Abstract
Gemcitabine, while a standard treatment of advanced pancreatic cancer (PaCa), alone is not very effective. New agents that are safe and effective are highly needed. Resveratrol is one such agent which is safe and multitargeted; and has been linked with suppression of survival, proliferation, invasion and angiogenesis of cancer. Whether resveratrol can sensitize PaCa to gemcitabine in vitro and in vivo was investigated. We established PaCa xenografts in nude mice, randomized into 4 groups, and treated with vehicle, gemcitabine, resveratrol and with combination. Modulation of NF-κB and markers of proliferation, angiogenesis and invasion were ascertained using electrophoretic mobility shift assay (EMSA), immunohistochemistry and western blot analysis. Resveratrol inhibited the proliferation of 4 different human PaCa cell lines, synergized the apoptotic effects of gemcitabine, inhibited the constitutive activation of NF-κB and expression of bcl-2, bcl-xL, COX-2, cyclin D1 MMP-9 and VEGF. In an orthotopic model of human PaCa, we found that resveratrol significantly suppressed the growth of the tumor (p < 0.001) and this effect was further enhanced by gemcitabine (p < 0.001). Both the markers of proliferation index Ki-67 and the micro vessel density CD31 were significantly downregulated in tumor tissue by the combination of gemcitabine and resveratrol (p < 0.001 vs. control; p < 0.01 vs. gemcitabine). As compared to vehicle control, resveratrol also suppressed the NF-κB activation and expression of cyclin D1, COX-2, ICAM-1, MMP-9 and survivin. Overall our results demonstrate that resveratrol can potentiate the effects of gemcitabine through suppression of markers of proliferation, invasion, angiogenesis and metastasis.
Keywords: apoptosis, chemoresistance, chemotherapeutic agents, NF-κB, pancreatic cancer
Pancreatic cancer (PaCa) is one of the most lethal cancers having an estimated 5-year survival rate with best treatments available today of ~4%. Almost 37,170 Americans were diagnosed with PaCa in the year 2007 and 33,370 died of the disease.1 Gemcitabine, the standard treatment of patients suffering from advanced PaCa, provides no statistically significant survival advantage.2 The combination of gemcitabine with erlotinib (EGFR inhibitor), platinum analogues, bevacizumab (antibody against VEGF), or celecoxib (COX-2 inhibitor) is in different phases of clinical trials.3
Various lines of evidence suggest that the transcription factor NF-κB and NF-κB-regulated gene products play a major role in growth, metastasis and chemo resistance of PaCa. First, NF-κB is constitutively active in PaCa4,5; second, AKT/PI3K which can activate NF-κB is active in PaCa6; third, overexpression of COX-2 regulated by NF-κB is frequently found in PaCa7; fourth, overexpression of cyclin D1 and VEGF, both regulated by NF-κB, has been reported in PaCa7; fifth, NF-κB has been linked with proliferation, invasion and angiogenesis8; and sixth, NF-κB activation in PaCa has been found to mediate chemoresistance,6 antiapoptosis,9 angiogenesis10 and metastasis.11 Thus agents that can modulate NF-κB and NF-κB-regulated gene products have potential for treatment of PaCa.
Resveratrol (trans-3,5,4′-trihydroxystilbene; Fig. 1a), a component of grapes, berries and peanuts has emerged as an “age-prolongation factor”12 and been demonstrated by our laboratory13 and others14 to be a potent blocker of the NF-κB pathway. Besides inhibiting the growth of a wide variety of tumor cells in culture,15 resveratrol and its derivative (KITC) has been shown to potentiate the cytotoxic effects of paclitaxel,16 cisplatin,17 velcade, thalidomide18,19 and gemcitabine.20 In animal studies, this stilbene was found to prevent a variety of carcinogen-induced cancers including breast,21 intestine,22 liver,23 esophagus24 and colon.25 Resveratrol also suppressed transgene-induced tumorigenesis in breast,26,27 and in prostate.28 Resveratrol was found to suppress the growth of a wide variety of transplanted tumors in rodents including hepatoma,29 neuroblastoma,30 gastric carcinoma,31 breast carcinoma,27 colon carcinoma,32 and leukemia.33 Additionally, resveratrol suppressed angiogenesis and metastasis in glioma,34 lung carcinoma,35 and breast cancer.26,27 This stilbene was found to sensitize tumors in mice to chemotherapeutic agents such as 5-fluorouracil.36 However the effect of resveratrol on the growth of human PaCa in animals is not known. Whether resveratrol can potentiate the effect of gemcitabine in PaCa, is also not known.
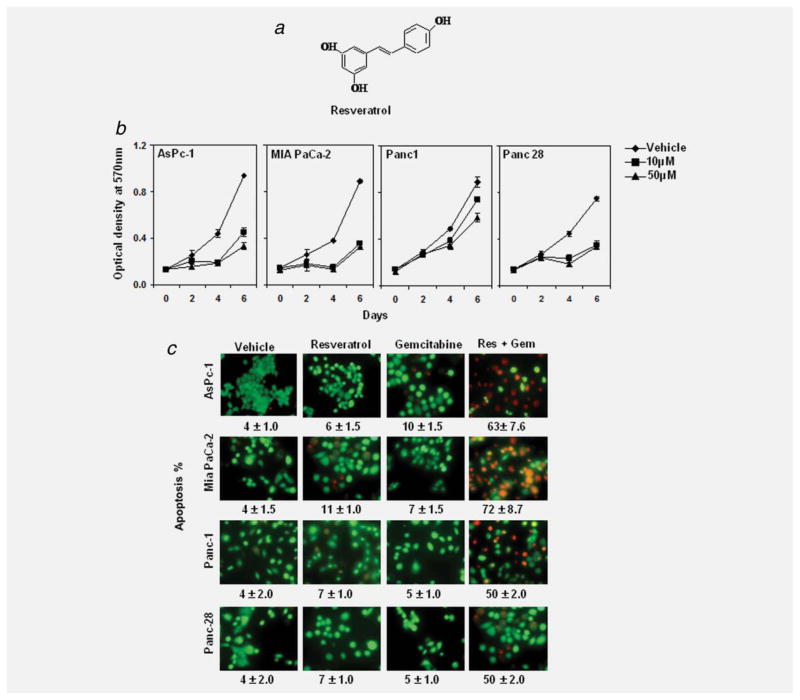
Figure 1.
