10]) genes have been found among families with early onset AD. APP gene duplications are also positively associated with the occurrence of early-onset FAD [11, 12]. Another classic hallmark of AD, neurofibrillary tangles (NFTs), are composed of hyperphosphorylated forms of the microtubule-associated protein tau (MAPT) [13]. The tau protein is encoded by the MAPT gene on chromosome 17. Whereas mutations in the APP gene are closely linked to familiar AD, no such relationship exists for MAPT variations and inherited AD.
Putative factors involved in AD pathogenesis
Amyloid-β (Aβ)
Aβ deposits are widely considered responsible for synaptic elimination and cell death (e.g., [14]). According to work by Braak and Braak [15, 16], Aβ plaque deposits originate first within isocortex, including basal portions of the frontal, occipital, and temporal lobes. In early stages these deposits are sparse and distributed. During later disease stages, the entire isocortex is burdened with dense Aβ plaques, and subcortical structures including the striatum, thalamus, and hypothalamus also host similar, although slightly lesser, levels. Notably, some structures remain mostly void of plaques even during late stages of disease (e.g., substantia nigra).
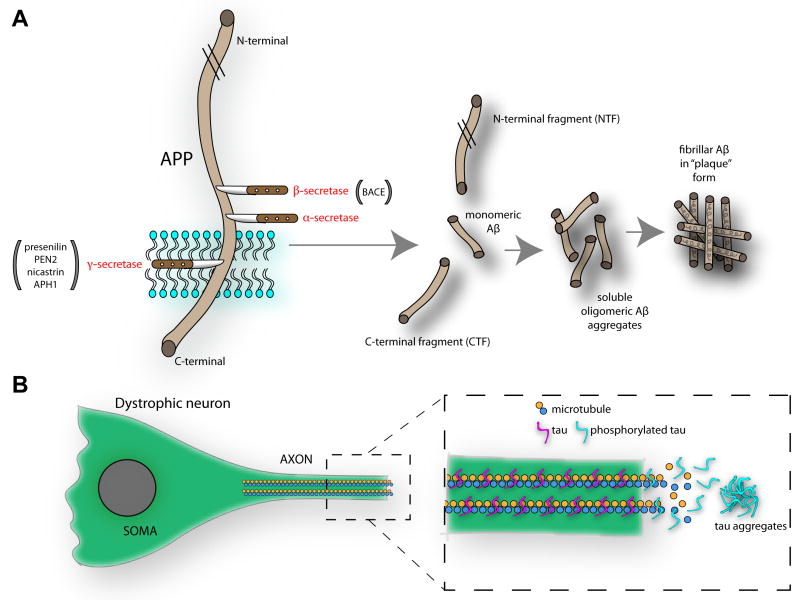
Aβ plaques are small (generally <80μm), spherical, deposits of the 38-43 aminoacid Aβ peptide [17, 18]. The generation of Aβ results from the proteolytic cleavage of APP (Figure 1A). APP is a transmembrane protein with a long extracellular N-terminal part and a short cytoplasmic C-terminus. Cleavage by α-or β-secretase generates N-terminal fragments and α- or β-carboxyl terminal fragments (CTFs). For Aβ to be produced, APP must be cleaved by β- and γ-secretase. Whereas β-secretase (BACE-1, β-site APP-cleaving enzyme [19-22]) cleaves APP within its N-terminal region, γ-secretase cleaves APP within its transmembrane domain, yielding the APP intracellular domain (AICD). Aβ is found in two major forms within the brain, Aβ40 (~90% of total Aβ) and Aβ42 (~10% of Aβ) [23]. Aβ42 was found to be the more toxic species of Aβ and this is supported by FAD mutations resulting in disproportionate Aβ42/Aβ40 ratios [24] which show severe toxicity and dysfunction. However, structure-function studies assessing how different conformations of Aβ impact vulnerable synapses are needed to assess these relationships [25, 26]. Following secretase-dependent metabolism, monomeric Aβ can form Aβ dimers, trimers, and oligomers. Soluble Aβ is secreted by cells depending upon endocytosis and synaptic activity [27-29]. As will be discussed in more detail later, secreted Aβ impacts cognitive function and behavior (in both positive and negative manners) by modulating synaptic function, however, the role for intracellular Aβ, β-cleaved fragments of APP, and the AICD in cellular dysfunction is gaining attention [30-32].
Figure 1. Pathways for Aβ and NFT aggregation in the brain.
(A) The membrane bound amyloid-β (Aβ) precursor protein (APP) undergoes proteolytic cleavage by β and γ secretases, under regulation by multiple factors including BACE and presenilin, PEN2 (presenilin enhancer 2), nicastrin, APH1 (anterior pharynx-defective 1). Following APP metabolism, multiple components are generated, including N- and C-terminal fragments (the later of which also contains the AICD [APP intracellular domain]), and monomeric Aβ that can aggregate to form oligomeric Aβ. The soluble oligomeric Aβ fragments can affect neural function either directly (see Figure 2) or indirectly by aggregating to form Aβ plaques which serve perhaps as storage sites for Aβ-related toxic factors. (B) The microtubule associated protein tau is normally tightly bound to microtubules where it aids in normal axonal transport. However, tau hyperphosphorylation reduces its affinity for microtubules, partly resulting in a breakdown in axonal structure and allowing the filamentous tau to aggregate. This pathological process is responsible for the dystrophic and ‘tangled’ neurons which account for neurofibrillary tangles.
APP metabolism is clearly important in AD pathogenesis. FAD-related gene mutations in APP, PS-1, and PS-2 are implicated in APP metabolism [33, 34]. Further, β- and γ- secretase inhibition reduces Aβ levels in the brain, resulting in reduced Aβ deposition and alleviation of behavioral deficits [35-37]. For instance, inhibition of γ-secretase with the compound DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) decreases cortical Aβ in a dose-dependent manner [36]. Compounds such as DAPT which aim to turn-down the cleavage of APP and consequentially the generation of Aβ have been considered an attractive source for disease modifying therapeutics (but see [38]).
These mechanisms serve as the foundation for the ‘amyloid cascade hypothesis’ of AD [39]. In accordance with this, Aβ levels increase throughout the brain during aging due to an inability of the brain to remove normally (versus pathogenically) produced Aβ. This may be exaggerated in cases of inherited ApoE4 [3, 4]. The accumulation of Aβ both impairs synaptic- and network-level processing of information and results in inflammatory and oxidative damage, which ultimately perturb cognition and behavior resulting in sporadic AD. A similar cascade hypothetically occurs in FAD, however, the increase in brain Aβ levels in these cases are due to missense mutations (APP, PS-1, PS-2).
Alzheimer’s is more than just Aβ
The fact that persons with relatively high levels of Aβ deposition may show normal cognitive function [40-42], combined with the failure of some anti-Aβ therapies to preserve or rescue cognitive function (e.g., [38]) suggests perhaps that not only is Aβ not universally neurotoxic, but also that mechanisms not solely reliant on Aβ also contribute to AD. Indeed, some literature suggests that Aβ’s role in AD is not toxic, but instead is a protective secondary response to the real culprit(s) of AD pathogenesis (for review see [43]). While this review focuses mostly on Aβ effects on neural activity and behavior, several alternative theories are important to discuss in relation to the pathogenesis of AD.
NFTs are inclusions composed mostly of hyperphosphorylated tau. Normally, tau is responsible for the stabilization of axonal microtubules critical for axonal transport and normal cell function and survival [44]. In some cases though tau changes in solubility and becomes hyperphosphorylated at particular residues (Figure 1B) [45]. Such pathology is observed in AD, Pick’s disease, and Frontal Temporal Dementia with Parkinsonism linked with chromosome 17 (FTDP-17) [46]. The hyperphosphorylation of tau reduces its affinity for microtubules, partly resulting in a breakdown in axonal structure and allowing the filamentous tau to aggregate (Figure 1B) [13, 45]. This pathological process is responsible for the dystrophic and ‘tangled’ neurons which account for NFTs. In the AD human brain, NFT pathology originates within the entorhinal cortex, and later is apparent in the hippocampus and neocortex [16, 47]. Tauopathies can be differentiated based upon the tau isoforms which constitute the NFTs. For example, in AD, 3R and 4R tau isoforms are observed, yet only the 3R tau isoform is observed in Pick’s disease [48]. Notably, at least in some conditions (e.g., frontotemporal dementia, FTD) the accumulation of tau is evidently independent of Aβ pathology [46].
Neurofibrillary dysfunction (i.e., NFTs) can be ‘induced’ in mice by placing human tau isoform mutations into the murine MAPT gene [49]. Studies of this mouse model have extended previous correlative work in humans, linking NFTs to AD-like cognitive dysfunction. Although the actual pathogenesis whereby hyperphosphorylation of tau results in cognitive and behavioral dysfunction is very unclear, it is expected that axonal malformations, including axonal swellings, would impede axonal transport of vital energy sources and materials within the cell, thus robbing the cell of its basic health.
