Abstract
Purpose
Radiation therapy is an integral part of the preoperative treatment of rectal cancers. However, only a minority of patients achieve a complete pathological response to therapy due to resistance of these tumors to radiation therapy. This resistance may be mediated by constitutively active pro-survival signaling pathways or by inducible/acquired mechanisms in response to radiation therapy. Simultaneous inhibition of these pathways can sensitize these tumors to radiation therapy.
Methods and Materials
Human colorectal cancer cells were exposed to clinically relevant doses of gamma rays and the mechanism of their radioresistance was investigated. We characterize the transcription factor nuclear factor-κB (NF-κB) activation as a mechanism of inducible radioresistance in colorectal cancer and use curcumin, the active ingredient in the yellow spice turmeric to overcome this resistance.
Results
Curcumin inhibited the proliferation and the post-irradiation clonogenic survival of multiple colorectal cancer cell lines. Radiation stimulated NF-κB activity in a dose- and time-dependent manner while curcumin suppressed this radiation-induced NF-κB activation via inhibition of radiation-induced phosphorylation and degradation of IκBα, inhibition of IKK activity, and inhibition of Akt phosphorylation. Curcumin also suppressed NF-κB regulated gene products (Bcl-2, Bcl-xL, inhibitor of apoptosis protein-2, cyclooxygenase-2, and cyclin D1).
Conclusions
Our results suggest that transient inducible NF-κB activation provides a pro-survival response to radiation that may account for development of radioresistance. Curcumin blocks this signaling pathway and potentiates the anti-tumor effects of radiation therapy.
Keywords: Curcumin, Radiation, NF-κB, Radiosensitization, Colorectal Cancer
Introduction
Rectal cancer frequently presents at a locally advanced stage where pre-operative chemoradiation therapy (CRT) is used to downstage the tumor, make it more resectable, and less likely to recur after surgery(1). It is known that response to CRT confers improved disease-free and overall survival rates, reduces the likelihood of local recurrence, and improves the chances of anal sphincter preservation at the time of surgery(2-5). Furthermore, complete pathological response to CRT confers the best clinical outcomes and permits selected patients to undergo less extensive resections, further minimizing toxicity of treatment(2, 6). Unfortunately, only about 20% of patients achieve complete pathological responses to pre-operative CRT(7). Efforts to improve this rate have focused on overcoming the resistance of colorectal cancers to radiation therapy by intensifying the radiation dose or by using radiosensitizing cytotoxic chemotherapy(7-10), neither of which has significantly improved outcomes to date and both of which are associated with increased toxicity.
The transcription factor nuclear factor kappaB (NF-κB) regulates several genes that induce cell proliferation, invasion, angiogenesis, metastasis, suppression of apoptosis and treatment resistance in a wide range of tumors(11). NF-κB plays a significant role in conferring a survival advantage to colorectal cancers and resistance to radiotherapy. Constitutive activation of NF-κB has been observed in colorectal cancer cells(12) but not in normal colorectal ductal epithelial cells(13, 14). Progressive increases in NF-κB levels correlate with transition of normal colonic epithelial cells to adenomas, dysplasia, and finally, invasive adenocarcinomas(14, 15). NF-κB promotes tumor growth via inhibition of apoptosis and induces proteins, such as cyclin D1 and cycloxygenase-2 (COX-2) that are implicated in colorectal carcinogenesis(16-18). Also, NF-κB plays a pivotal role in promoting radioresistance in colorectal cancer(12, 19-21). Taken together, these evidences suggest that NF-κB serves as a mediator of a pro-survival phenotype in colorectal cancer and its suppression could potentiate the radioresponse in colorectal cancer.
Curcumin (diferuloylmethane), a polyphenol from the rhizomes of Curcuma longa, is the major constituent of the yellow spice turmeric, a commonly used flavoring agent in Asian cooking. In a variety of tumor cells, curcumin has been reported to inhibit proliferation and angiogenesis, to induce apoptosis or cell cycle arrest, and to cause regression of tumors in preclinical models(22-29). In colorectal cancer specifically, curcumin has demonstrated potent anti-tumor activity and chemopreventive potential in vitro and in vivo(30, 31), however, its exact molecular mechanism of action remains elusive.
In the present study, we have investigated the role of curcumin in modulating the radioresponse of colorectal cancer cells in vitro. Curcumin enhanced the anti-tumor effects of radiation in a clonogenic assay. While dissecting the mechanism underlying this radiosensitization, we observed that sub-lethal doses of radiation induced NF-κB transiently in a dose-dependent manner in colorectal cancer cells and curcumin inhibited this response. This was mediated by inhibition of radiation-induced Akt phosphorylation, inhibitor of κB kinase (IKK) activation, inhibitor of κB alpha (IκBα) phosphorylation, and IκBα degradation. Curcumin also down-regulated NF-κB–regulated anti-apoptotic and colorectal carcinogenic gene products. Our studies help to elucidate a pro-survival, anti-apoptotic signaling response of surviving colorectal cancer cells to irradiation that is mediated by NF-κB activation and modulation of radioresponse by curcumin via inhibition of this inducible radioresistance pathway.