Resveratrol inhibits the growth and proliferation, enhances the apoptotic effects of gemcitabine. (a) Structure of resveratrol. (b) MTT assay results showed suppression of cell proliferation in 4 pancreatic cancer cell lines tested by resveratrol. Data shown are representative of 3 independent sets of experiments. (c) Live/Dead assay results indicated that resveratrol (Res, 10 μM) potentiates gemcitabine (Gem, 100 nM)-induced cytotoxicity. Data indicated as percentage proportions of apoptotic pancreatic cancer cells. Data are a representative of 3 independent experiments. Values are mean ± SD of independent experiments. The photographs were taken at the magnification of ×20.
In the present report, we investigated the effect of resveratrol on the growth of human PaCa in culture and in an orthotopic mouse model of PaCa. We investigated effects of resveratrol and gemcitabine on cell proliferation of representative cells of various kinds of pancreatic cancer (e.g., AsPC-1 is derived from pancreatic adenocarcinoma; MIA PaCa-2 and Panc-28 are pancreatic carcinoma while Panc-1 represents pancreatic duct cell carcinoma). We also examined the effect of this stilbene in combination with gemcitabine both in vitro and in vivo. The present work will show that the downregulation of NF-κB and NF-κB-linked gene products by resveratrol is one of the mechanisms of inhibition of tumor growth and angiogenesis of PaCa. Such studies have the potential to improve the treatment of PaCa.
Material and Methods
Reagents
Resveratrol (>98% pure) was supplied by Wuxi Gorunjie Technology, China. The antibodies against bcl-2, bcl-xL, COX-2, cyclin D1, ICAM-1, c-myc and procaspase-3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against VEGF and Ki-67 (rabbit monoclonal clone SP6) was from Neomarkers (Fremont, CA); antibodies against CXCR4 and p65 (for immuno histochemical analysis) were from Abcam, and MMP-9 from Cayman chemicals. Antibodies against survivin and β-actin were procured from Sigma (Sigma-Aldrich Corp. St. Louis, MO). Mouse CD31 monoclonal antibody was obtained from Pharmingen, San Diego, CA. The liquid DAB+ substrate chromogen system-HRP used for immuno histochemistry was obtained from Dako Cytomation (Carpinteria, CA). Penicillin, streptomycin, RPMI 1640 and fetal bovine serum (FBS) were obtained from Invitrogen (Grand Island, NY). Gemcitabine (Gemzar) from Eli Lilly was stored at 4°C and dissolved in sterile PBS on the day of use. D-luciferin potassium salt (Xenogen, Hopkinton, MA) was dissolved in sterile PBS at 40 mg mL−1 concentration.
Cell lines
The pancreatic cancer cell lines AsPC-1 (pancreatic adenocarcinoma; ATCC No: CRL-1682), MIA PaCa-2 (pancreatic carcinoma; ATCC No: CRL-1420) and Panc-1 (pancreatic duct cell carcinoma; ATCC No: CRL-1469) were obtained from the American Type Culture Collection (Manassas, VA). Panc-28 (pancreatic carcinoma) cell line was kindly provided by Dr. Shrikanth Reddy (The University of Texas M. D. Anderson Cancer Center, Houston, TX).37 All cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin.
Proliferation assay
The effect of resveratrol on proliferation of PaCa cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method as described previously.38 Briefly, the cells (2,500 per well) were incubated with resveratrol in triplicate in a 96-well plate for 2, 4 or 6 days at 37°C. MTT solution (5 mg mL−1) was added to each well and the plate was incubated for 2 hr at 37°C. The lysis buffer (20% SDS and 50% dimethyl formamide) was added, and the cells were further incubated for overnight at 37°C. The absorbance of the cell suspension was measured at 570 nm using an MRX Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA). This experiment was repeated 3 times independently, and the statistical analysis was done to obtain the final values.
Apoptosis assay
To determine whether resveratrol can potentiate the apoptotic effects of gemcitabine in pancreatic cancer cells, we used a Live/Dead assay kit (Molecular Probes, Eugene, OR), which determines intracellular esterase activity and plasma membrane integrity. Briefly, cells (5,000 per well) were incubated in a chamber slide, pretreated with resveratrol for 4 hr, and then treated with gemcitabine for 24 hr. Cells were then stained as per manufactures protocol. Cell viability was determined under a fluorescence microscope by counting live (green) and dead (red) cells. This experiment was repeated 3 times independently and the statistical analysis was done.
Animals
Male athymic nu/nu mice (4-weeks-old) were obtained from the breeding section of the Department of Experimental Radiation Oncology at University of Texas M. D. Anderson Cancer Center. The 4 animals per cage were housed in standard mice plexi glass cages in a room maintained at constant temperature and humidity under 12 hr light and dark cycle and fed regular autoclave chow diet with water ad libitum. None of the mice had any lesions, and all were tested pathogen-free. Our experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at UTMDACC.
