Abstract
Chronic cocaine use is associated with enhanced cue reactivity to drug stimuli. However, it may also alter functional connectivity (fcMRI) in regions involved in processing drug stimuli. Our aims were to evaluate the neural regions involved in subjective craving and how fcMRI may be altered in chronic cocaine users. Fourteen patients with a confirmed diagnosis of cocaine abuse or dependence (CCA) and 16 gender, age, and education-matched healthy controls (HC) completed a cue reactivity task and a resting state scan while undergoing functional magnetic resonance imaging. CCA showed increased activation compared to HC in left dorsolateral prefrontal and bilateral occipital cortex in response to cocaine cues but not to appetitive control stimuli. Moreover, CCA also showed increased activation within the orbital frontal cortex (OFC) for cocaine cues relative to the appetitive stimuli during a hierarchical regression analysis. A negative association between subjective craving and activity in medial posterior cingulate gyrus (PCC) was also observed for CCA. CCA exhibited increased resting state correlation (positive) between cue-processing seed regions (OFC and ventral striatum), and negative connectivity between cue-processing regions and PCC/precuneus. These alterations in fcMRI may partially explain the neural basis of increased drug cue salience in CCA.
Keywords: Cocaine, Cocaine Cue, Craving, Functional Connectivity, fMRI
1. Introduction
Neuroadaptations secondary to longstanding drug use are a major cause of drug addiction and may compromise the addict’s ability to suppress drug seeking when exposed to cues associated with drug use (Kalivas and O’Brien, 2008; Kalivas and Volkow, 2005). Unfortunately, in the case of cocaine dependence, few effective pharmacologic treatments exist (Poling et al., 2007). Further identifying the networks involved in the maladaptive decision to use cocaine upon cue exposure may help development of more effective treatments.
Research suggests an abnormal functional organization in addictive brains in which there is enhanced salience of drug-related cues but weakened strength of cognitive control (Baler and Volkow, 2006). In chronic cocaine abusers (CCA), the cue-elicited functional magnetic resonance imaging (fMRI) literature demonstrates that cocaine cues activate the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), posterior cingulate cortex (PCC), amygdala, orbitofrontal cortex (OFC), thalamus, insula, dorsal striatum (DS), and ventral striatum (VS) (Bonson et al., 2002; Childress et al., 1999; Duncan et al., 2007; Garavan et al., 2000; Grant et al., 1996; Kilts et al., 2004; Kosten et al., 2006; Maas et al., 1998; Wang et al., 1999; Wexler et al., 2001). However, the reliability of these activations across studies is mixed (Wilson et al., 2004).
One potential reason for mixed findings is that previous studies have not isolated the unique contribution of subjective craving to neural activation during cue exposure. Instead, post-scan subjective craving ratings are typically correlated with neural activation, resulting in activation of the DLPFC, ACC, amygdala, insula, and caudate (Grant et al., 1996; Maas et al., 1998; Sinha et al., 2005; Wang et al., 1999). Subjective craving is an important predictor of clinical outcomes and relapse risk when it is elicited in the context of drug cues (Drummond et al., 2000; Waters et al., 2004), although a consistent association between subjective craving and relapse behavior has not always been observed (Rosenberg, 2009; Tiffany and Carter, 1998). Dissociating subjective craving and cocaine cue processing networks has yet to be done in CCA, and may provide important information about the neurobiological substrates responsible for relapse.
Recent studies suggest that persistent functional changes within motivational networks associated with addiction can be measured through functional connectivity (fcMRI) analyses (Daglish et al., 2003; Gu et al., 2010; Hong et al., 2009; Ma et al., 2009; Tomasi et al., 2010). Resting-state fcMRI analyses examine low frequency oscillations in the blood-oxygen level dependent response occurring spontaneously in spatially distributed networks (Fox and Raichle, 2007). Despite being task-independent, they may provide information about critical brain networks overseeing basic sensory processes, memory, executive function, and salience detection (Fox and Raichle, 2007; Smith et al., 2009). Two recent studies investigating differences in functional connectivity in CCA reported decreased connectivity between mesocorticolimbic regions previously implicated in addiction (Gu et al., 2010; Tomasi et al., 2010).