Methods and Materials
Cells and Reagents
Colorectal cancer cell lines HCT116, HT29, and SW620 were kindly provided by Dr. Ray Meyn (MD Anderson Cancer Center). HCT116 cells were cultured in DMEM:F12 (50:50). HT29 cells were cultured in DMEM and SW620 cells were cultured in L-15. All media were supplemented with 10% FBS and 1% Penicillin-streptomycin (Invitrogen, Grand Island, NY). Curcumin was procured from LKT laboratories (St. Paul, MN). Antibodies to IκBα, inhibitor of apoptosis protein (IAP)-2, cyclin D1, and Bcl-XL were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to COX-2 was from BD Biosciences (San Diego, CA). Anti phospho-IκBαSer32/36 and anti phospho-AktSer473 were from Cell Signaling (Beverly, MA). Anti-Bcl-2 antibody was from DakoCytomation (Carpinteria, CA) and anti-β-actin was from Sigma (St. Louis, MO). Millimolar (mM) stock solutions of curcumin in dimethyl sulfoxide (DMSO) were stored at -20°C. For every experiment, the appropriate curcumin stock was diluted 1:1000 in culture medium immediately prior to use to obtain the respective micromolar (μM) concentrations. The control groups received DMSO diluted accordingly (final DMSO concentration was 0.1% in all groups).
In vitro cytotoxicity
The effect of curcumin on tumor cells was assessed using the XTT cell proliferation kit (Roche Applied Science, Indianapolis, IN). Briefly, cells were seeded in 96-well plates (3,000 cells per well) and grown overnight. Next day, the medium was aspirated and cells were exposed to different concentrations of curcumin. Following 4 h, curcumin was aspirated; the wells were rinsed and replenished with fresh medium. After further 48 h of culture, cell viability was determined by XTT assay. Results are expressed as percent cell viability for each concentration of curcumin with respect to DMSO controls.
Clonogenic survival
Cells were treated with vehicle control (DMSO) or 25 μM curcumin for 4 h and then irradiated with a 137Cs unit (cylindrical source 4.5 cm diameter × 5.3 cm length) at room temperature (dose rate 3.1 Gy/min). Following 3 h, curcumin was washed, cells were trypsinized and specific cell densities were re-plated in 60-mm petridishes and were incubated for colony formation for 10-14 days. Colonies were counted after staining with 0.5% alcoholic crystal violet. The fraction surviving a given treatment was calculated with respect to the survival of unirradiated controls (cells treated with DMSO or curcumin alone).
Electrophoretic mobility shift assays (EMSA)
NF-κB activation was assessed by EMSA as described previously(32). Briefly, nuclear extracts of treated cells were incubated with 32P-end-labeled double-stranded NF-κB oligonucleotide (15 μg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat, 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′ (boldface indicates NF-κB binding sites) for 30 min at 37°C, and the DNA-protein complex was resolved on 6.6% native PAGE. The radioactive bands on dried gels were quantified by a PhosphorImager using ImageQuant software (v 5.1; Molecular Dynamics, Sunnyvale, CA).
Immunobloting
Whole cell lysates were fractionated by SDS-PAGE, the proteins were electrotransferred to nitrocellulose membranes and probed with appropriate antibody. The blots were next probed with appropriate horseradish peroxidase conjugated secondary antibodies (Santa Cruz, CA) and developed by enhanced chemiluminescence using ECL™ (GE healthcare, Piscataway, NJ).
Kinase Assay
The effect of curcumin on radiation-induced IKK activation was assessed by an immunocomplex kinase assay(33). Briefly, the IKK complex from whole-cell extracts was precipitated with IKK-α antibody followed by treatment with protein A/G-Sepharose beads (Pierce Chemical, Rockford, IL) for 2 h. The beads were then washed with lysis buffer and assayed in a kinase assay mixture (50 mM HEPES, pH 7.4, 20 mM MgCl2, 2 mM dithiothreitol, 20 mCi [γ-32P]ATP, 10 mM unlabeled ATP) with the substrate glutathione S-transferase-IκBα (GST-IκBα ; amino acids 1-54) at 37°C. The reaction was terminated after 30 min by boiling the assay mixture with SDS sample buffer. The protein was resolved on 10% SDS-PAGE, the radioactive bands were visualized on dried gels with a PhosphorImager.
Statistics
All the experiments were done at least in triplicates unless otherwise mentioned. Results are expressed as mean ± standard error (SE). Comparisons between groups were performed by two-tailed Student's t tests. Representative data of one of the three independent experiments is shown.
