Abstract
Purpose
To investigate whether symptom burden before and during preoperative chemoradiation therapy (CRT) for rectal cancer predicts for pathological tumor response.
Methods and Materials
Fifty-four patients with T3/T4/N+ rectal cancers were treated on a phase II trial using preoperative capecitabine and concomitant boost radiotherapy. Symptom burden was prospectively assessed prior to (baseline) and weekly during CRT by patient self-reported questionnaires, the MD Anderson Symptom Inventory (MDASI) and Brief Fatigue Inventory (BFI). Survival probabilities were estimated using Kaplan-Meier's method. Symptom scores according to tumor downstaging (TDS) were compared using Students t-tests. Logistic regression was used to determine whether symptom burden levels predicted for TDS. Lowess curves were plotted for symptom burden across time.
Results
Among 51 patients evaluated for pathological response, 26 patients (51%) had TDS. Fatigue, pain, and drowsiness were the most common symptoms. All symptoms increased progressively during treatment. Patients with TDS had lower MDASI fatigue scores at baseline and at completion (week 5) of CRT (p=0.03 for both) and lower levels of BFI ‘usual fatigue’ at baseline.
Conclusion
Lower levels of fatigue at baseline and completion of CRT were significant predictors of pathological tumor response gauged by TDS, suggesting that symptom burden may be a surrogate for tumor burden. The relationship between symptom burden and circulating cytokines merits evaluation to characterize the molecular basis of this phenomenon.
Keywords: Rectal cancer, neoadjuvant chemoradiation, fatigue, down-staging, tumor response
Introduction
Cancer patients undergoing chemoradiation therapy (CRT) suffer from multiple non-specific tumor-related and treatment-related symptoms (1-4). Contemporary symptom research has focused on symptoms as a contributor to morbidity in cancer care. Research has shed light on the impact of patterns, temporal profiles, severity, and co-occurrence (clustering) of symptoms on quality of life and functional interference in patients (2-5). Symptom burden, the distress caused by these symptoms, has been used as a tool for measuring global quality of life after treatment (1, 2, 6). In many studies evaluating interventions, improvements in quality of life have been noted among patients who respond to treatment (7-10). These studies uniformly suggest that tumor response to treatment reduces symptom burden, the subjective counterpart of tumor burden.
However, in contrast to treatment response predicting the severity of symptoms, whether symptom burden can have a direct impact on tumor response to treatment is inadequately characterized. Some researchers have documented that baseline symptom burden correlated with overall outcome, such as survival and recurrence-free survival (11-17). Often, in these instances, symptom burden is a surrogate for overall performance status and its influence on overall survival may be less due to tumor response than due to medical co-morbidities and other confounders. Among patients without significant impairment of performance status, it would be instructive to understand whether symptom burden could directly influence pathological tumor response rather than overall outcomes. To our knowledge, this correlation has not been previously documented, particularly in rectal cancer. Therefore, we undertook this prospective assessment of symptoms in a group of uniformly treated patients with rectal cancer and correlated pre-treatment and during-treatment symptoms with pathological treatment outcomes at the time of surgery.
Patients and Methods
The University of Texas M. D. Anderson Cancer Center institutional review board approved the study, and each patient gave written informed consent before being recruited onto the trial. The study was conducted according to the principles of the Declaration of Helsinki and good clinical practice guidelines.
Study design including patient accrual, patient evaluation and staging procedures have been reported previously (18). Fifty-four patients with T3/T4/N+ rectal cancers were treated on a Phase II trial using preoperative capecitabine and concomitant boost radiotherapy. Median age was 56.7 years (range, 21.3 – 78.7). All patients had newly diagnosed rectal cancer with excellent performance status (ECOG 0-1).
