Abstract
Seasonal courtship signals, such as mating calls, are orchestrated by steroid hormones. Sex differences are also sculpted by hormones, typically during brief sensitive periods. The influential organizational-activational hypothesis [1] established the notion of a strong distinction between long-lasting (developmental) and cyclical (adult) effects. While the dichotomy is not always strict [2], experimental paradigms based on this hypothesis have indeed revealed long-lasting hormone actions during development and more transient anatomical, physiological and behavioral effects of hormonal variation in adulthood. Sites of action during both time periods include forebrain and midbrain sensorimotor integration centers, hindbrain and spinal cord motor centers, and muscles. African clawed frog (Xenopus laevis) courtship vocalizations follow the basic organization-activation pattern of hormone-dependence with some exceptions, including expanded steroid-sensitive periods. Two highly-tractable preparations—the isolated larynx and the fictively calling brain—make this model system powerful for dissecting the hierarchical action of hormones. We discuss steroid effects from larynx to forebrain, and introduce new directions of inquiry for which Xenopus vocalizations are especially well-suited.
Keywords: Frog, Xenopus, vocalization, courtship, reproduction, androgen, estrogen, steroids, central pattern generator, CPG, larynx
1. Introduction: Hierarchical control of reproductive behaviors by steroid hormones
Steroid-responsiveness varies across the life-span of the organism, with hormone actions falling into two broad categories, organization and activation [1]. In this framework, hormones are thought to “organize” neural and muscular tissues during discrete developmental sensitive periods, endowing animals with the capacity to generate specific behaviors. These organized circuits are “activated” in adulthood, typically producing cyclical changes via the same or overlapping targets. The action of a hormone at a single time on a given tissue can depend on previous hormone action on that target or on a different target.
Early studies, primarily in mammals, focused on hypothalamic and preoptic targets of steroid hormones [3; 4; 5]. However, androgens in particular have also been shown to act on hindbrain and spinal cord motoneurons and the muscles that they innervate, as well as hindbrain interneurons that participate in generating appropriate behavior patterns (central pattern generators, or CPGs) [6; 7; 8]. A hindbrain CPG may be organized by hormones during development, and then later activated by hormones in adulthood. The CPG may also require descending inputs from other hormone-dependent brain centers in order to function properly. Behavioral assays using classical hormone manipulation techniques (for example, castration, with and without hormone replacement) cannot distinguish the site of action of hormones and are blunt tools for dissecting temporal cascades of endocrine effects. Morphological changes in specific CNS regions that correlate with altered behavioral patterns have been identified [9; 10; 11], and one possibility is that they contribute to behavioral modification, but the causal link can be difficult to prove. Neuronal activity patterns with and without hormonal modulation as well as the locations and timing of underlying cellular changes are usually not known. The relation of changes in discrete circuit elements to behavior presents an additional challenge.
We have approached these issues by examining the action of steroid hormones on the neurons and muscles that generate vocal patterns in the South African clawed frog, Xenopus laevis. Vocal behaviors are exquisitely sensitive to endocrine action with marked effects during development and in adulthood [12]. Brain regions that express receptors for pituitary and steroid hormones have been identified [7; 13; 14]. Relating hormone-induced cellular changes to behavioral alterations has been advanced by insights obtained from two reduced preparations. One is the isolated vocal organ, or larynx, in which nerve stimulation in vitro can elicit sounds that closely match songs recorded in vivo [15]. This peripheral “vox in vitro” preparation allows direct tests of how hormone manipulations alter the functional output of the final common pathway, independent of hormone actions on other vocal elements in the CNS. The second is an isolated brain preparation from which nerve activity corresponding to distinct call-types can be recorded [16]. This central preparation has identified the functional components of the hindbrain vocal CPG and facilitates teasing apart the relative contributions of the CPG and descending inputs from midbrain and forebrain sensorimotor integration centers. X. laevis exhibits sex-specific vocal responses that are appropriate to given vocal contexts. Understanding how context is decoded from acoustic features of different calls in different hormonal states should reveal neuroendocrine mechanisms that underlie the coordination of courtship.
2. Xenopus laevis vocal behaviors are hormone-dependent and sexually distinct
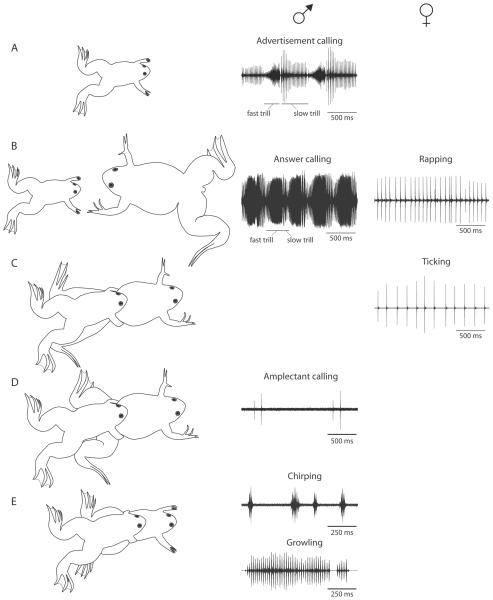
The basic unit of Xenopus calls is a click, a brief, sharp sound produced by a single, effective contraction of intrinsic laryngeal muscles. By modifying click intensity and temporal patterns, adult frogs can produce a sexually differentiated vocal repertoire consisting of seven distinct call types (Fig. 1). Five calls are unique to males, one is female-specific, and one is shared by the sexes. Calls are produced in specific social contexts. The most common male call, the advertisement call, is biphasic, consisting of alternating fast and slow trills [17]. Males produce advertisement calls spontaneously in isolation or when paired with conspecifics [18; 19]. The calls attract females and establish vocal dominance between males [18; 19; 20; 21]. Males respond to vocalizations of a conspecific with the answer call, a form of advertisement call in which the slow trill is shortened and the intensity modulation of the fast trill enhanced [18]. Chirping, growling and male ticking are produced during male-male encounters. Males growl or tick when clasped and chirp when interacting with, or clasping, other males; none of these calls accompanies male-female interactions. The amplectant call is produced when a male clasps a female or another male. The female call rapping is produced in response to imminent oviposition; rapping attracts males and functions as a vocal aphrodisiac, eliciting answer calling by males [19]. In contrast, non-receptive females tick when clasped by a male; ticking suppresses male calling [19] and is part of a suite of behaviors that induces release. Unlike males, females do not communicate vocally with each other. All of the calls described above have been recorded in ponds in South Africa [18]. Most vocally-evoked responses have been shown to also be elicited by playbacks of the appropriate vocalization. Male calling is distinct from that of females in many ways, including the rate of click trills (advertisement call, answer call, chirping and growling are rapid; rapping and ticking are slow), and the production of temporally complex vocalizations (advertisement and answer calls are biphasic and intensity modulated; rapping and ticking are monophasic and monotonous).
Figure 1.
The context-dependent X. laevis vocal repertoire. Drawings in left column depict behavioral context. Examples of male (middle column) and female (right column) vocalizations in each situation are shown. A, Adult males produce advertisement calls in isolation. These songs consist of intensity modulated fast (~60 Hz) and slow (~30 Hz) click trills that are repeated in a stereotyped alternating pattern of short (~200 ms) fast trills and longer (~800 ms) slow trills. Hz: clicks/sec. B, A sexually receptive female (larger body size, with noticeable cloaca, shown to the right of a male) ready to oviposit produces ~12 Hz click trains called rapping. These vocalizations elicit a modified version of the advertisement call—the answer call—in males. Answer calls are distinguished from advertisements calls by elongated fast trills and shortened slow trills and enhanced intensity modulation. C, Sexually unreceptive females convey their sexual status to males both physically—by extending the hindlimbs—and acoustically, by producing a 4 Hz release call, ticking. Together, these behaviors silence the male and facilitate the release of the female. D, Sexually receptive females clasped by males flex the hind limbs to permit oocyte fertilization and are typically silent. Males produce a low-intensity amplectant call that consists of repeating brief 2 – 4 click trills. Click rates within a trill are ~4 – 10 Hz, and trills are repeated about once per second. E, All male calls are used between males, however, two calls exclusively accompany male-male interactions. Chirps, brief fast (~60 – 80 Hz) click trills, are common either without physical contact, or are produced by a clasping male. Clasped males growl (~50 – 80 Hz) or tick (~4 – 8 Hz; not shown).