Results
Curcumin inhibited proliferation of colorectal cancer cells
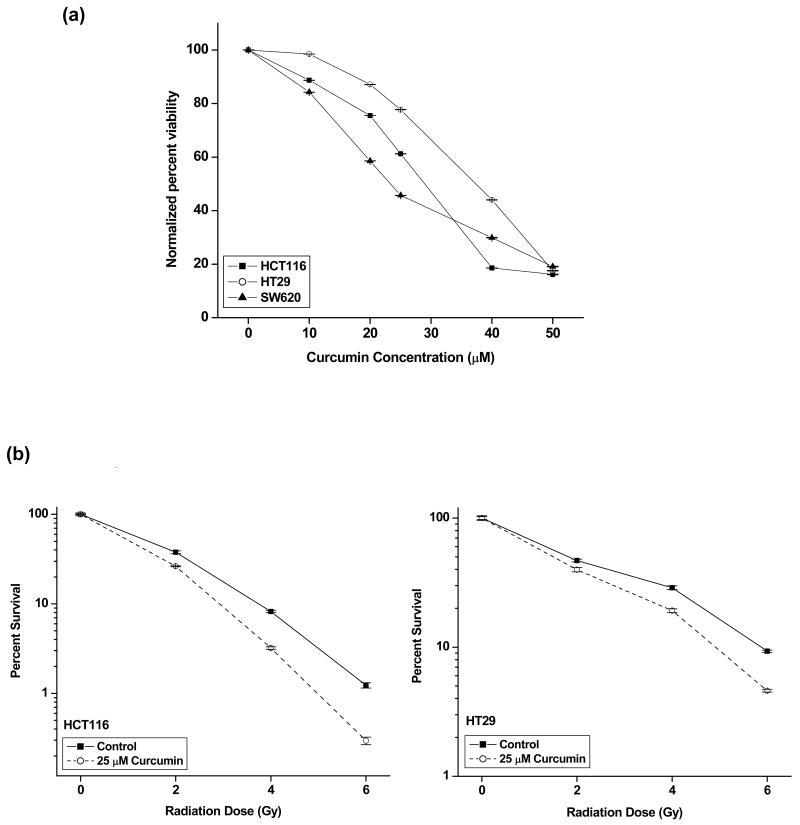
The cytotoxic effects of curcumin alone on colon cancer cell lines were determined using XTT assay. Curcumin inhibited the proliferation of all the cell lines in a dose-dependent manner (Fig. 1a). HCT116 and SW620 cells were equally sensitive to curcumin (IC50 27.7 ± 0.6 μM and 23.4 ± 1.3 μM, respectively; p = 0.144) whereas HT29 was least sensitive (IC50 37.4 ± 1.06 μM). p value
Figure 1. Curcumin augments radiation-induced cytotoxicity in colon cancer cells.
(a) Cells (3 × 104/mL) were exposed to different doses of curcumin in 96-well plate for 4 h after which the curcumin was washed and fresh medium was added to cells. Viability was assessed by XTT assay (Roche) after 48 h. The percent viability was calculated with respect to DMSO-treated controls. Points, mean of quadruplicates for each concentration; bars, SE. (b) HCT116 and HT29 cells were exposed to 25 μM curcumin for 4 h, and irradiated at indicated doses. Cells were re-plated for clonogenic assay 3 h post-radiation. The enhancement in radiosensitivity by curcumin was assessed on the basis of percent cell survival in comparison with the controls (cells irradiated with DMSO). Points, mean of the sextuplicates, bars, SE.
Curcumin sensitized colon cancer cells to radiation
Treatment with curcumin (25 μM) significantly enhanced the intrinsic tumor radiosensitivity of both HCT116 and HT29 as assessed by clonogenic survival assays (Fig. 1b). The dose enhancement ratio (DER) calculated at 10% surviving fraction was 1.28 (HCT116) and 1.18 (HT29) respectively. This dose (25 μM) was chosen for further experiments as higher doses were independently toxic to cells and lower doses had less appreciable radiosensitization effects (data not shown).
Radiation induced dose- and time-dependent NF-κB activation
Tumor cells were treated with graded doses of radiation (0-10 Gy) and incubated for 3 h before harvesting and preparation of nuclear extracts for assessing NF-κB activation. Radiation activated NF-κB in a dose-dependent manner in all three cell lines (Fig. 2a, 2c and 2e). Radiation dose inducing maximum NF-κB activation (10 Gy), was chosen for further studies. To discern the time course of NF-κB induction post-irradiation, cells were exposed to 10 Gy and harvested after different time intervals post irradiation. Marginal induction of NF-κB was seen in cells harvested immediately after irradiation (Fig. 2b) which increased significantly within 1 h. These NF-κB levels peaked at 3 h and started declining gradually after 6 h (Fig. 2b, 2d, and 2f).
Figure 2. Radiation induced NF-κB in colorectal cancer cells.