Treatment
Radiotherapy was administered to an initial pelvic field using a three-field technique (PA, right and left lateral fields) on a belly board and 18 MV photons. The pelvis was treated to 45 Gy in 25 fractions (1.8 Gy / fraction) prescribed to the 95% isodose line. Concomitant boost treatment was administered during the last week of scheduled radiotherapy, for an additional five fractions (1.5 Gy / fraction) delivered at least 6 hours after the initial pelvis treatment. The boost field encompassed the primary tumor volume and adjacent perirectal lymph nodes (including other involved nodes according to the clinical situation) with a 2 to 3 cm margin. Capecitabine was administered at 825 mg/m2 orally twice daily for the duration of radiotherapy with the initial dose starting approximately 1-2 hours before radiotherapy. Surgical operation was performed at 6 to 10 weeks after completing CRT. The definitive surgical techniques included low anterior resection with mesorectal resection techniques, proctectomy and coloanal anastomosis, abdomino-perineal resection or pelvic exenteration.
Study End Points
The primary endpoint was tumor response as assessed by pathological complete response (pCR) and tumor downstaging (TDS) at the time of surgery. pCR was defined as absence of cancer cells in the resection specimen on histopathologic review of the tumor region in its entirety. TDS was determined by comparing the initial tumor stage and pathological tumor stage after surgery. The secondary objectives included rates of local control (LC), disease-free survival (DFS), and overall survival (OS).
Symptom Assessment
One of the shortcomings of using a patient self-reporting system for symptom assessment is the subjectivity of symptom rating and reporting (2). Patients generally report their own sensations according to their own scale of symptom burden. Furthermore, patients are often reluctant to report symptoms because they don't want to be a bother to their physician or to be disqualified from a clinical trial. The availability of standardized assessment tools routinely incorporated into clinical practice would overcome some of these shortcomings. In our study, symptom burden was prospectively assessed prior to (baseline) and weekly during CRT by patient self-reported questionnaires, the MD Anderson Symptom Inventory (MDASI) and the Brief Fatigue Inventory (BFI). The MDASI is a multiple-symptom assessment tool that has been developed, characterized, and validated at our institution for reliable longitudinal assessment of symptom severity during cancer therapy (4). This questionnaire includes a list of 13 symptoms rated on an 11-point scale (0-10), with “0” indicating “not present” and “10” denoting “as bad as you can imagine” (4). The additional six interference items of MDASI are rated using a similar 11-point scale, with “0” representing “did not interfere” and “10” indicating “interfered completely”. The interference items include general activity, mood, work, walking, relations with other people, and enjoyment of life. The BFI is a tool to rapidly assess the severity and impact of cancer-related fatigue (3). The BFI is a list of three different ways of assessing fatigue (fatigue right now, usual fatigue during previous 24 hours, and worst level of fatigue during previous 24 hours) and the 6 interference items caused by patients' fatigue. The interference items are the same as those in the MDASI.
Statistical analysis
The actuarial rate of LC, DFS, and OS were calculated from time of study registration and estimated non-parametrically using Kaplan-Meier's product limit method. Baseline symptom scores (MDASI and BFI) according to TDS were compared using t-test. Logistic regression was used to determine whether symptom burden levels predicted for pathological response. Lowess curves were plotted for symptom burden across time. All tests were two-sided and a p-value of 0.05 or less was considered to be statistically significant. All analyses were performed using JMP software for Windows (version 5.1.2, SAS Institute, Cary, NC) and SPSS software for Windows (version 12.0, SPSS Inc, Chicago, IL).
Results
Tumor responses
Pathological response data was available for the 51 patients who underwent surgery. Twenty six patients (51%) achieved TDS and 9 patients (18%) achieved pCR. Patient characteristics, sorted by TDS, are listed in Table 1. The two groups were statistically similar for age, gender, performance status, tumor stage, pathology, hemoglobin level, and the level of carcinoembryonic antigen.