2.1 Androgen-dependent organization of the vocal pathways
Sexual differentiation of the vocal system begins at metamorphosis and proceeds throughout juvenile development, typically eleven months for males and two years for females raised under laboratory conditions [22; 23; 24; 25]. During this period, females rarely vocalize, but when they do, calls are indistinguishable from adult female ticking [26]. In contrast, maturing males produce rudimentary vocalizations that fall into three broad categories. The first closely resembles adult female ticking (Fig. 2A). Males also produce short, slow (~20 – 50 Hz) trills (Fig. 2, B and C). Finally, some juvenile males produce advertisement-like calling with alternating fast and slow trills that frequently include silent gaps between trill phases (Fig. 2, C and D), a feature absent from the calls of adult males (Fig. 1A). The masculinization of the vocal pathways relies on secretion of testicular androgens, also required for calling in adulthood [27].
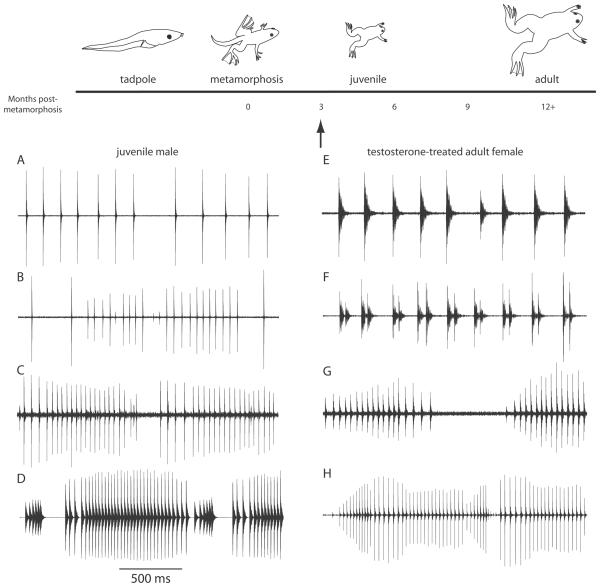
Figure 2.
Development of male-typical vocalizations. Male-like vocalizations are produced by juvenile males long before reaching sexual maturity (~11 months post-metamorphosis, PM). A, Female-like ticking produced by a young juvenile male. B, Example of defeminized calling in a juvenile male; click rates are faster than female calls (>20 Hz), but do not exhibit sustained advertisement call-like rates. C, Juveniles can produce distinct isolated slow trill-like calls. In this example, a few fast trill-like clicks are apparent between the more fully-formed slow trills. D, Example of two consecutive juvenile advertisement calls. Note the silent periods between fast and slow trills. All juvenile male calls (A-D) produced by animals approximately three months PM, or younger (arrow). E, Before testosterone-treatment, adult females tick. F, As early as 2 weeks post-treatment, testosterone implanted females (TFs) begin producing doublet and triplet clicks. G, Isolated slow trill-like calls are produced by TFs after longer periods. H, Within 8 weeks, TFs can produce advertisement like calls, albeit often with silent gaps following fast trills.
Juvenile male vocal “babbling” thus precedes the emergence of the highly stereotyped advertisement calls of adults and could contribute to the functional organization of the male vocal pathway. If so, the development of the CPG that controls vocal patterns may be both activity- and hormone-dependent. If the rate of CPG development is activity-dependent, activity in juveniles could drive earlier attainment of the ability to attract females. Alternatively, the production of rudimentary calling may not be necessary for the proper organization of the vocal CPG, per se, but could rather represent asynchronous maturation of different levels of the vocal pathway. For instance, it is possible that forebrain or midbrain centers that can initiate vocal behaviors become functional before the CPG or larynx are completely developed. Activity could drive vocal production before the hindbrain and neuromuscular effectors are capable of producing normal calls.
As described above, juvenile males and females are initially both capable of producing the female release call, ticking. Later, males gain the ability to produce advertisement calls without losing the capacity to produce ticks. Advertisement calling indistinguishable from that of adult males can be induced in adult females given testicular implants as juveniles [27]. Thus, male-typical vocal patterns can be induced regardless of chromosomal sex, and hormone treatment of females appears to mimic male developmental patterns. Interestingly, testosterone treatment of adult females induces a similar unfolding of vocal development [28; 29]. Initially the ticking of testosterone-treated females (TFs) becomes irregular and includes doublet and triplet clicks (Fig. 2F). This first vocal change thus includes a marked reduction in the minimum interval between clicks. Next, as in developing males, TFs produce slow, trill-like calls (Fig. 2G). Finally, TFs begin producing advertisement-like calls with alternating fast and slow phases. While the calls of adult TFs are clearly masculinized, they never attain a completely male-like quality [28; 29]. In addition, the amount of calling in adult TFs [27; 28] is significantly less than that of adult males. Whether a male deprived of androgen from the end of metamorphosis (either via castration or treatment with anti-androgens) would retain the capacity to respond to androgen as an adult has not been determined. Nonetheless, the observation that females, even as adults, can produce male-like advertisement calls argues that hormone responsiveness is not confined to a sharply delimited developmental window. Click acoustical features, maximum click rates, and long periods of sustained calling do not fully masculinize in adult TFs suggesting that these traits undergo permanent developmental changes during juvenile stages. Below, we describe some anatomical and physiological properties of the hindbrain and vocal organ that respond to endocrine manipulations. Because the isolated larynx and brain remain highly functional in vitro, we have exploited these preparations as a means of directly relating hormone-dependent changes to altered behavioral output.
2.2 Hormonal activation of adult behavior
In adult male X. laevis, vocal production depends on circulating androgens, as in other frogs [30; 31; 32]. Vocal output is dramatically reduced one month after gonadectomy, and is virtually eliminated after one year [17]; androgen replacement restores calling in castrated males. Thus at least some components of the vocal pathway must require androgen stimulation in adulthood to activate calling. Somewhat surprisingly, complete restoration of calling to castrated males was only achieved by gonadotropin administration to androgen-replaced males. Gonadotropin has been shown to act directly on the CNS and its behavioral effects could be due to action in the caudal ventral striatum—now referred to as the central amygdala (CeA) [33]—or the anterior preoptic area (APOA), the two brain regions in the vocal pathway that express gonadotropin receptors [14]. These results indicate that steroid-sensitive forebrain nuclei (both contain estrogen targets [34]) are candidates for a role in vocal activation.
More recently, intensive behavioral assays of castrated males revealed sparse residual calling between five and eighteen months after gonadectomy [35]. Only a small fraction of these animals still produced fast trills, and even those calls were acoustically abnormal: truncated or with reduced sound intensity. Many castrated males produced only isolated slow trills, a pattern that has not been observed in mature males [17; 18; 19], but does resemble vocal patterns observed in developing juvenile males [26] and testosterone-treated females [28]. Changes in components of the vocal circuit that could be responsible for these behavioral alterations include decreased input from the CeA, a degraded CPG, a demasculinized larynx, or a combination. These can be explored, as described below, using the two in vitro preparations—the isolated larynx and the fictively calling isolated brain—in which changes in activity of the vocal muscles and CPG, respectively, can be attributed to hormone-dependent changes in vocal behavior.
3. The sexually dimorphic larynx
Male and female call characteristics differ markedly. Females produce slow (2 – 20 Hz) monotonous calls, while males exhibit faster (up to 70 Hz) click rates with complex temporal dynamics (Fig. 1). What are the relative contributions of the vocal muscles, laryngeal synapse and CNS circuit elements to the observed sex differences in behavior? The sound-production mechanism in Xenopus is simplified relative to terrestrial vertebrates because it is not tied to actual inspiration and expiration. Bilateral contraction of paired laryngeal muscles produces opening of two, normally apposed, cartilaginous discs. Tension transients produced in the tendon connecting the muscles to the discs, when of sufficient magnitude, are associated with a click [15].
The X. laevis larynx remains functional in vitro. A single bilateral electrical stimulus delivered to the laryngeal nerves of the isolated larynx results in a single click with similar acoustic structure to that produced by actual frogs [15]. Tension transients and sounds produced by isolated male and female larynges reveal that functional properties of laryngeal muscles are highly sexually differentiated. For example, a 33 Hz train of stimulus pulses (faster than any female call) delivered via the nerve to the isolated female larynx results in a single click at the beginning of the train, followed by silence and fused muscle tension (fused tension: the muscle does not relax completely, thought required for producing another click). In contrast, the same rate of stimulation of male larynges produces a click following every pulse. Even at fast trill-like stimulus pulse rates, male larynges produce a click for each pulse, while tension records from female muscles exhibit partial tension transients insufficient to produce sound (compare Fig. 3A to B). Tension, EMG and click amplitudes increase progressively within a stimulus train in males, but not in females. In males, potentiation correlates with progressively increased click intensities within a fast trill [15]. The physiological characteristics of male and female larynges are well-matched to the demands of their sex-specific vocalizations: slower, monotonous trills in females and faster, intensity modulated trills in males. Are these sex differences due to sex differences in exposure to hormones and, if so, when and how do hormones exert their effects?
Figure 3.
