Abstract
Objective
To examine associations between exposure to maternal diabetes in utero and body mass index (BMI) growth trajectories from birth through 13 years of age among a diverse cohort of youth.
Study design
Mixed linear effects models were constructed to assess differences in BMI and BMI growth velocity from birth through 13 years of age for 95 subjects exposed to diabetes in utero and 409 unexposed subjects enrolled in a retrospective cohort study.
Results
The overall BMI growth trajectory (adjusted for sex and race/ethnicity) was not significantly different for exposed and unexposed subjects from birth through 26 months of age (p=0.48). However, the overall growth trajectory from 27 months of age through 13 years differed by exposure status (p=0.008), adjusted for sex and race/ethnicity. The difference was primarily due to a significantly higher BMI growth velocity among exposed youth between 10–13 years, increasing by 4.56 kg/m2 compared to 3.51 kg/m2 in the unexposed (p=0.005). Control for demographic variables, socioeconomic factors and maternal pre-pregnancy BMI did not alter the observed associations.
Conclusions
Exposure to maternal diabetes in utero accelerates BMI growth in late childhood thus increasing long-term obesity risk.
Keywords: Gestational diabetes, fetal overnutrition, fetal exposure to diabetes, childhood obesity, childhood BMI, growth trajectory
The rapid increases in childhood obesity observed worldwide herald an alarming forecast for future burden of hypertension, diabetes and cardiovascular disease (1–3). Significant research has suggested the existence of critical periods for the development of childhood obesity. Fetal life, the time period of greatest developmental plasticity, has been suggested to be one such important period (4). Indeed, several pregnancy factors have been associated with greater obesity in the offspring, including maternal pre-pregnancy BMI (5, 6), gestational weight gain (7, 8), and gestational diabetes (9–12).
The possibility that intrauterine exposure to maternal diabetes could place offspring at increased risk for obesity and related metabolic consequences later in life has generated considerable interest. Maternal diabetes is a recognized risk factor for excess fetal growth, macrosomia and increased fetal adiposity (13, 14). However, less is known about the pattern of postnatal growth in offspring exposed to maternal diabetes. In unexposed children, body mass index (BMI) increases rapidly during the first 9–12 months of life, then declines reaching a minimum at 4 to 6 years of age before beginning a gradual increase throughout adolescence and most of adulthood (15). Cross-sectional analyses have suggested that Pima Indian offspring exposed to diabetes in utero experience a period of catch-down growth between one to two years of age followed by higher BMI throughout childhood and adolescence compared to unexposed Pima youth (16–18). However, the extent and pattern of the influence of in utero diabetes exposure on the childhood BMI trajectory is unknown. The study of entire growth trajectories, rather than cross-sectional analyses across specific time points, allows for a better understanding of how and when fetal exposures influence postnatal growth through childhood as a whole, and therefore may generate information about optimal time periods for focused preventative interventions.
To address this, our study explored BMI growth trajectories from birth through 13 years of age among a diverse cohort of children exposed and unexposed to maternal diabetes in pregnancy, adjusting for the effects of potential confounders.
METHODS
This report uses data from a retrospective cohort study conducted in Colorado: Exploring Perinatal Outcomes among Children (EPOCH). Participants were 6–13 year old offspring of singleton pregnancies, born at a single hospital in Denver between 1992 and 2002, whose biological mothers were members of the Kaiser Permanente of Colorado Health Plan (KPCO), and who were still KPCO members and living in Colorado over the study period (2006–2009).
For this analysis, eligible participants were children exposed to diabetes in utero (exposed group) and a random sample of children not exposed to diabetes in utero without intrauterine growth restriction, defined as birth weight for gestational age score < the 10th percentile (unexposed group). The study was approved both by the Colorado Multiple Institutional Review Board and Human Participant Protection Program. All participants provided written informed consent and youth provided written assent.
