Abstract
The ability of the cell to generate sufficient energy through oxidative phosphorylation and to maintain healthy pools of mitochondria are critical for survival and maintenance of normal biological function, especially during periods of increased oxidative stress. Mitochondria in most cardiovascular cells function at a basal level that only draws upon a small fraction of the total bioenergetic capability of the organelle; the apparent respiratory state of mitochondria in these cells is often close to state 4. The difference between the basal and maximal activity, equivalent to state 3, of the respiratory chain is called the reserve capacity. We hypothesize that the reserve capacity serves the increased energy demands for maintenance of organ function and cellular repair. However, the factors that determine the volume of the reserve capacity and its relevance to biology are not well understood. In this study, we first examined whether responses to 4-hydroxynonenal (HNE), a lipid peroxidation product found in atherosclerotic lesions and the diseased heart, differ between vascular smooth muscle cells, adult mouse cardiomyocytes, and rat neonatal cardiomyocytes. In both types of cardiomyocytes, oxygen consumption increased after HNE treatment, while oxygen consumption in smooth muscle cells decreased. The increase in oxygen consumption in cardiomyocytes decreased the reserve capacity and shifted the apparent respiratory state closer to state 3. Neonatal rat cardiomyocytes respiring on pyruvate alone had a fourfold higher reserve capacity than cells with glucose as the sole substrate, and these cells were more resistant to mitochondrial dysfunction induced by 4-HNE. The integration of the concepts of reserve capacity and state-apparent are discussed along with the proposal of two potential models by which mitochondria respond to stress.
Keywords: mitochondria, reserve capacity, respiratory state, 4-hydroxynonenal, aldehydes, heart disease
1. Introduction
Recent studies have indicated that the bioenergetic reserve capacity (also called spare respiratory capacity) is critical for maintaining cellular function during acute and chronic stress. The concept of reserve capacity has been examined in the context of the failing heart in vivo [15], in cardiomyocytes treated with oxidized lipids [18], in endothelial cells subjected to oxidative stress [11], and in neurons subjected to glutamate toxicity or inhibition of electron transport [7, 41]. In these studies, breaching of the maximal respiratory capacity—leading to loss of the reserve capacity—was predictive of cell death or organ dysfunction. However, it has been difficult to test the reserve capacity hypothesis directly beyond simple usage in cells with high energy demand such as skeletal or cardiac muscle. In addition, the biological processes that both satisfy the basal bioenergetic requirements of the cell and regulate the increased utilization of the respiratory capacity are not clear. The advent of new technologies that have supported the concept of reserve capacity have also allowed for the measurement of the “apparent respiratory state” of mitochondria in intact cells under controlled conditions [14]. Despite the evolution of these ideas, their integration in the context of cell biology remains challenging and is the subject of this short overview.
The respiratory state of the cell is likely regulated at multiple levels. At face value, the balance of ATP demand with substrate availability would appear to be the major modulator regulating respiratory state in intact cells. However, the generation of reactive oxygen species, cell signaling, intracellular Ca2+, and the redox state of the electron transport chain are also variables influencing respiratory activity. Studies in isolated mitochondria have been useful for examining changes in respiration due to modifications in respiratory chain components or intramitochondrial metabolic enzymes; yet, these studies may not relate directly to changes in respiratory function in the intact cell. For example, isolated mitochondria experiments typically use an excess of substrate (usually for one complex only). Addition of ADP to mitochondria incubated with substrate alone increases oxygen consumption and is used to measure state 3 respiration. This is different, on many fronts, from respiration occurring in intact cells and organs. In cells and tissues, substrate is generally not saturating and comes from multiple sources (e.g., glycolysis, fatty acids) leading to input of electrons at more than one point in the respiratory chain. In addition, intracellular adenine nucleotides are the largest regulators of mitochondrial respiration [9]; ADP in the cytoplasm is in the µM range and in equilibrium with the creatine kinase reaction [34]. Hence, mitochondria in cells respond to dynamic changes in ATP/ADP ratios, and this is generally not encompassed in experiments with isolated mitochondria, where higher levels of ADP are generally used. Also, cell signaling and Ca2+ play a major role in respiratory responses to stress, and control over these regulators cannot be readily recapitulated in isolated mitochondrial preparations.
Previous studies have shown that cells normally consume oxygen in an intermediate state between state 3 and 4, what we have called state apparent. For example, bovine endothelial cells were measured to respire at state 3.6 basally and to respire at a near maximal respiratory state of 3.1 when challenged with oxidants [11]. The mitochondria in cells can then populate a wide range of “respiratory states” and under basal conditions often have a considerable “reserve respiratory capacity.” When the respiratory state is taken toward state 3, the reserve capacity is utilized. Engagement of the reserve capacity also occurs upon exposure of cardiac myocytes to the lipid peroxidation product 4-hydroxynonenal (HNE) [18] and in smooth muscle cells treated with diamide [20]; in both cases, this can lead to bioenergetic collapse and cell death. Although the studies cited above underscore the importance of the reserve capacity, many questions remain. For example, it is not known how other cardiovascular cells, e.g., aortic smooth muscle cells, respond to products of lipid peroxidation; do they increase their rate of oxygen consumption after electrophilic insults as do more excitable cells such as cardiomyocytes? This is important because lipid peroxidation products accumulate in the diseased vasculature and heart [12, 17, 28, 36, 39]. Hence, understanding bioenergetic responses of these different cell types could give insights into the mechanisms of cardiovascular disease and potential therapeutic treatments. In addition, the response of myocytes to HNE has only been examined in neonatal myocytes [18]. Do adult cardiac myocytes respond in a similar manner to oxidative stress? How does modulation of the reserve capacity affect the response of the cell to stress? How does the concept of reserve capacity relate with the mitochondrial respiratory state, and does this change the way we think about mitochondrial and cellular responses to stress?
In this overview, we illustrate some key experimental data that address some of these questions. We found that the response of cardiovascular cells to electrophilic stress differs widely based on cell type and that respiratory substrates modulate the reserve capacity, apparent respiratory state, and the bioenergetic responses of the cells to stress induced by the oxidized lipid HNE. The implications for these findings are discussed and separate models of mitochondrial responses to stress are proposed.
2. Methods
2.1. Materials
All reagents were from Sigma (St. Louis, MO, USA) and of the highest grade offered. HNE was prepared on site as previously described [35].
2.2. Cell isolation and culture
Neonatal rat cardiac myocytes (NRCMs) were isolated from 1- or 2-day–old Sprague-Dawley rats and cultured as described previously [1, 22, 23, 30]. Primary adult mouse cardiomyocytes (AMCMs) were isolated as described in [26]. Primary rat aortic smooth muscle cells (SMCs) were isolated and cultured as described in [19].