Research suggests lateral OFC may be primarily involved in processing reward associated cues, whereas medial OFC may be more involved in the hedonic response to reward (Elliott et al., 2010; Kringelbach, 2005; Kringelbach and Rolls, 2004). VS has strong anatomical connections with OFC (Haber et al., 2006) and is involved in acquiring and expressing cue-elicited drug seeking and encoding salient events (Everitt and Robbins, 2005). The involvement of VS and lateral OFC in reward cue processing render them excellent candidates for examining fcMRI differences within the cocaine cue- processing network. We predicted that there would be increased connectivity within the cocaine cue-processing network in CCA as a result of increased utilization of this circuit following drug exposure.
The current study employed a novel task to investigate the contributions of cue-elicited versus subjective craving elicited brain activation. Recently abstinent CCA and matched healthy controls (HC) continuously rated their subjective craving during videos depicting drug usage and an appetitive control while undergoing fMRI, with hierarchical regression determining the potential contribution of subjective craving ratings. The second aim investigated whether CCA exhibited altered fcMRI within the reward cue processing network, which we defined a priori using the lateral OFC and VS as seed regions based on previous literature (Kringelbach and Rolls, 2004).
2. Methods
2.1 Participants
Sixteen subjects with a confirmed diagnosis of chronic cocaine abuse and/or dependence and 16 gender, age, and education-matched HC were recruited. Two CCA were excluded from the study because of excessive head motion (three standard deviations) compared to the rest of their cohort. Informed consent was obtained according to institutional guidelines at the University of New Mexico. All CCA participants were abstinent from cocaine for a minimum of three days prior to their MRI scan (confirmed by urine screen: One Step Multi-Drug, Multi-Line Screen Test) to allow for elimination of the majority of active cocaine metabolites. Thus CCA were unlikely to be in significant acute withdrawal (Walsh et al., 2009). CCA participants were excluded from the study if they had a history of DSM-IV opiate or sedative dependence, learning disorder, attention-deficit hyperactivity disorder, any major neurological condition, diagnosis of a schizophrenia spectrum disorder, or any contraindications for MRI. HC were excluded based on similar criteria, with the additional criteria of any history of diagnosed psychiatric disorders (with the exception of a remote history of substance abuse). For additional clinical details, see the online supplementary materials.
2.2 Clinical Assessment
Participants completed a battery of measures, including the Fagerstrom Test for Nicotine Dependence (FTND), the Cocaine Craving Questionnaire (Brief-NOW and Brief-GENERAL forms) (CCQ-N and CCQ-G) (Heinz et al., 2006), and the Structured Clinical Interview for DSM Disorders I Module E (SCID-I-E) for substance abuse and dependence. In addition, the Timeline Followback calendar was used to determine cocaine usage during the previous 30 days.
To reduce redundancy amongst similar neuropsychological measures, composite indices were calculated for the domains of memory, processing speed, executive functioning, and attention (see the online supplementary materials). The Wechsler Test of Adult Reading (WTAR) provided an estimate of pre-morbid intelligence. Measures of emotional status [State-Trait Anxiety Index (STAI) and Beck Depression Inventory-Second Edition (BDI)] were also assessed.
2.3 Tasks
All participants completed two tasks. In the first task, participants viewed 14 videos (see acknowledgments and supplementary materials) depicting cocaine consumption (e.g., someone snorting powder) or preparation (e.g., someone preparing a syringe). Participants also viewed 14 videos depicting food consumption (e.g., someone eating ice cream) or preparation (e.g., roasting tomatoes). Video presentation was pseudo-randomized, with average video duration equal to 12 seconds. Participants were instructed to continuously rate their desire to use cocaine on an 8-point Likert scale (0–7) as their cravings changed. The rating scale was presented at the bottom of the screen, and anchors reminded participants of scalar values (0 = None at All; 7 = Very Strong). Participants could increase (middle finger button) or decrease (index finger button) their craving ratings throughout the experiment, and rating levels were updated at a 50 Hz frequency. Subjects practiced the task prior to entering the scanner environment.
For the connectivity analyses, participants maintained fixation on a centrally presented cross (visual angle = 0.92°) for approximately five minutes, which occurred after the craving task.
High resolution TI anatomic (voxel size = 1×1×1 mm3) and whole brain echo-planar images (TR = 2000 ms; TE = 29 ms; flip angle = 750°; voxel size = 3.75×3.75×4.55 mm3) were collected on a 3T Siemens Trio scanner (supplementary material).