The Aβ and NFT pathways integrate in several manners. Multiple lines of evidence suggest that Aβ upregulates NFTs. Double-transgenic tau/APP mice, overexpressing human mutations of both APP (Tg2576 line) and tau (JNPL3 line), have more NFTs than tau mutants alone [50]. Further, intracerebral injection of Aβ [51] or anti-Aβ immunotherapy [52] in tau mutant mice result in altered levels of NFTs, with a positive correlation between Aβ level and NFTs in both studies. On the other hand, tau levels can mediate the neurotoxic impact of Aβ [53]. Highly compelling evidence for this comes from recent work showing that Aβ-dependent axonal transport deficits do not occur in absence of the MAPT gene [54]. Also, phosphorylated tau may modulate Aβ-dependent synaptic defects [55] as will be discussed in more detail later. These lines of evidence allow integration of two prominent AD pathogenic pathways into a more cohesive picture.
Deficient endosomal-lysosomal trafficking can induce cellular dysfunction and even the cell death typical of AD. Abnormalities of endosomes and endocytosis occur at the earliest stages of AD [56] as evidenced by the upregulation of the class of endocytosis-related genes (i.e., rab4, rab5, and rab7) early in the disease within regions most prominently impacted by AD [57]. Further evidence links these endocytic pathway alterations to upstream dependence on APP and βCTF and to downstream consequences on neurotrophin signaling and cholinergic nerodegeneration [32, 58, 59]. As reviewed in detail elsewhere [60, 61], A D-associated genes and disease risk factors contribute to reduced autophagy, and thus impede the normal clearance of damaged organelles and proteins, activated caspases, tau, and even Aβ which may all independently contribute to the neurodegeneration typical of the disease. Enhancing lysosomal proteolysis can partially restore normal cell function and cognition in APP transgenic mice [62, 63].
Oxidative damage and cerebral neuro-inflamation are also important to discuss in the context of understanding how AD-relevant pathological features impact neural circuit function and behavior. Whereas oxidative stress is evident even prior to Aβ deposition [64, 65], microglia-related inflammation proceeds as an apparent function of amyloidogenesis (for review see [66]). Activated microglia are typically observed surrounding Aβ deposits in AD and activated microglia further produce reactive oxygen species [67, 68]. Indeed, oxidative damage markers are increased in brains of persons with AD and in APP mouse models [68, 69]. Oxidative damage and neuro-inflamation are thus considered major factors in the pathogenesis of AD [70], though perhaps secondary to amyloidogenesis (at least in FAD cases). The therapeutic reduction of activated microglia, through for example NSAIDs (non-steroidal anti-inflammatory drugs), is a topic of interest for clinical intervention [71-73].
Summary on AD pathogenesis
Given the manifest purpose of this review to discuss and provoke thoughts on how to better link AD pathogenesis with impairments in synaptic information processing and behavior, we will focus on Aβ modulation of these factors since, as reviewed below, Aβ is a prime culprit in directly modulating synaptic transmission in both FAD and sporadic AD. However, we emphasize that all of the factors discussed above interplay to create the state that we call ‘AD’. A new synthesis of the events leading to AD was recently proposed by Herrup [70] and we suggest this as a reference for those wishing to gain a more gestalt view of AD pathogenesis.
Linking AD pathogenesis to neural dysfunction
Among all of the previously listed pathological features characteristic of AD, it is important to keep in mind that it is the loss of synaptic function, not the accumulation of Aβ or NFTs, nor the occurrence of neuroinflamation and oxidative damage, which likely underlies cognitive and behavioral dysfunction in AD. Within this section we will examine evidence for how synaptic function may be impaired in AD.
Background into electrophysiological studies
Synaptic changes have been at the heart of studies into learning and memory since the late 19th century when Ramon y Cajal postulated the neuron theory [74]. Since then, persistent, activity-dependent changes have been frequently observed within synapses – forming the cellular basis for learning and memory [75]. This is most often modeled within the hippocampal network [76] (Figure 2A). In this model, strong, high-frequency stimulation (termed ‘tetanus’) of Schaffer collateral fiber input to neurons in the CA1 region of the hippocampal formation can evoke long-term changes in post-synaptic neurons (the pyramidal cell neurons), indicated by an enhancement in the excitatory post-synaptic potential (EPSP) termed long-term potentiation (LTP). LTP anatomically coincides with an increase in spine numbers and enlarged spines [77]. Weak or infrequent Schaffer collateral stimulation on the other hand fails to facilitate the granule cell EPSP response. The strong, frequent stimulation is necessary to remove the magnesium blockade of the postsynaptic glutamatergic NMDA receptor (N-methyl-d-aspartic acid) [78] and thus allow entry of calcium ions through the NMDA receptor into the cell (Figure 2B) [79]. While AMPA receptors (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) normally underlie unfacilitated glutamatergic transmission, their membrane expression is upregulated following tetanus and can further support LTP [80]. On the other hand, NMDA receptor activation with persistent sub-threshold stimulation can result in long-term depression (LTD), a reduction in the EPSP amplitude which coincides with a reduction in synaptic contacts [81]. LTP and LTD can function together in an experience-dependent manner to homeostatically adjust synaptic strength based on the history of activity at a given synapse. These forms of synaptic plasticity are generally homosynaptic, i.e., affecting the activated synapses and not affecting other synapses within that cell’s dendritic tree [82]. However, in some cases the induction of LTP at one synapse can facilitate the expression of LTP at neighboring synapses [83]. This type of plasticity, wherein the correlation of pre-synaptic activity with strong post-synaptic depolarization results in strengthening of that synapse, is commonly referred to as Hebbian plasticity, and is considered an effective way of storing experience-dependent information in a neural circuit [75].
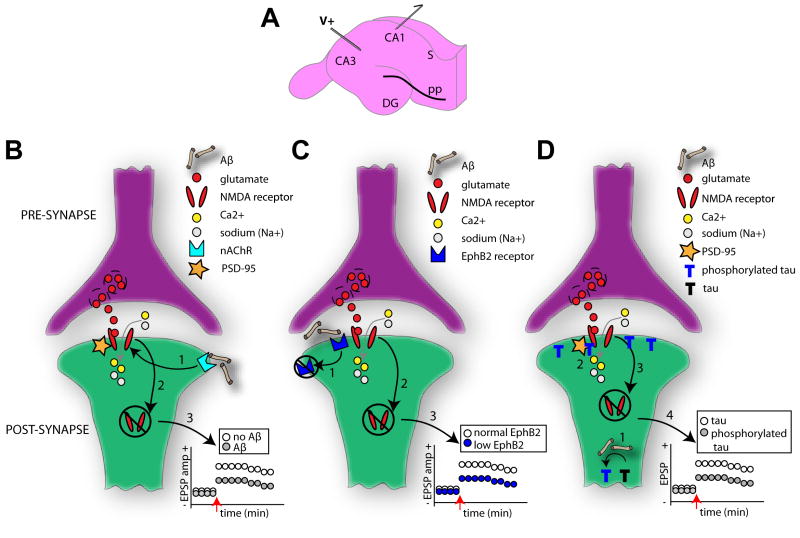
Figure 2. Multiple mechanistic factors contribute to synaptic dysfunction.
(A) Diagram of the hippocampal slice preparation which is commonly used to elucidate mechanisms of learning and memory. Regions CA1, CA3, dentate gyrus (DG) and subiculum (S) are indicated. In this example, a traditional paradigm with electric stimulation (V+) of the Schaffer collaterals and postsynaptic recordings (outgoing arrow) from the hippocampal CA1 pyramidal cells is depicted. Long-term potentiation (LTP) in these synapses is NMDAR (N-methyl-d-aspartic acid receptor) dependent and subject of vast research regarding AD-related cognitive deficits [182]. Several notable models regarding AD mechanisms on cellular learning and memory exist. NMDARs normally allow the influx of calcium (Ca2+) and sodium (Na+) ions upon the binding of glutamate. In one model (B), however, the binding of soluble Aβ to postsynaptic membrane-bound α-7 nicotinic (nAChR) receptors (1) results in the eventual destabilization of the neuronal PDZ protein, PSD-95, which anchors the NMDARs to the synaptic membrane. This destabilization of the NMDAR yields its ultimate internalization (2). This internalization of membrane bound NMDARs reduces the strength of the synapse (for instance less long-term potentiation LTP) (3). In related model (C) [87], Aβ oligomers bind to the receptor tyrosine kinase EphB2 (1), preventing the normal EphB2 – NMDA receptor interaction known important for stabilization of NMDA receptors on the membrane and induction of LTP [88]. EphB2-dependent loss of surface NMDARs (2) thereby reduces synaptic strength (3). Tau is also thought to impact synaptic strength in an Aβ-dependent manner (D) [55]. Hyperphosphorylation of tau, due to Aβ, results in the aggregation of phosphorylated tau in dendritic spines (1). This may lead to reduced stabilization of NMDARs to the PSD complex (2) and ultimately their internalization (3). In this manner phosphorylated tau may reduce synaptic strength (4). Across all of these models the enhanced NMDAR internalization will entail reduced surface expression of AMPA receptors (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and a major change in synaptic efficacy, and ultimately spine loss [89].