2.3. Measurement of cellular energetics
Cellular energetics were measured in cells using a Seahorse Bioscience XF24 extracellular flux analyzer (Billerica, MA), as described previously [18]. For measuring energetics in NRCMs and SMCs, the media was changed to unbuffered DMEM (Mediatech, Manassas, VA) supplemented with 4 mM glutamine containing either pyruvate (1 mM), D-glucose (5.5 mM), or both 1 h before energetic assays. For CMCs, the myocytes were resuspended in modified Tyrode’s solution containing 135 mM NaCl, 4.4 mM KCl, 1.0 mM MgCl2, 1.2 mM KH2PO4, 0.5 mM CaCl2, 11 mM glucose, 10 mM 2,3-butanedione monoxime (BDM), and 30 mM taurine, pH 7.4, and allowed to settle in the XF24 plates prior to assay. Data are expressed as the rate of oxygen consumption (OCR) in pmol/min or the rate of extracellular acidification (ECAR) in mpH/min.
2.4. Statistical analyses
Data are reported as means ± SEM. Comparisons between two groups were performed with Student’s t-test. One-way analysis of variance (ANOVA) with Bonferroni correction or repeated measure ANOVA were used where appropriate. The null hypothesis was rejected when p < 0.05.
3. Results and Discussion
3.1. How do different cardiovascular cell types respond to electrophilic stress?
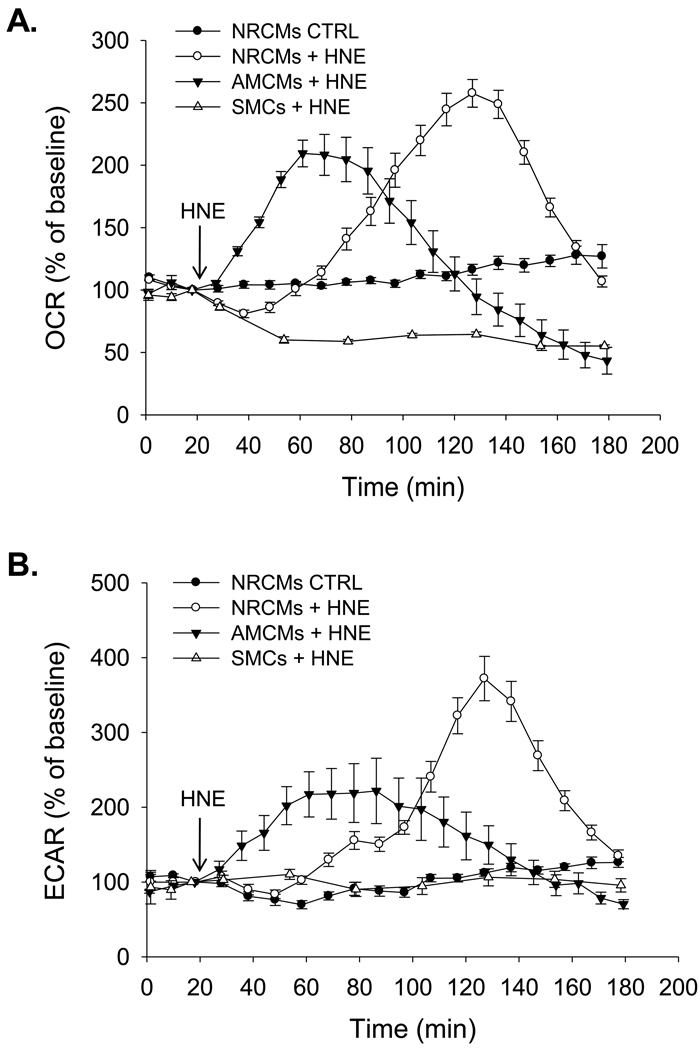
We first addressed this question by examining the bioenergetic responses of neonatal cardiomyocytes (NRCMs), adult mouse cardiomyocytes (AMCMs), and rat aortic smooth muscle cells (SMCs) to the electrophilic lipid HNE. HNE is derived in vivo from the oxidation of arachidonate and linoleate and can deplete intracellular glutathione levels and/or covalently modify proteins, leading to cell damage and death [13]. Interestingly, electrophiles such as HNE accumulate to quite high levels in both the diseased vasculature and heart [12, 17, 28, 36, 39], yet the mechanisms by which cells in these tissues handle electrophile stress are unclear. As shown in Fig. 1A, NRCMs increased their rate of oxygen consumption approximately 1 h after exposure to 20 µM HNE. This is comparable to previous findings showing the concentration-dependent responses of these cells to HNE [18]. For clarity, only one control trace (with untreated NRCMs) is shown; in all cell types, the baseline was stable in untreated cells. Adult cardiac myocytes, however, began increasing their rate of oxygen consumption almost immediately after HNE exposure, indicating that although bioenergetic responses between the two myocyte types were similar, the adult myocytes respond more quickly to the electrophilic insult. It should be noted that the assay conditions for adult myocytes were different than for NRCMs and SMCs. For adult myocytes, we used a modified Tyrode’s solution containing 11 mM glucose and no pyruvate as the running medium, which differs from the typical DMEM running medium containing 5.5 mM glucose and 1 mM pyruvate. Nevertheless, the general response to stress, i.e., the increase in oxygen consumption upon HNE addition, was similar between the two myocyte types.
Figure 1. Bioenergetic responses of different cardiovascular cells to electrophile stress.
Extracellular flux analysis of oxygen consumption and glycolysis in neonatal rat cardiomyocytes (NRCMs), adult mouse cardiomyocytes (AMCMs), and rat aortic smooth muscle cells (SMCs). Three baseline measurements of oxygen consumption rate (OCR; panel A) or extracellular acidification rate (ECAR; panel B) were recorded from isolated cardiomyocytes or SMC. Vehicle (DMEM) or HNE was then injected to a final concentration of 20 µM and the measurements then continued for the indicated times. n = 3–5 per group; Repeated measure one-way ANOVA indicated that the response of the groups in panels A and B to HNE differed with respect to time (p<0.05).
The underlying reasons for this increase in oxygen consumption remain unknown. It does not appear to be due to increased beating (twitching) of the myocytes because BDM, an inhibitor of cross-bridge formation that decreases or prevents myocyte contraction, does not reverse or inhibit the change in oxygen consumption. In both myocyte types the rate of oxygen consumption subsequently decreased below baseline levels which we ascribe to mitochondrial damage. This damage phase is associated with cell death, as described in [18], which has been shown to occur via Ca2+ overload [29] and by causing mitochondrial permeability transition [38]. Interestingly, the response of smooth muscle cells differed from the myocytes. Almost immediately after injection of 20 µM HNE, oxygen consumption in these cells declined to ~50% of their baseline rate (Fig. 1A), indicating that the energetic responses associated with the adaptive phase in myocytes do not occur in smooth muscle cells.