2.4 Craving Task: Image Processing and Statistical Analyses
Functional images were generated using Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Time series images were spatially registered to the second EPI image of the first run to minimize effects of head motion, temporally interpolated to correct for slice-time acquisition differences and de-spiked. Separate regressors for the food and cocaine videos (identical for all subjects) were created by convolving the experimental time-course for each condition with a double gamma variate function (S-Figure 1). Moreover, two additional regressors were created for each participant based on their subjective craving response (if present). The first regressor modelled each button press as a single event regardless of subjective craving amplitude level (i.e., a 1 was modelled the same as a 5) or direction of change (i.e., increase or decrease in craving). The second regressor resembled a step function, with each button press signalling the onset of either increasing or decreasing subjective levels of craving (i.e., craving magnitude modelled).
Two voxel-wise multiple regression analyses were conducted for all participants. A high-pass filter was included in all multiple regressions to remove low-frequency drifts. The first analysis entered all four regressors (if subjective craving was present) simultaneously into a single model. This was done to parse out variance associated with the videos and self-report of craving, which was expected to be different across the two groups. Next, a hierarchical multiple regression analysis was performed in which the food video regressor was entered first into the model, followed by the cocaine video regressor, the button-press regressor and finally the subjective craving regressor (if latter regressors were present). The incremental change in variance (R2) captured by the addition of each successive regressor could be calculated, converted to a signed correlation coefficient equivalent to a semi-partial correlation ( ), and finally to a z-score using Fisher’s method. The hierarchical regression achieved two objectives. The first was to identify regions exhibiting unique activation during the drug videos relative to the food videos within each group. Second, it identified unique variance modelled by the subjective craving response. The most conservative approach was adopted by entering the craving regressor last in the model.
The resulting beta values (standard regression) and z-scores (hierarchical regression) were transformed to a standard stereotaxic coordinate space (Talairach and Tournoux, 1988) using a 12 degree-of-freedom affine transformation and spatially smoothed using a 8 mm Gaussian kernel. Group differences (CCA versus HC) in cue (cocaine and food videos) reactivity and fcMRI were directly contrasted using independent sample t-tests. Z-scores (unique variance for different regressors) from hierarchical regression were compared against baseline separately for each group using a two-tailed t-test.
2.5 fcMRI: Image Processing and Statistical Analyses
Individual anatomical images (T1) were segmented into maps of cerebral spinal fluid (CSF), white matter, and gray matter (Zhang et al., 2001). Resultant CSF and white matter masks were used to obtain an average time-series for these tissues during the extended resting state runs for each individual. Finally, six movement parameters, the ROI-based time-series for CSF, the ROI-based time-series for white matter, a constant, and a linear term were entered into the regression against extended resting state time-series to remove noise variance associated with each of these variables (Fox et al., 2005). Resultant data were then transformed to a standard stereotaxic coordinate space (Talairach, 1988).
The residual resting-state time courses were averaged across a 12 mm diameter sphere for a priori determined regions in lateral OFC and VS. Seeds were chosen a priori based on published literature (Kringelbach and Rolls, 2004) rather than deriving seed regions from the craving task to avoid bias and to increase the generalizability of findings. Resultant correlation coefficients were then converted to z-scores using Fisher’s method.
Group-wise comparisons were performed with independent samples t-tests. For all whole-brain contrasts (fcMRI and cue-reactivity maps), a significance threshold corresponding to p < .005 was applied in combination with a minimum cluster size threshold of 32 native voxels to correct for false positives (p < .05) based on 10,000 Monte Carlo simulations (Forman et al., 1995). Region of interest analyses (connectivity analyses) were also performed to interrogate the a priori hypothesis of increased fronto-striatal connectivity, which were corrected (p < .05) at a spatial size of 20 native voxels (see the online supplementary materials).
3. Results
3.1 Neuropsychological and Clinical Data
Neuropsychological testing indicated a significant group difference on pre-morbid intelligence (t28 = −2.8, p < .01), with CCA exhibiting lower estimates than HC. Therefore, pre-morbid intelligence was used as a covariate for all other behavioral and functional analyses. ANCOVAs suggested higher scores (F2,27 = 4.3, p < .05) on the BDI for CCA than HC (although both were within the normal range) and a trend towards higher scores on the STAI Trait (F2,27 = 3.8, p = .06). The two groups did not differ on measures of STAI State or on FTND scores (p > .10). As expected, compared to HC, CCA demonstrated increased craving on scores for the CCQ-G (t28 = −5.9 p < .01) and CCQ-N (t28 = −6.1, p < .01). A MANCOVA suggested no group differences on neuropsychological composite scores (Table 1).
Table 1.