Sexually differentiated contractile properties of the larynx. A, Tension transients are measured from the laryngeal tendon following 10 Hz (left) and 70 Hz (right) stimulus trains applied to the laryngeal nerve. The progressive increase in tension in response to a 10 Hz train is mediated by weak neuromuscular synapses and the resulting recruitment of muscle fibers within a train. At 70 Hz (the upper limit for fast trill click rates), muscle tension fully relaxes between each stimulus pulse. B, Laryngeal synapses in females are strong resulting in large tension values after a single stimulus pulse and little potentiation within a 10 Hz train. Contractile and relaxation rates of female laryngeal muscle fibers are slow, leading to mostly fused tension during stimulation trains at male call rates. C, Larynges of TFs are masculinized; more potentiation is seen compared with intact-females, and tension transients become fully masculinized. D, Long-term castration (more than five months) leads to a decrease in potentiation during 10 Hz trains, and an increase in fused tension in response to 70 Hz stimulus trains.
3.1 Hormone-dependent development of sexually differentiated laryngeal properties
Fiber number
What specific properties contribute to the observed differences in contractility of male and female laryngeal muscles? The number of adult male muscle fibers is significantly higher than in females [36]. At metamorphosis, a difference in fiber number is not apparent, but rather develops over the next 6 months (Fig. 4A). Muscle fiber addition requires proliferation and fusion of myoblasts and androgen has been shown to evoke myogenesis in juvenile larynges [37]. Male-typical fiber numbers are maintained even three years after castration [38]. The development of male-like fiber numbers can be blocked by treatment with the anti-androgen flutamide [36] or by castration [39] at metamorphosis. Female fiber number can only be partially masculinized by testosterone (T) treatment at metamorphosis [39], dihydrotestosterone (DHT) in early juveniles [40], or by testicular implants (but not T) in adulthood [38].
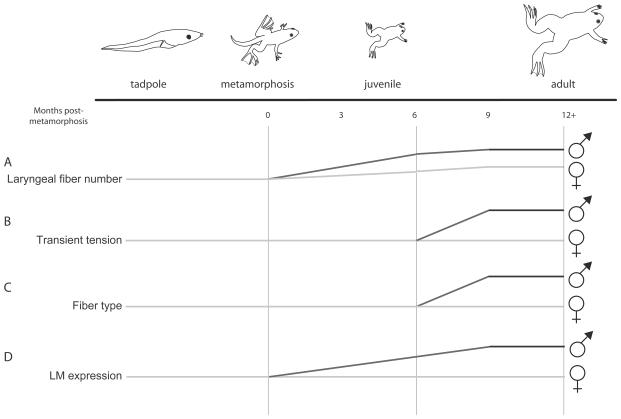
Figure 4.
Patterns of androgen-dependent masculinization of laryngeal traits. A, Laryngeal fiber number increases in both males and females during post-metamorphosis (PM) life. Males add muscle fibers faster than females between 0 and 9 months PM at which point male numbers are similar to adult values. B-C, Transient tension and muscle fiber twitch type become masculinized starting at 6 months PM; both are fully masculinized before adulthood. D, Expression of laryngeal myosin (LM) begins increasing in males after metamorphosis, and peaks by about 9 months PM.
One possible explanation for failure to fully masculinize female fiber numbers with T is that androgen secretion in male tadpoles increases the number of myoblasts capable of producing muscle fibers during juvenile stages. DHT treatment increases fiber numbers in tadpoles [41], although this relative increase is not maintained. DHT treatment in tadpoles may have organized the capacity for female attainment of male fiber numbers but lower circulating androgens of females prevented fiber number increase. If so, exposing female tadpoles to DHT and then implanting testes at metamorphosis or adulthood should permit fully masculine fiber numbers. The onset of androgen action on the larynx is determined by rising titers of thyroxine late in tadpole development [42]. Androgen action during late tadpole development could set the maximum adult fiber number.
Transient and fused tension
In early juvenile stages, trains of nerve stimulation lead to laryngeal muscle fused tension in both sexes [22]. As development proceeds, transient tension increases in males but not females [22]. Castration prevents increases in transient tension [43]. In some studies, tension profiles were partially masculinized by brief (4 weeks) testosterone treatment in adult females [15]; in other cases, similar periods of testosterone treatment fully masculinized juvenile [43] and adult [29] female larynges. Potter et al. [29] showed that both twitch rise and relaxation times decreased in TFs, accounting for the observed decrease in fused tension between stimulus pulses (Fig. 3C). Thus, the ability of laryngeal muscles to contract at male-like rates is androgen-dependent and is normally acquired during juvenile stages (Fig. 4B). This ability however is not limited to males or a particular developmental stage.
Twitch type and laryngeal myosin expression
Sex differences in muscle tension reflect differences in laryngeal muscle fiber types. Using histological techniques to assay myosin ATP-ase activity and oxidative capacity [44; 45; 46; 47], Sassoon et al. [48] found that all adult male laryngeal muscle fibers are fast twitch and fatigue-resistant, while female fibers are mostly slow twitch, with only a small population of fast twitch fibers. The finding that male fibers are both fast twitch and low-fatigue suggests that androgen-directed larynx development is permissive for producing fast trills for long uninterrupted periods. Until six months after metamorphosis, both male and female larynges have an adult female-like composition: mostly slow twitch [22]. Males have nearly achieved adult male-like numbers at this time suggesting that the larynx of juveniles adds both fast and slow-twitch fibers during this period [39]. The percentage of slow-twitch fibers then starts to decline in males reaching adult values (0%) at 9 months after the end of metamorphosis (Fig. 4C). The fast twitch fibers in the adult male larynx all express a larynx-specific myosin heavy chain (LM) whose expression is much weaker in adult female muscle [49]. Myosin heavy chain type predicts twitch speed in Xenopus muscle fibers [50] and LM expression is presumed to contribute to the all fast twitch character of adult male laryngeal muscle.
Expression of LM increases in male juveniles from metamorphosis until it is fully masculinized around nine months after metamorphosis (Fig. 4D) [49]. Long-term (five months) testosterone treatment of adult females partially masculinizes fiber types. Testosterone treatment in juvenile males and females, however, induces rapid (five weeks) and nearly complete masculinization of fiber type [48]. Also, castration of juvenile males halts the development of male-typical fiber type [43]. Thus, juvenile male and female larynges are highly sensitive to androgen, which establishes male-typical fast twitch profiles. DHT treatment of juvenile females elevates LM expression, while castrating juvenile males decreases expression [51]. Thus, both fiber twitch type and LM expression (presumed to account for adult twitch type) are androgen-sensitive in juveniles which likely accounts for the observed sexual dimorphisms.
Studies in another species of Xenopus (X. tropicalis) whose genome has been completely sequenced (allowing assessment of relative contributions of different myosin heavy chain isoforms) supports the idea that the androgen sensitivity of LM expression accounts for sex differences in muscle contractile properties. Male X. tropicalis produce rapid calls and all fibers are fast-twitch [52]. The family of myosin heavy chain genes includes an embryonic myosin expressed only in the larynx (xtLM) whose expression is androgen-regulated [52]. Treating juveniles with androgen for two weeks shifts the relative expression of this LM from 0.3% to 52%.
Studies in X. laevis (B. T. Nasipak and D. B. Kelley, submitted for publication) suggest that proliferation of a specific population of myoblastic cells, satellite cells, is required for fiber-type switching. Unlike other myoblastic populations, satellite cells [53] are found even in adult muscle where they can contribute to muscle repair [54]. These cells are identified via expression of the transcription factor, Pax7. One hypothesis for the relation between proliferation and conversion of muscle fibers in males from slow to fast during late juvenile development (Fig. 4C) is that the Pax7-expressing myoblasts also express LM. When enough of these satellite cells fuse with an existing fiber, myosin heavy chain expression switches to the LM form and all fibers then become fast twitch (Fig. 5).
Figure 5.