Table 1. Patient characteristics by tumor downstaging.
| Characteristics | No. of patients (%) | |
|---|---|---|
| TDS - NO | TDS - YES | |
| Age | ||
| Median, years (range) | 55.0 (21.3 – 76.8) | 58.1 (30.7 – 78.7) |
| Gender | ||
| Male | 15 (60) | 16 (61.5) |
| Female | 10 (40) | 10 (38.5) |
| ECOG performance status | ||
| 0 | 22 (88) | 22 (84.6) |
| 1 | 3 (12) | 4 (15.4) |
| Tumor stage by EUS | ||
| T2, N+ | 0 (0) | 1 (3.9) |
| T3, N0 | 10 (40) | 12 (46.1) |
| T3, N+ | 14 (56) | 12 (46.1) |
| T4, N+ | 1 (4) | 1 (3.9) |
| Histologic differentiation | ||
| Well differentiated | 1 (4) | 1 (3.9) |
| Moderately differentiated | 21 (84) | 21 (80.7) |
| Poorly differentiated | 3 (12) | 4 (15.4) |
| Hemoglobin level | ||
| Median (range) | 13.9 (10.8 – 16.0) | 14.0 (11.1 – 16.6) |
| CEA level | ||
| Median (range) | 3.1 (1.1 – 19.7) | 2.8 (0.7 – 65.5) |
Abbreviations: TDS = Tumor downstaging; ECOG = Eastern Cooperative Oncology Group; EUS = Endoscopic ultrasound; CEA = Carcinoembryonic antigen.
Local control and survival
The median clinical follow-up was 1.8 years (range, 0.7-3.1) and radiographic follow-up was 1.8 years (range, 0.2-3.0). To date, using an intent-to-treat analysis, one patient developed a local recurrence, one patient had local persistence that progressed locally at 1.5 years, no patient failed in regional lymph nodes and 9 patients developed distant metastases. The actuarial rate of local control was 93% at 2 years. The actuarial 2 year DFS and OS were 76% and 98%, respectively.
Patterns of symptom burden and its relation to tumor response
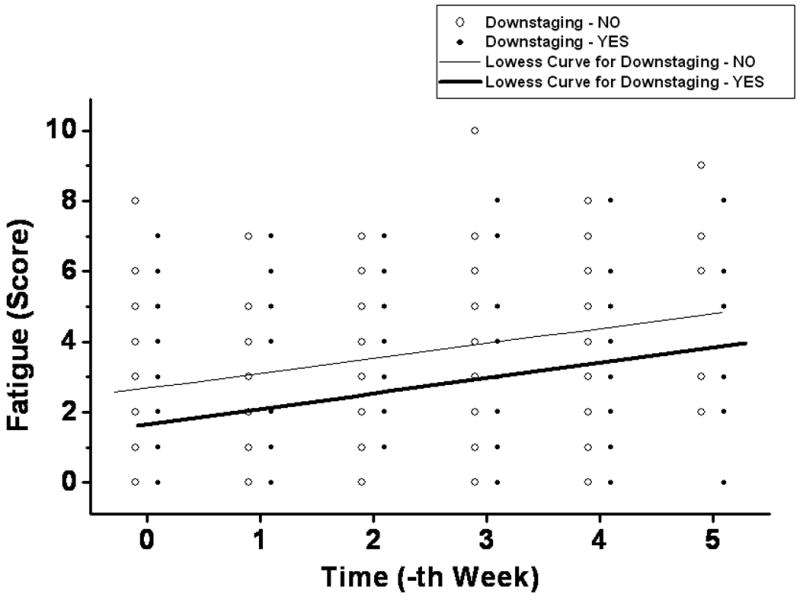
Temporal profiles of different MDASI symptoms during the preoperative chemoradiation are plotted in Figure 1. Fatigue, pain, and drowsiness were the predominant symptoms in their severity. Among baseline MDASI scores, only the fatigue score correlated with TDS (Table 2). A similar trend was not observed for other symptom burden parameters of pain, sleep disturbance, appetite, nausea, or feeling of sadness. Low MDASI fatigue scores at baseline and at completion of preoperative CRT predicted for TDS (Table 3 and Figure 2). Patients with TDS had lower levels of fatigue at baseline and at completion (week 5) of CRT compared to those without TDS (p=0.03 for both). Individual fatigue scores and Lowess curve fit across time also demonstrated this correlation with TDS (Figure 3), with patients with TDS displaying lower scores throughout the entire course of CRT.