Demographic and clinical characteristics of chronic cocaine abuse/dependence (CCA) and healthy control (HC) participants.
| CCA | HC | |||||
|---|---|---|---|---|---|---|
| Mean | SD (+/−) | Mean | SD (+/−) | p value | Cohen’s d | |
| Demographic | ||||||
| Age | 37.14 | 8.97 | 36.38 | 8.77 | p > .10 | −0.09 |
| Education | 12.5 | 1.98 | 13.44 | 1.90 | p > .10 | 0.48 |
| HQ | 67.21 | 71.48 | 90.10 | 16.04 | p > .10 | 0.44 |
| Clinical | ||||||
| BDI-II*▲ | 48.81 | 8.40 | 43.04 | 4.55 | p < .05 | −0.85 |
| STAI-State▲ | 49.61 | 10.54 | 45.53 | 8.02 | p > .10 | −0.43 |
| STAI-Trait▲ | 55.79 | 13.64 | 46.56 | 8.61 | p = .06 | −0.81 |
| FTND | 2.63 | 2.07 | 1.71 | 2.22 | p > .10 | −0.43 |
| CCQ-G* | 3.09 | 1.38 | 1.06 | .16 | p < .01 | −2.07 |
| CCQ-N* | 3.02 | 1.30 | 1.04 | .15 | p < .01 | −2.15 |
| Neuropsych | ||||||
| WTAR* | 45.00 | 11.38 | 54.94 | 7.89 | p < .01 | 1.02 |
| Attention▲ | 44.16 | 4.22 | 46.07 | 4.81 | p > .10 | 0.42 |
| Memory▲ | 54.65 | 8.60 | 52.65 | 9.33 | p > .10 | −0.22 |
| PS▲ | 48.49 | 4.89 | 46.79 | 5.96 | p > .10 | −0.31 |
| EF▲ | 48.45 | 9.83 | 48.69 | 4.72 | p > .10 | 0.03 |
Note: HQ = handedness quotient; BDI-II = Beck Depression Inventory-Second Edition; STAI = State-Trait Anxiety Index; FTND = Fagerstrom Test for Nicotine Dependence; CCQ-G = Cocaine Craving Questionnaire-General; CCQ-N = Cocaine Craving Questionnaire-Now; WTAR = Wechsler Test of Adult Reading; PS = processing speed; EF = executive function. Demographic data are raw scores (above solid line), whereas clinical and Neuropsychological measures (below solid line) are T-scores.
Denotes significant result
Means, standard deviations, and effect sizes for neuropsychological and some clinical indices reported following correction for WTAR as covariate at 50.30.
3.2 Behavioral Data
Cue-elicited craving behavioral analyses were restricted to CCA as only 2 HC reported any degree of craving during the fMRI video task. A 2 × 2 [Time (run 1 versus run 2) × Video (cocaine versus food)] mixed-measures ANOVA was performed on the mean maximum rating for cocaine craving during each video type. Results indicated a significant effect of video (F1,13 = 5.8, p < .05), with greater craving ratings during cocaine (2.3 +/− .4) than food (1.1 +/−.3) videos. A similar analysis was conducted for the baseline state (i.e., fixation) following video presentation, which suggested that subjective levels of craving continued into the baseline state following the cessation of the drug videos (see the online supplementary materials).
3.3 Cue-elicited Functional Comparisons
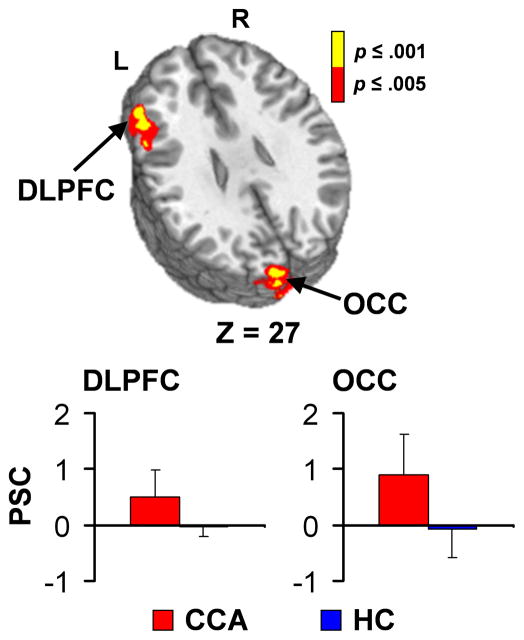
Two MANOVAs comparing rotational and translational motion parameters suggested that there were no group differences in head motion (p > 0.10). Group-wise comparisons of cue-elicted brain activation for both food and cocaine videos were conducted using estimates of pre-morbid intelligence as a covariate. There were no group differences in response to the food cues. However, CCA exhibited increased activation compared to HC within the left DLPFC (BAs 9/46) and predominantly left occipital cortex (BA17/18/19; Figure 1) for the cocaine cues.