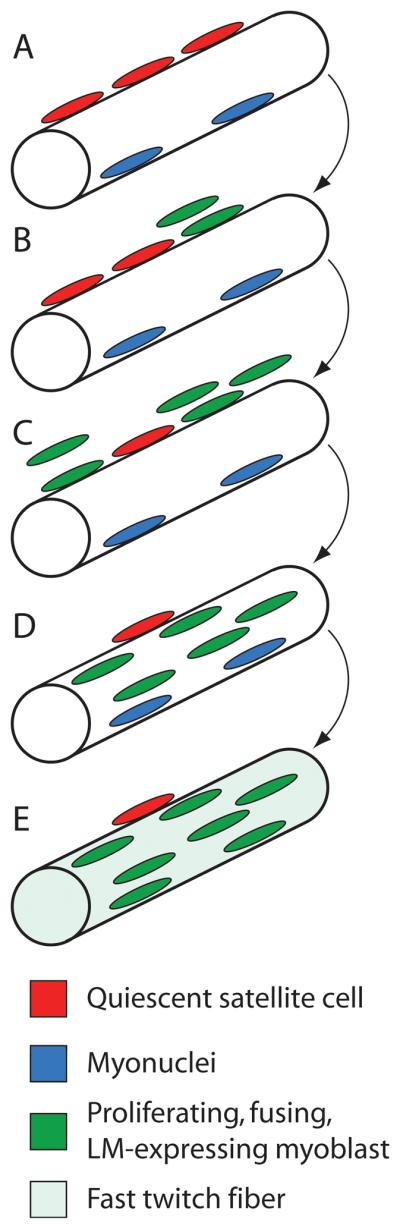
Proposed mechanism for fiber type switching in male Xenopus larynges. A, A multinucleated (blue), laryngeal muscle fiber with adjacent, quiescent (non-proliferating), Pax-7 positive satellite cells (red). B-C, Satellite cells start to proliferate and produce fusion-competent, myoblast progeny (green). D, These LM-expressing myoblasts fuse with the parent muscle fiber. E, At a critical ratio of LM-expressing (green) to non-expressing (blue) myonuclei, myosin heavy chain expression switches to the LM isoform and the muscle fiber is fast twitch.
Taken together the available evidence suggests that expression of a larynx-specific myosin heavy chain is responsible for the contractile properties of adult male laryngeal muscle fibers. Both male-specific fiber numbers and LM expression depend on androgen secretion, most likely because a different sub-population of myoblasts is responsible for each. One population (early myoblasts) may require androgen during tadpole stages for survival and amplification (an organizational effect). Androgen would then promote further proliferation during early juvenile stages and ongoing fusion to form new fibers. The second population (satellite cells) would determine LM expression during late juvenile stages via fusion with existing myoblasts to switch fiber types. The existence of two independent myoblast populations would explain why androgen treatment in juvenile females greatly masculinized LM expression while only slightly increasing fiber numbers.
Synaptic strength
The intensity of male muscle contractions increases progressively (potentiates) during stimulus trains delivered to the nerve, while female contractions potentiate very little (Fig. 3, compare B to A). Some male muscle fibers require a few nerve stimuli to produce action potentials while others require more, leading to progressive increases in the numbers of muscle fibers that contract [55]. The sex difference in potentiation also reflects a sexually differentiated laryngeal synapse. Quantal content (representing the probability of neurotransmitter release from the pre-synaptic terminal) of adult female synapses is higher than in adult males [56]. Quantal content is not sexually dimorphic in juveniles, but estrogen-treatment can increase quantal content in juveniles of both sexes and adult males, while DHT-treatment decreases quantal content of juvenile females [57]. Ovariectomy prevents the development of female-typical quantal content, and leads to defemininization of mature larynges [58]. Paradoxically, gonadotropin-induced elevated estrogen levels correlate with decreases in quantal content [59]. One possibility is that synapse strengthening occurs via genomic mechanisms, while acute synapse weakening by estrogens occurs through a non-genomic signaling pathway. Together, these data indicate that muscle potentiation in males, as observed during stimulus trains and in vivo fast trills, occurs because the weak synapses of males strengthen with use and also because progressively more fibers contract. Estrogen-strengthened synapses in females may allow females to continue to call during gonadotropin surges with corresponding synapse weakening.
In summary, sexually differentiated larynges contribute to behavioral differences between the sexes. Sex differences in fiber number, fiber type, and LM expression are absent until after metamorphosis (see Fig. 4). Sex differences in all traits (except quantal content) are induced by endogenous androgens in males, or can be (partly or fully) masculinized by exogenous androgen exposure in juvenile and adult females, though masculinization tends to be faster and more complete in juveniles. At metamorphosis, male and female larynges both highly express androgen receptor (AR) [60]. Androgen secretion itself does not become sexually differentiated until mid-juvenile periods [61]. Female AR expression decreases more steeply during juvenile development than males, possibly accounting for some of the emerging sex differences [60].
Thus masculine traits in the larynx develop during juvenile stages and are androgen-dependent. In the case of fiber number and twitch type, androgen-induced changes are permanent (as is the case for classical organizational effects in mammals). Other traits, such as transient tension and synaptic strength, depend on hormone secretion in adulthood (as is the case for classical activational effects in mammals). While many traits are more hormone-sensitive during development, all traits (fiber number, tension, LM expression, twitch type, synaptic strength) can be masculinized to some extent in adult females. That females can produce advertisement-like vocalizations with only partially masculinized laryngeal properties suggests that no single male-typical trait is absolutely essential for advertisement calling. Rather, the fully mature male larynx is exquisitely well-adapted for generating long periods of fast, intensity-modulated advertisement calls. The most prominent female-typical laryngeal characteristic—a strong vocal synapse—may ensure that the slower female calls are expressed prominently, even without the fiber recruitment available to males.
3.2 Hormonal regulation of adult male and female larynges
In adulthood, male laryngeal muscles continue to express ARs and hormonal activation of the vocal pathways is partly mediated in the larynx. Watson and Kelley (1992) found that long-term castration resulted in a slight (~15%) increase in fused (non-transient) tension (in response to 71 Hz nerve stimulation) in male larynges. More recently, Zornik and Yamaguchi [35] found more fused tension (~ 47%) at a similar stimulation rate (Fig. 3D), but a few animals were still able to produce some, albeit abnormal, fast trills (either truncated in duration or with decreased sound intensity). These findings suggest that even significant fused tension does not entirely eliminate fast trills. Two male muscle fiber properties—fiber number and twitch type—are permanent and not demasculinized following castration [38; 48], indicating that these properties are not responsible for the increased tension. Expression of LM, which is androgen-sensitive during juvenile periods (described above), may remain dependent on circulating androgens to maintain fully masculinized levels. The decrease in potentiation in larynges of castrated males [35] (Fig. 3D) may be due to alteration in strength of the laryngeal synapse.
4. Vocalizations are produced by a sexually differentiated hindbrain CPG
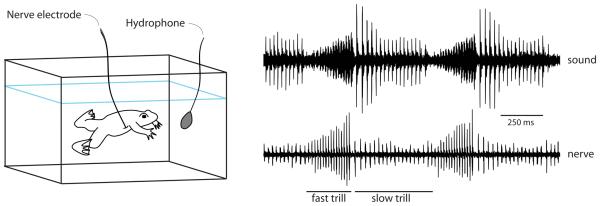
While the larynges of male and female X. laevis are well-suited to support their respective call types, how are they driven by nerve activity in the CNS? En passant recordings from the laryngeal nerve in males and females during actual calling [62] reveal activity that is strikingly similar to the actual sounds produced—each vocal click is immediately preceded by a compound action potential (CAP) in the laryngeal nerve (Fig. 6). Thus behavioral output is mostly driven by inputs from the CNS, with the larynges of each sex able to transduce nerve signals into sound.
Figure 6.
Nerve activity matches sound production. Yamaguchi and Kelley [62] recorded laryngeal nerve activity from singing frogs (depicted at left). Example sound oscillograms (top, right) of male advertisement calls show that each click is preceded by a compound action potential (CAP) on the laryngeal nerve (bottom, right). Thus, the pattern of clicks produced by the larynx is dictated by inputs from the brain.
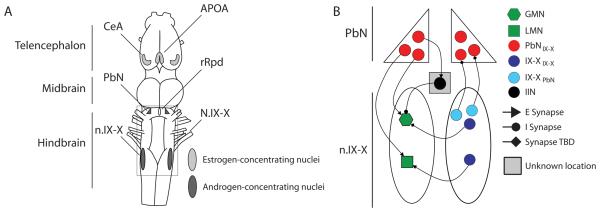
How are the vocal patterns themselves generated? Axons in the laryngeal nerve (N. IX-X) originate in neurons of motor nucleus IX-X (n.IX-X) in the caudal medulla, which also contains interneurons [63]. The primary input to n.IX-X comes from the parabrachial nucleus (PbN) of the rostral hindbrain (previously called the dorsal tegmental area of the medulla, or DTAM, in X. laevis [63; 64; 65; 66] or pretrigeminal nucleus in Rana pipiens [67]). Neurons in the PbN project bilaterally to n.IX-X, contacting both motor neurons and interneurons [65]. Interneurons in n.IX-X also send robust bilateral inputs rostrally, back to the PbN (summarized in Fig. 7). These two reciprocally connected vocal nuclei concentrate androgens and express AR in X. laevis [7; 13] and are thought to collectively generate vocal patterns in the terrestrial frog Rana pipiens [67].
Figure 7.