Exposure definition
Physician-diagnosed maternal diabetes status was ascertained from the KPCO Perinatal database, an electronic database linking the neonatal and perinatal medical record. The database contains data that define delivery events for each woman. Gestational diabetes (GDM) is coded as present if diagnosed through the standard KPCO screening protocol (described below) and absent if screening was negative. Since the 1990s, KPCO has routinely screened for GDM in all non-diabetic pregnancies using a two-step standard protocol. At 24–28 weeks, all pregnant women are offered screening with a 1-h 50-g oral glucose tolerance test (OGTT). Patients with blood glucose value ≥140 mg/dl undergo a diagnostic 3-hour 100-g diagnostic OGTT. GDM is diagnosed when two or more glucose values during the diagnostic OGTT meet or exceed the criteria for a positive test, as recommended by the National Diabetes Data Group (19). The KPCO screening and diagnostic protocols have remained constant over time. Exposure to diabetes in utero was defined as presence of pre-existent diabetes or GDM diagnosed during the index pregnancy. In addition, birth weight, birth length, gestational age, and maternal pre-pregnancy weight were obtained from the database.
Childhood height and weight measurements
All participants were invited to a research office visit in which standard anthropometric measures were recorded. Current height and weight were measured in light clothing and without shoes. Weight was measured to the nearest 0.1 kg using a portable electronic SECA scale. Height was measured to the nearest 0.1 cm using a portable SECA stadiometer with a fine-gauge knitting needle. Height and weight were measured and recorded twice, and an average was taken. Scales and stadiometers were calibrated every two months using standard weights for scales, and aluminum measuring rod for the stadiometer. Previously recorded measures of recumbent length (up to age 2 years), standing height (after the child is able to stand) and weight from pediatric office visits were abstracted from the KPCO medical record. For children with an enrollment gap, medical records from non-KPCO providers were obtained. Weight and height measurements at provider offices were obtained with similar instruments over time but were taken under less standardized conditions than in a research setting. The median number of BMI measurements for subjects was 10 (ranging from 1 to 35). BMI was calculated as kg/m2.
Other measurements
Race/ethnicity was self-reported using 2000 U.S. Census-based questions and categorized as Hispanic (any race), non-Hispanic white (NHW), or non-Hispanic African-American (AA). Maternal pre-pregnancy body mass index (BMI, kg/m²) was calculated from the KPCO measured weight before the last menstrual cycle preceding pregnancy and height collected at the in-person research visit. Maternal level of education and total household income at the time of birth were self-reported during the research office visit. Pubertal development at the time of the EPOCH visit was assessed by child-self report with a diagrammatic representation of Tanner staging adapted from Marshall and Tanner (20) and youth were categorized as Tanner < 2 (pre-pubertal) and ≥ 2 (pubertal).
Statistical Analysis
Mixed effects linear models were constructed to assess differences in average BMI and BMI growth velocity for subjects exposed and unexposed to DM in utero. This modeling approach allows for intrasubject correlation of repeated measures on subjects and accounts for an unbalanced design in the number of BMI observations on each subject and the age (time) at which they were collected. Due to the change in use of recumbent length to standing height around the age of 2 years, two separate growth curves were developed to model the BMI trajectory over time. The first model was fit for the infancy period from birth through 26 months and a second model for the childhood period from 27 months to 13 years. An iterative process was used to determine the degree of polynomial in age for both its random and fixed effects. Two, three, and four degree polynomial functions were explored by adding linear, quadratic, and cubic terms. Both final models used a quadratic polynomial for the fixed effects of age on BMI and linear random effect. A spline with a single knot at 11 months was included in the infancy period model (from birth through 26 months) which allowed a quadratic function before and after the knot. The best fit was determined based on each model’s ability to predict BMI at specific ages (6 months, 1 year, 2 years, etc.) compared to a categorical linear effects model.
The BMI growth model for the infancy period (birth-26 months) for the ith subject at time j (i.e., age in months) took the form:
The terms b0i + b1i are the subject specific random intercept and random slope for the ith subject and Iage(ij) >11month is an indicator variable for ages of 11 months and older. The BMI growth model for the childhood period (27 months – 13 years) took the form:
Covariates for the base model included exposure to diabetes in utero, sex and race/ethnicity as fixed effects, and the fully adjusted model added gestational age, maternal age, maternal pre-pregnancy BMI, maternal education and household income at birth as fixed effects. The average BMI during the infancy and childhood periods, as well as BMI growth velocity during specific age ranges were estimated for exposed and unexposed subjects from the base model.