Figure 1.
This figure depicts the regions exhibiting differences in activation between chronic cocaine abuser (CCA) and healthy control (HC) groups during presentation of cocaine cues. Significant clusters included the left dorsolateral prefrontal cortex (DLPFC) and left occipital cortex (OCC). Warm colors indicate greater activation for CCA compared to HC (red: .001 < p ≤ .005; yellow: p ≤ .001). Axial (z) slice location is provided according to the Talairach atlas (L= left and R = right). Bar graphs present percent signal change (PSC) values for each cluster (red bars = CCA, blue bars = HC).
3.4 Hierarchical Regression with CCA Group
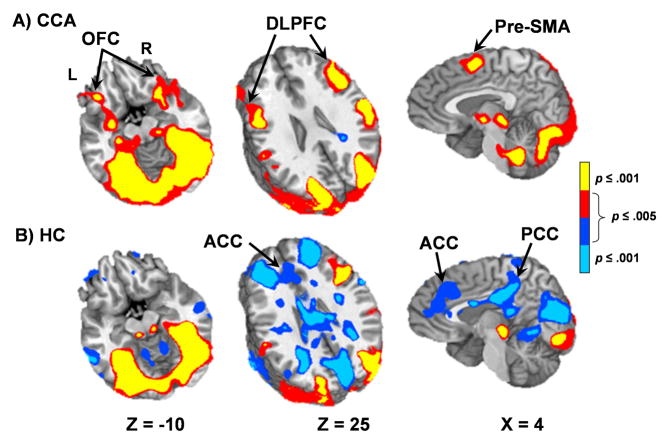
The unique variance associated with the cocaine video (after entering the food video) was also examined separately for both HC and CCA through a hierarchical multiple regression. HC exhibited widespread deactivation of the default-mode network (Raichle et al., 2001) during the presentation of cocaine videos, which was absent in CCA (Figure 2). Principal regions of deactivation included the ACC (BAs 24/32), PCC (BAs 23/29/30/31, angular gyrus (BAs 39/40), inferior and middle temporal gyri (BAs 20/21) and middle and superior frontal gyri (BAs 8/9/10). In contrast to HC, cocaine videos resulted in additional activation for CCA within the bilateral OFC (BAs 10/11/47), DLPFC (BAs 9/46), pre-supplementary motor area (pre-SMA; BAs 6/8), insulae (BA 13), parahippocampal gyri (BAs 27/28/35) extending into the amygdalae, and anterior thalamus.
Figure 2.
This figure presents the regions exhibiting unique variance associated with the cocaine videos in the hierarchical regression analysis for chronic cocaine abuser (CCA; Panel A) and healthy control (HC; Panel B) groups. Cocaine videos elicited activation (warm colors; red: .001 < p ≤ .005, yellow: p ≤ .001) within the frontal craving network areas for CCA, including bilateral orbital frontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC), with minimal deactivation of the default-mode network. In contrast, deactivation (cool colors; blue: .001 < p ≤ .005, cyan: p ≤ .001) of the default-mode network was prominent for HC and included the anterior cingulate gyrus (ACC) and posterior cingulate gyrus (PCC). Axial (z) and sagittal (x) slice locations are provided according to the Talairach atlas (L= left and R = right).
Although 13/14 CCA reported experiencing some cue induced cravings, the relationship between subjective ratings and the presentation of the cocaine videos was highly variable across participants (correlation = 0.0 – 0.92; see supplemental results). However, only a single cluster within the bilateral PCC (BAs 29/30/31) and medial visual areas (BAs 17/18/19/23/30) demonstrated a negative association with subjective craving levels (S-Figure 2).
The monitoring of subjective craving states likely introduced additional cognitive demands. Therefore, a separate analysis (see the online supplementary materials) was conducted (CCA only) in which the cocaine video regressor was entered last into the hierarchical regression following the other three regressors (food video, motor response and subjective craving) to statistically control for motor, attention and decision making processes involved in subjective ratings. These results (see S-Figure 3) were subsequently compared to the previously described model where the cocaine video was entered second following the food video. However, the only regions that did not exhibit significant unique activation when cocaine videos were entered last into the hierarchical regression were the bilateral insulae and pre-SMA.