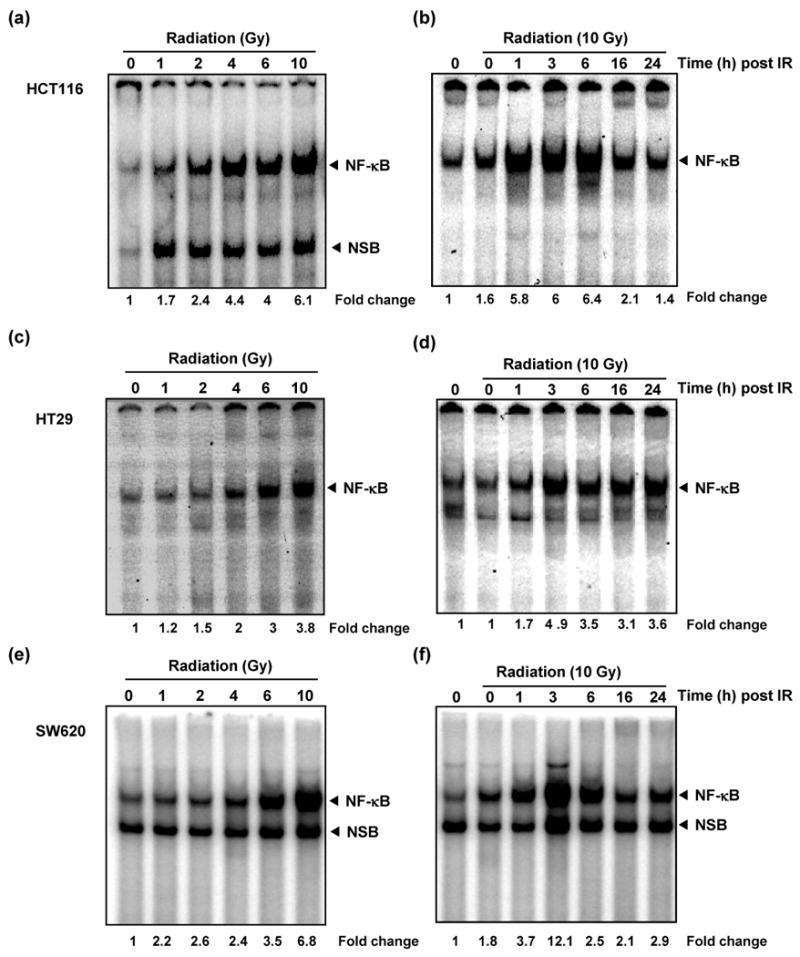
Dose dependent increase in NF-κB activity was observed in HCT116 (a), HT29 (c), and SW620 (e) cells. Colon cancer cells (2 × 105/mL) were exposed to graded doses of radiation and harvested 3 h after irradiation. Nuclear extracts were probed for NF-κB activation by EMSA. The time course of radiation-induced NF-κB activation after 10 Gy irradiation in HCT116 (b), HT29 (d), and SW620 (f). Cells (2 × 105 /mL) were exposed to radiation, harvested post-radiation at indicated time points, and probed for NF-κB. Activation of NF-κB was expressed as fold increase of DNA-bound protein over untreated controls (basal levels). The band labeled NF-κB represents the p65:p50 heterodimer, the predominant functionally active NF-κB dimer whereas the lower band is a non-specific band formed by the inactive NF-κB homodimer (p50:p50)
Curcumin suppressed radiation-induced NF-κB activation
Cells treated with DMSO (control) or curcumin (25 μM) for 4 h were exposed to single dose of radiation (10 Gy) and harvested at different time points post-radiation for assessing NF-κB activation. Radiation induced NF-κB activation within 30 min, and the NF-κB levels increased with increasing incubation time, peaking at 3 h post-irradiation (Fig. 3a). Curcumin effectively inhibited NF-κB at all these time points (Fig. 3a). Based on this time course study, we examined effect of curcumin on NF-κB activation in these cell lines. Cells treated with DMSO or curcumin (10 and 25 μM) for 4 h were exposed to single dose of radiation (10 Gy) and harvested 3 h post-radiation. In all these cells, radiation induced significant levels of NF-κB in the untreated cells (Fig. 3 b-d). Curcumin treatment significantly inhibited this NF-κB activation in all these cells at both 10 and 25 μM.
Figure 3. Inhibition of radiation-induced NF-κB activation by curcumin.