RESULTS
A total of 95 children exposed to diabetes in utero (87 offspring of GDM mothers and 8 of mothers with type 1 diabetes) and 409 unexposed youth participated in the EPOCH study and had complete data on variables of interest. The participants were 49.0% non-Hispanic white, 42.5% Hispanic and 8.5% African American. At the time of birth, 96.8% of the mothers had at least a high school education and 53.9% had a household income greater than $50,000/year. The average age of the child at study visit was 9.5±1.7 and 10.6±1.3 years for exposed and unexposed youth, respectively (p<.0001). Additionally, 30% of exposed subjects self-reported a Tanner stage ≥ 2 at the study visit, indicating they had begun puberty, compared to 50% of unexposed subjects (p=0.0005). Mothers of exposed offspring were, on average, older at delivery than those of unexposed offspring (33.1±5.2 vs. 30.0±5.4, p=0.03), and had a higher pre-pregnancy BMI (27.7±6.2 vs. 25.7±6.6, p=0.01).
Figure 1 shows the modeled BMI growth trajectories for the infancy period from birth through 26 months for youth exposed and unexposed to DM in utero. Over this age range, neither the average BMI from birth to 26 months (p=0.53), nor the BMI growth trajectories (p=0.48) were significantly different for exposed and unexposed youth. The BMI growth trajectory for the childhood period from 27 months to 13 years is presented in Figure 2. Exposed youth had significantly higher average BMI over this range (p=0.01) and an accelerated BMI growth trajectory (p=0.008) compared with unexposed youth.
Figure 1.
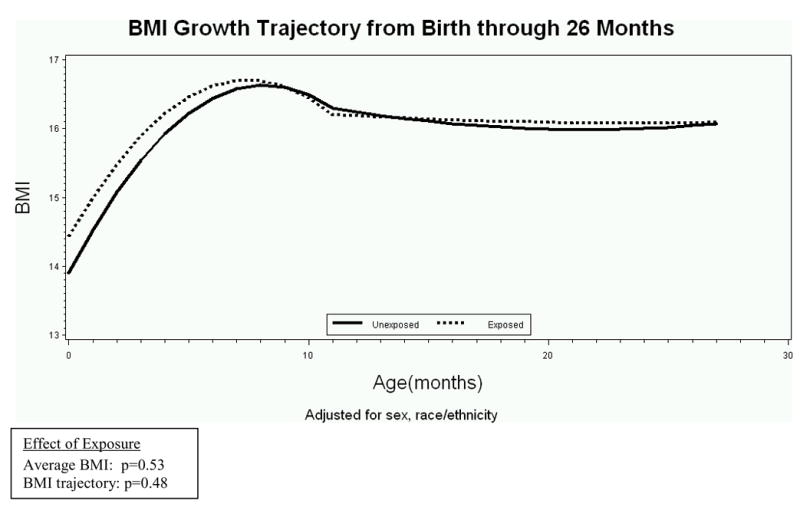
Mean BMI curves for youth both exposed and unexposed to maternal diabetes in utero from birth - 26 months of age, adjusted for sex and race/ethnicity.
Figure 2.
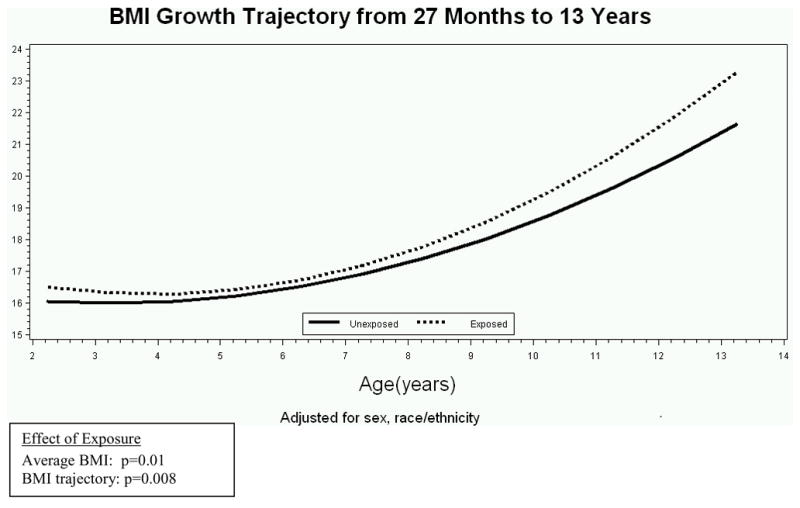
Mean BMI curves for youth both exposed and unexposed to maternal diabetes in utero from 27 months of age to 13 years, adjusted for sex, race/ethnicity.