Aβ and the synaptic loss
LTP and LTD studies within the hippocampus have provided vast information on the modulation of synaptic function by Aβ. This research has contributed to the synthesis of the Aβ-dependent synaptic degradation theory (for reviews see [84, 85]). Multiple variants regarding the intracellular mechanisms behind Aβ-dependent synapse loss exists. In one model, the binding of oligomeric Aβ to postsynaptic membrane-bound α-7 nicotinic receptors (nAChR) renders neighboring NMDA receptors inactive and ultimately they internalize following a sequence of intracellular signaling events (Figure 2B) [86]. Perhaps complimentary, recent findings suggest direct influences of Aβ oligomers on the receptor tyrosine kinase EphB2 [87], which prevents the normal EphB2 – NMDA receptor interaction, important for stabilization of NMDA receptors on the membrane and induction of LTP [88] (Figure 2C). An additional model [55] proposes that the phosphorylation of tau by Aβ results in more phosphorylated tau accumulating in the synapse (Figure 2D). From there the phosphorylated tau, similar to the previous models, weakens the membrane stability of the NMDA receptors. Across all of these models, the internalization of membrane bound NMDA receptors reduces the strength of the synapse and its ability to express NMDA-dependent plasticity, impacting normal cell-cell communication [86]. This cascade, if persistent, could have detrimental effects on the cell including complete synapse loss. Indeed, Aβ-dependent internalization and endocytosis of AMPA receptors results in the withdrawal or loss of dendritic spines [89]. As an example of this, Shankar and colleagues [90] extracted oligomeric Aβ from the cerebral cortex of humans with AD and examined the modulation of LTP and LTD in the mouse hippocampus. The authors found that Aβ oligomers reliably inhibited LTP, enhanced LTD, and reduced the density of dendritic spines. The effect of Aβ on LTP and LTD was dose dependent and could be blocked with bath application of antibodies to the Aβ N-terminus [90]. Thus, soluble oligomeric Aβ robustly alters synaptic function.
While soluble Aβ is clearly important in modulating synaptic activity (e.g., [90]), the physical presence of Aβ plaque deposits also impacts normal cellular function and synaptic activity. As gross evidence of this, an approximately 50% reduction in spine density is observed within a 20μm region surrounding plaques in Tg2576 APP mutant mice [14]. Further, in AD human tissue, neural processes neighboring Aβ plaques display deviated pathways, seemingly to avoid encroaching plaques which could result in a several millisecond delay in axonal transmission speed [91]. Such a delay could potentially disrupt temporal summation by target neurons and, when summed globally, could substantially disrupt temporal patterns of neural firing. Additionally, highly atypical cellular responses can be observed around Aβ plaques. Busche and colleagues [92] found a mix of suppressed and hyperexcited responses in different populations of cells which surrounded Thioflavin-S positive Aβ plaques (representing β-sheet fibrillar Aβ deposits) in APP model mice. Such hyperactivity within some neurons was associated with a reduction in GABAergic (γ-Aminobutyric acid) inhibition. Hyperactive neurons were found only within proximate regions of plaques. Thus, Aβ does not apparently result in a simple decrease in synaptic activity as the synaptic loss and cell death studies [84, 93] would lead one to believe. Similar recent work in APP mice has shown that abnormal Ca2+ waves among astrocytes can be observed spreading as far as 200 μm from Aβ plaques [94] and that neuritic calcium homeostasis is impaired in mice with, but not without, plaques [95] (but see [96] for an example of how APP expression in cultured neurons inhibits endogenous calcium oscillations). Thus, whether it is a direct toxic effect of Aβ or other APP-related neurotoxic factors (e.g., CTF’s [32]), for which the plaques could be a reservoir, the physical presence of the Aβ plaque has serious implications for neural processing.
These Aβ-dependent changes in synaptic activity can themselves directly impact the synthesis and secretion of Aβ [27, 29]. Increased synaptic vesicle release serves to enhance the accumulation of Aβ within and around the synapse. The persistent mechanisms of Aβ on the synapse, such as the above described NMDA-dependent mechanisms (Figure 2), go on to depress excitatory neurotransmission. Thus, Aβ-induced changes in synaptic activity engage an Aβ-dependent positive feed-back loop which results in the eventual synaptic loss and anatomical modifications (e.g., spine retraction) observed in AD.
Neural synchrony: a simple measure for network dysfunction in AD
A popular avenue of neural analysis in both humans and AD mouse models are measures of neural synchrony [97] using scalp electroencephalogram (EEG) or local field potential (LFP) recordings (Figure 3). These methods reflect summed neural events across large spatial regions and thus activity from thousands of cells. While lacking in spatial precision, these network-level analyses are advantageous when studying principles of information processing. For instance, the encoding of an odor memory requires information to travel from the olfactory bulb, to the olfactory cortex, to the entorhinal cortex, and finally to the hippocampus [98]. An understanding of complete system function therefore must include multi-site recordings which can assay large-scale network function such as that provided by EEG and LFP recordings.
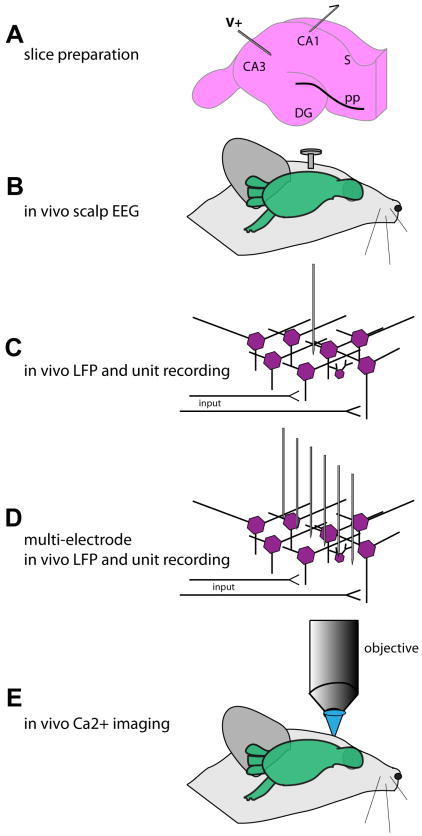
Figure 3. Physiological methods in the study of AD.
A wealth of methods are available in the study of neural circuits in AD models, yet each with its unique strengths and weaknesses. The most common tool in AD model physiology is the hippocampal slice preparation (A) which, while allowing precise recording sites and pharmacological interventions, is in an isolated neural circuit far disconnected from possible behavioral read-outs. Allowing simultaneous physiological and behavioral recordings are the methods depicted in B-E. Whereas in vivo scalp EEG (electroencephalography) presents with relative ease of data collection in comparison to the more precise methods in C, D, and E, it lacks spatial resolution. In contrast, whereas spatially and temporally precise methods shown in C, D, and E allow high levels of resolution, the data collection and analysis can be very time consuming, making the task to interpret large populations of data exceptionally time consuming. LFP = local field potential.
The characteristic EEG in AD contains a shift towards lower frequency rhythms in the power spectrum (a ‘slowing’) and a reduction in fast rhythm coherence [99]. In one early example, 55 out of 71 patients with various forms of dementia had abnormal EEG activity [100]. Further, the authors found that the degree of slowing in the EEG activity was associated with the magnitude of cognitive impairment. EEG studies relating EEG measures with AD pathogenesis are less common though. However, literature looking at EEG throughout stages of AD may allow inference regarding pathogenesis. First, EEG changes appear sensitive to transitions from mild cognitive impairment (MCI) to AD [101], perhaps reflecting a reduction in GABAergic inhibition [92, 102]. Second, during early onset AD, there is an increase in theta power and through disease progression there is a reduction in alpha power [103]. Finally, enhanced theta power and lower beta power in some cranial regions are significantly correlated with upcoming cognitive decline [104]. These differences in cortical EEG power may reflect differential vulnerability of circuits to pathology. Combining what was previously discussed in terms of the synaptic loss hypothesis and events related to neural activity regarding Aβ, these results suggest a possible utility of EEG in inferring Aβ deposition (and possibly other AD-related pathologies including NFTs) within the brains of AD patients. However, such methods would not provide the sophistication in terms of spatial resolution to understand precise relationships between pathology and circuit, cognitive, and behavioral dysfunctions.
Rodent LFP studies provide enhanced resolution to assess impacts of Aβ presence on circuit function. The hippocampal circuit, as commonly studied in AD models, relies upon a network level LFP theta rhythm to entrain neural information [105, 106]. In one recent study, Aβ was injected into the hippocampus of rats and theta rhythm in the septum measured to understand how hippocampal Aβ changes the spread of information to the septum within the theta frequency range [107]. This design is optimal since any aberrant activity in the septum must be due to the Aβ in the hippocampus, whereas, within APP transgenic mice with widespread Aβ deposition, the exact source of dysfunction is unclear. The authors found that Aβ within the hippocampus resulted in a reduction in LFP theta power in this model [107].
Another AD-relevant group of investigations utilizing EEG and LFP recordings come from studies on epileptic activity. AD humans [108] and APP transgenic mice display epileptic seizures which can be observed behaviorally (visible convulsions) and by looking for epileptiform activity with EEG or LFP recordings [109-111]. Anatomically, APP mouse brains display atypical innervation by hippocampal mossy fiber collaterals of inhibitory basket cells [110], a circuit especially implicated in seizure activity. The occurrence of seizure activity seems likely due to fibrillar, but not oligomeric Aβ [109], however, this is quite surprising given the impact of oligomeric Aβ in synaptic function as described above (e.g., [90]). This epilepsy-relevant model presents not only a unique opportunity to understand how Aβ influences circuit function, but also why seizure prevalence is greater in AD than in normal aged individuals [108].