Changes in glycolysis after HNE exposure were measured by monitoring the extracellular acidification rate (ECAR), which is indicative of glycolytic flux [14]. In other studies, we have used glycolysis inhibitors or non-metabolizable substrates to verify that ECAR is indeed a surrogate marker for glycolytic activity [33]. As shown in Fig. 1B, glycolytic flux increased after HNE treatment in NRCMs and AMCMs. Mirroring oxygen consumption rates, the rates of glycolytic flux in myocytes later decreased below baseline. Smooth muscle cells showed no change in glycolytic activity with HNE treatment.
3.2. What drives the bioenergetic response of myocytes to electrophile stress?
In many studies to date (see [7, 11, 18, 20, 33]), a straightforward bioenergetic assay (also called a mitochondrial function test or stress test) has been used to examine cellular energetics. This assay uses inhibitors of respiratory chain components and uncoupling agents to examine and quantify ATP-linked oxygen consumption, non-ATP-linked oxygen consumption (proton leak), maximal respiratory capacity, and non-mitochondrial oxygen consumption. Oligomycin, an inhibitor of mitochondrial ATP synthase, is used to examine coupling efficiency and can allow for the calculation of the percentage of basal oxygen consumption that is related to ATP demand and that which can be ascribed to proton leak. Proton leak occurs primarily at two places: through mitochondrial anion carriers or through the lipid bilayer [21]. It should be noted that oligomycin induces a state 4-like respiratory condition which increases the mitochondrial membrane potential (ΔΨ) [6], resulting in higher proton leak through the mitochondrial membrane [4, 5]. Thus, the proton leak values determined in these energetic assays will be a slight overestimate of the actual leak, and the ATP-linked oxygen consumption rates will be a slight underestimate of the real value. Nevertheless, these measurements are useful for examining mitochondrial responses to a given treatment and comparing treatment groups.
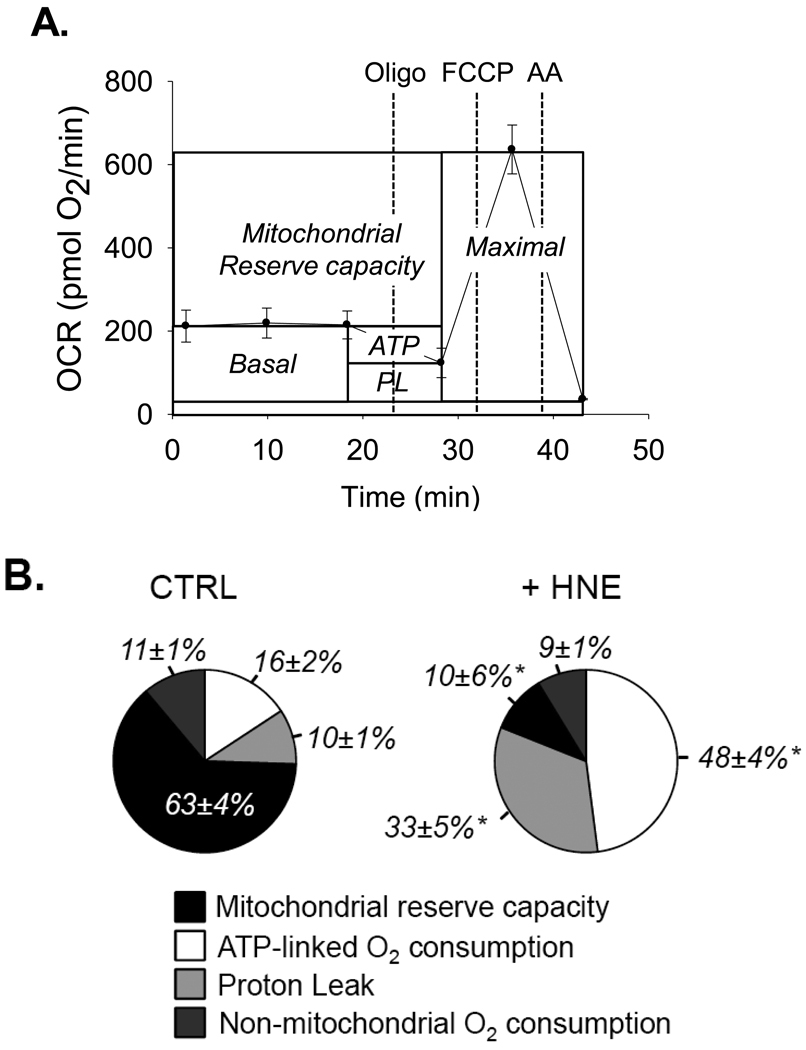
As shown in Fig 2A, sequential addition of oligomycin, the protonophore FCCP, and the electron transport chain inhibitor antimycin A after baseline measurements can not only allow for the determination of ATP-linked oxygen consumption rates, proton leak, maximal respiratory rates, and non-mitochondrial oxygen consumption rates, but also, by subtraction of the basal rate from the maximal rate, can be used to measure the mitochondrial reserve capacity. As shown in Fig. 2B, exposure of NRCMs to 20 µM HNE increases ATP-linked oxygen consumption and proton leak, leading to diminishment of the reserve capacity. Therefore, increases in ATP demand and decreases in mitochondrial efficiency are likely the primary drivers of the response of cardiomyocytes to HNE resulting in increased oxygen consumption and utilization of the reserve capacity. In an integrated model of cardiomyocyte function, it was shown through metabolic analysis that mitochondrial respiration has the highest response coefficients with respect to cytoplasmic ATP [9]. So, one scenario is that HNE-induced damage to proteins in the cytosol leads to depletion of cytoplasmic ATP (e.g., by increasing ATP utilization by the proteasome, etc.) and increases overall ATP demand. Ultimately, a combination of regulatory mechanisms, including sarcolemmal, cytoplasmic, and mitochondrial processes, likely contribute to the control of energy supply and demand in the heart and underlie the observed effects.
Figure 2. Measurement of bioenergetic parameters in myocytes using XF technology.
(A) Mitochondrial function assay in primary cardiomyocytes: After three baseline OCR measurements, oligomycin (1 µg/ml), FCCP (1 µM) and antimycin A (10 µM) were injected sequentially. OCR measurements were recorded after each injection. ATP-linked oxygen consumption (ATP) and the OCR due to proton leak (PL) can be calculated using the basal OCR rate and the oligomycin-insensitive rate. Injection of the uncoupling agent FCCP is used to determine the maximal respiratory capacity, and injection of antimycin A allows for the measurement of non-mitochondrial oxygen consumption. The reserve capacity is calculated by subtracting the maximal rate of oxygen consumption by the basal OCR. (B) Pie charts illustrating the effects of HNE on parameters of mitochondrial function. After three baseline rates, HNE was injected to 10 µM. After approximately 2h, oligomycin, FCCP, and antimycin A were injected sequentially and parameters of mitochondrial function were calculated as described in panel A. n = 3–4 per group, *p<0.05.