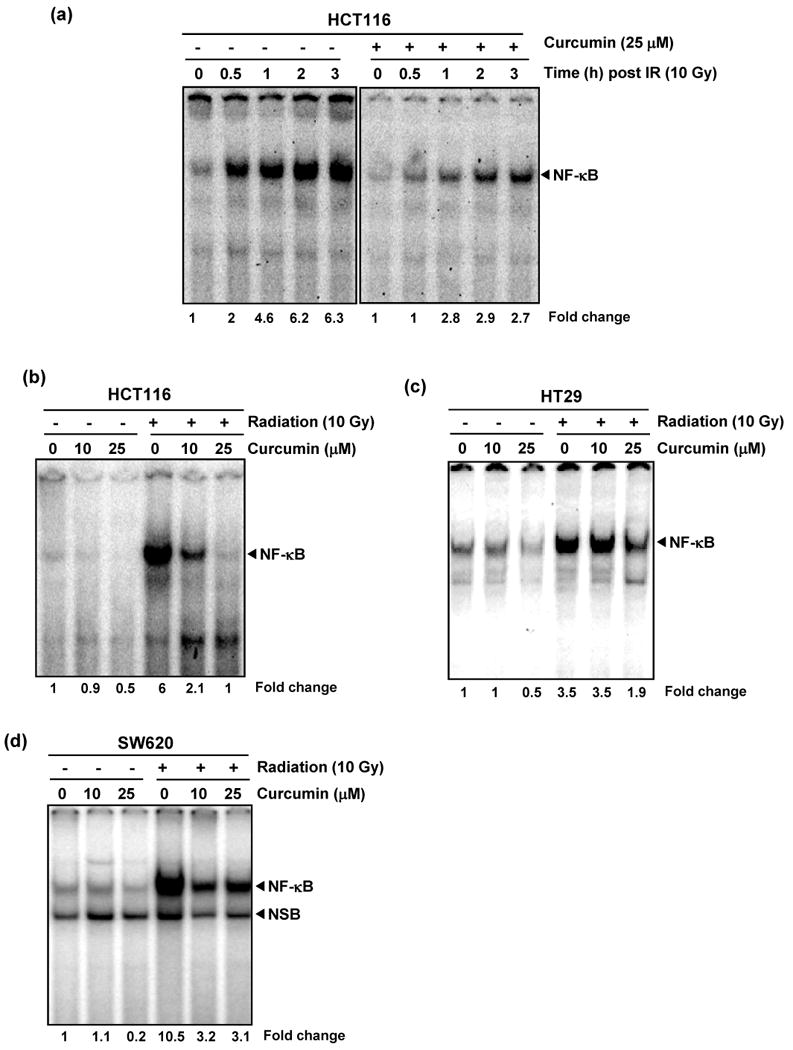
Curcumin inhibited radiation-induced activation of NF-κB. (a) HCT116 cells were incubated with curcumin (25 μM) for 4 h, exposed to radiation (10 Gy) and nuclear extracts prepared at indicated time points post IR were probed for NF-κB. Consistent suppression of radiation-induced NF-κB activity was noted at all time points (b-d). Suppression of radiation-induced NF-κB was also observed in HCT116 (b), HT29 (c) and SW620 (d) cells at both 10 and 25 μM concentrations, with a greater response observed for higher doses curcumin.
Curcumin inhibited radiation-induced phosphorylation and degradation of IκBα, activation of IKK, and activation of Akt
To investigate the mechanism of radiation-induced NF-κB activation and curcumin's action along this pathway, we interrogated the canonical pathway leading up to degradation of IκBα and release of cytoplasmic NF-κB for nuclear translocation and DNA-binding. HCT116 was chosen for further studies as the degree of induction of NF-κB following radiation (6-fold change) was neither as low as HT-29 (3.5-fold) nor as high as SW-620 (10.5-fold), curcumin was moderately effective as an inhibitor of NF-κB and it is a commonly used colorectal cell line with intact wild-type p53 that senses and regulates downstream effects of radiation-induced DNA damage. Cells were exposed to 6 and 10 Gy of radiation and harvested at different times post-irradiation. Radiation induced IκBα degradation in a dose dependent manner (extent of degradation being higher in 10 Gy treated cells than in 6 Gy) (Fig. 4a). In cells pre-treated with curcumin (25 μM), this radiation induced IκBα degradation was significantly inhibited (Fig. 4b).
Figure 4. Curcumin inhibits radiation-induced activation of proteins upstream of NF-κB.
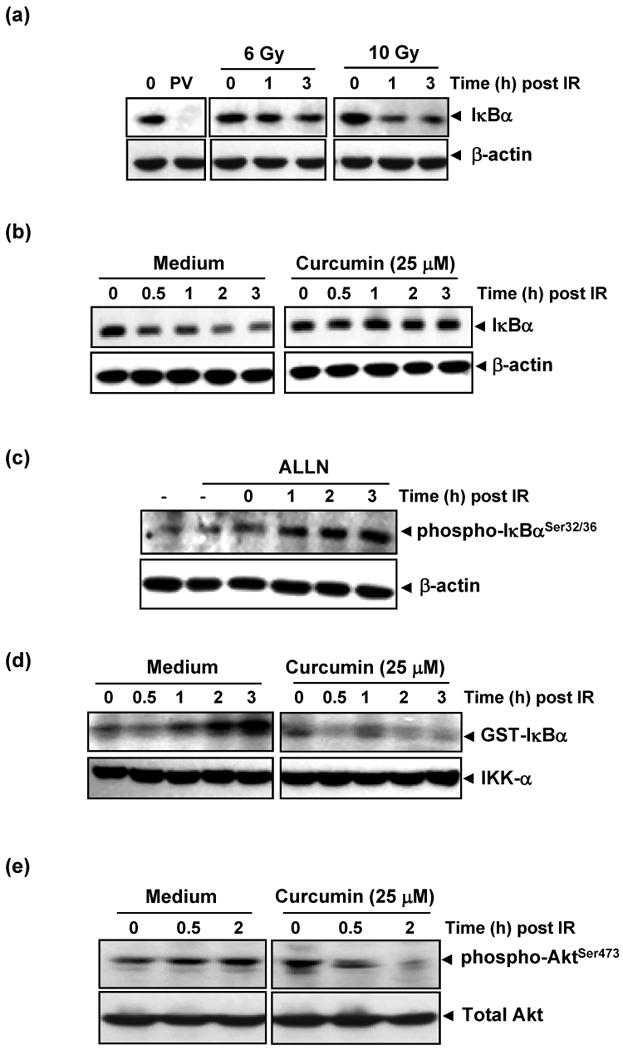
(a) Radiation induced IκBα degradation. HCT116 cells were exposed to 6 or 10 Gy of radiation and incubated for the indicated times. Pervanadate (PV; 50μM), a protein tyrosine phosphatase inhibitor, which induces IκBα degradation via tyrosine phosphorylation was positive control. Cytoplasmic extracts were prepared and subjected to immunoblotting using anti-IκBα antibody. (b) Pre-treatment with curcumin abrogated radiation-induced degradation of IκBα. HCT116 cells were incubated with 25 μM curcumin for 4 h prior to radiation (10 Gy) and cytoplasmic extracts were immunoblotted as described above. (c) Radiation induced phosphorylation of IκBα. HCT116 cells were incubated with 50 μg/ml ALLN for 30 min, exposed to radiation (10 Gy), and cytoplasmic extracts obtained at different time points were subjected to immunoblot analysis using phospho-specific anti-IκBα antibody (Ser32/36) (d) Curcumin inhibited radiation-induced IKK activation. HCT116 cells were pre-treated with or without 25 μM curcumin for 4 h and the IKK complex from whole-cell extracts was precipitated with