The vocal nuclei of X. laevis. A, Diagram of the Xenopus brain (anterior is up) shows known vocal nuclei. In the telencephalon, the central amygdala (CeA) and anterior preoptic area (APOA) are estrogen targets and have known connections with hindbrain vocal nuclei, which consist of the parabrachial nucleus (PbN) in the rostral medulla, and the caudal laryngeal motor nucleus, n. IX-X. B, Details of hindbrain circuit (box in A). Motor neurons that innervate the larynx are located in cranial motor nucleus n. IX-X which also contains glottal motor neurons (involved in breathing, but not calling) and premotor interneurons. Commissural interneurons (IX-XIX-X neurons) connect n.IX-X bilaterally. The major descending input to n.IX-X comes from PbN, with direct inputs to both motor neurons and interneurons (PbNIX-X). These nuclei are reciprocally connected, with n.IX-X neurons projecting back to PbN (IX-XPbN). Electrical stimulation of PbN results in spiking of laryngeal motor neurons and inhibition of glottal motor neurons. This latter connection must be mediated via inhibitory interneurons, though their location has yet to be identified.
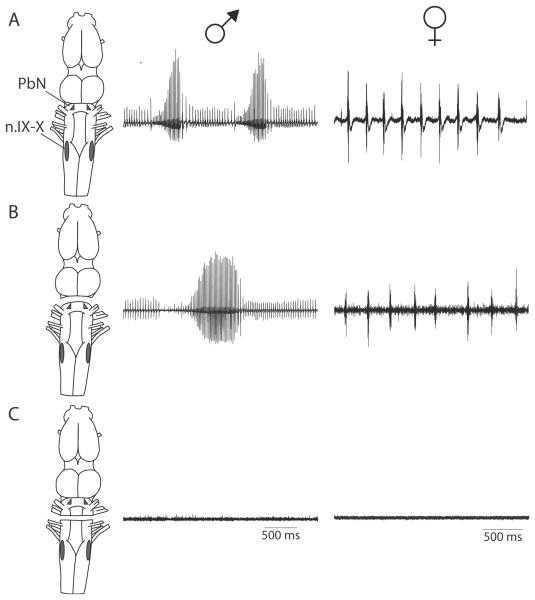
In an isolated brain preparation from X. laevis, serotonin (5-HT) bath-application elicits patterns of nerve CAPs nearly identical to those recorded by Yamaguchi and Kelley [62] in calling frogs in vivo (compare Fig. 6 to Fig. 8A) [16]. In these experiments, transections severing the connections between the PbN and n.IX-X eliminated 5-HT-induced fictive vocalizations (Fig. 8C). In contrast, fictive calls can still be elicited after transecting immediately anterior to the PbN (Fig. 8B), thus removing the forebrain and midbrain but preserving connections between the PbN and n.IX-X [68]. Thus, vocalizations are generated by a hindbrain CPG that includes the PbN and n.IX-X.
Figure 8.
Identifying the vocal CPG. A, Bath-application of serotonin (5-HT) to isolated male or female brains elicits fictive advertisement calling or ticking, respectively, that can be recorded as CAPs in the laryngeal nerve. B, Transections placed immediately anterior to PbN do not eliminate fictive calling, but transections caudal to PbN (C) do. Local field potential recordings in PbN reveal that vocal activity is eliminated following transections caudal to PbN (not shown), indicating that the vocal CPG encompasses neurons in n.IX-X and PbN, and that the reciprocal connections between these nuclei are required for vocal pattern generation.
In this section, we describe sexually differentiated elements within the hindbrain CPG, and discuss the effects of hormone manipulation on these elements. We finish by describing recent studies that indicate an androgen requirement for long-term maintenance of the vocal CPG.
4.1 Steroids organize the vocal hindbrain
Vocal motor neurons
The larynx is innervated by a group of motor neurons resident in n. IX-X in the caudal hindbrain. During advertisement calling, these neurons appear to be progressively recruited to fire action potentials, accounting for the increased amplitude of compound action potentials (CAPs) recorded from the laryngeal nerve of singing males (Fig. 8A) [62]. During rapid trills, such as advertisement calling, nerve CAPs are highly synchronous (resembling a single action potential); during slower trills, such as ticking and amplectant calling, CAPs are less synchronous and these features are also present in nerve recordings from fictively singing isolated brains (Fig. 9; compare A and B to C and D). In general, calls with faster click rates are associated with more synchronous CAPs, while slow click rates are driven by less synchronous CAPs. Males exhibit both CAP types, leading to the suggestion that the motor nucleus comprises at least two functional motor neuron groups. Because females and males both produce asynchronous CAPs, while synchronous CAPs are unique to males, asynchronous motor neuron firing may represent the default phenotype. All laryngeal motor neurons concentrate androgens, but a subset may respond by expressing cellular changes (such as expression of specific ion channels) that underlie CAP synchrony.
Figure 9.
Synchrony of motor neuron firing is call-specific. During in vivo nerve recordings, Yamaguchi and Kelley (2000) found that CAP duration was inversely related to the interval between each CAP (and resulting click). This property is preserved in vitro. Fictive nerve activity in response to 5-HT application reveals that fast trill CAPs are shorter than those observed during fictive slow trills. Fictive amplectant CAPs, whose rates are 5 – 10 Hz, are much longer than fast and slow trill CAPs, but similar in duration to fictive ticking CAPs.
The number of laryngeal motor neurons in males is about twice that of females [23; 38; 69]. This difference originates during tadpole stages (thus preceding sex differences in muscle fiber number) and is due to sex differences in ontogenetic cell death [69] as is the case for other sexually dimorphic motor neuron populations in mammals [70]. Cell death is triggered by secretion of thyroid hormone and mitigated by androgen secretion [71]. Male tadpoles treated with anti-androgen have female motor neuron numbers at metamorphosis while androgen-treated females have male-like numbers [41]. Androgen treatment of adult females does not reverse or reduce these differences, indicating that differences in motor neuron number are permanently organized during development [38]. Another sexual dimorphism is the size of motor neuron cell bodies, which are larger in males; unlike motor neuron number, however, androgen treatment can masculinize female cell body size in as little as one week [29].
Patch clamp recordings from a vocal slice preparation revealed that the functional properties of male and female vocal motor neurons differ [72]. Male motor neurons have lower input resistances, are strongly adapting and initiate spikes at reliable, short latencies. Female motor neurons are slowly adapting and translate differences in membrane depolarization into graded differences in firing rate. These differences are accompanied by sexually differentiated expression of various currents. In particular, the hyperpolarization-activated depolarizing cation current (Ih), is found almost exclusively in male vocal motor neurons and could contribute to the latency and reliability of firing. Modeling suggests that a low-threshold potassium (IKL) current accounts for sex differences in firing patterns. One possibility is that spike precision in male motor neurons (endowed by Ih, IKL or both) contributes to the production of synchronous CAPs during fast rate male calls; Ih and IKL expression may be under direct control of androgens.
Premotor neurons
Laryngeal motor neurons receive monosynaptic, glutamatergic (AMPA) excitatory inputs from the PbN [65] as well as excitatory inputs from interneurons in contralateral n.IX-X (Fig. 7) [73]. In addition to interneurons (located rostrally), n.IX-X also includes glottal motor neurons. These are inhibited during evoked activity in the PbN. This characteristic may segregate the glottal opening required for breathing from song, which occurs without respiration.
Stimulation of the PbN (or contralateral n.IX-X) in isolated male brains results in synchronous CAPs in the laryngeal nerve [65; 73]. The resulting CAPs are similar in duration to those observed in vivo during male fast trills. Because suprathreshold electrical stimulation likely produced synchronous spiking of most premotor neurons, a possible explanation is that synchronicity of male fast trill CAPs is a function of precisely timed premotor neuron inputs. Therefore, in addition to spike timing (dictated by motor neuron membrane properties), another feature that might control CAP duration is the timing of premotor inputs. Stimulation experiments in females and recordings of premotor neurons during fictive calling can determine the degree to which premotor synchronicity contributes to CAP duration.
Tract tracing studies have identified few anatomical differences in the CPG circuitry between the sexes. Following injections of fluorescent dextran amines into either PbN or n.IX-X, retrograde labeling in the corresponding nucleus was generally less robust in females than in males [64]. While females may have fewer interneurons than males, less robust labeling could also be due to less efficient dye transport in females. The hindbrain circuit for both sexes encompasses the same nuclei and similar patterns of connectivity. Thus, the marked functional differences between male and female vocal patterns must arise from physiological properties of individual neuron types.