3.3. What regulates the apparent respiratory state and how does this relate with reserve capacity?
Using the bioenergetic assay described above, one can also extrapolate information regarding the intracellular mitochondrial respiratory state. It may be important to first define the differences between respiratory states measured in isolated mitochondria and the stateapparent calculated from measurements in intact cells. In isolated mitochondria, state 4 is defined by the rate of oxygen consumption in the presence of substrate and lack of ADP. In state 4, mitochondria have a more reduced electron transport chain, demonstrate relatively low electron transport chain activity, and generate more ROS. Under these conditions, the rate of oxygen consumption is constrained by the rate of proton leak back across the mitochondrial inner membrane. Hence, the majority of oxygen consumed by state 4 mitochondria is due to proton leak. [31]. In a cellular context, the addition of oligomycin is equivalent to state 4 respiration in the mitochondrion since all mitochondrial oxygen consumption linked to ATP synthesis is prevented.
When ADP is added to isolated mitochondria in the presence of substrate (inducing state 3 respiration), translocation of H+ back across the membrane (which lowers the Δp) through the ATP synthase occurs at a rate limited by the availability of oxidizable substrates and regulatory components of the electron transport chain. In state 3, the electron transport chain is in a more oxidized state and is more active; ΔΨ is lower than that found in state 4 mitochondria, and mitochondria generate near maximal amounts of ATP. Control over oxygen consumption is an amalgam of the ability of the respiratory chain to transport electrons, the availability of oxidizable substrate, substrate transport into mitochondria, and ATP turnover reactions [31]. Defining a state 3 equivalency in intact cells using a protonophore (such as FCCP) can be problematic since this will change metabolic respiratory control. This is much different than in isolated mitochondria preparations, where substrate conditions can be more well-controlled. Nevertheless, using protonophores to increase oxygen consumption to the near state 3 rate provides a unique opportunity to divulge key regulatory elements of bioenergetic function in cells which cannot be revealed by experiments with isolated mitochondria.
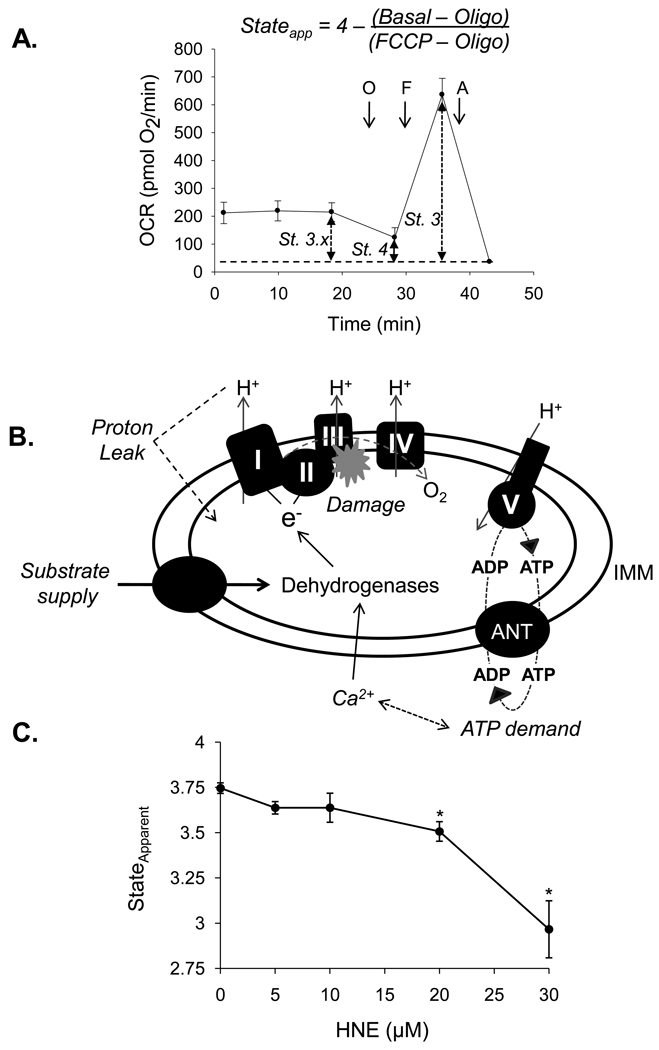
As described in [11] and shown in Fig. 3A, the basal rate of oxygen consumption (stateapparent) is substantially lower than the maximal rate measured upon addition of FCCP, and we presume this represents the mean respiratory state of mitochondria in the cell. The equation for calculating the respiratory stateapparent, initially described by Dranka et al. [11], is shown above Fig. 3A. When the cell is quiescent, ATP demand is minimal and the organelles on average will have a stateapparent close to 4.0. As cellular ATP demands increase, the stateapparent proceeds toward state 3. In the case of a stress that pushes the ATP demand to 3.2–3.0, the cell approaches its maximal respiratory rate and depletes its reserve capacity. This region is where the mitochondrial engine “redlines,” i.e., where mitochondria reach their maximum rate of turnover and are more likely to incur damage. In nearly all instances tested to date, breaching of this maximal rate has led to bioenergetic failure. For instance, a maximal rate of oxygen consumption was achieved by catecholamine infusion in a swine model of heart failure, leading to a significant reduction in phosphocreatine/ATP levels [15], and bioenergetic failure and cell death occurs in isolated myocytes treated with HNE once the maximal respiratory capacity is reached [18].
Figure 3. Effects of electrophile stress on the mitochondrial respiratory stateapparent in cardiomyocytes.
Defining the mitochondrial respiratory stateapparent using the mitochondrial function assay: (A) After three baseline oxygen consumption rate (OCR) measurements, oligomycin (1 µg/ml), FCCP (1 µM) and antimycin A (10 µM) are injected sequentially, and OCR measurements are recorded after each injection. The basal rate of oxygen consumption describes the average respiratory state of mitochondria in the cell, which is described here as state 3.x. Exposure of cells to oligomycin puts mitochondria in state 4, and FCCP is then used to increase the respiration rate to a maximal rate comparable to state 3. The equation shown above the figure can then be used to calculate the respiratory stateapparent, where Basal represents the basal OCR, Oligo represents the OCR after oligomycin exposure, and FCCP represents the FCCP-stimulated OCR. The non-mitochondrial rate of oxygen consumption should be subtracted from all values prior to the calculation. (B) Schematic illustrating the modules that regulate reserve capacity and mitochondrial state apparent. Factors regulating respiration rate are italicized. (C) Effects of HNE on the respiratory stateapparent in cardiomyocytes: NRCMs were treated with HNE at the concentrations indicated and subjected to the energetic assay shown in panel A. n = 3–4 per group, *p<0.05.