3.5 Resting State fcMRI Analysis
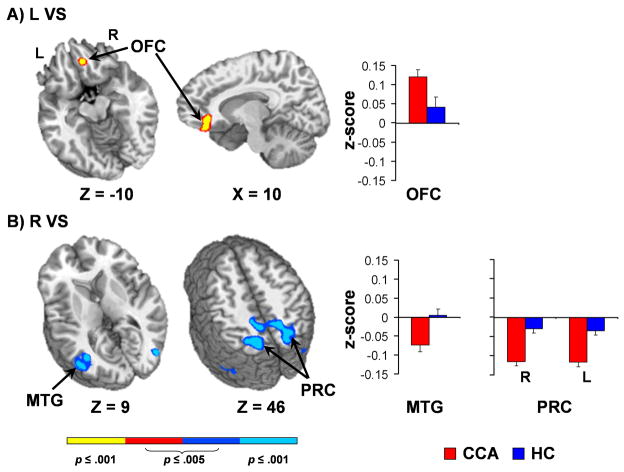
Increased connectivity (i.e., positive correlations) between the left VS seed and right OFC, extending into rostroventral ACC (BAs 10/11//32), was observed for CCA relative to HC (Figure 3A) in our region of interest analyses. Whole brain analyses (Figure 3B) indicated that CCA demonstrated increased anticorrelations for several bilateral posterior cortical regions including precuneus and PCC (BAs 7/31), middle temporal gyrus (BA 37), inferior parietal lobule (BA 40) and occipital cortex (BA 19) across multiple seeds (right VS, LOFC1 and LOFC2).
Figure 3.
This figure presents regions showing differential functional connectivity for chronic cocaine abusers (CCA; red) and healthy controls (HC; blue) based on seeds in the ventral striatum (VS). Panel A presents increased positive correlations between the left (L) VS and right orbital frontal cortex (OFC) for CCA. Panel B presents regions showing increased anti-correlations with the right (R) VS for CCA, including the middle temporal gyrus (MTG) and precuneus (PRC), which were also observed for several other seeds (see Table 2). Bar graphs indicate mean z-score within these clusters for CCA (red bars) and healthy control (blue bars) groups. Axial (z) and sagittal (x) slice locations are provided according to the Talairach atlas (L= left and R = right).
4. Discussion
Consistent with previous findings (Bonson et al., 2002; Garavan et al., 2000; Grant et al., 1996; Kilts et al., 2004; Kosten et al., 2006; Maas et al., 1998; Sinha et al., 2005; Wang et al., 1999), CCA exhibited increased activation in the left DLPFC and occipital cortex for cocaine cues relative to HC. In addition, hierarchical analyses indicated that cocaine videos were associated with increased activation within the OFC, DLPFC, amygdalae and anterior thalamus in CCA relative to an appetitive control stimulus. Hierarchical analyses also suggested that specifically modeling subjective craving responses captured additional unique variance within the PCC (negative association) and medial visual areas for CCA. Finally, CCA exhibited increased connectivity between the ventral striatum and OFC, as well as higher anti-correlations between these structures and the precuneus, PCC and ventral visual stream.
Our functional results showed greater DLPFC activation in response to cocaine videos for CCA compared to HC, consistent with previous literature implicating DLPFC in motivational decision making and inhibitory control (Barraclough et al., 2004; Fassbender et al., 2006). In addition, OFC, DLPFC, amygdalae and thalamic activation were also found to be associated with cocaine videos in a hierarchical analysis in CCA. Increased activation in the thalamus (Garavan et al., 2000; Sinha et al., 2005) and amygdala (Childress et al., 1999; Grant et al., 1996; Kilts et al., 2004) has also been previously reported for CCA following cue exposure. In addition, the OFC has previously been implicated in reward cue processing in HC (Kringelbach, 2005; Kringelbach and Rolls, 2004) and in response to cocaine videos (Garavan et al., 2000; Grant et al., 1996) and scripts (Bonson et al., 2002; Wang et al., 1999) in CCA. Collectively, these results emphasize the role that these structures play in response to cue exposure, although they do not necessarily indicate increases in subjective craving levels.
Specifically, although CCA endorsed increased craving on clinical measures and during exposure to cocaine stimuli, they continued to report craving during the baseline periods following the drug cues. Moreover, the relationship between cue exposure and subjective cravings exhibited considerable variation across subjects. These findings are consistent with previous work suggesting that cue time course and subjective craving time course do not perfectly coincide (Wexler et al., 2001), and that subjective craving may be elicited by triggers other than cue presentation (Drummond et al., 2000; Rosenberg, 2009).