Orthotopic implantation of MIA PaCa-2 cells in nude mice
Our in vitro studies demonstrated that MIA PaCa-2 cells were more sensitive to the combination treatment of gemcitabine and resveratrol. Thus we decided to select this pancreatic cancer cell line for in vivo studies. MIA PaCa-2 cells were stably transfected with luciferase as described previously.39 Luciferase-transfected MIA PaCa-2 cells were harvested from subconfluent cultures after a brief exposure to 0.25% trypsin and 0.2% EDTA. Trypsinization was stopped with medium containing 10% FBS. The cells were washed once in serum-free medium and resuspended in PBS. Cell viability was determined by trypan blue method. Only suspensions consisting of single cells, with >90% viability, were used for the injections. Mice were anesthetized with ketamine–xylazine solution (Sigma-Aldrich, St. Louis, MO), a small left abdominal flank incision was made, and MIA PaCa-2 cells (2 × 106) in 50 μL PBS were injected into the subcapsular region of the pancreas using a 27-gauge needle and a calibrated push button-controlled dispensing device (Hamilton Syringe Co., Reno, NV). The abdominal wound was closed in 1 layer with wound clips (Braintree Scientific, Braintree, MA).
Experimental protocol
After 1 week of implantation, mice were randomized into the following treatment groups (n = 8) based on the bioluminescence measured after the first IVIS imaging: (i) untreated control (vehice only; ethanol: PBS mixture, 1:3); (ii) resveratrol alone (40 mg kg−1, once daily p.o.); (iii) gemcitabine alone (25 mg kg−1, twice weekly by i.p. injection); and (iv) combination of resveratrol (40 mg kg−1), once daily p.o., and gemcitabine (25 mg kg−1), twice weekly by i.p. injection. Tumor volumes were monitored weekly by the bioluminescence IVIS Imaging System 200 using a cryogenically cooled imaging system coupled to a data acquisition computer running Living Image software (Xenogen Corp., Alameda, CA). Before imaging, animals were anesthetized in an acrylic chamber with 2.5% isoflurane/air mixture and injected i.p with 40 mg mL−1 D-luciferin potassium salt in PBS at a dose of 150 mg kg−1 body weight. After 10 min of incubation with luciferin, mice were placed in a right lateral decubitus position and a digital grayscale animal image was acquired followed by acquisition and overlay of a pseudo color image representing the spatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. Mice were imaged on 7, 14, 21, 28 and 35 days after tumor implantation. Therapy was continued for 4 weeks and animals were sacrificed 1 week later. Primary tumors in the pancreas were excised and the final tumor volume was measured as V = 2/3πr3, where r is the mean of the 3 dimensions (length, width and depth). The final tumor volumes were initially subjected to one-way ANOVA and then later compared among groups using unpaired Bonferroni multiple comparison tests. Half of the tumor tissue was formalin fixed and paraffin embedded for immuno-histochemistry and routine H&E staining. The other half was snap frozen in liquid nitrogen and stored at −80°C. H&E staining confirmed the presence of tumor(s) in each pancreas.
Preparation of nuclear extract from tumor samples
Pancreatic tumor tissues (75–100 mg/mouse) from control and experimental mice were minced and homogenized using a Dounce homogenizer and the homogenate was incubated on ice for 1 hr in 0.5 mL of ice-cold buffer A [10 mmol L−1 HEPES (pH 7.9), 1.5 mmol L−1 KCl, 10 mmol L−1 MgCl2, 0.5 mmol L−1 DTT, 0.5 mmol L−1 phenylmethylsulfonyl fluoride (PMSF)]. The homogenate was centrifuged at 16,000g at 4°C for 10 min. The resulting nuclear pellet was suspended in 0.2 mL of buffer B [20 mmol L−1 HEPES (pH 7.9), 25% glycerol, 1.5 mmol L−1 MgCl2, 420 mmol L−1 NaCl, 0.5 mmol L−1 DTT, 0.2 mmol L−1 EDTA, 0.5 mmol L−1 PMSF, and 2 μg mL−1 leupeptin] and incubated on ice for 2 hr with intermittent mixing. The suspension was then centrifuged at 16,000g at 4°C for 15 min. The supernatant (nuclear extract) was collected and stored at −80°C until use. Protein concentration was measured by the Bradford assay with BSA as the standard.
Assay of NF-κB activation in pancreatic cancer cells and tumor samples
To assess NF-κB activation, we prepared nuclear extracts from pancreatic cancer cell lines and tumor samples and carried out electrophoretic mobility shift assays (EMSA) essentially as described previously.40 Briefly, nuclear extracts prepared from pancreatic cancer cells (1 × 106/mL) and tumor samples were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligo nucleotide (15 μg of protein with 16 fmol of DNA) from the HIV long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCT GGGGACTTTC CAGGGAGGCGTGG-3′; italic type indicates NF-κB -binding sites) for 30 min at 37°C. The resulting DNA-protein complex was separated from free oligo nucleotide on 6.6% native poly acrylamide gels. The dried gels were visualized, and radioactive bands were quantitated using a Phosphor Imager (Molecular Dynamics, Sunnyvale, CA) with Image Quant software.
Immuno-histochemical analysis of p65, VEGF and COX-2 in tumor samples
The nuclear localization of p65 and expression of COX-2, MMP-9 and VEGF were examined using an immunohistochemical method described previously.38 The pancreatic cancer tumor samples were embedded in paraffin and fixed with paraformaldehyde. After being washed in DPBS, the slides were blocked with protein block solution (Dako Cytomation) for 20 min and then incubated overnight with rabbit polyclonal anti-human p65, mouse monoclonal anti-human VEGF, and anti-COX-2 antibodies (1:100, 1:50 and 1:75, respectively). After the incubation, the slides were washed and then incubated with biotinylated link universal antiserum followed by horseradish peroxidase-streptavidin conjugate (LSAB+ kit). The slides were rinsed, and color was developed using 3,3′-diaminobenzidine hydrochloride as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin and mounted with DPX mounting medium for evaluation. Pictures were captured with a Photometric Cool SNAP CF color camera (Nikon, Lewisville, TX) and Meta Morph version 4.6.5 software (Universal Imaging, Downingtown, PA).
Ki-67 immunohistochemistry
Formalin-fixed, paraffin-embedded sections (5 μm) were stained with anti-Ki-67 antibody as described previously.38 Results were expressed as percentage of Ki-67 positive cells ± SE per 40× magnification. A total of ten 40× fields were examined from 3 tumors of each of the treatment groups. The values were initially subjected to one-way ANOVA and then later compared among groups using unpaired Student’s t test.