Table I shows the BMI growth velocity of subjects exposed and unexposed to DM in utero, as well as the number of BMI data points available in each age-group period. Based on the quadratic spline model from birth through 26 months (adjusted for sex and race/ethnicity) we estimate that, on average, offspring of diabetic pregnancies gained 2.19 kg/m2 and unexposed offspring gained 2.70 kg/m2 in the first 9 months of life, a difference of −0.51 kg/m2 that was not statistically significantly (p=0.12). Between 9 and 12 months of age, the model reflected a decrease in BMI with exposed infants losing, on average, −0.05 kg/m2 compared to a loss of −0.36 BMI in the unexposed (p=0.03). The negative BMI growth trajectory continued from 13–26 months with no significant differences by exposure. Based on the quadratric model for the older ages (adjusted for sex, race/ethnicity) we found that, on average, the growth trajectory decreased from 27 months through 3 years by −0.22 kg/m2 and −0.02 kg/m2 for exposed and unexposed youth, respectively (p=0.19). The BMI growth velocity increased from ages 4 years though 6 years, similarly for exposed and unexposed youth (0.35 kg/m2 and 0.40 kg/m2, respectively, p=0.73) and continued to accelerate from 7 through 9 years, increasing by 1.73 kg/m2 and 1.44 kg/m2 in exposed and unexposed, respectively, (p=0.13). Between 10–13 years, the BMI growth velocity was significantly higher among the exposed youth, increasing by 4.56 kg/m2 compared to 3.51 kg/m2 among the unexposed youth (p=0.005).
Table 1.
BMI growth velocity of subjects exposed and unexposed to DM in utero, by age-group*
| Unexposed (n=409) | Exposed (n=95) | Difference | P | |||
|---|---|---|---|---|---|---|
| Age group | # obs | β (SE) | # obs | β (SE) | β (SE) | |
| Birth | 374 | 77 | ||||
| Birth – 8m | 826 | 2.70 (0.17) | 245 | 2.19 (0.29) | −0.51 (0.33) | 0.12 |
| 9–12m | 43 | −0.36 (0.05) | 11 | −0.05 (0.14) | 0.31 (0.15) | 0.03 |
| 13–26m | 677 | −0.17 (0.18) | 201 | −0.10 (0.34) | 0.07 (0.38) | 0.85 |
| 27m–3y | 311 | −0.02 (0.07) | 93 | −0.22 (0.13) | −0.20 (0.15) | 0.19 |
| 4–6y | 500 | 0.40 (0.06) | 150 | 0.35 (0.12) | −0.05 (0.14) | 0.73 |
| 7–9y | 632 | 1.44 (0.08) | 207 | 1.73 (0.17) | 0.29 (0.19) | 0.13 |
| 10–13y | 867 | 3.51 (0.15) | 157 | 4.56 (0.34) | 1.05 (0.37) | 0.005 |
| Total | 4230 | 1141 | ||||
Adjusted for sex, race/ethnicity.