Figure 1. Temporal patterns of MDASI scores over the course of treatment.
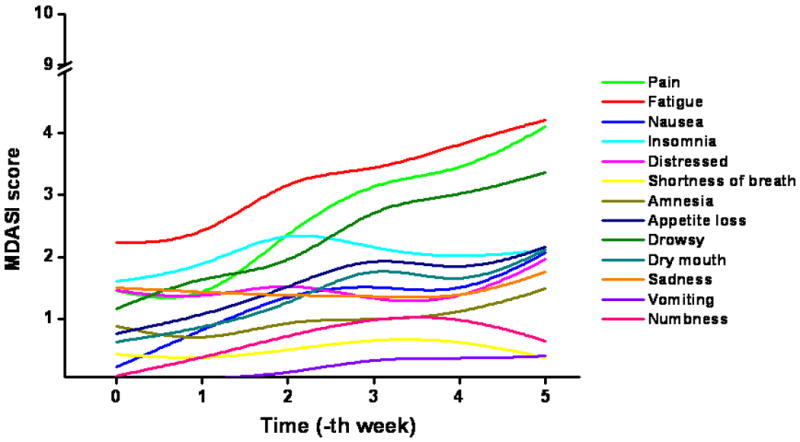
Table 2. Baseline MDASI scores by tumor downstaging.
| MDASI item | Score (mean ± SE) | p-value | |
|---|---|---|---|
| TDS – NO | TDS – YES | ||
| Pain | 1.75 ± 0.54 | 1.10 ± 0.54 | 0.40 |
| Fatigue | 3.05 ± 0.51 | 1.59 ± 0.47 | 0.04 |
| Nausea | 0.30 ± 0.16 | 0.18 ± 0.15 | 0.59 |
| Insomnia | 1.85 ± 0.50 | 1.32 ± 0.48 | 0.44 |
| Distressed (Upset) | 1.50 ± 0.43 | 1.29 ± 0.42 | 0.72 |
| Shortness of breath | 0.20 ± 0.32 | 0.68 ± 0.31 | 0.29 |
| Amnesia | 1.00 ± 0.39 | 0.76 ± 0.37 | 0.66 |
| Lack of appetite | 0.75 ± 0.41 | 0.77 ± 0.40 | 0.97 |
| Drowsy (Sleepy) | 1.25 ± 0.36 | 1.05 ± 0.34 | 0.68 |
| Dry mouth | 0.45 ± 0.34 | 0.86 ± 0.32 | 0.38 |
| Sadness | 1.45 ± 0.46 | 1.43 ± 0.43 | 0.98 |
| *Vomiting | - | - | NA |
| *Numbness (Tingling) | - | - | NA |
Abbreviations: MDASI = MD Anderson Symptom Inventory; TDS = Tumor downstaging.
For vomiting and numbness, no patient reported the score other than zero.
Table 3. Multiple logistic regression of weekly fatigue level predicting tumor response.
| Time point | Fatigue score (mean ± SE) | Odds ratio | p-value | |
|---|---|---|---|---|
| TDS – NO | TDS – YES | |||
| Baseline | 3.05 ± 0.51 | 1.59 ± 0.47 | 1.38 | 0.034 |
| Week 1 | 2.76 ± 0.45 | 1.71 ± 0.45 | 1.34 | 0.062 |
| Week 2 | 3.33 ± 0.45 | 3.32 ± 0.47 | 1.05 | 0.694 |
| Week 3 | 3.84 ± 0.57 | 3.06 ± 0.58 | 1.17 | 0.245 |
| Week 4 | 4.22 ± 0.53 | 3.63 ± 0.56 | 1.17 | 0.298 |
| Week 5 | 5.17 ± 0.65 | 3.38 ± 0.62 | 1.51 | 0.03 |
Abbreviations: TDS = Tumor downstaging.