CD31 staining for micro vessel density
Ethanol-fixed, paraffin-embedded sections (5 μm) were stained with rat anti-mouse CD31 monoclonal antibody as described previously.38 Areas of greatest vessel density were then examined under higher magnification (100×) and counted. Any distinct area of positive staining for CD31 was counted as a single vessel. Results were expressed as the mean number of vessels ± SE per high-power field (100×). A total of 20 high-power fields was examined and counted from 3 tumors for each of the treatment groups. The values were initially subjected to 1-way ANOVA and then later compared among groups using unpaired Student’s t test.
Protein extraction and western blot analysis
Pancreatic tumor tissues (75–100 mg/mouse) from control and experimental mice were minced and incubated on ice for 30 min in 0.5 mL of ice-cold whole-cell lysate buffer (10% NP-40, 5 mol L−1 NaCl, 1 mol L−1 HEPES, 0.1 mol L−1 EGTA, 0.5 mol L−1 EDTA, 0.1 mol L−1 PMSF, 0.2 mol L−1 sodium orthovanadate, 1 mol L−1 NaF, 2 μg mL−1 aprotinin, 2 μg mL−1 leupeptin). The minced tissue was homogenized using a Dounce homogenizer and centrifuged at 16,000g at 4°C for 10 min. The proteins were then fractionated by SDS-PAGE, electro transferred to nitrocellulose membranes, blotted with each antibody and detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). The Western blot signals were quantitated by densitometric analysis using Total Lab Nonlinear Dynamic Image analysis software (Nonlinear USA).
Results
The purpose of this study was to determine whether resveratrol, a stilbene (see chemical structure, Fig. 1a) might have a role in the treatment of PaCa either alone or in combination with gemcitabine and if so, through what mechanism. We used 4 different well-characterized human PaCa cell lines derived from various types of pancreatic cancer e.g., AsPC-1 is derived from pancreatic adenocarcinoma; MIA PaCa-2 and Panc-28 are pancreatic carcinoma while Panc-1 represents pancreatic duct cell carcinoma. To facilitate the monitoring of tumor growth in animals, one of these cell lines, MIA PaCa-2 was stably transfected with luciferase reporter and used in the orthotopic transplant model in mice.
Resveratrol inhibits growth, synergizes the effect of gemcitabine and down regulates constitutive NF-κB activation and NF-κB-regulated gene products
We first investigated the effect of resveratrol on the proliferation of 4 different PaCa cell lines. Resveratrol inhibited the growth of all 4 human pancreatic cancer cells (AsPC-1, MIA PaCa-2, Panc-1 and Panc-28) in a dose- and time-dependent manner (Fig. 1b).
Whether resveratrol can potentiate the effect of gemcitabine against these 4 cell lines was also examined. We employed an esterase staining assay (live/dead assay) to establish whether resveratrol can potentiate the apoptosis induced by gemcitabine. As shown in Figure 1c, the dose of resveratrol (10 μM) or gemcitabine (100 nM) that had minimum effect on apoptosis alone produced synergistic apoptosis when combined.
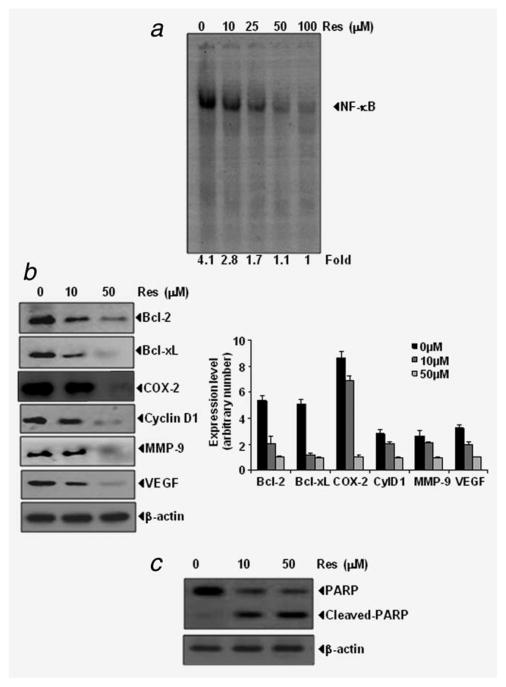
How resveratrol potentiates the effects of gemcitabine was also investigated. NF-κB has been shown to be constitutively expressed in these cell lines and causes resistance to apoptosis. Whether resveratrol causes down regulation of constitutive NF-κB activation in MIA PaCa-2 cells was examined by using a DNA binding assay. Results show that the treatment with resveratrol inhibited NF-κB activation in a dose-dependent manner (Fig. 2a).
Figure 2.
(a) EMSA results showing that resveratrol suppresses the constitutive activation of NF-κB in a dose-dependent manner. The MIA PaCa-2 (1 ×106) cells were treated with indicated concentration of resveratrol for 4 hr, nuclear extracts were prepared and assayed for NF-κB activation by EMSA. Data are a representative of 2 independent experiments. (b) Left, resveratrol suppresses the constitutive expression of gene products involved in antiapoptosis, proliferation, metastasis and angiogenesis; right, quantitation of protein levels and (c), resveratrol induces the cleavage of PARP. The MIA PaCa-2 (1 × 106) cells were treated with indicated concentrations of resveratrol for 24 hr. At the termination of experiment whole cell lysate was prepared and western blot was performed.
Whether resveratrol downregulates the NF-κB-regulated gene products was also examined. We found that resveratrol suppressed the constitutive expression of anti apoptotic (bcl-2, bcl-xL), proliferative (COX-2, cyclin D1), metastatic (MMP-9) and angiogenic (VEGF) protein expression in a dose-dependent manner in MIA PaCa-2 cells (Fig. 2b). Resveratrol also induces the cleavage of PARP in MIA PaCa-2 cells (Fig. 2c).