There are many factors contributing to respiratory rate, apparent respiratory state, and the reserve capacity. In an intact cell, those factors will be more varied and complex than in isolated mitochondria. Glycolysis, fatty acid oxidation, and Kreb’s cycle activity will all control respiratory rate by supplying NADH. This is illustrated in Fig. 3B as the supply arm. In addition, proton leak and ATP turnover (ATP demand) will lead to increased oxygen consumption and will modulate the apparent respiratory state of the cell. Periods of increased activity, for instance in a cardiomyocyte under stress, may also raise Ca2+ and ATP demand, leading to activation of matrix dehydrogenase enzymes and increased NADH levels. Lastly, damage to mitochondrial respiratory chain components could decrease the ability of the respiratory chain to support electron transport, decreasing reserve capacity and potentially increasing the stateapparent (because the maximal rate would be lower, making the respiratory state more close to state 3).
To determine the effects of HNE on the stateapparent, NRCMs were treated with HNE at the concentrations indicated in Fig. 3C and subjected to the energetic assay shown in Fig. 3A. In this assay, the 30 µM HNE treatment group reached their maximal respiratory capacity quickly, leading to bioenergetic failure and “redlining” of the mitochondrial machinery. At this concentration of HNE, the NRCMs die relatively quickly [18], further supporting the idea that reserve capacity and stateapparent can be used as indicators or predictors of how well the cell might deal with stress.
3.4. What are the effects of respiratory substrates on the reserve capacity and stateapparent in cardiac myocytes?
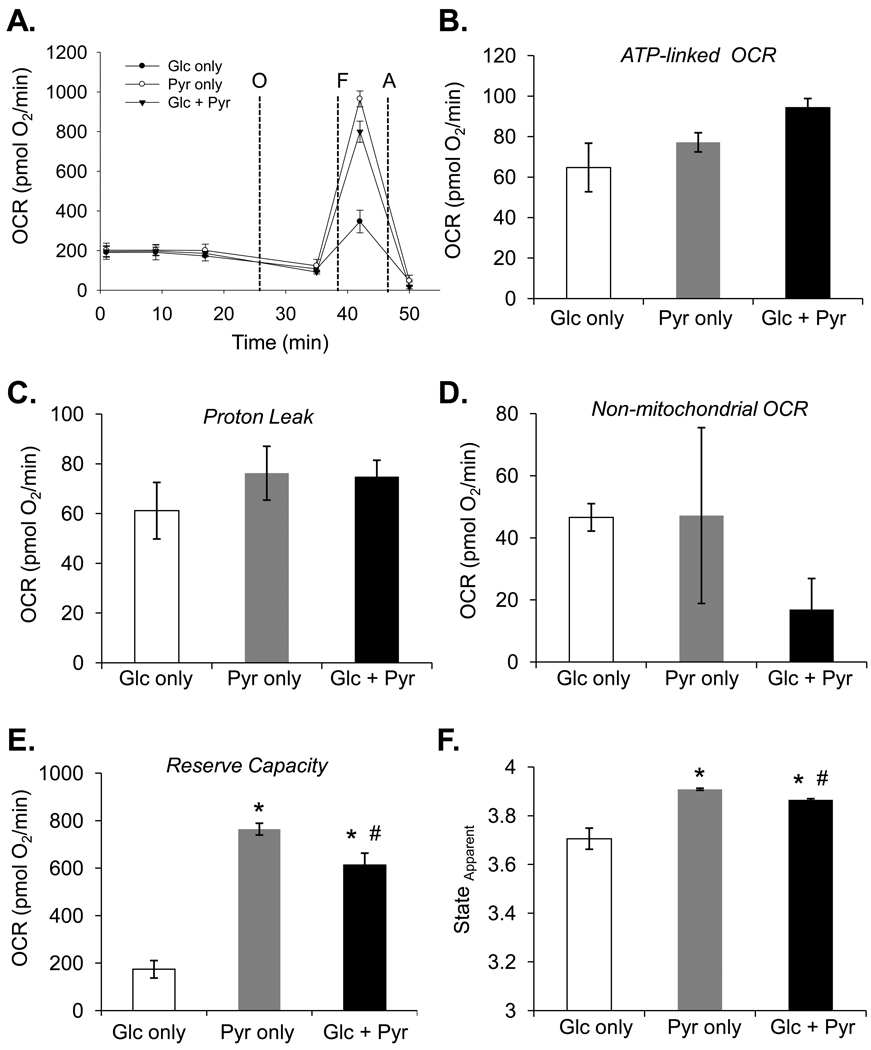
While the assembly and abundance of the respiratory complexes or relative damage to respiratory chain components are factors regulating electron flow and mitochondrial oxidative capacity, the ability to generate reducing equivalents through the efficient delivery of substrates to mitochondria is clearly a critical factor regulating cellular respiratory capacity under basal conditions. In synaptosomes, inhibitors of mitochondrial pyruvate transport or electron transport chain inhibitors were used to examine maximal respiratory capacity and the propensity of mitochondria to undergo failure [7]. In cardiomyocytes, it was shown that the combined action of adenine nucleotides and Ca2+ account for the regulation of respiration [9], and these effectors can regulate substrate delivery. To examine how myocytes respond to simple changes in substrate, the bioenergetic assays described in Fig. 3 were applied to NRCMs given glucose alone, pyruvate alone, or a combination of glucose and pyruvate immediately prior to the bioenergetic assay. As shown in Fig. 4A, cells in each substrate condition had identical basal oxygen consumption rates. Upon treatment with oligomycin, no significant differences in ATP-linked oxygen consumption (Fig. 4B) or proton leak (Fig. 4C) were observed, although a trend toward an increase in ATP demand was apparent in cells having both glucose and pyruvate as substrates. Interestingly, cells in medium containing pyruvate as the sole substrate showed a threefold higher maximal respiratory capacity (i.e., the FCCP-stimulated rate) than cells with glucose alone (Fig. 4A), and glucose in combination with pyruvate showed a lower maximal rate of respiration than cells having just pyruvate as the substrate. The reason why glucose lowers maximal capacity may be due to compensatory increases in glycolysis and/or negative regulation of oxidative phosphorylation by glycolytic intermediates, e.g., through the Crabtree effect [10]. The increase in maximal respiratory capacity in cells having pyruvate as a sole substrate therefore equates to a nearly fourfold increase in mitochondrial reserve capacity compared with cells having glucose as the sole substrate and a ~15% higher capacity than cells respiring with glucose and pyruvate present (Fig. 4E). Calculations of the stateapparent under each condition showed that cells in pyruvate alone were respiring near state 3.9 while cells cultured in only glucose were near 3.7 (Fig. 4F). A modest, but significant change in respiratory state was noted between cells having pyruvate alone as compared with cells in pyruvate + glucose.
Figure 4. Substrate-dependent changes in mitochondrial function.