β expresses change over age range in kg/m2
Table II presents the parameter estimates for the fixed effects of exposure to diabetes in utero from the fully adjusted model (Model 2) controlled for sex, race/ethnicity, gestational age, maternal age, maternal pre-pregnancy BMI, maternal education and household income at birth. From birth through 26 months of age, higher BMI was independently associated with male sex (p=0.0001), higher gestational age (p=0.004), higher pre-pregnancy BMI (<.0001), higher household income (p=0.04), and older maternal age (p=0.02), but not with exposure to diabetes in utero (adjusted p=0.21). However, from 27 months to 13 years of age, exposure to diabetes in utero was associated with an average higher BMI (adjusted p=0.02) and an altered BMI growth trajectory (adjusted p=0.009) among the exposed, an effect that was not attenuated by controlling for demographic variables (sex, race/ethnicity) socioeconomic factors (maternal education, income, maternal age), or a surrogate marker of genetic predisposition to obesity (maternal pre-pregnancy BMI). Other factors associated with higher childhood BMI between 27 months of age and 13 years were male sex (p=0.02), and pre-pregnancy BMI (p=0.03).
Table 2.
Estimated effects of exposure to diabetes in utero and selected covariates on childhood BMI growth velocity, from the fully adjusted model
| Birth to 26 months spline | 27 months to 14 years | |||
|---|---|---|---|---|
| Parameter | β (SE) | P | β (SE) | P |
| Sex (male) | 0.53 (0.14) | 0.0001 | 0.37 (0.17) | 0.02 |
| Race/ethnicity | ||||
| Hispanic | 0.18 (0.15) | 0.24 | 0.07 (0.18) | 0.70 |
| AA | 0.03 (0.26) | 0.91 | 0.18 (0.31) | 0.55 |
| Household income | −0.30 (0.14) | 0.04 | −0.08 (0.18) | 0.65 |
| Maternal education (<high school) | −0.66 (0.45) | 0.14 | −0.58 (0.58) | 0.32 |
| Gestational age (/week) | 0.09 (0.03) | 0.004 | 0.06 (0.04) | 0.08 |
| Maternal age | −0.03 (0.01) | 0.02 | −0.02 (0.02) | 0.31 |
| Pre pregnancy BMI | 0.04 (0.01) | <.0001 | 0.03 (0.01) | 0.03 |
|
Overall effect of exposure | ||||
| Average BMI | 0.21 | 0.02 | ||
| BMI trajectory | 0.13 | 0.009 | ||
AA= African-American
Results of the fully adjusted model. Separate growth curves are presented for the infancy period of birth to 26 months and childhood period of 27 months to 14 years. See statistical methods for form of the models.
DISCUSSION
In a multi-ethnic population from Colorado, we found that exposure to diabetes in utero was associated with an altered growth trajectory in children (27 months through 13 years of age, p=0.008), in particular a higher BMI growth velocity starting at ages 10 to 13 years (p=0.005). The effect of exposure on the BMI growth trajectory in childhood was independent of potential confounders such as demographic (sex, race/ethnicity) and developmental characteristics (gestational age), or markers of other in utero exposures (maternal age, income and education, pre-pregnant BMI). Importantly, no significant differences in growth trajectories were noted in infancy and early childhood. This study used longitudinal analysis to examine the impact of in utero diabetes exposure on BMI growth trajectories from birth to adolescence.
Our findings are consistent with the long-term patterns of accelerated BMI growth associated with in utero diabetes exposure reported in other studies. Silverman et al (21) observed a period of catch-down growth among a population of NHW and AA offspring of diabetic pregnancies compared to offspring of non-diabetic pregnancies in Chicago. The initial period of poor growth or catch-down weight in the first year of life was followed by a higher BMI in adolescence among offspring of diabetic mothers relative to non-exposed peers. Among Pima Indian youth, Touger et al (18) reported a change in weight z score between birth and 1.5 years of age of −0.56 vs. 0.12, (p<0.01) for exposed verses unexposed offspring suggesting early catch-down growth, but a higher weight z score at age 7.7 years of 1.26 in exposed vs. 0.00 in unexposed children (p<0.01). In an earlier study, Dabelea et al (17) reported no differences in mean BMI among Pima Indian sib-pairs exposed and unexposed to diabetes in utero at ages 5–8 years, but significantly higher BMI levels in exposed siblings at ages 9–12 years, which persisted throughout adolescence into young adulthood. Our study did not identify specific periods of catch-down growth in exposed versus unexposed infants; the overall growth trajectory up to 26 months of age was not significantly different between the two groups. Interestingly, in a recent follow up study of a randomized clinical trial in Australia, treatment of mild GDM did not result in BMI differences in offspring at 4–5 years of age (22). All the data summarized above suggested that the long-term effects of GDM exposure on childhood obesity become apparent later during childhood (e.g., during puberty). Our findings are consistent with previous reports and provide novel and direct evidence that exposure to diabetes in utero results in accelerated BMI growth starting in late childhood.