Resveratrol inhibits the growth of orthotropic implanted PaCa in nude mice
We examined the therapeutic potential of resveratrol and gemcitabine either alone or in combination on the growth of orthotopically implanted human pancreatic cells in nude mice. The experimental protocol is depicted in Figure 3a. We decided to use MIA PaCa-2 cells for in vivo studies because first, we found MIA PaCa-2 relatively more sensitive from rest of cells and second, MIA PaCa-2 is stably transfected with luciferase. MIA PaCa-2 cells were implanted in the pancreas tails of nude mice. After a week, based on the initial IVIS image we randomized animals into 4 groups and started the treatment per the experimental protocol. The treatment was continued for 4 weeks and animals were sacrificed 6 weeks after tumor cell injection. The IVIS imaging was done on every week after the tumor implantation (Fig. 3b left panel).
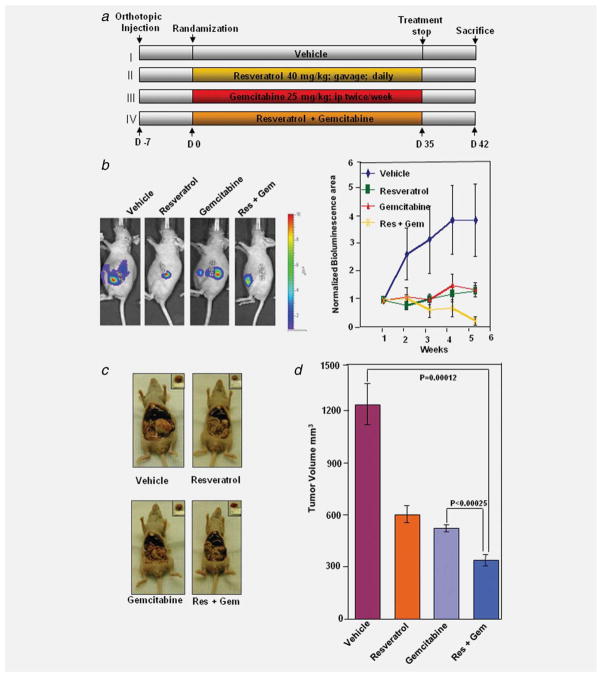
Figure 3.
Resveratrol potentiates the effect of gemcitabine to inhibit the growth of pancreatic cancer in nude mice. (a) Schematic representation of experimental protocol described in Material and Methods. Group I was given with Vehicle (100 μL, p.o., daily) and PBS (100 μL, i.p., twice weekly), Group II was given with resveratrol (40 mg kg−1, p.o., daily), Group III was given with gemcitabine (25 mg kg−1, i.p., twice weekly), and Group IV was given with resveratrol (40 mg kg−1, p.o., daily) and gemcitabine (25 mg kg−1, i.p., twice weekly; n = 8). (b) Left panel, bioluminescence IVIS images of orthotopically implanted pancreatic tumors in live, anesthetized mice. Right panel, measurements of photons per second depicting tumor volume at various time points using live IVIS imaging at the indicated times (n = 8). (c) Necropsy photographs of mice bearing orthotopically implanted pancreatic tumors; (d) tumor volumes in mice measured on the last day of the experiment at necropsy using Vernier calipers and calculated using the formula V = 2/3πr3 (n = 6).
The bioluminescence imaging results showed a gradual increase in tumor volume in the control group (Fig. 3b, right panel) as compared with rest of the groups. The tumor volume in the combination of resveratrol and gemcitabine group was significantly lower than resveratrol alone group (p < 0.01) or gemcitabine alone group (p < 0.05). Gemcitabine alone treatment was shown to be effective as resveratrol (p < 0.001 when compared to control; p > 0.05 when compared to resveratrol alone group) (Fig. 3b, right panel).
We found that resveratrol alone when given orally at 40 mg kg−1 significantly inhibited the growth of the tumor (p < 0.001 when compared to control) (Fig. 3d). Gemcitabine alone was as effective as resveratrol (p < 0.001 when compared to control; p > 0.05 when compared to resveratrol alone group); and the combination of the two agents were more effective in reducing the tumor burden. The tumor volume in the combination of resveratrol and gemcitabine group was significantly lower than resveratrol alone group (p < 0.01) or gemcitabine alone group (p < 0.01) (Fig. 3d).
Resveratrol inhibits CD31 and Ki-67 expression
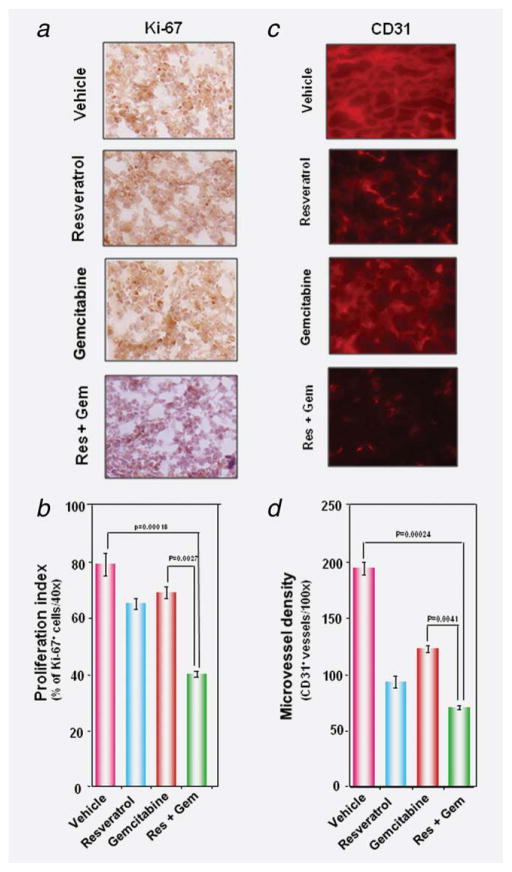
While Ki-67-positive index is used as a marker for cell proliferation, the CD31 index is a marker for micro vessel density. Whether resveratrol and gemcitabine modulate these markers, was examined. Figures 4a and 4b shows that both resveratrol (p < 0.05) and gemcitabine (p < 0.05) alone significantly down regulated the expression of Ki-67 in PaCa tissue and the combination of the 2 was most effective (p < 0.01). Similarly when examined for CD31, we found that both agents significantly reduced the CD31 expression as compared to control group (Fig. 4c) and two together were most effective (p < 0.01 when compared to gemcitabine alone) (Fig. 4d).