4.2 Steroid-dependent masculinization of the vocal CPG
Temporal patterns of X. laevis vocalizations are distinct in males and females, and development of male-typical calls requires androgens. The CPGs generating male vocal patterns must, therefore, also be masculinized by androgens during juvenile development, either naturally in intact males, or by exogenous hormone treatment in castrated male or female juveniles [27]. Female vocalizations can also be partly masculinized by testosterone treatment in adulthood [28; 29]. The fictively calling isolated brain preparation offers a powerful tool for discerning the process of CPG masculinization at the level of individual neurons. Whole-cell recordings in this preparation could uncover distinct membrane properties that support the masculinization of vocal patterns, perhaps including specific androgen-regulated ion channels as have been described in some fish [74; 75; 76]. One reasonable hypothesis is that stages in the masculinization of calls in T-treated females reflect the appearance of Ih or IKL, two male-typical motor neuron conductances. Similarly, upregulation of NMDA-R expression in PbN neurons, necessary for fast trill production [66], may also support the switch from isolated slow trill production to biphasic calling in both juvenile males and TFs [26; 29]. The role of specific ion channels in the physiological properties of male and female motor neurons can be tested directly by transfection of cDNAs in the vocal slice preparation.
4.3 Androgen-dependent maintenance of the vocal CPG in adulthood
The prevailing experimental paradigm for determining the role of steroid hormones in a behavior is castration with and without hormone replacement. Wetzel and Kelley [17] found that castration completely eliminated calling in males, and androgen treatment with gonadotropin reinstated vocal behaviors to pre-castration levels. These kinds of studies, however, do not identify the locus (forebrain, midbrain, hindbrain, muscles) of castration-induced vocal loss. Descending midbrain or forebrain vocal initiation signals might be eliminated, androgens may be absolutely required for the function of the hindbrain vocal CPG (both the PbN and vocal motor neurons express AR), or vocal muscles may be deactivated during androgen deprivation, thus preventing the translation of normal CPG output into sound. To distinguish between these sites of action, Zornik and Yamaguchi [35] compared vocalizations of castrated and control males to two in vitro tissue preparations, the isolated larynx [15] and the fictively calling isolated brain [16]. Similar to the results of Wetzel and Kelley [17], calling was nearly abolished five months after castration. Demasculinization of laryngeal contractile properties (described above) alone could not be responsible for this effect because a few castrates still produced occasional advertisement-like calls. Instead, the blockade of calling must be due either to a deactivation of the CPG itself or brain regions that initiate its activity.
Fictive vocalizations in the isolated brain are elicited by binding of 5-HT to 5-HT2C-like receptors in the hindbrain [68]. This experimental paradigm allows us to bypass any upstream vocal initiation centers and directly activate the CPG. Castration reduces the level of fictive calling. In castrates, both fictive fast and slow trills were slower than those produced by control brains (Fig. 10, compare C to A). While androgen deprivation did not completely inactivate the vocal CPG, circulating androgens may maintain CPG function over long time periods. Though biphasic vocalizations were rare in vivo, they were reliably recorded in vitro. Hindbrain CPGs in brains from castrated males are still capable of producing biphasic trills. We conclude that decreased calling in castrated males is due to the absence of vocal initiation by upstream vocal initiation centers and that this lack of initiation can be overridden by exogenous 5-HT application acting on the hindbrain CPG.
Figure 10.
Hormones control the output of the vocal CPG. A, Fictive advertisement calls produced by brains from adult males are highly similar to activity recorded during in vivo calling, with alternating fast and slow trills and each call lasting approximately one second. B, Bath-application of 5-HT to brains from young juvenile males elicits a pattern of nerve CAPs resembling fictive calling from adult males, though CAP rates tend to be slightly slower, and calls tend to be produced one at a time, not for long uninterrupted periods. C, Some brains from castrated males continue to generate biphasic fictive advertisement calls. As shown in the example, these can be produced in a relatively continuous manner, however, silent gaps between fast and slow trills are common, and both fast and slow trill CAP rates are slower than in intact male brains. D, Fictive vocal activity recorded from TFs is qualitatively comparable to that of adult males, though CAP rates are not fully masculinized.
Because Wetzel and Kelley [17] observed vocal silencing within one month after castration, the activational effects of androgens on initiation centers must degrade relatively quickly, within a few weeks. In contrast, CPG functions also deteriorate after gonadectomy, but these changes occur more slowly, over many months, indicating that androgens may function to maintain CPG activity over the lifetime of the animal. Such castration-induced changes may simply reflect a steroid-dependent maintenance of organizational changes that occurred during development. Alternatively, the observed CPG degradation by Zornik and Yamaguchi [35] may be mediated by the down-regulation of a different suite of gene products. Future studies, including experiments with hormone-replaced castrates, should uncover which gene products are necessary for vocal maintenance in adulthood.
5. Vocal initiation
Hindbrain and serotonin
As described above, males produce few, if any, vocalizations one month after castration, and are virtually silent between five months and one year after gonadectomy [17; 35]. This effect can be partially overcome in vitro by directly activating the hindbrain CPG with 5-HT bath-application, thus inducing fictive vocalizations in animals that did not call in vivo. Androgen-sensitive vocal initiation must thus arise upstream of the hindbrain CPG; where? One possibility is the rostral raphe nuclei (rRpd) which project to both CPG nuclei—PbN and n.IX-X. As is the case for the neurons located rostrally in n.IX-X, the rRpd also expresses 5-HT2C-like receptors, which are known to be involved in vocal initiation [77]. By elevating endogenous 5-HT levels using a selective serotonin reuptake inhibitor (SSRI), Yu and Yamaguchi [68] confirmed that endogenous 5-HT can activate the vocal CPG. The raphe nuclei are the primary source for serotonin in the brain [78], making activity in this region a strong candidate for participating in vocal initiation.
Activating 5-HT2C-like receptors in either males or females elicits sex typical vocal patterns in the nerve (fictive advertisement calling in males, fictive ticking in females), indicating that raphe-mediated vocal initiation is not itself sexually differentiated [77]. Because androgen targets have not been identified in the raphe [13; 79], the induction of calling in sex-specific patterns may instead be mediated by inputs from steroid-sensitive midbrain or forebrain centers to the raphe. Candidates for activating calling include the midbrain tegmentum (the midbrain periaqueductal grey is also implicated in vocalization in many species from fish to primates) [80; 81; 82; 83] and the central amygdala (CeA), which is reciprocally connected with both the PbN and the midbrain tegmentum [33].
Forebrain and midbrain; hormonal sensitivity
Are upstream vocal initiation centers involved in the decreased vocal behavior of castrated males? One possibility is that a decrease in circulating androgens results in deactivated forebrain nuclei that elicit male calling (via deactivation of AR-expressing neurons in the PbN that project to the CeA and/or midbrain tegmentum), blocking a normal path for activation of calling. While neither upstream region accumulates T or DHT [79; 84], both are reciprocally connected with the PbN which is an androgen target and thus could be indirectly activated and deactivated by circulating hormone levels.
The central amygdala (CeA) concentrates estradiol, but does not express androgen receptors [7; 13; 34; 84]. Neurons in the CeA accumulate radioactivity after exposure to tritiated T but not DHT, suggesting that they are labeled due to aromatization of the androgen to estradiol (DHT is not aromatizable). The role of estrogen in male vocal behaviors has not been studied directly. However, one study may provide indirect evidence that estrogen affects male calling [17]. Males were castrated and androgen-replaced; those given testosterone, aromatizable to estrogen, showed elevated calling at lower concentrations than those treated with the non-aromatizable dihydrotestosterone. At higher concentrations, differences were no longer apparent between the two androgens. At these supraphysiological levels, other androgen-sensitive regions (such as the CPG) may overcome an activation barrier usually lowered by forebrain and midbrain activity. Although speculative, the existence of estrogen binding regions within vocal forebrain nuclei indicates a possible site for steroid-dependent vocal control. The APOA also concentrates estradiol and is indirectly linked to the vocal circuit via connections with the CeA [64]. Anatomical studies thus provide evidence that estrogens could affect calling in males, though more detailed studies will be required to confirm this hypothesis. In the terrestrial frog, Rana pipiens, Schmidt [85] found that APOA stimulation could elicit fictive vocalizations in an in vitro brain preparation. Due to its close proximity to CeA, it would be difficult to confirm the role of APOA using such an approach, however, the results indicate that some ventral forebrain region can induce activity in the anuran vocal CPG.
Acoustic activation and suppression of calling
Advertisement calling in male frogs is strongly modulated by auditory input. For example, female rapping stimulates calling while ticking suppresses calling [19; 86]. Auditory activity can be recorded from the CeA in terrestrial frogs [87]; the CeA receives input from the central nucleus of the thalamus in X. laevis, which receives input from the inferior colliculus (ICo, nucleus LTOR)—the major auditory midbrain nucleus in X. laevis [7; 88]. The majority of neurons in the ICo respond to temporal features of click trains (T. M. Elliott, J. Christensen-Dalsgaard, and D. B. Kelley, submitted for publication). An outstanding question here is whether circulating hormones influence the sensitivity of auditory regions; LTOR accumulates both androgens and estrogens [34; 79]. Notably, the CeA in X. laevis is over 97% GABAergic [89]. While input from the CeA to the PbN could directly suppress calling (as occurs in response to ticking), the role of this nucleus in stimulating calling (as occurs in response to rapping) could involve disinhibition.