The mechanisms underlying the accelerated BMI growth trajectory in childhood among offspring of diabetic pregnancies are the object of extensive research. Several mechanisms that are not mutually exclusive may explain this association. They include genetic predisposition and shared familial factors, as well as specific intrauterine effects (i.e., fuel-mediated teratogenesis, also known as fetal overnutition). For example, exposure to maternal diabetes in utero may modulate delivery of lipid substrates to the fetus, resulting in adipocyte dysregulation and fatty acid accumulation (23). More research is needed in this area because distinguishing between specific intrauterine mechanisms and general familial (genetic and nongenetic) factors is important for the development of randomized trials aimed at testing effective interventions.
In our study, differences in pubertal development between exposed and unexposed offspring did not explain differences in growth trajectories (data not shown), and exposed offspring were in fact less likely to have begun puberty by the time of the EPOCH visit. However, the role of pubertal development as a potential mediator or modifier of the long-term consequences of exposure to diabetes in utero on childhood adiposity patterns requires additional study and prospective follow up of this cohort.
We believe that our data have important public health implications. Efforts to intervene or treat obese adults have generally been unsuccessful, and thus, identifying mechanisms and critical periods that influence obesity risk in future generations represent an important opportunity to develop targeted prevention efforts to break the vicious cycle of obesity. Importantly, the Bogalusa Heart Study demonstrated that cardiovascular risk factors, including obesity, track from childhood into adulthood (24). Our data suggest that exposure to diabetes in utero results in accelerated BMI growth during late childhood years. Further follow up of this cohort is necessary to determine if the accelerated growth trajectory continues into teenage and early adult period. Because no differences in growth trajectories were observed early in life, the perinatal and early childhood periods may represent windows of opportunity for targeted efforts aimed at preventing the increased risk of obesity associated with in utero exposure, manifesting in late childhood/early pubertal years. Such preventive strategies among high risk children include promotion of breastfeeding, which has been associated with reduced risk of obesity in late adolescence and adulthood (25), encouragement of physical activity, and promotion of healthy foods, such as fruits, vegetables, whole-grain breads and cereals, low fat diary products and no sweetened drinks.
Our study has several limitations. We did not have sufficient data points on each subject to construct individual growth curves or accurately estimate whether exposure to diabetes in utero influences the age at adiposity rebound, another marker of increased risk for later-life obesity (26). Our study, like others (27) used maternal pre-pregnancy BMI as a proxy for genetic predisposition. This is problematic because maternal obesity is a risk factor for the exposure considered in this study (27, 28) and may represent over adjustment. However, in our population, adjustment for maternal pre-pregnant BMI did not eliminate the observed relationship between exposure to diabetes in utero and increased growth trajectory during late childhood, suggesting that the observed association is not completely accounted for by genetic predisposition to obesity. We were unable to assess the impact of maternal hyperglycemia less severe than the cutoff for diagnosis of GDM. Offspring exposed to less extreme levels of hyperglycemia in utero were captured in our unexposed group, thus possibly biasing our results towards the null. The relatively young age of our cohort prevented assessment of accelerated growth after puberty, when differences are likely to be more pronounced (29, 30): only 30% of exposed youth and 50% of unexposed youth reported a Tanner stage greater than 2, indicating they had begun puberty. Prospective follow up of this population is necessary to understand the impact of exposure to diabetes in utero on BMI trajectories during teen and early adult years.
Our study also has important strengths. First, the longitudinal analysis utilizing mixed linear effects models made efficient use of the data, allowing us to explore more than just linear changes in BMI between two time periods. Our methods represent a novel approach to assess the influence of in utero diabetes exposure on childhood growth. Additional strengths include the ethnically diverse cohort including NHW, Hispanic and AA youth and our validated exposure, assessed without concern for recall bias.