A hierarchical regression approach was adapted to isolate variance associated with subjective craving after statistically controlling for cue exposure. However, this analysis did not reveal any additional activation in traditional areas associated with craving, which may have resulted from the conservative statistical approach that was adopted (i.e., craving regressor entered last). Instead, a negative correlation was observed between the subjective craving regressor and activation in the PCC and medial visual cortex. Previous studies have also reported an inverse relationship between subjective craving during stress imagery and PCC activation (Li et al., 2005), although others have found that PCC activation predicts relapse (Kosten et al., 2006). Such conflicting results emphasize the need for future study on the role of the PCC in addiction.
The hierarchical regression also indicated deactivation of the default-mode network during the presentation of cocaine videos for HC but not for CCA (Buckner et al., 2008; Raichle et al., 2001). This was unexpected, as the default-mode network generally exhibits task-induced deactivations during attention-demanding tasks (McKiernan et al., 2003) and has previously been resulted in greater task induced deactivations in CCA compared to HC (Tomasi et al., 2007; Tomasi et al., 2010). In our hierarchical analysis, subjective craving ratings were negatively associated with activation in the PCC, one of the default-mode’s central hubs (Buckner et al., 2008). Therefore, the lack of task-induced deactivations within the default-mode in CCA may have been secondary to the persistent presence of craving beyond the video. This could have also been due to the potential effect of psychoactive medications on default-mode network functional connectivity (Greicius et al., 2008). In addition, CCA may indeed be characterized by differences in functional connectivity within the default-mode network, as has been reported for several other neuropsychiatric conditions (Broyd et al., 2009).
Current results suggest that the relationship between regions of the cocaine cue processing network may also be altered in the absence of external stimuli, as has been recently reported by others (Gu et al., 2010; Tomasi et al., 2010). However, whereas previous work indicated decreased connectivity between selected mesocorticolimbic regions in CCA relative to HC (Gu et al., 2010; Tomasi et al., 2010), we observed increased fcMRI between the ventral striatum and OFC. The increased connectivity between reward and reward cue processing regions like VS (Everitt and Robbins, 2005) and medial OFC (Elliott et al., 2010; Kringelbach, 2005; Kringelbach and Rolls, 2004), with regions which have a larger role in motivational decision making and inhibitory control like rostroventral ACC (Beckmann et al., 2009; Margulies et al., 2007), may mediate the increased salience of drug cues and the greater potential for drug cues to trigger drug use. However, increased connectivity between regions involved in reward cue processing and motivation like OFC, ACC, and VS has also been reported in healthy controls (Beckmann et al., 2009; Di Martino et al., 2008; Margulies et al., 2007). Therefore, future studies should determine whether alterations in functional connectivity may also be directly related to the magnitude of the task-evoked BOLD response following cue exposure in CCA.
By contrast, the increased anti-correlation between cue processing regions and PCC, precuneus and visual cortex in CCA compared to HC may mediate the increased ability of cocaine cues to trigger subjective craving. Precuneus has been previously implicated in mediating craving resistance (Brody et al., 2007), and PCC, which is activated by cocaine cues in other studies (Duncan et al., 2007; Garavan et al., 2000; Kilts et al., 2004; Kosten et al., 2006), has been implicated in motivation and flexibility during decision making (Daw et al., 2006; Maddock, 1999; Mohanty et al., 2008). The increased anti-correlation between cue processing seed regions and precuneus/medial PCC in CCA compared to HC may mediate their increased perseveration on drug-related cues and craving.
Current findings are limited by several factors. Foremost, the current sample size was small. Therefore, null effects should be interpreted with caution. Second, current results may have been biased by other clinical differences (depression, co-morbid use of other substances, presence of pharmacological agents, etc.) between the two populations. Specifically, benzodiazepines may decrease prefrontal activation during tasks requiring memory and attention (Coull et al., 1999; Sperling et al., 2002), and may decrease amygdalar, insular, and cingulate gyrus activation to stress cues (Paulus et al., 2005; Schunck et al., 2011; Schunck et al., 2010). SSRIs may decrease OFC, amygdalar, parahippocampal and ventral striatal activation to rewarding, aversive, or other emotional stimuli (McCabe et al., 2010; Murphy et al., 2009; Windischberger et al., 2009). Unfortunately, these limitations are not easily controlled in most clinical studies of substance users, and are more likely representative of a true clinical sample of cocaine users. Third, our craving task by design involved the active rating of subjective experience rather than just passively viewing stimuli, which likely involved the recruitment of additional neuronal resources. However, the only regions which demonstrated a change in activation when cocaine videos were entered last into the regression were the insulae and pre-SMA. Finally, current fcMRI analyses were conducted following the presentation of the craving task, which may have resulted in residual activation within this circuit (Beckmann et al., 2005; Greicius et al., 2009; Toro et al., 2008), and thus connectivity findings could have been influenced by subjective craving.