antibody against IKK-α. IKK activity was analyzed by an immune complex kinase assay. The fusion protein GST-IκBα (glutathione S-transferase-IκBα) that was subsequently immunoblotted serves as a substrate for IKK. (e) Pre-treatment with curcumin inhibited radiation-induced activation of Akt. In experiments similar to those above, cytoplasmic extracts of HCT116 cells treated with and without curcumin prior to radiation were immunoblotted with anti phospho-Akt Ser473 antibody.
IκBα is phosphorylated at Ser 32/36 or Tyr 42 residues before degradation by polyubiquitination. To determine whether radiation signals phosphorylation of IκBα, cells were incubated with a proteasome inhibitor (ALLN) for 30 min before exposure to 10 Gy radiation. Phosphorylation of IκBα was monitored at different time intervals post irradiation. Corresponding to the previously noted temporal pattern of IκBα degradation, increasing levels of phosphorylated IκBα were noted at a similar time frame (Fig. 4c) confirming that radiation phosphorylates Ser 32/36 residues of IκBα.
Since the most common effector molecule for phosphorylation and subsequent degradation of IκBα is IKK(34), we investigated the role of radiation and curcumin on IKK activation. As seen in Fig. 4d, IKK activation occurred within 2 h following radiation and this activation was almost completely suppressed by curcumin.
One of the known activators of IKK is Akt/PKB, which also mediates tumor cell resistance via induction of pro-survival signaling. Hence, we investigated the effects of curcumin on Akt activation. Radiation induced phosphorylation of AktSer473 in HCT116 cells and curcumin significantly inhibited this phosphorylation (Fig. 4e). Taken together, these results suggest that curcumin blocks proliferative signals by targeting both IKK activation and AKT phosphorylation.
Curcumin suppressed NF-κB regulated gene expression
Because NF-κB regulates the expression of the anti-apoptotic and proliferative proteins such as IAPs(35, 36), Bcl-xL(37), Bcl-2(38), cyclin D1(39) and COX-2(40), we examined whether curcumin can modulate the expression of these anti-apoptotic gene products in HCT116 cells. Immunoblot analysis showed that radiation induced these anti-apoptotic proteins and that curcumin suppressed them (Fig. 5). It is of particular note here that although radiation did not induce COX-2 levels in these cells, the constitutive levels of this protein were completely suppressed by curcumin.
Figure 5. Curcumin inhibits NF-κB regulated gene products.
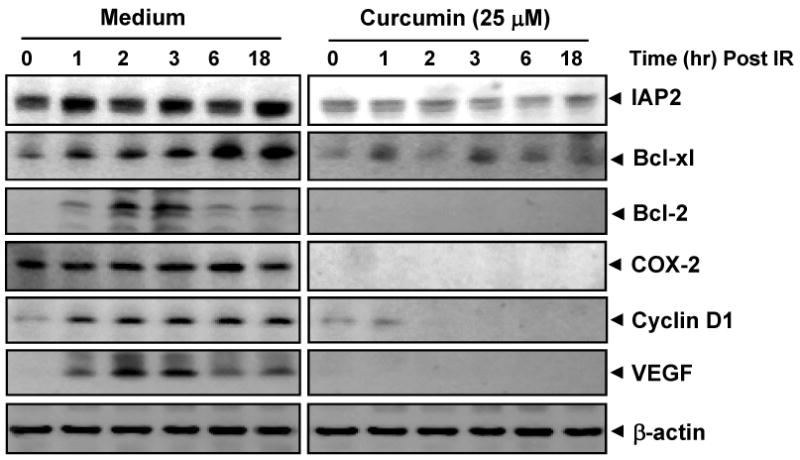
Curcumin suppresses radiation-induced expression of NF-κB-dependent anti-apoptotic (IAP2, Bcl-2, Bcl-XL,), inflammatory (COX-2), proliferative (cyclin D1) and angiogenic (VEGF) gene products. Cells were pre-incubated with 25 μM curcumin (4 h), exposed to radiation (10 Gy), and harvested post-radiation at indicated time points for immunoblot analysis.