But what about studies of neural function in a behaving model?
Studies of multielectrode single-unit electrophysiology in behaving models are unfortunately even less common. These studies are valuable in that they can probe deep brain structures implicated in AD with single or multi-cell resolution. Within the rat hippocampus, the greatest determinate of cellular firing is the animals’ location in space [112]. A recent study examined the activity of these ‘place cells’ in aged (16 months old) Tg2576 APP mice [113] and found that place cell firing is disrupted. In particular, an increased place field size (the region in physical space wherein the place cell is active) was found in APP mice versus nontransgenic controls. The authors hypothesize that this deficit in place cell activity may underlie known spatial memory deficits in APP mice, and in fact the deficits in place cell firing correlated with spatial memory behavior in these mice [113]. While not exhaustive in how different conformations of Aβ may play roles in this dysfunction, this study is a fantastic example of applying existing ethologically relevant neurocognitive assays, advanced electrophysiological tools, and AD models to understand disease-relevant dysfunction.
Despite the implication of most work on Aβ and neural function, Aβ is not inherently bad. In fact, Puzzo and colleagues recently showed that picomolar levels of Aβ42 monomers and oligomers actually facilitates normal hippocampal LTP [114]. This and other studies which will be discussed later, point towards a multiplexed function for Aβ within neural networks. Whereas too little or too much Aβ negatively impacts synaptic transmission and neural function, a median, nonpathological amount of Aβ within the brain (and local at the synapse) serves a necessary physiological function.
Alternate sources of neural dysfunction in AD
After all this discussion on the regulation of synaptic activity by Aβ, what role is there left for modulation by other pathological factors? As mentioned earlier, hyperphosphorylation of tau results in a breakdown in axonal transport and an aggregation of tau [13, 45]. Teasing apart the effects of tau and Aβ in AD has been difficult since both are hallmark pathologies of the disease [5]. A pivotal recent study by Ittner and colleagues [115] reported that the cellular effects of Aβ are at least in part mediated by tau. Δtau74 mice overexpressing truncated tau (only expressing the projection domain of the human tau 40 isoform) and tau knock-out mice (tau -/-) display atypical postsynaptic targeting of the tyrosine-protein kinase Fyn. Previous work showed that Fyn reductions in APP mouse models reduces Aβ-dependent neurotoxicity [116]. Ittner and colleagues report that this mistargeting of Fyn prevents and even perhaps protects from normal NMDA receptor-mediated Aβ excitotoxicity. In fact, crossing tau-/- or Δtau74 mice with APP23 mice reduced the severity of pharmacologically-induced seizures [115]. Thus, in addition to Aβ-induced exaggeration of tau in APP models [52, 117, 118], tau can also exaggerate the effects of Aβ on neural function.
Recent findings also suggest that presynaptic PS-1 expression modulates LTP [119, 120] with reductions in presynaptic PS-1 associated with weaker LTP. Depleted presynaptic PS-1 levels impair normal Ca2+ release from the endoplasmic reticulum store which ultimately impairs neurotransmitter release probability and thus LTP [119]. These results highlight the impact of pre- and not only post-synaptic mechanisms (as mostly implied in the synaptic loss theory) in AD pathophysiology.
Perhaps explaining the occurrence of LTD in APP models is work on the small GTPase rab5 and its involvement in AMPA receptor internalization. Endosomal-related genes, including rab5, are upregulated in AD-vulnerable brain areas [57]. In the postsynaptic terminal, rab5 activation removes both GluR1 and GluR2 AMPA receptor subunits, thereby facilitating LTD [121]. This facilitates the subsequent increase in rab5 resulting in an activity-dependent scenario where more synaptic activation results in the progressive removal of AMPA receptors from the postsynapse [121]. While this cascade could be considered Aβ-independent, the fact that Aβ can also result in hyperexcitability which consequentially will facilitate rab5 accumulation suggests that these two factors may in some situations indirectly interact to exaggerate pathophysiology. Indeed, APP-dependent apoptosis is at least partially modulated by rab5 [122] and the spatial pattern of rab5 gene expression is associated with high levels of Aβ deposition [57]. Determining whether rab5 changes precede or are a consequence of other AD-related factors (Aβ, βCTF, or AICD elevations) will be an important step in understanding the occurrence of LTD in the disease. Analogous evidence may come from work in Down syndrome, a form of early-onset AD, wherein rab5 changes are driven by βCTF, independently of Aβ (or γ-secretase) [32].
Summary on dysfunctional neural activity in AD
The results of the above studies reflect that synaptic dysfunction can span multiple levels of processing in the brain. While loss of single synapses may go mostly unnoticed during daily functioning, similar losses on a greater scale (hundreds to thousands of synapses) would dramatically alter the dynamics of the cell, the local circuit of which the cell is a part, and all inter-connected regions (e.g., [123]). Long-term dysfunction across these levels may lead to remodeling of vital circuits and thus will entail serious consequences for brain function. Thus, while changes in synaptic strength, membrane dynamics, and spike timing are all critical to local circuit function, it is likely not wise to ignore network-level phenomenon (e.g., neural synchrony) widely-considered important for normal cognitive function [97, 124]. Further, while single cell analyses will provide important and precise clues to AD mechanisms, network-level events may be more feasible for broad, sweeping studies into the impact of particular pathogenic features on neural function. This is especially true when trying to relate function with behavior as alluded among the behavioral studies discussed below.
AD pathogenesis and behavioral dysfunction
Behavioral dysfunction in relation to Aβ
A strong relationship exists between AD biomarkers and the severity of cognitive and behavioral dysfunction [125, 126]. Though sometimes discrepancies with this relationship are reported. Work in AD mouse models is overall supportive in concluding that the spatiotemporal patterns of Aβ are highly correlated with the magnitude of behavioral dysfunction (e.g., [127-131]). Similar to the AD electrophysiological methods, most behavioral assays in AD mouse models are based upon impairments in learning and memory. Research in an early APP mouse model, the PDAPP mouse [132], revealed that spatial memory in a water maze is negatively associated with the accumulation of Aβ deposits in the hippocampus [133]. In this study, PDAPP mice showed age-progressive deficits in water maze behavior, but not object recognition [133]. This finding is intriguing since the regions of the brain responsible for these two behaviors, which were differentially impacted by Aβ deposition, are somewhat overlapping. Regardless, this research started a land-slide of studies linking Aβ deposition in transgenic models with clinically-relevant learning and memory impairments. Occurring around the same time, another APP transgenic mouse line, the Tg2576 mouse [130], also showed that Aβ progression (Aβ40 and Aβ42) was associated with a reduction in spatial reference memory. Importantly, and supportive of the physiological effects discussed above, these data reflect a common theme which has been upheld over the years in AD model research, namely that dysfunction can occur even prior to the development of fibrillar Aβ plaques. Thus, soluble Aβ or other APP metabolites are focal points of much research today. In fact, a recent study by Gandy and colleagues using a new model of mice, expressing the E693Q APP mutant, which produces oligomeric Aβ without parenchymal fibrillar Aβ deposition, reports that elevations in soluble oligomeric Aβ is the prime culprit underlying behavioral disruption (spatial memory in Morris water maze) [134].
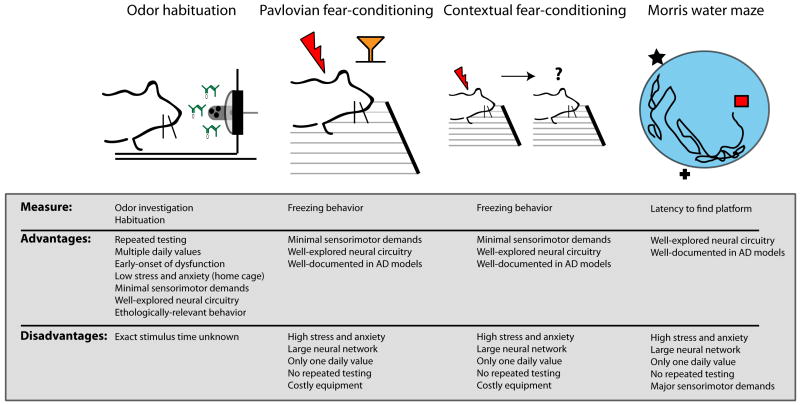
Sensory dysfunction is common in AD [135-138]. Olfactory sensory dysfunction is particularly evident in AD mouse models, including those of both tau [139] and APP [129, 140]. A recent study by our group [129] reported that APP Tg2576 mice display age-dependent progressive loss of olfactory sensory function. In this study, mice were screened in an odor habituation assay which is an ethologically relevant task that relies upon the animal’s intrinsic attractivity to odors to extrapolate measures of odor investigation, odor habituation over repeated short-interval presentations, and even a crude measure of odor discrimination (Figure 4). This task is potentially advantageous to other more-traditional tasks used for phenotyping mice since, as among other things, it allows repeated testing of the same animal (Figure 4). Also, supporting the use of the odor habituation task, another recent work has shown that the odor habituation task yields more significant results when comparing APP with WT mice than Pavlovian fear-conditioning [141]. The odor habituation task revealed that olfactory dysfunction is detectable at just 3 months of age in Tg2576 mice. Also, at 3 months of age, aggregated non-fibrillar Aβ was detected within the outer-most layer of the olfactory bulb (the glomerular layer), but nowhere else in the brain. Thus, the results of this study suggest that early-life olfactory dysfunction is likely attributable to this initial deposition of Aβ within the olfactory bulb [129]. However, other neurotoxic factors related to APP processing (e.g., βCTFs, AICDs), which coincide with the accumulation of Aβ may also play a role in this model [32, 142]. These studies are especially important in AD models since olfactory sensory dysfunction, including deficient abilities to discriminate and recognize odors [143-145], is a prevalent early-indicator of AD and may contribute to not only understanding AD pathogenesis but also enhancing diagnostic sensitivity for the disease [146]. Future studies which examine the neural correlates of impaired odor habituation behavior will provide unique insights into the behaviorally-relevant modulation of synaptic activity by AD pathogenic factors, such as Aβ.