Extracellular flux analysis of cardiomyocytes in medium containing pyruvate and/or glucose: (A) Bioenergetic function assay in cardiomyocytes having glucose (Glc only), pyruvate (Pyr only), or glucose and pyruvate (Glc + Pyr) as substrate. ATP-linked OCR (B), proton leak (C), non-mitochondrial OCR (D), reserve capacity (E), and the mitochondrial respiratory stateapparent (F) were calculated from the assay in panel A. n = 5 per group, *p<0.05 vs. Glc only group; #p<0.05 vs Pyr only group.
3.5. Do changes in the reserve capacity and stateapparent affect the cardiomyocyte response to electrophilic stress?
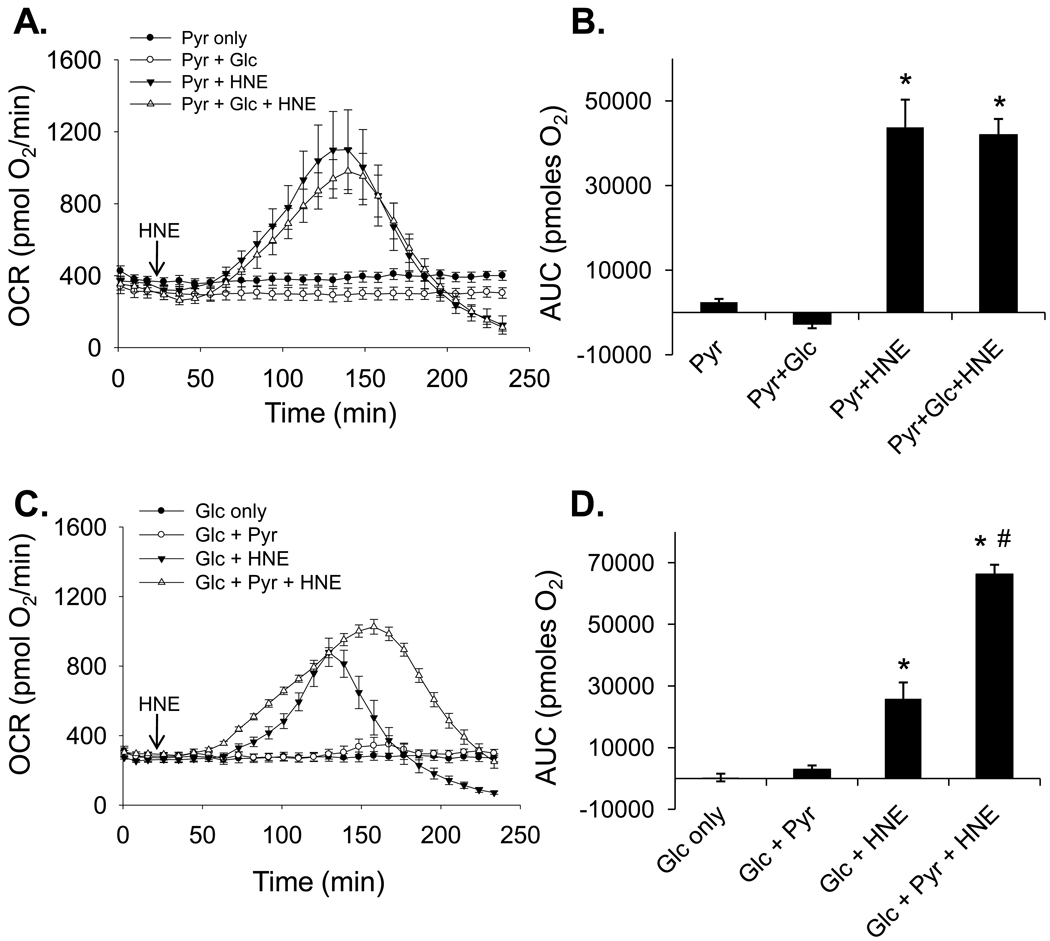
To test whether modulating the reserve capacity and stateapparent affect the bioenergetic response to stress, we examined oxygen consumption in NRCMs treated with HNE under the different substrate conditions discussed above. As shown in Fig. 5A and C, cells cultured in the various substrate conditions showed steady rates of oxygen consumption at baseline. However, when treated with HNE, cells cultured in pyruvate alone showed a large increase in oxygen consumption that was largely unaffected by the presence of glucose (Fig. 5A and B). As shown in Fig. 5C, cells cultured in glucose alone utilized less oxygen in response to HNE treatment than cells having pyruvate and more quickly reached bioenergetic failure. The OCR values of cells having glucose as the sole substrate fell well below baseline approximately 50 min earlier than cells having pyruvate in the medium (Fig. 5C). The area under the curve (AUC) analyses in Fig. 5B and D show that pyruvate increases overall oxygen consumption, which is likely used to maintain energy levels during the exposure to HNE. These findings support the view that a larger reserve capacity and a lower baseline respiratory state (i.e., a resting stateapparent nearer to 4) confer an advantage by allowing cells to increase oxygen consumption (which is used to generate ATP [18]) and to tolerate electrophilic insults for a longer period of time.
Figure 5. Effects of substrate on the bioenergetic response of cardiomyocytes to HNE.
Extracellular flux analysis of cardiomyocytes exposed to HNE in the presence of glucose and/or pyruvate. (A) One hour prior to energetic assays, the culture medium was removed and changed to medium containing pyruvate alone (1 mM; Pyr only) or pyruvate in the presence of glucose (5.5 mM; Pyr + Glc). After three baseline OCR measurements, HNE was injected to a final concentration of 20 µM in one of the pyruvate only groups (Pyr + HNE) and in one of the groups containing both pyruvate and glucose (Pyr + Glc + HNE), and rates were recorded for the indicated time. (B) Area under the curve (AUC) analysis of oxygen consumption rates from panel A: After baseline measurements, the AUC was measured to indicate the total amount of oxygen consumed in each group. (C) Cells were incubated in medium containing glucose alone (5.5 mM; Glc only) or glucose in the presence of pyruvate (Glc + Pyr). After three baseline OCR measurements, HNE was injected to a final concentration of 20 µM in one of the Glc only groups (Glc + HNE) and in one of the groups containing both glucose and pyruvate (Glc + Pyr + HNE), and rates were recorded for the indicated time. (D) Area under the curve (AUC) analysis of oxygen consumption rates from panel C: After baseline measurements, the AUC was measured to indicate the total amount of oxygen consumed in each group. n = 5 per group, *p<0.05 vs. cells not treated with HNE; #p<0.05 vs. Glc + HNE group.
3.6. How do these findings and concepts change the way we think about the mitochondrial response to stress?
With these concepts in mind, it is intriguing to ponder the molecular mechanisms that regulate mitochondrial stress responses. “Which cell signaling pathways regulate reserve capacity and stateapparent?”; “How does glycolysis regulate mitochondrial respiration?”; and “How do mitochondria in state 4 handle oxidative stress compared to state 3-respiring mitochondria?” are three questions that remain inadequately addressed. Regarding cell signaling, it appears that the Akt pathway can regulate reserve capacity because stimulation of aortic smooth muscle cells with platelet-derived growth factor increases the mitochondrial reserve capacity, and this stimulation is inhibitable with PI3K or Akt inhibitors [33]. In this context, increased glycolytic flux could feed pyruvate to mitochondria to increase respiratory capacity. However, here we show that the presence of glucose decreases maximal respiratory capacity in NRCMs, suggesting that the regulation is cell-type specific.