Gonadotropin and vocal initiation
An intriguing result from the Wetzel and Kelley study [17] was that androgen-replaced castrates sang more when injected with human chorionic gonadotropin (hCG), indicating non-gonadal sites of action. Recently, Yang et al. [14] found luteinizing hormone (LH) receptor expression in the ventral forebrain, including CeA and APOA. These data support the hypothesis that one or both of these nuclei are involved in regulating or initiating male calling, and suggest that gonadotropin might act in concert with gonadal steroids to exert their full effects.
6. Summary and conclusions
The powerful effects of gonadal steroids on the developing and adult nervous system make essential contributions to the expression of male- and female-typical courtship and reproductive behaviors. These hormones act on multiple sites ranging from the cerebral cortex, through the hypothalamus and preoptic area to motor neurons and muscle. Action at different times in development include the production and survival of hormone-targets and their neural partners as well as the establishment—through dendritic branching, axonal trajectories and ion channel expression—of the neural circuitry underlying behavioral expression and male-female differences. Disentangling which hormone acts where and when, to do what, is a dauntingly complex task. We've approached the neuroendocrine role of gonadal steroids through a series of informative reduced preparations which allow us to disentangle some of the levels of control that are important in our model system, courtship song in X. laevis. As is likely the case for many other reproductive behaviors (including copulatory behaviors of rodents and courtship songs of birds and fish), the neural machinery for vocalizations includes a hindbrain pattern generator. In X. laevis this CPG can be activated by elevated levels of the neuromodulator serotonin. The actual sex-specific output of the CPG, however, is not determined by serotonin but rather by exposure to gonadal steroids during development and adulthood. Even the vocal organ, whose cellular properties are largely established during development via androgenic regulation of cell number and type, retains some plasticity in adulthood notably in the regulation of synaptic strength by estradiol and the modulation of contraction and relaxation rates by androgen. Sex differences in ion channel expression in vocal motor and premotor neurons are also likely to be shaped by androgens (e.g. ion channels in the electric organs of fish, [74]).
The major sites of action for gonadal steroids in the hindbrain CPG are the neurons in n.IX-X (which include vocal motor neurons and neurons projecting to PbN and contralateral n.IX-X) and the PbN. These neurons and their synaptically connected circuit elements are thus the most likely candidates for the sexually differentiated features of the CPG that are sculpted and maintained by gonadal steroids. In the actual animal, vocal behaviors are activated and suppressed by acoustic stimuli: the song of other males and females. These stimuli are temporally distinctive and key features of songs are represented in the auditory midbrain, which itself is sensitive to estrogens and androgens. Reproductive state is also affected by the pituitary hormone, gonadotropin, which in addition to its classical role in stimulating gonadal steroid secretion, acts directly on forebrain auditory centers. These findings open the door to understanding the neuroendocrine control of the sensorimotor integration performed by neural circuit elements and required for effective communication and reproduction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–8. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisk RD. Diencephalic placement of estradiol and sexual receptivity in the female rat. Am J Physiol. 1962;203:493–6. doi: 10.1152/ajplegacy.1962.203.3.493. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff DW. Autoradiographic localization of radioactivity in rat brain after injection of tritiated sex hormones. Science. 1968;161:1355–6. doi: 10.1126/science.161.3848.1355. [DOI] [PubMed] [Google Scholar]
- 5.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 6.Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–5. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1976;165:487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- 9.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–70. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 10.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96:7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci. 2009;29:6558–67. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang EJ, Kelley DB. Hormones and the regulation of vocal patterns in amphibians: Xenopus laevis vocalizations as a model system. Academic Press; 2009. [Google Scholar]
- 13.Perez J, Cohen MA, Kelley DB. Androgen receptor mRNA expression in Xenopus laevis CNS: sexual dimorphism and regulation in laryngeal motor nucleus. J Neurobiol. 1996;30:556–68. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Yang EJ, Nasipak BT, Kelley DB. Direct action of gonadotropin in brain integrates behavioral and reproductive functions. Proc Natl Acad Sci U S A. 2007;104:2477–82. doi: 10.1073/pnas.0608391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias ML, Kelley DB. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J Neurosci. 1987;7:3191–7. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci. 2007;27:1485–97. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- 18.Tobias ML, Barnard C, O'Hagan R, Horng S, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Animal Behaviour. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc Natl Acad Sci U S A. 1998;95:1870–5. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picker MD. Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour. 1983;84:74–90. [Google Scholar]
- 21.Tobias ML, Corke A, Korsh J, Yin D, Kelley DB. Vocal competition in male Xenopus laevis frogs. Behav Ecol Sociobiol. 2010;64:1791–1803. doi: 10.1007/s00265-010-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobias ML, Marin ML, Kelley DB. Development of functional sex differences in the larynx of Xenopus laevis. Dev Biol. 1991;147:251–9. doi: 10.1016/s0012-1606(05)80022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley DB, Dennison J. The vocal motor neurons of Xenopus laevis: development of sex differences in axon number. J Neurobiol. 1990;21:869–82. doi: 10.1002/neu.480210605. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MA, Kelley DB. Androgen-induced proliferation in the developing larynx of Xenopus laevis is regulated by thyroid hormone. Dev Biol. 1996;178:113–23. doi: 10.1006/dbio.1996.0202. [DOI] [PubMed] [Google Scholar]
- 25.Edwards CJ, Yamamoto K, Kikuyama S, Kelley DB. Prolactin opens the sensitive period for androgen regulation of a larynx-specific myosin heavy chain gene. J Neurobiol. 1999;41:443–51. [PubMed] [Google Scholar]
- 26.Yamaguchi A, Munoz MM, Bose TO, Oberlander JG, Smith S. Sexually distinct development of vocal pathways in Xenopus laevis. Dev Neurobiol. 2010;70:862–74. doi: 10.1002/dneu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson JT, Kelley DB. Testicular masculinization of vocal behavior in juvenile female Xenopus laevis reveals sensitive periods for song duration, rate, and frequency spectra. J Comp Physiol A. 1992;171:343–50. doi: 10.1007/BF00223964. [DOI] [PubMed] [Google Scholar]
- 28.Hannigan P, Kelley DB. Androgen-induced alterations in vocalizations of female Xenopus laevis: modifiability and constraints. J Comp Physiol A. 1986;158:517–27. doi: 10.1007/BF00603797. [DOI] [PubMed] [Google Scholar]
- 29.Potter KA, Bose T, Yamaguchi A. Androgen-induced vocal transformation in adult female African clawed frogs. J Neurophysiol. 2005;94:415–28. doi: 10.1152/jn.01279.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wada M, Gorbman A. Relation of mode of administration of testosterone to evocation of male sex behavior in frogs. Horm Behav. 1977;8:310–9. doi: 10.1016/0018-506x(77)90005-8. [DOI] [PubMed] [Google Scholar]
- 31.Burmeister SS, Wilczynski W. Social context influences androgenic effects on calling in the green treefrog (Hyla cinerea) Horm Behav. 2001;40:550–8. doi: 10.1006/hbeh.2001.1723. [DOI] [PubMed] [Google Scholar]
- 32.Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J Comp Physiol A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- 33.Moreno N, Gonzalez A. Central amygdala in anuran amphibians: neurochemical organization and connectivity. J Comp Neurol. 2005;489:69–91. doi: 10.1002/cne.20611. [DOI] [PubMed] [Google Scholar]
- 34.Morrell JI, Kelley DB, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. II. Estradiol. J Comp Neurol. 1975;164:63–77. doi: 10.1002/cne.901640106. [DOI] [PubMed] [Google Scholar]
- 35.Zornik E, Yamaguchi A. Vocal pathway degradation in gonadectomized Xenopus laevis adults. J Neurophysiol. doi: 10.1152/jn.00883.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sassoon D, Kelley DB. The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am J Anat. 1986;177:457–72. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- 37.Sassoon D, Segil N, Kelley D. Androgen-induced myogenesis and chondrogenesis in the larynx of Xenopus laevis. Dev Biol. 1986;113:135–40. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- 38.Watson JT, Robertson J, Sachdev U, Kelley DB. Laryngeal muscle and motor neuron plasticity in Xenopus laevis: testicular masculinization of a developing neuromuscular system. J Neurobiol. 1993;24:1615–25. doi: 10.1002/neu.480241206. [DOI] [PubMed] [Google Scholar]