Figure 4. The odor habituation task as a robust behavioral assay in AD model research.
Whereas traditional behavioral assays to phenotype AD model mice have yielded insights into disease pathogenesis, including Pavlovian and contextual fear-conditioning tasks, and the Morris water maze task, novel behavioral models may offer several advantages. The odor habituation task [129], for instance, provides behavioral screening within less than 20 minutes/mouse in their home cage. Further, this assay allows for repeated (longitudinal) testing, relies upon a well-explored neural circuitry (the rodent olfactory system), and imposes minimal sensorimotor demands. This task and others like it which provide numerous advantages over more commonly used assays will allow substantial progress in AD model phenotyping, tracking pathological progression, and in evaluating candidate therapeutics. Such tasks will be especially valuable when combined with simultaneous monitoring of neural activity (such as those described in the later portion of Fig 3).
Manipulations of FAD models, either genetic or pharmacological, have yielded additional insights into the role of Aβ levels and Aβ conformations in behavioral dysfunction. Acute and chronic treatment methods, including Aβ immunization therapy [147-151], and a variety of other methods [65, 152, 153] (just to list a small portion), have revealed rescues of behavioral dysfunction in AD mouse models, yet which do not always correlate with decreases in pathology. Crossing of mouse models so that AD-related mutations are co-expressed within the same mouse has revealed the often compounding effects of FAD mutations on Aβ deposition and behavior. APP transgenic mice crossed with PS-1 FAD mutant mice deposit Aβ earlier in life and by 12 months of age show greater Aβ deposition than the APP mutant mouse alone [154, 155]. In contrast, genetic methods to enhance lysosomal proteolysis to eliminate potentially toxic proteins including Aβ [141], can preserve normal behaviors. Whereas in a 6 month old TgCRND8 mouse, which overexpresses APP with two FAD APP mutations shows high levels of intracellular protein accumulation, including Aβ, accelerated extracellular Aβ deposition, and significant deficits in fear learning and odor habituation behavior, these pathologies are ameliorated and behavior is preserved to WT levels in CBKO/TgCRND8 mice [141]. Whether this preservation is extended into models which serve to remediate Aβ aggregation [156, 157] is yet to be determined.
AD model mice also display abnormal sociosexual and motivated behaviors [158-160]. These findings are potentially interesting when thinking about social behavior issues in AD (e.g., agitation, aggression). The execution of social and motivated behaviors also require coordination of numerous levels of sensory input along with internal states (attention/arousal levels). Thus, these studies present a potentially unique glance at dysfunction in AD. For instance, nest construction is an essential affiliative behavior important for social interaction which also contributes to the survival of offspring. Building nests requires coordinated chewing and pulling of materials with the teeth and forelimbs, as well as the grouping of material into the nest. APP transgenic mice do not construct normal nests [159, 160]. This deficit appears to be induced by mutant APP gene overexpression, but is also exaggerated throughout aging [159] which suggests that it is also influenced by the accumulation of Aβ or perhaps other neurotoxic factors related to APP over expression. In another study, Park and colleagues found that male APP mice display enhanced copulatory behavior both before and after castration. Further, continued copulation in male mice following castration was highly associated with an upregulation of the APP gene in the hypothalamus [158].
Aβ also plays a supportive role in normal behavior [114, 161]. At least two different groups have shown that Aβ is necessary to support learning and memory-based tasks [114, 161]. Similar conclusions may perhaps be drawn from the work discussed above by Park and colleagues on male sexual behavior where greater APP expression seemingly facilitated sexual behavior [158]. Thus, across all of these studies it’s important to consider Aβ not as a static entity (toxic or non-toxic), but that variations in not only Aβ conformation, but also concentration are important in mediating differential effects on behavior.
Behavioral dysfunction in tau mutant mice
Mice overexpressing human tau isoforms also develop progressive behavioral impairments, however, distinctly different from APP mice. Tau mutants are often reported to be free of dysfunction in most commonly used learning and memory tasks despite pathological accumulation in the hippocampus [162]. However, a strong motor-based phenotype is apparent in tau models. For instance, the JNPL3 mouse develops motor deficits and eventually hind-limb paralysis [163]. Further, inhibiting tau phosphorylation, but not removal of NFTs, with a kinase inhibitor (K252A) preserved normal motor function in JNPL3 mice [163]. In addition to motor deficits, tau mutant mice are impaired in olfactory habituation behavior [139] (similar to that mentioned earlier in APP transgenic mice). Further, novel recent work by Scattoni and colleagues has revealed an early social behavior phenotype in the tau mutant mouse P301S [164]. By taking advantage of the natural tendency for mouse pups to emit ultrasonic vocalizations (10-100 kHz) upon maternal separation, the authors revealed that tau mutant pups emit greater abnormally high amounts of ultrasonic vocalizations. Thus, similar to APP mutant mice, tau mutants display a diverse repertoire of behavioral phenotypes which span a variety of bases (cognitive, sensory, motor, and social).
Reflecting the above discussed interactions between Aβ and tau, recent work has also shown that overexpression of tau along with APP, accomplished with a tau/APP cross, may be beneficial in preserving cognitive and motor impairments [165]. The differential role of tau and Aβ in inducing often unique behavioral deficiencies possibly reflects aspects of spatial deposition of such mutations. Alternatively, neural circuits responsible for these behaviors may be especially vulnerable to particular pathologies. For instance, the hippocampal and olfactory systems may be especially vulnerable to Aβ-mediated cell dysfunction (both behaviors are highly correlated with Aβ deposition) whereas motor systems may be more vulnerable to tau pathology. Experiments to tease out these questions will be important in clarifying the roles of these pathologies in AD-relevant dysfunction.
Alternative mechanisms of behavioral dysfunction
Several novel models mimicking the clinically-relevant deficits observed in AD and even some aspects of AD-like pathology are especially interesting in relation to understanding the impact of AD pathogenesis on behavioral dysfunction. One example of this, the FeCy25 mouse [31], over-expresses the APP intracellular domain (AICD), and even though no changes in APP processing or Aβ levels are observed, displays progressive impairments in working memory. Despite the stability of Aβ levels, FeCy25 mice display heightened hyperphosphorylation and aggregation of tau and neurodegeneration. Thus, it is clear that independent of Aβ, the AICD alone can modulate behavior [31]. How the AICD-overexpression directly modulates neural activity in behaviorally-relevant manners remains unresolved.
A need for modeling direct relationships between AD pathology and neuronal and behavioral dysfunction: Future quests
The ever-progressing development of refined AD models, in combination with sophisticated methods to record from identified neural populations, presents an exciting time whereby classic molecular studies can be paired with physiological analyses of neural function in manners to further the understanding of AD-relevant mechanisms. These studies can go far beyond simple assessments of molecular expression, LTP, and behavior – as is typical in AD animal-model research. After all, what does knowing animal X has more Aβ in the hippocampus, and that this type of animal also has deficient hippocampal LTP really tell us about AD pathogenesis? Additionally, could it be that these multistep studies are a likely contributor to the failure of so many putative AD treatments to pass from pre-clinical to clinical success? Indeed, hippocampal LTP, the most commonly studied physiological trait in AD models, is not linked with all forms of learning and memory, cognitive or behavioral processes. NMDA receptor mutant mice show differential abilities for learning and memory (e.g., [166-168]). Thus, perhaps it is not surprising that methods which base outcomes on changes in LTP/LTD have not stood the test of time in providing serious translational understandings.
What can be done to more tightly link molecular characteristics of AD, be it Aβ, tau, cell death, or neuroinflamation, with behavioral dysfunction? The solution may be more precise circuit analyses which closely link circuit function with strictly defined behaviors. Future research on specific cell types which combines genetic model systems, molecular tools, physiological read-outs, and neurocognitive assays will reveal tight understandings between cognitive and behavioral dysfunction in a manner which will expedite and optimize therapeutic interventions. These explorations will be even more fruitful in assessing impact of certain circuit dysfunction when combined with methods to overexpress mutations of APP, MAPT, or the presenilins within particular neural cell types.