In summary, our findings add to the literature describing the neural mechanisms by which environmental cues trigger craving and drug use in individuals with drug dependence (Bechara, 2005; Kalivas and Volkow, 2005). Our findings suggest increased fcMRI between regions involved in attribution of incentive salience (OFC and VS), as well as higher anti-correlations between selected seeds and regions (PCC and precuneus) implicated in motivational decision making (Daw et al., 2006) and resisting craving (Brody et al., 2007). Continuing to clarify how these different regions underlie compulsive drug seeking behavior is imperative as we move forward to develop effective treatments for cocaine dependence.
Supplementary Material
Table 2.
Voxel-wise group comparisons of seed-based correlation results for significant seeds only.
| Seed | Region | Side | BA | X | Y | Z | Volume (ml) | Z score | |
|---|---|---|---|---|---|---|---|---|---|
| HC | CCA | ||||||||
| R LOFC1 | PCC and precuneus | M | 5/7/31 | 9.0 | −49.9 | 50.7 | 5.083 | −0.002 | −0.079 |
| Precuneus | L | 7/19 | −18.6 | −65.4 | 44.5 | 4.089 | −0.007 | −0.070 | |
| Middle temporal and supramarginal gyrus | L | 39/40 | −35.2 | − 49.0 | 32.5 | 2.566 | −0.006 | −0.067 | |
| R LOFC2 | PCC and precuneus | M | 7/31 | 2.2 | −53.8 | 43.7 | 4.874 | −0.052 | −0.117 |
| R VS | PCC and precuneus | M | 7/31 | 8.9 | −55.0 | 45.0 | 5.708 | −0.030 | −0.115 |
| Precuneus | L | 7 | −19.7 | −59.8 | 47.3 | 4.803 | −0.036 | −0.117 | |
| Middle temporal and occipital gyrus | L | 19/37/39 | −39.1 | −62.2 | 0.3 | 4.939 | 0.005 | −0.074 | |
| Middle and superior occipital gyrus | R | 19 | 32.7 | − 75.8 | 17.3 | 4.474 | −0.003 | −0.091 | |
| L VS* | Orbital frontal gyrus | R | 10/11/ 32 | 10.5 | 35.1 | −11.4 | 1.461 | 0.041 | 0.120 |
Note: Side refers to the hemisphere showing activation where L = left and R = right hemisphere, and M = medial. The Brodmann area (BA), the center of mass in Talairach coordinates (X, Y, Z), and the volume are specified for each area of activation. Significant results were obtained for the lateral orbital frontal cortex (LOFC) and ventral striatum (VS) seeds.
Results from region of interest analysis.
Footnotes
Supplementary materials for this article, containing further information on methods and results, can by found by accessing the online version of this paper at http://dx.doi.org by entering doi:...
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Dolan RJ. Dissociating neuromodulatory effects of diazepam on episodic memory encoding and executive function. Psychopharmacology (Berl) 1999;145:213–222. doi: 10.1007/s002130051051. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage. 2003;20:1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;95(Suppl 2):S247–255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20:198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Foxe JJ, Garavan H. Mapping the functional anatomy of task preparation: priming task-appropriate brain networks. Hum Brain Mapp. 2006;27:819–827. doi: 10.1002/hbm.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31:355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2009;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clin Psychol Rev. 2009;29:519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Schunck T, Mathis A, Erb G, Namer I, Hode Y, Demazières A, Luthringer R. One milligram of lorazepam does not decrease anxiety induced by CCK-4 in healthy volunteers: investigation of neural correlates with BOLD MRI. J Psychopharmacol. 2011;25:52 – 59. doi: 10.1177/0269881110367449. [DOI] [PubMed] [Google Scholar]
- Schunck T, Mathis A, Erb G, Namer IJ, Demazieres A, Luthringer R. Effects of lorazepam on brain activity pattern during an anxiety symptom provocation challenge. J Psychopharmacol. 2010;24:701–708. doi: 10.1177/0269881109104864. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp Clin Psychopharmacol. 2009;17:205–216. doi: 10.1037/a0016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, Gerstl F, Fink M, Moser E, Kasper S. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2009;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.