Figure 4.
Resveratrol enhances the effect of gemcitabine against tumor cell proliferation and angiogenesis in pancreatic cancer. (a) Immunohistochemical analysis of proliferation marker Ki-67 indicates the inhibition of pancreatic cancer cell proliferation in resveratrol either alone or in combination with gemcitabine-treated groups of animals. (b) Quantification of Ki-67 positive cells as described in Material and Methods. Data represented mean ± SE. p < 0.001 versus control; p < 0.01 versus gemcitabine. (c) Immunohistochemical analysis of CD31 for micro vessel density in pancreatic cancer tumors indicates the inhibition of angiogenesis by either resveratrol alone and in combination with gemcitabine; (d) quantification of CD31+ micro vessel density as described in Material and Methods. Data represented mean ± SE. p < 0.001 versus control; p < 0.01 versus gemcitabine. The photographs were taken at the magnification of ×40.
Resveratrol inhibited the constitutive NF-κB activation and NF-κB-regulated gene products in PaCa
We evaluated the effect of resveratrol and gemcitabine on NF-κB levels in pancreatic tumor tissues. Figure 5a shows that resveratrol either alone or in combination with gemcitabine was quite effective in suppressing the constitutive expression of NF-κB in pancreatic cancer tissue. Gemcitabine alone had no significant effect on constitutive NF-κB in PaCa tissue.
Figure 5.
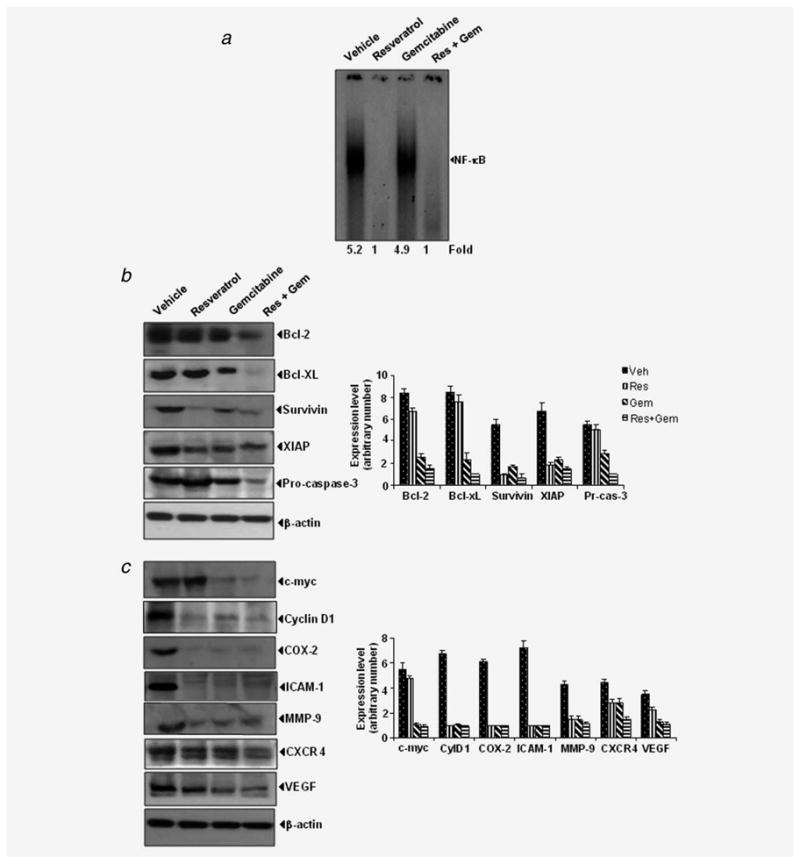
Effects of resveratrol and gemcitabine against expression of NF-κB and NF-κB-regulated gene products in pancreatic cancer tissue samples. (a) Detection of NF-κB by DNA binding assay (EMSA) in orthotopic tumor tissue samples showed the inhibition of NF-κB by resveratrol. (b,c) Left, western blot showing that combination of resveratrol and gemcitabine inhibit the expression of NF-κB-dependent gene products in pancreatic tumor tissues. Right, quantitation of protein levels. Samples from 3 animals in each group were analyzed and representative data are shown.
PaCa showed overexpression of proliferative (c-myc, cyclin D1, COX-2), survival (bcl-2, bcl-xL, survivin, XIAP), invasion (ICAM-1, MMP-9, CXCR4) and angiogenesis (VEGF) gene products, all under the control of NF-κB. Whether resveratrol and gemcitabine can modulate the expression of these NF-κB-regulated gene products, was examined by western blot analysis. We found that resveratrol alone significantly down regulated the expression of cyclin D1, COX-2, ICAM-1, MMP-9 and survivin (Figs. 5b and 5c). It did not significantly affect CXCR4, bcl-2, bcl-xL or VEGF expression. Treatment with both resveratrol and gemcitabine was needed to down regulate CXCR4, bcl-2, bcl-xL and VEGF expression. Thus the combination of gemcitabine and resveratrol was effective in down regulating the overexpression of proliferative (c-myc, cyclin D1, COX-2), survival (bcl-2, bcl-xL, survivin, XIAP), invasion (ICAM-1, MMP-9, CXCR4) and angiogenesis (VEGF) gene products.