Discussion
The primary objective of this study was to determine whether curcumin can sensitize colorectal cancer cells to radiation and to investigate the mechanism of action of this radiosensitization. We used colorectal cancer cells HCT116 (wild type p53, mutant K-ras), HT29 cells (mutant p53, wild type K-ras), and SW620 (mutant p53 and K-ras). Curcumin inhibited proliferation and the post-irradiation clonogenic survival of these cell lines. Transient dose-dependent radiation-induced NF-κB was suppressed by curcumin via inhibition of radiation-induced phosphorylation and degradation of IκBα, inhibition of IKK activity, and inhibition of Akt phosphorylation. Curcumin also suppressed NF-κB regulated gene products (Bcl-2, Bcl-xL, IAP-2, COX-2, cyclin D1 and VEGF). Our results suggest that transient radiation-inducible NF-κB activation provides a pro-survival response to radiation that may account for development of radioresistance. Curcumin blocks this signaling pathway and potentiates the anti-tumor effects of radiotherapy.
The identification of inducible radioresistance in colorectal cancer mediated via the NF-κB pathway is of considerable interest, because this pathway could then serve as a target for developing molecular-based radiosensitization protocols for radioresistant tumors. Activation of NF-κB in cancer cells has been shown to attenuate apoptosis induced by radiation(21). Inhibition of NF-κB, therefore, has the potential to improve radiotherapeutic efficacy by enhancing radiation-induced cell kill. NF-κB can be inhibited by multiple strategies including the use of a modified form of IκBα(19, 20, 41, 42), decoy of NF-κB(43), or proteosome inhibitors(20), all of which have shown promising activity in preclinical studies. One concern with highly specific NF-κB targeting strategies is the likelihood that this could lead to immunosuppression and greater susceptibility to infections and/or lymphoproliferative disorders as has been documented with the use of tumor necrosis factor α (TNF-α) antagonists in autoimmune diseases (44). In contrast, curcumin has a broader spectrum of activity, that possibly cause radiosensitization via multiple mechanisms including inhibition of TNF-α-mediated NF-κB activation(45) (Supplementary Fig. 1) and Akt-mediated inhibition of the MDM2 oncogene(46). Furthermore, the more potent inhibition of inducible NF-κB than constitutive NF-κB and the lack of any toxicity in clinical studies so far suggest that curcumin may have a wide therapeutic window.
The identification of curcumin as a radiosensitizer in colorectal cancer is also of considerable interest, because of its affordability, ease of oral administration, and lack of toxicity in clinical use. In human trials of its safety, doses as large as 12 gm/day have been tolerated with minimal clinical toxicity(47). It has shown clinical activity in a small colorectal polyp prevention study in patients with familial adenomatous polyposis syndrome who had not undergone a prophylactic colectomy and had failed other chemopreventive treatments(48). Lastly, an oral dose of 3.6 gm of curcumin achieves pharmacologically efficacious levels of curcumin in colorectal cancers with negligible distribution of the parent drug outside the gut(49).
An improved understanding of the complex multi-step colorectal carcinogenesis process at the molecular level and the aberrant homeostatic pathways defining resistance to therapies has led to the increasing use of novel targeted agents inhibiting pathways mediating tumor proliferation/ survival and/or tumor angiogenesis. While some of these agents have shown promising activities in the treatment of metastatic colorectal cancer, similar improvements in outcomes following their integration into preoperative CRT have not been observed so far. In general, these approaches have addressed pre-existing or de novo chemo- and radio- resistance pathways using highly targeted agents that are prone to being circumvented by downstream signaling via redundant pathways. In contrast to these constitutively active pathways, the relative contribution of acquired and/or inducible resistance pathways to the overall problem of chemo- or radio-resistance has not been actively investigated. In this study, we report a novel strategy for improving rectal cancer therapy, based on combining radiation therapy with curcumin, which inhibits radiation-induced NF-κB in cancer cells. Our studies help to elucidate a pro-survival, anti-apoptotic signaling response of colorectal cancer cells to irradiation mediated by signaling via the NF-κB pathway and one of the mechanisms by which curcumin modulates radioresponse by targeting this inducible radioresistance pathway.
Supplementary Material
Acknowledgments
This work was supported by the Clayton Foundation for Research (to B.B.A.).