Directions for future pre-clinical AD research
1) Enhance diversity of model systems and circuits
Focusing upon the hippocampal circuit which is so well-explored seems advantageous in some regards. However, perhaps the fact that clear relationships between disease pathology and behavior have not been established is telling us something? While learning and memory impairments are a serious component of AD symptomatology, this may not be the ‘gold standard’ method whereby to understand disease pathogenesis. Other methods which are more closely tied to circuit function and behavior might open doors that allow insights into novel mechanisms and relationships. Additionally, methods which simply uncover novel behavioral and circuit-level dysfunction may lead to exciting new findings. One promising model which may meet these demands is the odor habituation task [129]. Also, of consideration for this is the recent emergence of sexual and social behavior screening in relation to pathological expression [158-160, 164]. Given the reliance of rodents on sensory cues and their predisposition to engage in social behaviors these particular avenues of research in AD rodent models may be ideal. Exploring the extent of disease-relevant molecular perturbation on behaviorally-relevant circuit function in these novel models will surely lead to exciting new discoveries.
2) Reduce the use of multi-step experiments
Chaining analyses from mouse to mouse, with each mouse providing different clues to the question at hand likely presents opportunities for unfortunately problematic conclusions. This may be especially evident when comparing in vivo pharmacological outcomes. The failure for many pre-clinical results to translate into success in human studies suggests that while chained experiments may provide clues regarding the most superficial and grossly-obvious disease features, they may not sufficiently reflect the in toto state of the system necessary for proper daily function and survival.
Instead, measuring circuit function in behaving, or at least awake animals will allow direct relationships to be formed between pathogenesis, neural activity, candidate therapeutic approaches, and behavior. Investigations into network activity versus the function of isolated cells is especially important given the importance of network-level function to cognition and behavior, and the relationship of network-level activity to AD (e.g., [40, 99, 169]). Several groups have already begun to make major strides in understanding disease mechanisms through this systems-level approach [92, 110, 113] and those following in their footsteps will undoubtedly offer exceptional insights into not only basic neuroscience mechanisms, but also disease function and therapeutic strategies.
An example of multi-faceted analyses in the same individual mouse include directly addressing the contributions of Aβ deposits to neural processing and behavior by combined in vivo Ca2+ imaging with simultaneous Aβ plaque imaging in behaving mice. Ca2+ imaging of cells loaded with Ca2+ sensitive dyes has provided exceptional insights into the basic principles of neural processing [170-175]. Ca2+ imaging yields superior ability to collect data from hundreds of cells, over a large spatial region at a single time in vivo, making it superior to current multi-electrode methods. Further, the temporal resolution of Ca2+ imaging is several orders of magnitude higher than alternative imaging methods, especially in comparison to fMRI (functional magnetic resonance imaging). In fact, modern analysis methods even allow for the deconvolution of action potential firing from Ca2+ imaging data [176, 177]. Thus Ca2+ imaging in vivo, and especially in awake-behaving animals, wherein Ca2+ responses can be matched with behavioral responses [177-179], presents as a potential major tool in understanding the impact of AD pathological features on circuit function (Figure 3).
3) Devote more energy to the validation of translational models
A wealth of phenotyping tools is available for use in AD rodent models. While all may provide clues to disease mechanisms, not all are easily translatable into the clinical setting. One example of this is the Morris water maze for spatial memory [180]. This task has elucidated numerous basic principles of hippocampal circuit function and is fairly sensitive in phenotyping AD mouse models. However, especially after taking into account inherent confounding variables [181], it is not clear that this test provides translational relevant information. Behavioral tests which directly monitor the function of identified circuits in the brain in mice and can be adapted to the clinical setting are needed to allow direct translational spread of knowledge gained pre-clinically. The same applies to tools that monitor neural activity. Methods such as EEG, LFP, and fMRI in awake animals offer much promise in translating knowledge directly into clinical studies. Ideally, these studies would focus on neural circuits which are most early impacted by disease pathogenesis (e.g., entorhinal cortex and olfactory cortex). Further development and large-scale validation of all of these methods and how they relate to AD molecular markers is needed to allow insights which will stand the test of time clinically.
Conclusions
The science of understanding the molecular basis of AD is at an exciting place in time. It is clear that Aβ and other pathological features of the disease (e.g., tau, βCTFs, endosomal dysfunction) impact neural function which ultimately precipitates aberrant network-level activity. Aβ especially plays multiple critical roles in synapse loss and network dysfunction which seem to impact cognition and behavior. However, direct links between the steps within this network-dysfunction → behavioral dysfunction cascade are very unclear. These gaps in knowledge preclude not only our understanding of basic AD pathogenesis, but also the development of disease-targeted treatments. Given the importance of information processing across a distributed network, versus within isolated brain slices, future studies assessing in vivo network function will be critical to our understanding of AD mechanisms. The ever improving development of AD-like model animals, in combination with the increasing sophistication of rodent behavioral and physiological methods paves the way for great advances in AD research to be made.
Acknowledgments
We would like to thank two anonymous reviewers for kindly providing most thoughtful insights and critiques which improved this review.
Contributor Information
Daniel W. Wesson, Emotional Brain Institute, Nathan S. Kline Institute for Psychiatric Research, New York University School of Medicine; Orangeburg, NY 10962 USA. dwesson@nki.rfmh.org
Ralph A. Nixon, Center for Dementia Research, Nathan S. Kline Institute for Psychiatric Research, New York University School of Medicine; Orangeburg, NY 10962 USA
Efrat Levy, Center for Dementia Research, Nathan S. Kline Institute for Psychiatric Research, New York University School of Medicine; Orangeburg, NY 10962 USA.
Donald A. Wilson, Emotional Brain Institute, Nathan S. Kline Institute for Psychiatric Research, New York University School of Medicine; Orangeburg, NY 10962 USA
References
- 1.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100(3):1160–8. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimers Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Association; Washington, D.C: 2009. [Google Scholar]
- 3.Corder E, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Roses A. Apolipoprotein E is a relevant susceptibility gene that affects the rate of expression of Alzheimer’s disease. Neurobiology of Aging. 1994;15(Suppl 2):S165–7. doi: 10.1016/0197-4580(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer A, Stelzmann R, Schnitzlein H, Murtagh F. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clinical Anatomy. 1907;8(6):429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 6.Levy E, et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–6. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 7.Bertram L, Lill C, Tanzi R. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Cruts M, Hendriks L, Van Broeckhoven C. The presenilin genes: a new gene family involved in Alzheimer disease pathology. Hum Mol Genet. 1996;5(Spec No):1449–55. doi: 10.1093/hmg/5.supplement_1.1449. [DOI] [PubMed] [Google Scholar]
- 9.Clark RF, et al. The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat Genet. 1995;11(2):219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- 10.Sherrington R, et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum mol Genet. 1996;5(7):985–988. doi: 10.1093/hmg/5.7.985. [DOI] [PubMed] [Google Scholar]
- 11.Cabrejo L, et al. Phenotype associated with APP duplication in five families. Brain. 2006;129(11):2966–2976. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- 12.Rovelet-Lecrux A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genetics. 2006;38(1):24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 13.Kosik K. Tau protein and neurodegeneration. Molecular neurobiology. 1990;4(3):171–179. doi: 10.1007/BF02780339. [DOI] [PubMed] [Google Scholar]
- 14.Spires TL, et al. Dendritic Spine Abnormalities in Amyloid Precursor Protein Transgenic Mice Demonstrated by Gene Transfer and Intravital Multiphoton Microscopy. J Neurosci. 2005;25(31):7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Frequency of Stages of Alzheimer-Related Lesions in Different Age Categories. Neurobiology of aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 17.Spillantini MG, Goedert M, Jakes R, Klug A. Different configurational states of beta-amyloid and their distributions relative to plaques and tangles in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(10):3947–3951. doi: 10.1073/pnas.87.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and biophysical research communications. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 19.Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402(6761):537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 21.Yan R, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402(6761):533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 22.Hussain I, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Molecular and cellular neurosciences. 1999;14(6):419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 23.Lewczuk P, et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiology of Aging. 2004;25(3):273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 24.Borchelt DR, et al. Familial Alzheimer’s Disease-Linked Presenilin 1 Variants Elevate A[beta]1-42/1-40 Ratio In Vitro and In Vivo. Neuron. 1996;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 25.Kuperstein I, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO journal. 2010;29(19):3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwald J, Riek R. Biology of Amyloid: Structure, Function, and Regulation. Structure. 2010;18(10):1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37(6):925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 28.Cirrito JR, et al. Endocytosis Is Required for Synaptic Activity-Dependent Release of Amyloid-[beta] In Vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirrito JR, et al. Synaptic Activity Regulates Interstitial Fluid Amyloid-[beta] Levels In Vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 30.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-[beta] in Alzheimer’s disease. Nat Rev Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 31.Ghosal K, et al. Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proceedings of the National Academy of Sciences. 2009;106(43):18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, et al. Alzheimers-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proceedings of the National Academy of Sciences. 2010;107(4):1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price DL, Sisodia SS. MUTANT GENES IN FAMILIAL ALZHEIMER’S DISEASE AND TRANSGENIC MODELS. Annual Review of Neuroscience. 1998;21(1):479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 34.Steiner H, Capell A, Leimer U, Haass C. Genes and mechanisms involved in beta-amyloid generation and Alzheimer’s disease. European archives of psychiatry and clinical neuroscience. 1999;249(6):266–270. doi: 10.1007/s004060050098. [DOI] [PubMed] [Google Scholar]