Whether modulation of nuclear NF-κB, COX-2, VEGF and MMP-9 can also be detected by immunohistochemical methods was examined. As shown in Figure 6 these gene products were substantially down regulated in resveratrol-treated samples. The down regulation was less impressive with gemcitabine alone. The immunohistochemical data supports the data obtained from western blot.
Figure 6.
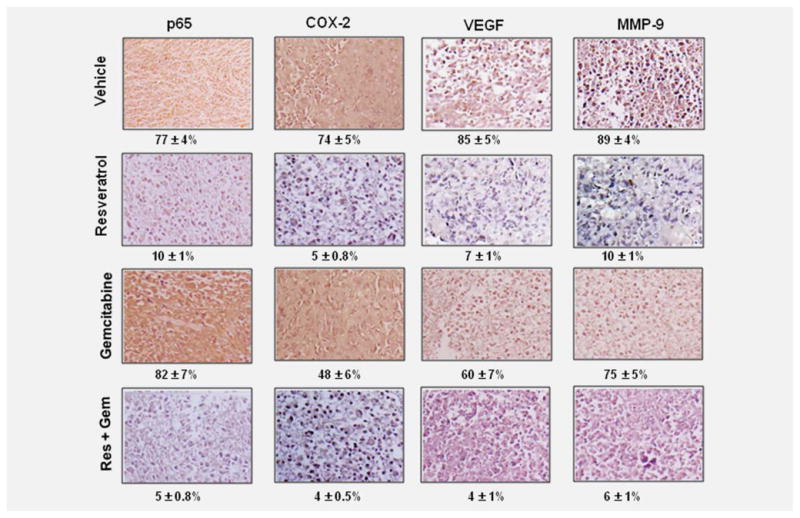
Immuno histochemical analysis of nuclear p65, COX-2, MMP-9 and VEGF showed the inhibition of NF-κB, COX-2, MMP-9 and VEGF by either resveratrol alone or in combination with gemcitabine. Percentage, positive staining for the given biomarker. The photographs were taken at the magnification of ×40.
These results collectively indicate that resveratrol suppress the activation of NF-κB thereby inhibiting the expression of genes involved in proliferation, survival, invasion and angiogenesis.
Discussion
Over the years numerous targeted agents have been tested in combination with gemcitabine including erlotinib, platinum analogues, bevacizumab and celecoxib,3 but they have proved toxic and ineffective. Because cancer is a multigenic disease, most targeted therapies alone have not and will not have much future. In fact, most recent advances in cancer drug development have been in the area of multiple targeting, once referred to as “dirty drugs.” Resveratrol, on the other hand is a compound derived from a Chinese traditional medicine (Polygonum cuspidatum), red grape skin, berries and peanuts; is highly safe agent and has the ability to modulate multiple cell signaling pathways.15 Because of its ability to activate the protein deacetylase enzyme silent information regulator 2/sirtuin 1 (SIRT1), resveratrol has emerged recently as an agent that can prolong the life span, probably through the delay of the onset of most chronic illnesses including cancer.41 The work from our laboratory showed for the first time that resveratrol is a potent inhibitor of the NF-κB pathway,13 which has been closely linked to inflammation and cancer.8 The aim of this study was to determine whether resveratrol has potential either alone or in combination with gemcitabine in the treatment of pancreatic cancer, one of the most lethal cancers. Whether examined cell culture or in animal model, resveratrol alone inhibited the growth of PaCa and the presence of gemcitabine enhanced its activity.
We found that the proliferation of all 4-cell lines (MIA PaCa-2, AsPC-1, Panc-1 and Panc-28), which exhibit K-ras and p53 mutations,42 was inhibited by resveratrol. Our results are in agreement with a previous report that resveratrol can suppress the proliferation of AsPC-1 and Panc-1 cells.43 We also found for the first time that resveratrol when used in combination with gemcitabine, is highly effective in inducing apoptosis in all 4 cell lines. We found that downregulation of NF-κB, which is constitutively activated in PaCa, could be one of the mechanisms. We also showed that bcl-xL, bcl-2, COX-2, cyclin D1, VEGF and MMP-9 which are constitutively expressed by PaCa cells, were down regulated by resveratrol. These gene products may contribute to the effect of resveratrol in PaCa. Golkar et al. showed that macrophage inhibitory cytokine (MIC-1), a member of the transforming growth factor beta (TGF-beta) super family, is up-regulated in resveratrol-treated PaCa cells contributes to its activity,44 thus suggesting that more than one mechanism may be involved in the anti proliferative activity of resveratrol.
We also found that in an orthotopic mouse model resveratrol effectively suppressed the growth of PaCa. In this model, resveratrol was found to be as effective as gemcitabine in inhibiting the tumor volume. When the two agents were used together, maximum abrogation of tumor volume was observed. Although our study is the first to report the effect of resveratrol alone on PaCa in mouse model, these results are consistent with previous reports which showed in rodents the growth suppressive effects of resveratrol alone against hepatoma,29,36 neuroblastoma,30,34 gastric carcinoma,31 breast carcinoma,26,27 colon carcinoma,32 and leukemia.33 The effects of resveratrol against PaCa were slightly but significantly enhanced by gemcitabine in animals. This is in agreement with previous reports that inhibition of growth of tumors in mice by resveratrol is enhanced by 5-fluorouracil36 and gemcitabine.20 When examined for the mechanism by which resveratrol manifests its effects against PaCa in animal models, we found that the proliferation marker Ki-67 as well as micro vessel density indicator CD31 were down regulated by resveratrol. Further investigation, also revealed the down regulation of NF-κB and NF-κB-regulated cyclin D1, COX-2, survivin, ICAM-1, MMP-9 and VEGF.