- 39.Marin ML, Tobias ML, Kelley DB. Hormone-sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development. 1990;110:703–11. doi: 10.1242/dev.110.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias ML, Marin ML, Kelley DB. The roles of sex, innervation, and androgen in laryngeal muscle of Xenopus laevis. J Neurosci. 1993;13:324–33. doi: 10.1523/JNEUROSCI.13-01-00324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson JC, Watson JT, Kelley DB. Androgen directs sexual differentiation of laryngeal innervation in developing Xenopus laevis. J Neurobiol. 1994;25:1625–36. doi: 10.1002/neu.480251213. [DOI] [PubMed] [Google Scholar]
- 42.Robertson JC, Kelley DB. Thyroid hormone controls the onset of androgen sensitivity in the developing larynx of Xenopus laevis. Dev Biol. 1996;176:108–23. doi: 10.1006/dbio.1996.9990. [DOI] [PubMed] [Google Scholar]
- 43.Tobias ML, Marin ML, Kelley DB. Temporal constraints on androgen directed laryngeal masculinization in Xenopus laevis. Dev Biol. 1991;147:260–70. doi: 10.1016/s0012-1606(05)80023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nachlas MM, Tsou KC, De Souza E, Cheng CS, Seligman AM. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957;5:420–36. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- 45.Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–2. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- 46.Burke RE. Motor units: physiological/histochemical profiles, neural connectivity and functional specializations. Amer. Zool. 1978;18:127–134. [Google Scholar]
- 47.Nemeth P, Hofer HW, Pette D. Metabolic heterogeneity of muscle fibers classified by myosin ATPase. Histochemistry. 1979;63:191–201. doi: 10.1007/BF00644541. [DOI] [PubMed] [Google Scholar]
- 48.Sassoon DA, Gray GE, Kelley DB. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci. 1987;7:3198–206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catz DS, Fischer LM, Moschella MC, Tobias ML, Kelley DB. Sexually dimorphic expression of a laryngeal-specific, androgen-regulated myosin heavy chain gene during Xenopus laevis development. Dev Biol. 1992;154:366–76. doi: 10.1016/0012-1606(92)90075-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lannergren J, Hoh JF. Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B Biol Sci. 1984;222:401–8. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- 51.Catz DS, Fischer LM, Kelley DB. Androgen regulation of a laryngeal-specific myosin heavy chain mRNA isoform whose expression is sexually differentiated. Dev Biol. 1995;171:448–57. doi: 10.1006/dbio.1995.1295. [DOI] [PubMed] [Google Scholar]
- 52.Baur LA, Nasipak BT, Kelley DB. Sexually differentiated, androgen-regulated, larynx-specific myosin heavy-chain isoforms in Xenopus tropicalis; comparison to Xenopus laevis. Dev Genes Evol. 2008;218:371–9. doi: 10.1007/s00427-008-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–7. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 55.Tobias ML, Kelley DB. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis. J Neurosci. 1988;8:2422–9. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tobias ML, Kelley DB, Ellisman M. A sex difference in synaptic efficacy at the laryngeal neuromuscular junction of Xenopus laevis. J Neurosci. 1995;15:1660–8. doi: 10.1523/JNEUROSCI.15-03-01660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tobias ML, Kelley DB. Sexual differentiation and hormonal regulation of the laryngeal synapse in Xenopus laevis. J Neurobiol. 1995;28:515–26. doi: 10.1002/neu.480280411. [DOI] [PubMed] [Google Scholar]
- 58.Tobias ML, Tomasson J, Kelley DB. Attaining and maintaining strong vocal synapses in female Xenopus laevis. J Neurobiol. 1998;37:441–8. [PubMed] [Google Scholar]
- 59.Wu KH, Tobias ML, Kelley DB. Estrogen and laryngeal synaptic strength in Xenopus laevis: opposite effects of acute and chronic exposure. Neuroendocrinology. 2001;74:22–32. doi: 10.1159/000054667. [DOI] [PubMed] [Google Scholar]
- 60.Fischer LM, Kelley DB. Androgen receptor expression and sexual differentiation of effectors for courtship song in Xenopus laevis. Seminars in Neuroscience. 1991;3:469–480. [Google Scholar]
- 61.Kang L, Marin M, Kelley D. Androgen biosynthesis and secretion in developing Xenopus laevis. Gen Comp Endocrinol. 1995;100:293–307. doi: 10.1006/gcen.1995.1160. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi A, Kelley DB. Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female african clawed frogs (Xenopus laevis) J Neurosci. 2000;20:1559–67. doi: 10.1523/JNEUROSCI.20-04-01559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zornik E, Kelley DB. Breathing and calling: neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol. 2007;501:303–15. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 64.Brahic CJ, Kelley DB. Vocal circuitry in Xenopus laevis: telencephalon to laryngeal motor neurons. J Comp Neurol. 2003;464:115–30. doi: 10.1002/cne.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zornik E, Kelley DB. Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J Neurosci. 2008;28:612–21. doi: 10.1523/JNEUROSCI.4754-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zornik E, Katzen AW, Rhodes HJ, Yamaguchi A. NMDAR-dependent control of call duration in Xenopus laevis. J Neurophysiol. 2010;103:3501–15. doi: 10.1152/jn.00155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behav Brain Res. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- 68.Yu HJ, Yamaguchi A. Endogenous serotonin acts on 5-HT2C-like receptors in key vocal areas of the brain stem to initiate vocalizations in Xenopus laevis. J Neurophysiol. 2010;103:648–58. doi: 10.1152/jn.00827.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kay JN, Hannigan P, Kelley DB. Trophic effects of androgen: development and hormonal regulation of neuron number in a sexually dimorphic vocal motor nucleus. J Neurobiol. 1999;40:375–85. [PubMed] [Google Scholar]
- 70.Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–9. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perez J, Kelley DB. Androgen mitigates axotomy-induced decreases in calbindin expression in motor neurons. J Neurosci. 1997;17:7396–403. doi: 10.1523/JNEUROSCI.17-19-07396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi A, Kaczmarek LK, Kelley DB. Functional specialization of male and female vocal motoneurons. J Neurosci. 2003;23:11568–76. doi: 10.1523/JNEUROSCI.23-37-11568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zornik E. Regulating breathing and calling in an aquatic frog: neuronal networks in the Xenopus laevis hindbrain. Department of Biological Sciences, Columbia University; New York City: 2006. p. 129. [Google Scholar]
- 74.McAnelly ML, Zakon HH. Androgen modulates the kinetics of the delayed rectifying K+ current in the electric organ of a weakly electric fish. Dev Neurobiol. 2007;67:1589–97. doi: 10.1002/dneu.20530. [DOI] [PubMed] [Google Scholar]
- 75.Liu H, Wu MM, Zakon HH. A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal. J Neurosci. 2008;28:9173–82. doi: 10.1523/JNEUROSCI.2783-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Few WP, Zakon HH. Sex differences in and hormonal regulation of Kv1 potassium channel gene expression in the electric organ: molecular control of a social signal. Dev Neurobiol. 2007;67:535–49. doi: 10.1002/dneu.20305. [DOI] [PubMed] [Google Scholar]
- 77.Yu HJ, Yamaguchi A. 5-HT2C-like receptors in the brain of Xenopus laevis initiate sex-typical fictive vocalizations. J Neurophysiol. 2009;102:752–65. doi: 10.1152/jn.90469.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–9. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 79.Kelley DB. Locations of androgen-concentrating cells in the brain of Xenopus laevis: autoradiography with 3H-dihydrotestosterone. J Comp Neurol. 1981;199:221–31. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- 80.Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- 81.Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–46. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 82.Shiba K, Umezaki T, Zheng Y, Miller AD. Fictive vocalization in the cat. Neuroreport. 1996;7:2139–42. doi: 10.1097/00001756-199609020-00015. [DOI] [PubMed] [Google Scholar]
- 83.Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. I. Testosterone. J Comp Neurol. 1975;164:47–59. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt RS. Neural correlates of frog calling. Masculinization by androgens. Horm Behav. 1983;17:94–102. doi: 10.1016/0018-506x(83)90019-3. [DOI] [PubMed] [Google Scholar]
- 86.Elliott TM, Kelley DB. Male discrimination of receptive and unreceptive female calls by temporal features. J Exp Biol. 2007;210:2836–42. doi: 10.1242/jeb.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mudry KM, Capranica RR. Evoked auditory activity within the telencephalon of the bullfrog (Rana catesbeiana) Brain Res. 1980;182:303–11. doi: 10.1016/0006-8993(80)91190-7. [DOI] [PubMed] [Google Scholar]
- 88.Edwards CJ, Kelley DB. Auditory and lateral line inputs to the midbrain of an aquatic anuran: neuroanatomic studies in Xenopus laevis. J Comp Neurol. 2001;438:148–62. doi: 10.1002/cne.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brox A, Puelles L, Ferreiro B, Medina L. Expression of the genes GAD67 and Distal-less-4 in the forebrain of Xenopus laevis confirms a common pattern in tetrapods. J Comp Neurol. 2003;461:370–93. doi: 10.1002/cne.10688. [DOI] [PubMed] [Google Scholar]