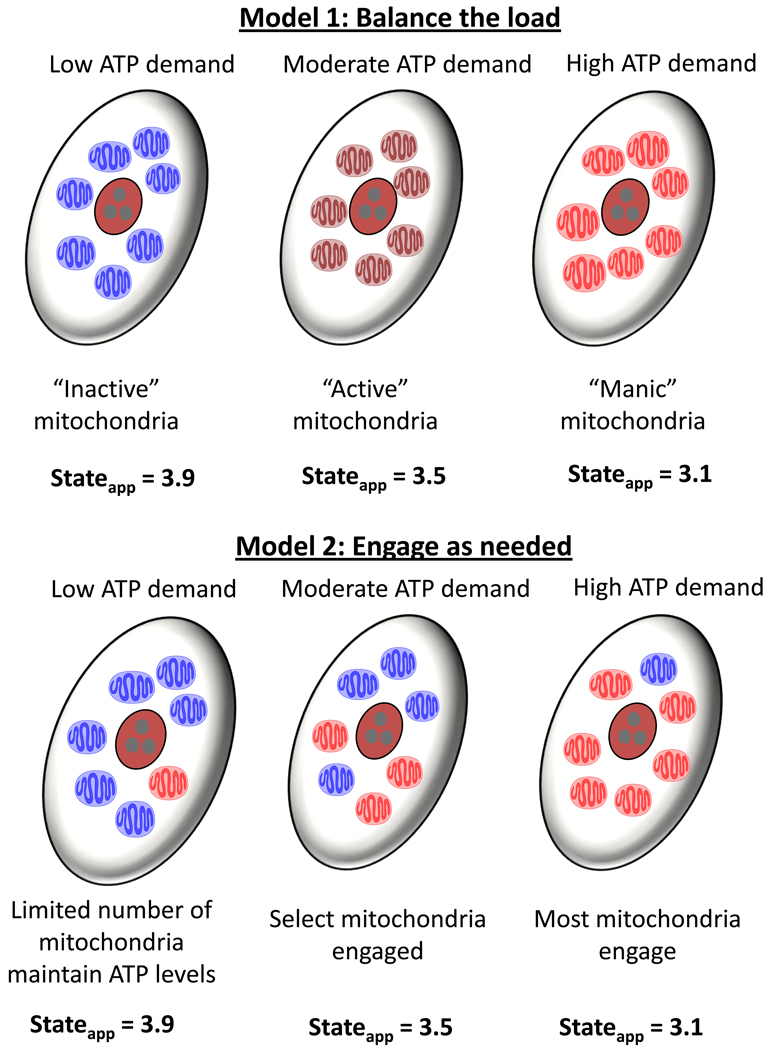
Another interesting question is, “Do all mitochondria in a cell respond to a given stress, or do certain subpopulations or mitochondrial networks provide the required amount of ATP for the cell?” We propose two simple models by which cardiomyocytes mitochondria respond to insults such as that induced by HNE. In the first model shown in Fig. 6, all mitochondria in a “resting” cell are relatively inactive. When the need for energy arises, these mitochondria respond collectively to increase ATP, thereby increasing the respiratory stateapparent to the midrange (e.g., state 3.5). Upon a strong challenge, these mitochondria then all reach a near maximal rate (e.g., state 3.1) to uphold energy requirements. We call this model the “Balance the load” model because all mitochondria endure the same stress and respond identically. The inherent problem with this model is that, should the respiratory state foster an increased ability to undergo damage (e.g., at state 3.1), there would be no remaining mitochondria left undamaged after the insult. In addition, adherence to this model suggests that no gradients of the stressor exist in the cell whether added exogenously or for aldehydes such as HNE that are produced endogenously; this seems unlikely.
Figure 6. Proposed models of mitochondrial responses to stress.
Two simple models by which mitochondria respond to insults that increase ATP demand. Model 1, “Balance the load” model: When the need for energy arises, mitochondria respond collectively to increase ATP. A moderate demand is matched by a commensurate change in the mitochondrial stateapparent to somewhere in the midrange (e.g., state 3.5). Upon a strong challenge, all mitochondria in the cell respond and increase respiration to near their maximal rate (e.g., state 3.1). Model 2, “Engage when needed” model: In this model, most mitochondria in cells with low ATP demand idle at a respiratory state near 4, and only a subset of mitochondria work at a respiratory state more toward 3 to uphold basal energy requirements. When a moderate demand for energy arises, the cell then engages other subsets of mitochondria to generate the required amount of ATP. Under these conditions, some mitochondria might be near state 3.9 while others are more near state 3.1, averaging a collective stateapparent of 3.5. During times of very high energy demand, more mitochondria engage, yet a subpopulation of mitochondria remains in a resting state.
The second model—“Engage when needed”—seems to circumvent these problems (Fig. 6). In this model, most mitochondria in “resting” cells are idling at a respiratory state near 4, and only a subset of mitochondria are working at a respiratory state more toward 3 to uphold basal metabolism. Therefore, one mitochondrion would potentially have less or more of a reserve capacity than another mitochondrion in the same cell. When a moderate demand for energy arises, the cell then engages other subsets of mitochondria to generate the required amount of ATP. Hence, under these conditions, some mitochondria might be near state 3.9 while others are closer to state 3.1, averaging to a stateapparent of 3.5. Now what happens when the demand for ATP increases further? In this model, more mitochondria engage, yet a few are still left in the resting mode.
Indeed, there appears to be evidence for this model. The first level of support lies in the knowledge that distinct subpopulations of mitochondria (e.g., subsarcolemmal and interfibrillar mitochondria) exist in the cardiomyocyte [24]. These mitochondria have been shown to be biochemically distinct, i.e., they oxidize substrates at different rates, differ in respiratory control, and exhibit differences in Ca2+ transport [32, 40]. Even though mitochondria within individual cells have been shown to be morphologically heterogeneous and unconnected (thus allowing them to have distinct functional properties) [8], they remain highly ordered and coordinate energy metabolism in the myocyte. An interesting observation regarding the spatio-temporal oscillations of cardiomyocyte mitochondria suggests that while Model 2 may predominate basally, a hybrid between model 1 and 2 may occur under conditions of stress. It was shown using rhodamine dye that perturbation of a few mitochondria in the cell leads to the appearance of mitochondrial networks oscillating in synchrony [25]. Under conditions of oxidative stress, the mitochondrial network locks into a low-frequency, high amplitude oscillatory mode [2, 3] which appears more analogous to Model 1 than Model 2. If this indeed occurs in vivo under conditions of stress, then it is imperative to understand whether some populations of mitochondria are more resistant to damage than others. Does “locking” into a certain respiratory state or mode relate to an increased propensity of mitochondria to be damaged? The further elucidation of how mitochondria respond to stress in intact cells appears to require the use of high resolution techniques, similar to that in Kurz et al. [25], that can differentiate between small changes in respiratory state in individual mitochondria.