Our results also show the overexpression of CXCR4 in tumor tissues and it’s down regulation by the combination regime of resveratrol and gemcitabine. There is evidence that overexpression of CXCR4 is associated with advanced stages of pancreatic cancer.45 However, we also observed differential responses of resveratrol on bcl-2 and bcl-xL when compared among in vitro and in vivo effects and these may be because of different niche. Our observations are also in agreement with reports that resveratrol suppresses angiogenesis and metastasis in glioma,30,34 lung carcinoma,35 and breast cancer.26,27 Our results overall suggest that resveratrol has significant potential for the treatment of pancreatic cancer and its effects are further enhanced by gemcitabine. Although resveratrol is known to exhibit antioxidant activity46,47 and combining several agents with antioxidant activity with chemotherapeutics is discouraged48; we found no evidence of antagonistic effects of resveratrol when combined with gemcitabine. Limited clinical trials suggest that resveratrol is quite safe in human.49 Based on these results, further studies are required to fully explore the potential of resveratrol as an anticancer agent in PaCa.
Acknowledgments
Dr. Aggarwal is the Ransom Horne Jr., Professor of Cancer Research. The authors are thankful to Walter Pagel for editing the manuscript.
Grant sponsor: Clayton Foundation
References
- 1.Cancer facts and figures. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha-Lima CM. New directions in the management of advanced pancreatic cancer: a review. Anticancer Drugs. 2008;19:435–46. doi: 10.1097/CAD.0b013e3282fc9d11. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 5.Weichert W, Boehm M, Gekeler V, Bahra M, Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP, Niesporek S, Jacob J, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97:523–30. doi: 10.1038/sj.bjc.6603878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, Schafer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 7.Aoki T, Nagakawa Y, Tsuchida A, Kasuya K, Kitamura K, Inoue K, Ozawa T, Koyanagi Y, Itoi T. Expression of cyclooxygenase-2 and vascular endothelial growth factor in pancreatic tumors. Oncol Rep. 2002;9:761–5. [PubMed] [Google Scholar]
- 8.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–63. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 10.Xiong HQ. Molecular targeting therapy for pancreatic cancer. Cancer Chemother Pharmacol. 2004;54 (Suppl 1):S69–S77. doi: 10.1007/s00280-004-0890-2. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–54. [PubMed] [Google Scholar]
- 12.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 13.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–19. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 14.Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–83. [PubMed] [Google Scholar]
- 15.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 16.Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 17.Rezk YA, Balulad SS, Keller RS, Bennett JA. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol. 2006;194:e23–e6. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–95. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 20.Bernhaus A, Ozsvar-Kozma M, Saiko P, Jaschke M, Lackner A, Grusch M, Horvath Z, Madlener S, Krupitza G, Handler N, Erker T, Jaeger W, et al. Antitumor effects of KITC, a new resveratrol derivative, in AsPC-1 and BxPC-3 human pancreatic carcinoma cells. Investig N Drugs. 2009;27:393–401. doi: 10.1007/s10637-008-9183-7. [DOI] [PubMed] [Google Scholar]
- 21.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–63. [PubMed] [Google Scholar]
- 22.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–7. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 23.Khanduja KL, Bhardwaj A, Kaushik G. Resveratrol inhibits N-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J Nutr Sci Vitaminol (Tokyo) 2004;50:61–5. [PubMed] [Google Scholar]
- 24.Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–6. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 25.Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 26.Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer. 2005;115:36–45. doi: 10.1002/ijc.20874. [DOI] [PubMed] [Google Scholar]
- 27.Shibata MA, Akao Y, Shibata E, Nozawa Y, Ito T, Mishima S, Morimoto J, Otsuki Y. Vaticanol C, a novel resveratrol tetramer, reduces lymph node and lung metastases of mouse mammary carcinoma carrying p53 mutation. Cancer Chemother Pharmacol. 2007;60:681–91. doi: 10.1007/s00280-007-0414-y. [DOI] [PubMed] [Google Scholar]
- 28.Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007;28:1946–53. doi: 10.1093/carcin/bgm144. [DOI] [PubMed] [Google Scholar]
- 29.Liu HS, Pan CE, Yang W, Liu XM. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol. 2003;9:1474–6. doi: 10.3748/wjg.v9.i7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136:57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280–4. doi: 10.3748/wjg.v11.i2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan MH, Gao JH, Lai CS, Wang YJ, Chen WM, Lo CY, Wang M, Dushenkov S, Ho CT. Antitumor activity of 3,5,4′-trimethoxystilbene in COLO 205 cells and xenografts in SCID mice. Mol Carcinog. 2008;47:184–96. doi: 10.1002/mc.20352. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Fan GX, Wang W, Li T, Yuan YK. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int Immunopharmacol. 2007;7:1221–31. doi: 10.1016/j.intimp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Chen JC, Chen Y, Lin JH, Wu JM, Tseng SH. Resveratrol suppresses angiogenesis in glioms: evaluation by color Doppler ultrasound. Anticancer Res. 2006;26:1237–45. [PubMed] [Google Scholar]
- 35.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, Lopez-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007;245:144–8. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–52. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma A, Guha S, Diagaradjane P, Kunnumakkara AB, Sanguino AM, Lopez-Berestein G, Sood AK, Aggarwal BB, Krishnan S, Gelovani JG, Mehta K. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14:2476–83. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 38.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 39.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 41.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 42.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Kloppel G, Kalthoff H, Ungefroren H, Lohr M, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of. K-ras, p53, p16 and. DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 43.Ding XZ, Adrian TE. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas. 2002;25:e71–e6. doi: 10.1097/00006676-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Golkar L, Ding XZ, Ujiki MB, Salabat MR, Kelly DL, Scholtens D, Fought AJ, Bentrem DJ, Talamonti MS, Bell RH, Adrian TE. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res. 2007;138:163–9. doi: 10.1016/j.jss.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 46.Sethi G, Ahn KS, Sung B, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. SH-5, an AKT inhibitor potentiates apoptosis and inhibits invasion through the suppression of anti-apoptotic, proliferative and metastatic gene products regulated by IkappaBalpha kinase activation. Biochem Pharmacol. 2008;76:1404–16. doi: 10.1016/j.bcp.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 48.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–83. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 49.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]