- 35.Comery TA, et al. Acute {gamma}-Secretase Inhibition Improves Contextual Fear Conditioning in the Tg2576 Mouse Model of Alzheimer’s Disease. J Neurosci. 2005;25(39):8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanz TA, et al. The gamma-Secretase Inhibitor N-[N-(3,5-Difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl Ester Reduces Abeta Levels in Vivo in Plasma and Cerebrospinal Fluid in Young (Plaque-Free) and Aged (Plaque-Bearing) Tg2576 Mice. Journal of Pharmacology and Experimental Therapeutics. 2003;305(3):864–871. doi: 10.1124/jpet.102.048280. [DOI] [PubMed] [Google Scholar]
- 37.Kounnas M, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67(5):769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nature reviews. Drug discovery. 2010;9(10):749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 40.Sperling R, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabinovici GD, Jagust WJ. AMYLOID IMAGING IN AGING AND DEMENTIA: TESTING THE AMYLOID HYPOTHESIS IN VIVO. Behav Neurol. 2009;21(1):117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aizenstein HJ, et al. Frequent Amyloid Deposition Without Significant Cognitive Impairment Among the Elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H-g, et al. Amyloid-B in Alzheimer Disease: The Null versus the Alternate Hypotheses. Journal of Pharmacology and Experimental Therapeutics. 2007;321(3):823–829. doi: 10.1124/jpet.106.114009. [DOI] [PubMed] [Google Scholar]
- 44.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iqbal K, et al. Mechanism of neurofibrillary degeneration in Alzheimer’s disease. Molecular neurobiology. 1994;9(1-3):119–123. doi: 10.1007/BF02816111. [DOI] [PubMed] [Google Scholar]
- 46.Lee VM, Goedert M, Trojanowski JQ. NEURODEGENERATIVE TAUOPATHIES. Annual Review of Neuroscience. 2001;24(1):1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 47.Price J, Davis P, Morris J, White D. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiology of Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 48.Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y. Vulnerable neuronal subsets in Alzheimer’s and Pick’s disease are distinguished by their tau isoform distribution and phosphorylation. Annals of neurology. 1998;43(2):193–204. doi: 10.1002/ana.410430209. [DOI] [PubMed] [Google Scholar]
- 49.Gotz J, et al. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO journal. 1995;14(7):1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis J, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 51.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Abeta 42 Fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 52.Oddo S, Billings L, Kesslak JP, Cribbs D, LaFerla F. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Roberson ED, et al. Reducing Endogenous Tau Ameliorates Amyloid ß-Induced Deficits in an Alzheimer’s Disease Mouse Model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 54.Vossel K, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198–198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoover B, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cataldo AM, et al. Endocytic Pathway Abnormalities Precede Amyloid {beta} Deposition in Sporadic Alzheimer’s Disease and Down Syndrome : Differential Effects of APOE Genotype and Presenilin Mutations. Am J Pathol. 2000;157(1):277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginsberg SD, et al. Regional Selectivity of rab5 and rab7 Protein Upregulation in Mild Cognitive Impairment and Alzheimer’s Disease. Journal of Alzheimer’s disease. 2010 doi: 10.3233/JAD-2010-101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cataldo AM, et al. App Gene Dosage Modulates Endosomal Abnormalities of Alzheimer’s Disease in a Segmental Trisomy 16 Mouse Model of Down Syndrome. J Neurosci. 2003;23(17):6788–6792. doi: 10.1523/JNEUROSCI.23-17-06788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salehi A, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51(1):29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Nixon RA, Yang D-S, Lee J-H. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4(5):590–9. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 61.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai L-H. Amyloid-Independent Mechanisms in Alzheimer’s Disease Pathogenesis. J Neurosci. 2010;30(45):14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller-Steiner S, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51(6):703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 63.Yang DS, et al. Reversal of Autophagy Dysfunction in the TgCRND8 Mouse Model of Alzheimer’s Disease Ameliorates Amyloid Pathologies and Memory Deficits. Brain. 2011;134(Pt 1):258–77. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VMY. Increased Lipid Peroxidation Precedes Amyloid Plaque Formation in an Animal Model of Alzheimer Amyloidosis. J Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Q, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. The Journal of neuroscience. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cameron B, Landreth GE. Inflammation, microglia, and alzheimer’s disease. Neurobiology of Disease. 2010;37(3):503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread Peroxynitrite-Mediated Damage in Alzheimer’s Disease. J Neurosci. 1997;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. Journal of neurochemistry. 1997;68(1):255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 69.Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Annals of the New York Academy of Sciences. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 70.Herrup K. Reimagining Alzheimer’s Disease--An Age-Based Hypothesis. J Neurosci. 2010;30(50):16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson BL, et al. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.014. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nature reviews Neurology. 2010;6(7):405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 73.Andersen K, et al. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? The Rotterdam Study. Neurology. 1995;45(8):1441–1445. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- 74.Ramon y, Cajal S. Neuron Theory or Reticular Theory? Nature Publishing Group; 1954. [Google Scholar]
- 75.Hebb DO. The organization of behavior: A neuropsychological theory. New York: John Wiley; 1949. [Google Scholar]
- 76.Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magee J, Johnston D. Plasticity of dendritic function. Current Opinion in Neurobiology. 2005;15(3):334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. The Journal of Physiology. 1983;334(1):33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 80.Man HY, et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25(3):649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 81.Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Progress in Neurobiology. 2001;65(4):339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- 82.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–41. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 83.Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450(7173):1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selkoe DJ. Alzheimer’s Disease Is a Synaptic Failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 85.Tanzi RE. The synaptic A[beta] hypothesis of Alzheimer disease. Nat Neurosci. 2005;8(8):977–979. doi: 10.1038/nn0805-977. [DOI] [PubMed] [Google Scholar]
- 86.Snyder E, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nature Neuroscience. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 87.Cisse M, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469(7328):47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takasu M, Dalva M, Zigmond R, Greenberg M. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295(5554):491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh H, et al. AMPAR Removal Underlies A[beta]-Induced Synaptic Depression and Dendritic Spine Loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shankar GM, et al. Amyloid-[beta] protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knowles RB, et al. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Busche MA, et al. Clusters of Hyperactive Neurons Near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science. 2008;321(5896):1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 93.Hof PR, Morrison JH. In: The cellular basis of cortical disconnection in Alzheimer’s disease and relating dementing conditions, Alzheimer’s disease. Terry RD, Katzman R, Bick KL, editors. Raven Press, Ltd.; New York: 1994. pp. 197–228. [Google Scholar]
- 94.Kuchibhotla K, Lattarulo C, Hyman B, Bacskai B. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323(5918):1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuchibhotla K, et al. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santos S, et al. Expression of human amyloid precursor protein in rat cortical neurons inhibits calcium oscillations. The Journal of neuroscience. 2009;29(15):4708–4718. doi: 10.1523/JNEUROSCI.4917-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uhlhaas PJ, Singer W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 98.Wilson DA, Stevenson RJ. Learning to smell : olfactory perception from neurobiology to behavior. ix. Baltimore: Johns Hopkins University Press; 2006. p. 309. [Google Scholar]
- 99.Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clinical neurophysiology. 2004;115(7):1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Weiner H, Schuster DB. The electroencephalogram in dementia; some preliminary observations and correlations. Electroencephalography and clinical neurophysiology. 1956;8(3):479–488. doi: 10.1016/0013-4694(56)90014-1. [DOI] [PubMed] [Google Scholar]
- 101.Jelic V, et al. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiology of Aging. 2000;21(4):533–540. doi: 10.1016/s0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 102.Palop JJ, Mucke L. Amyloid-[beta]-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schreiter-Gasser U, Gasser T, Ziegler P. Quantitative EEG analysis in early onset Alzheimer’s disease: correlations with severity, clinical characteristics, visual EEG and CCT. Electroencephalography and clinical neurophysiology. 1994;90(4):267–272. doi: 10.1016/0013-4694(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 104.Claus JJ, et al. Quantitative spectral electroencephalography in predicting survival in patients with early Alzheimer disease. Archives of neurology. 1998;55(8):1105–1111. doi: 10.1001/archneur.55.8.1105. [DOI] [PubMed] [Google Scholar]
- 105.Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci. 1982;2:1705–1711. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buzsaki G. Rhythms of the Brain. Oxford Press; 2006. [Google Scholar]