In summary, this study provides novel evidence of an altered childhood growth patterns for youth exposed to diabetes in utero. Among a diverse population of young children, exposure to diabetes in utero was associated with an overall higher growth trajectory in late childhood and accelerated BMI growth velocity starting at ages 10 to 13 years, relative to unexposed children. These results provide further support for the hypothesis that fetal exposure to a diabetic intrauterine environment influences childhood growth and obesity risk.
Acknowledgments
Supported by General Clinical Research 308 Centers Program (RO1 DK068001307 to D.D. and M01 RR00069). The study sponsor had no role in the study. The authors declare no conflict of interest.
Abbreviations
- BMI
Body Mass Index
- DM
diabetes mellitus
- EPOCH
Exploring Perinatal Outcomes Among Children
- T2D
type 2 Diabetes
- KPCO
Kaiser Permanente of Colorado
- GDM
gestational diabetic mothers
- OGTT
oral glucose tolerance test
- NHW
non Hispanic AA, African-American
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–81. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Jacques P, Dallal G, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. New England Journal of Medicine. 1992;327:1350–56. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 3.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 4.Freinkel N. Of pregnancy and progeny (review). Banting Lecture 1980. Diabetes. 1980;29:1023–36. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann KE, Bergmann RL, Von Kries R, Böhm O, Richter R, Dudenhausen JW, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27:162–72. doi: 10.1038/sj.ijo.802200. [DOI] [PubMed] [Google Scholar]
- 6.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116:1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36:361–77. doi: 10.1016/j.ogc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22:215–20. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettitt D, Baird H, Aleck K, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. NEJM. 1983;308:242–46. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 11.Dabelea D, Knowler W, Pettitt D. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9:83–9. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Pettitt D, Nelson M, Saad R, Bennett P, Knowler W. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 1993;16:310–5. doi: 10.2337/diacare.16.1.310. [DOI] [PubMed] [Google Scholar]
- 13.Catalano P, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 14.White P, Koshy P, Duckers J. Management of pregnancy complicating diabetes and of children of diabetic mothers. Med Clin North Am. 1953;1:1481–96. doi: 10.1016/s0025-7125(16)34963-x. [DOI] [PubMed] [Google Scholar]
- 15.Rolland-Cachera MF, Deheeger M, Guilloud-Bataille M. Tracking the development of adiposity from one month of age to adulthood. Annals of Human Biology. 1987;14:219–229. doi: 10.1080/03014468700008991. [DOI] [PubMed] [Google Scholar]
- 16.Pettitt DJ, Bennett WC, Knowler WC, Baird HR, Aleck KA. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy: long-term effects on obesity and glucose tolerance in the offspring. Diabetes. 1985;34:119–122. doi: 10.2337/diab.34.2.s119. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant siblings. Diabetes. 2000;49:2208–11. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 18.Touger L, Looker H, Krakoff J, Lindsay R, Cook V, Knowler W. Early growth in offspring of diabetic mothers. Diabetes Care. 2005;28:585–60. doi: 10.2337/diacare.28.3.585. [DOI] [PubMed] [Google Scholar]
- 19.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 20.Marshall W, Tanner J. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 21.Silverman B, Rizzo T, Green O, Cho N, Winter R, Ogata E, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40:121–5. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 22.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33:964–968. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katajima W, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97:776–80. doi: 10.1016/s0029-7844(01)01328-x. [DOI] [PubMed] [Google Scholar]
- 24.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Associations between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 25.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 26.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129–35. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 27.Gillman M, Rifas-Shiman S, Berkey C, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–e7. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 28.Kuh D, Ben Shlomo Y. A life course approach to chronic disease epidemiology: tracing the origins of ill-health from early to adult life. 2. Oxford: Oxford University Press; 1997. [Google Scholar]
- 29.De Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–6. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- 30.Naumova EN, Must A, Laird NM. Tutorial in biostatistics: evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–41. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]