Footnotes
Conflict of interest: No conflicts of interest
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 3.Janjan NA, Abbruzzese J, Pazdur R, et al. Prognostic implications of response to preoperative infusional chemoradiation in locally advanced rectal cancer. Radiother Oncol. 1999;51:153–160. doi: 10.1016/s0167-8140(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 4.Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Crane CH, Skibber JM, Feig BW, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517–524. doi: 10.1002/cncr.11075. [DOI] [PubMed] [Google Scholar]
- 6.Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60:1098–1105. doi: 10.1016/j.ijrobp.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan S, Janjan NA, Skibber JM, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Rodel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol. 2007;25:110–117. doi: 10.1200/JCO.2006.08.3675. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 10.Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer:phase I trial results. J Clin Oncol. 2006;24:656–662. doi: 10.1200/JCO.2005.04.1749. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Voboril R, Weberova-Voborilova J. Constitutive NF-κB activity in colorectal cancer cells: impact on radiation-induced NF-κB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–523. [PubMed] [Google Scholar]
- 13.Yu LL, Yu HG, Yu JP, et al. Nuclear factor-κB p65 (RelA) transcription factor is constitutively activated in human colorectal carcinoma tissue. World J Gastroenterol. 2004;10:3255–3260. doi: 10.3748/wjg.v10.i22.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima M, Morisaki T, Sasaki N, et al. Increased nuclear factor-κB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–681. [PubMed] [Google Scholar]
- 15.Yu HG, Yu LL, Yang Y, et al. Increased expression of RelA/nuclear factor-κB protein correlates with colorectal tumorigenesis. Oncology. 2003;65:37–45. doi: 10.1159/000071203. [DOI] [PubMed] [Google Scholar]
- 16.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 17.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 18.Charalambous MP, Maihofner C, Bhambra U, et al. Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-κB and IκBα in human colorectal cancer epithelial cells. Br J Cancer. 2003;88:1598–1604. doi: 10.1038/sj.bjc.6600927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukogawa T, Koyama F, Tachibana M, et al. Adenovirus-mediated gene transduction of truncated IκBα enhances radiosensitivity in human colon cancer cells. Cancer Sci. 2003;94:745–750. doi: 10.1111/j.1349-7006.2003.tb01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo SM, Tepper JE, Baldwin AS, Jr, et al. Enhancement of radiosensitivity by proteasome inhibition:implications for a role of NF- κB. Int J Radiat Oncol Biol Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 22.Tomita M, Kawakami H, Uchihara JN, et al. Curcumin (diferuloylmethane) inhibits constitutive active NF-κB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765–772. doi: 10.1002/ijc.21389. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Amin HM, Lai R, et al. Curcumin (diferuloylmethane) inhibits constitutive NF- κB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Siwak DR, Shishodia S, Aggarwal BB, et al. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IκB kinase and nuclear factor κB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Aggarwal BB, Shishodia S, et al. Nuclear factor-κB and IκB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 26.Wei SC, Lin YS, Tsao PN, et al. Comparison of the anti-proliferation and apoptosis-induction activities of sulindac, celecoxib, curcumin, and nifedipine in mismatch repair-deficient cell lines. J Formos Med Assoc. 2004;103:599–606. [PubMed] [Google Scholar]
- 27.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-κB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 28.Chan MM, Fong D, Soprano KJ, et al. Inhibition of growth and sensitization to cisplatin-mediated killing of ovarian cancer cells by polyphenolic chemopreventive agents. J Cell Physiol. 2003;194:63–70. doi: 10.1002/jcp.10186. [DOI] [PubMed] [Google Scholar]
- 29.Mehta K, Pantazis P, McQueen T, et al. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs. 1997;8:470–481. doi: 10.1097/00001813-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Villegas I, Sanchez-Fidalgo S, Alarcon de la Lastra C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol Nutr Food Res. 2008;52:1040–1061. doi: 10.1002/mnfr.200700280. [DOI] [PubMed] [Google Scholar]
- 31.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-κB-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 32.Takada Y, Khuri FR, Aggarwal BB. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-κB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-κB-regulated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:26287–26299. doi: 10.1074/jbc.M400963200. [DOI] [PubMed] [Google Scholar]
- 33.Sandur SK, Ichikawa H, Sethi G, et al. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-κB activation and NF-κB-regulated gene products through modulation of p65 and IκBα kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 35.You M, Ku PT, Hrdlickova R, et al. ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu ZL, McKinsey TA, Liu L, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamatani M, Che YH, Matsuzaki H, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NF-κB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 38.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 39.Guttridge DC, Albanese C, Reuther JY, et al. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto K, Arakawa T, Ueda N, et al. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 41.Wang CY, Cusack JC, Jr, Liu R, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 42.Yamagishi N, Miyakoshi J, Takebe H. Enhanced radiosensitivity by inhibition of nuclear factor kappa B activation in human malignant glioma cells. Int J Radiat Biol. 1997;72:157–162. doi: 10.1080/095530097143374. [DOI] [PubMed] [Google Scholar]
- 43.Kawamura I, Morishita R, Tsujimoto S, et al. Intravenous injection of oligodeoxynucleotides to the NF-κB binding site inhibits hepatic metastasis of M5076 reticulosarcoma in mice. Gene Ther. 2001;8:905–912. doi: 10.1038/sj.gt.3301478. [DOI] [PubMed] [Google Scholar]
- 44.Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 45.Chendil D, Ranga RS, Meigooni D, et al. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Zhang Z, Hill DL, et al. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 47.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.