- 107.Villette V, et al. Decreased rhythmic GABAergic septal activity and memory-associated theta oscillations after hippocampal amyloid-beta pathology in the rat. The Journal of neuroscience. 2010;30(33):10991–11003. doi: 10.1523/JNEUROSCI.6284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amatniek J, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 109.Minkeviciene R, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. The Journal of neuroscience. 2009;29(11):3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palop J, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lalonde R, Dumont M, Staufenbiel M, Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behavioural Brain Research. 2005;157(1):91–98. doi: 10.1016/j.bbr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 112.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 113.Cacucci F, Yi M, Wills T, Chapman P, O’Keefe J. Place cell firing correlates with memory deficits and amyloid plaque burden in Tg2576 Alzheimer mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(22):7863–7868. doi: 10.1073/pnas.0802908105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puzzo D, et al. Picomolar Amyloid-{beta} Positively Modulates Synaptic Plasticity and Memory in Hippocampus. J Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ittner L, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 116.Chin J, et al. Fyn Kinase Modulates Synaptotoxicity, But Not Aberrant Sprouting, in Human Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2004;24(19):4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lewis J. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 118.Gotz J. Tau and transgenic animal models. Brain research reviews. 2001;35(3):266–286. doi: 10.1016/s0165-0173(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 119.Zhang C, et al. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460(7255):632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang D, et al. Inactivation of presenilins causes pre-synaptic impairment prior to post-synaptic dysfunction. Journal of neurochemistry. 2010;115(5):1215–1221. doi: 10.1111/j.1471-4159.2010.07011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown T, Tran I, Backos D, Esteban J. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45(1):81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 122.Laifenfeld D, et al. Rab5 Mediates an Amyloid Precursor Protein Signaling Pathway That Leads to Apoptosis. J Neurosci. 2007;27(27):7141–7153. doi: 10.1523/JNEUROSCI.4599-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andrews-Hanna JR, et al. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freeman WJ. Mass action in the nervous system: Examination of the Neurophysiological Basis of Adaptive Behavior through the EE. New York: Academic Press; 1975. p. 496. [Google Scholar]
- 125.Villemagne VL, et al. Blood-Borne Amyloid-{beta} Dimer Correlates with Clinical Markers of Alzheimer’s Disease. J Neurosci. 2010;30(18):6315–6322. doi: 10.1523/JNEUROSCI.5180-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiology of Disease. 2009;35(2):128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gordon MN, et al. Correlation between cognitive deficits and A[beta] deposits in transgenic APP+PS1 mice. Neurobiology of aging. 2001;22(3):377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 128.Montgomery KS, et al. Novel age-dependent learning deficits in a mouse model of Alzheimer’s disease: Implications for translational research. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.003. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wesson DW, Levy E, Nixon RA, Wilson DW. Olfactory Dysfunction Correlates with β-Amyloid Plaque Burden in an Alzheimer’s Disease Mouse Model. J Neurosci. 2010;30(2):505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 131.Westerman MA, et al. The Relationship between Abeta and Memory in the Tg2576 Mouse Model of Alzheimer’s Disease. J Neurosci. 2002;22(5):1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F [beta]-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 133.Chen G, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408(6815):975–9. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 134.Gandy S, et al. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Annals of neurology. 2010;68(2):220–230. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cacace AT. Aging, Alzheimer’s Disease, and Hearing Impairment: Highlighting Relevant Issues and Calling for Additional Research. Am J Audiol. 2007;16(1):2–3. doi: 10.1044/1059-0889(2007/001). [DOI] [PubMed] [Google Scholar]
- 136.Cronin-Golomb A, Rizzo J, Corkin S, Growdon J. Visual function in Alzheimer’s disease and normal aging. Ann N Y Acad Sci. 1991;640:28–35. doi: 10.1111/j.1749-6632.1991.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 137.Murphy C. Loss of Olfactory Function in Dementing Disease. Physiology & Behavior. 1999;66(2):177–182. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- 138.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in Neurodegenerative Disease: A Meta-analysis of Olfactory Functioning in Alzheimer’s and Parkinson’s Diseases. Arch Neurol. 1998;55(1):84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 139.Macknin JB, Higuchi M, Lee VMY, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human [tau] protein. Brain Research. 2004;1000(1-2):174–178. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 140.Guérin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiology of Aging. 2009;30(2):272–283. doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 141.Yang DS, et al. Reversal of Autophagy Dysfunction in the TgCRND8 Mouse Model of Alzheimer’s Disease Ameliorates Amyloid Pathologies and Memory Deficits. Brain. 2010 doi: 10.1093/brain/awq341. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pimplikar S. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. International journal of biochemistry & cell biology. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knupfer L, Spiegel R. Differences in olfactory test performance between normal aged, Alzheimer and vascular type dementia individuals. International Journal of Geriatric Psychiatry. 1986;1(1):3–14. [Google Scholar]
- 144.Doty RL, Shaman P, Applebaum SL, Giberson R, Sikorski L, Rosenberg L. Smell identification ability: Changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 145.Murphy C, Nordin S, Jinich S. Very early decline in recognition memory for odors in Alzheimer’s disease. Aging, Neuropsychology, and Cognition. 1999;6:229–240. [Google Scholar]
- 146.Wesson DW, Wilson DA, Nixon RA. Should olfactory dysfunction be used as a biomarker of Alzheimer’s disease? Expert Review of Neurotherapeutics. 2010;10(5):633–635. doi: 10.1586/ern.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serrano-Pozo A, et al. Beneficial effect of human anti-amyloid-{beta} active immunization on neurite morphology and tau pathology. Brain. 2010;133(5):1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thakker DR, et al. Intracerebroventricular amyloid-B antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proceedings of the National Academy of Sciences. 2009;106(11):4501–4506. doi: 10.1073/pnas.0813404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Janus C, et al. A[beta] peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 150.Dodart J-C, et al. Immunization reverses memory deficits without reducing brain A[beta] burden in Alzheimer’s disease model. Nat Neurosci. 2002;5(5):452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 151.Schenk D, et al. Immunization with amyloid-[beta] attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 152.Jiang Q, et al. ApoE Promotes the Proteolytic Degradation of A[beta] Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zelcer N, et al. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proceedings of the National Academy of Sciences. 2007;104(25):10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holcomb L, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 155.Borchelt DR, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 156.Mi W, et al. Cystatin C inhibits amyloid-[beta] deposition in Alzheimer’s disease mouse models. Nat Genet. 2007;39(12):1440–1442. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- 157.Kaeser SA, et al. Cystatin C modulates cerebral [beta]-amyloidosis. Nat Genet. 2007;39(12):1437–1439. doi: 10.1038/ng.2007.23. [DOI] [PubMed] [Google Scholar]
- 158.Park JH, Bonthius PJ, Tsai H-W, Bekiranov S, Rissman EF. Amyloid {beta} Precursor Protein Regulates Male Sexual Behavior. J Neurosci. 2010;30(30):9967–9972. doi: 10.1523/JNEUROSCI.1988-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wesson DW, Wilson DA. Age and gene overexpression interact to abolish nesting behavior in Tg2576 amyloid precursor protein (APP) mice. Behavioural Brain Research. doi: 10.1016/j.bbr.2010.08.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deacon R, et al. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behavioural Brain Research. 2008;189(1):126–138. doi: 10.1016/j.bbr.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 161.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learning & Memory. 2009;16(4):267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tanemura K, et al. Neurodegeneration with Tau Accumulation in a Transgenic Mouse Expressing V337M Human Tau. J Neurosci. 2002;22(1):133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Le Corre S, et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scattoni M, et al. Early behavioural markers of disease in P301S tau transgenic mice. Behavioural Brain Research. 2010;208(1):250–257. doi: 10.1016/j.bbr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 165.Morgan D, et al. Apparent behavioral benefits of tau overexpression in P301L tau transgenic mice. Journal of Alzheimer’s disease. 2008;15(4):605–614. doi: 10.3233/jad-2008-15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wilson MA, Tonegawa S. Synaptic plasticity, place cells and spatial memory: study with second generation knockouts. Trends in neurosciences. 1997;20(3):102–106. doi: 10.1016/s0166-2236(96)01023-5. [DOI] [PubMed] [Google Scholar]
- 167.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87(7):1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- 168.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 169.Buckner RL, et al. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Regehr WG, Connor JA, Tank DW. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature. 1989;341(6242):533–6. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- 171.Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32(4):723–35. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 172.Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5(7):657–64. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- 173.Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron. 2001;31(6):903–12. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 174.Knopfel T, Diez-Garcia J, Akemann W. Optical probing of neuronal circuit dynamics: genetically encoded versus classical fluorescent sensors. Trends Neurosci. 2006;29(3):160–6. doi: 10.1016/j.tins.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 175.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. PNAS. 2003;100(12):7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yaksi E, Friedrich RW. Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nat Methods. 2006;3(5):377–83. doi: 10.1038/nmeth874. [DOI] [PubMed] [Google Scholar]
- 177.Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10(5):631–9. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 178.Komiyama T, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464(7292):1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 179.Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6(4):e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 181.Schellinck HM, Cyr DP, Brown RE, Jane Brockmann TJRMNKEW-EJCMH, Leigh WS. Advances in the Study of Behavior. Academic Press; 2010. How Many Ways Can Mouse Behavioral Experiments Go Wrong? Confounding Variables in Mouse Models of Neurodegenerative Diseases and How to Control Them; pp. 255–366. [Google Scholar]
- 182.Colino A, Malenka RC. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. Journal of neurophysiology. 1993;69(4):1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]