Because it has been shown that mitophagy removes damaged mitochondria [37], it would also be interesting to learn how damaged mitochondrial components are specifically identified and removed by autophagy and how this affects energetics. Autophagy has been shown to occur under conditions associated with oxidative stress, e.g., in atherosclerotic lesions and diseased heart tissue [16, 27]. It could be that stress in a distinct mitochondrial network in the cell is managed during the post-insult period by mitophagy. In this scenario, the network may undergo recurrent mitochondrial fission to segregate damaged mitochondrial machinery and membranes for their selective removal by autophagy. In previous studies, we have found that lipid peroxidation products such as HNE and acrolein activate mitophagy in vascular smooth muscle cells; in this context, autophagy removed HNE-modified proteins and promoted survival after the electrophile insult [19]. We speculate that mitophagy plays an important role in regulating the long-term energetic integrity of the cell, while the reserve capacity is a measure of the ability of the cell to respond acutely to oxidative insults.
3.7. Conclusions
In this study, we found that the mitochondrial response to electrophiles is cell-type specific and that substrates such as pyruvate and glucose affect the reserve capacity and mitochondrial responsiveness to HNE. To integrate the concepts of reserve capacity, stateapparent, and mitochondrial responses to stress, we propose two possible mitochondrial models, one in which the collective whole of mitochondria respond to stress and one in which a more compartmentalized response is envisaged. Our future interests lie in understanding how respiratory state affects the propensity of cardiomyocyte mitochondria to withstand or succumb to stress (e.g., damage caused by electrophilic aldehydes) and elucidating the role of mitochondria and autophagy in heart disease.
Acknowledgments
Funding: BGH was supported by a grant from the NIH-NCRR (P20 RR024489) and a University of Louisville Clinical and Translational Science Pilot Program grant (20020). SPJ was supported by grants from the NIH-NHLBI (R01 HL083320, R01 HL094419), American Heart Association National Center Scientist Development Grant (0535270N), NIH-NCRR (P20 RR024489), and Kentucky Science and Engineering Foundation grant (KSEF-1677-RDE-011). VDU was supported by National Institutes of Health Grants (ES10167 and DK 75865).
Abbreviations
- AMCMs
adult mouse cardiomyocytes
- BDM
2,3-butanedione monoxime
- DMEM
Dulbecco's modified Eagle's medium
- ECAR
extracellular acidification rate
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- HNE
4-hydroxynonenal
- NRCM
neonatal rat cardiomyocyte
- OCR
oxygen consumption rate
- SMCs
smooth muscle cells
- XF
extracellular flux
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akao M, Ohler A, O'Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- 2.Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, O'Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J. 2006;91:4317–4327. doi: 10.1529/biophysj.106.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand MD. The proton leak across the mitochondrial inner membrane. Biochim Biophys Acta. 1990;1018:128–133. doi: 10.1016/0005-2728(90)90232-s. [DOI] [PubMed] [Google Scholar]
- 5.Brown GC. The leaks and slips of bioenergetic membranes. FASEB J. 1992;6:2961–2965. doi: 10.1096/fasebj.6.11.1644259. [DOI] [PubMed] [Google Scholar]
- 6.Brown GC, Lakin-Thomas PL, Brand MD. Control of respiration and oxidative phosphorylation in isolated rat liver cells. Eur J Biochem. 1990;192:355–362. doi: 10.1111/j.1432-1033.1990.tb19234.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortassa S, O'Rourke B, Winslow RL, Aon MA. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys J. 2009;96:2466–2478. doi: 10.1016/j.bpj.2008.12.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Ruiz R, Averet N, Araiza D, Pinson B, Uribe-Carvajal S, Devin A, Rigoulet M. Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate. A possible role in Crabtree effect induction? J Biol Chem. 2008;283:26948–26955. doi: 10.1074/jbc.M800408200. [DOI] [PubMed] [Google Scholar]
- 11.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935–H943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 14.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K, Hou M, Ye Y, Wu X, Mansoor A, From AH, Ugurbil K, Bache RJ, Zhang J. Oxidative capacity in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H541–H548. doi: 10.1152/ajpheart.01142.2002. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu SQ, Bhatnagar A, Srivastava S. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J. 2009;417:513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 20.Hill BG, Higdon AN, Dranka BP, Darley-Usmar VM. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation. Biochim Biophys Acta. 2010;1797:285–295. doi: 10.1016/j.bbabio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ Res. 2003;93:697–699. doi: 10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 23.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 24.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–C1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Spatio-temporal oscillations of individual mitochondria in cardiac myocytes reveal modulation of synchronized mitochondrial clusters. Proc Natl Acad Sci U S A. 2010;107:14315–14320. doi: 10.1073/pnas.1007562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Hill BG, Gu Y, Cai J, Srivastava S, Bhatnagar A, Prabhu SD. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am J Physiol Heart Circ Physiol. 2007;293:H3673–H3684. doi: 10.1152/ajpheart.00284.2007. [DOI] [PubMed] [Google Scholar]
- 27.Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Kusano K, Nakamura Y, Kakishita M, Ohta K, Nagase S, Yamamoto M, Miyaji K, Saito H, Morita H, Emori T, Matsubara H, Toyokuni S, Ohe T. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105:2867–2871. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Miura D, Kusano KF, Fujimoto Y, Sumita-Yoshikawa W, Fuke S, Nishii N, Nagase S, Hata Y, Morita H, Matsubara H, Ohe T, Ito H. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J Card Fail. 2009;15:709–716. doi: 10.1016/j.cardfail.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholls DG, Ferguson SJ. Bioenergetics 3. Boston: Academic Press; 2002. [Google Scholar]
- 32.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 33.Perez J, Hill BG, Benavides GA, Dranka BP, Darley-Usmar VM. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem J. 2010;428:255–267. doi: 10.1042/BJ20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira HL, Belzacq AS, Haouzi D, Bernassola F, Cohen I, Jacotot E, Ferri KF, El Hamel C, Bartle LM, Melino G, Brenner C, Goldmacher V, Kroemer G. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–4316. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 39.Waddington EI, Croft KD, Sienuarine K, Latham B, Puddey IB. Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis. 2003;167:111–120. doi: 10.1016/s0021-9150(02)00391-x. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein ES, Benson DW, Fry DE. Subpopulations of human heart mitochondria. J Surg Res. 1986;40:495–498. doi: 10.1016/0022-4804(86)90221-0. [DOI] [PubMed] [Google Scholar]
- 41.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]