Figure 2. Mean value of weekly fatigue score according to tumor downstaging.
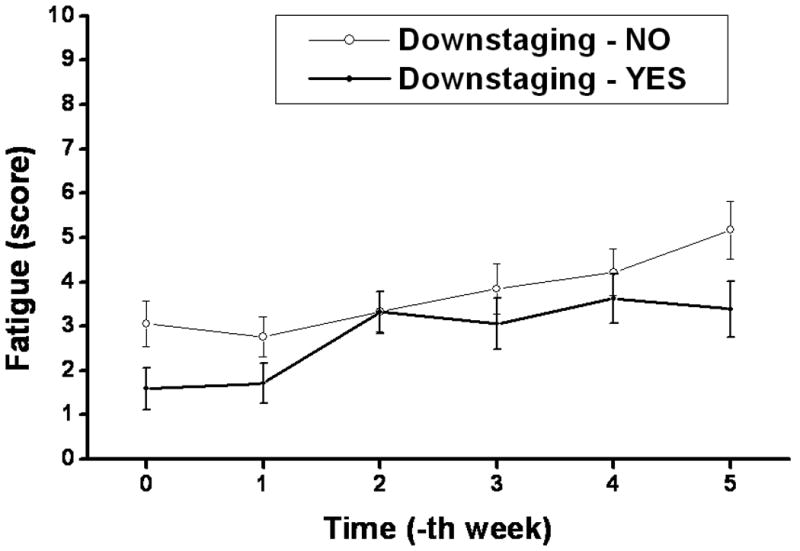
Figure 3. Lowess fit of weekly fatigue scores according to tumor downstaging.
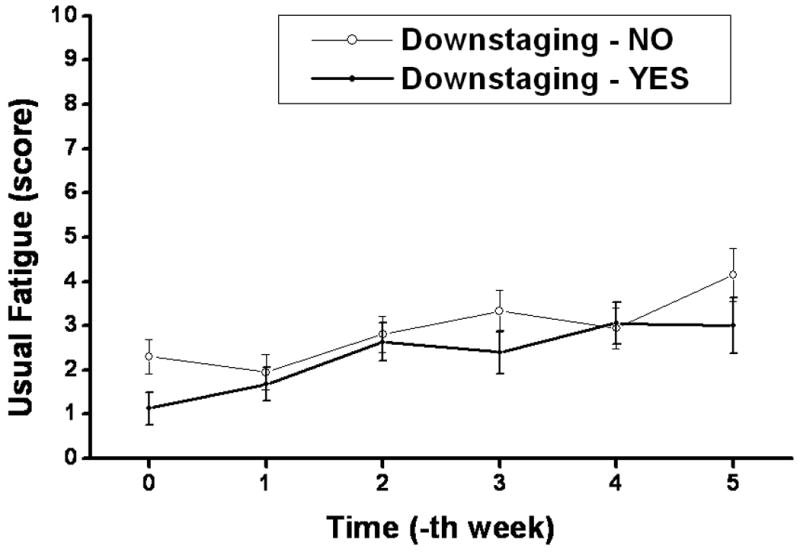
Longitudinal patterns of different BFI scores during the course of CRT are plotted in Figure 4. A pattern of MDASI fatigue score was plotted simultaneously with BFI scores. All BFI scores, besides the scores on interference with enjoyment of life and mood, showed co-occurrence with MDASI fatigue scores across time. Among baseline BFI scores, the level of ‘usual fatigue’ during the previous 24 hours was the only factor which correlated with TDS (Table 4). The mean values of weekly BFI ‘usual fatigue’ score according to TDS are plotted in Figure 5. Patients with TDS had lower levels of BFI ‘usual fatigue’ at baseline compared to those without TDS (p = 0.03); however, there was no significant correlation between BFI ‘usual fatigue’ at completion of CRT and TDS.
Figure 4. Temporal patterns of MDASI fatigue score and BFI scores over the course of treatment.
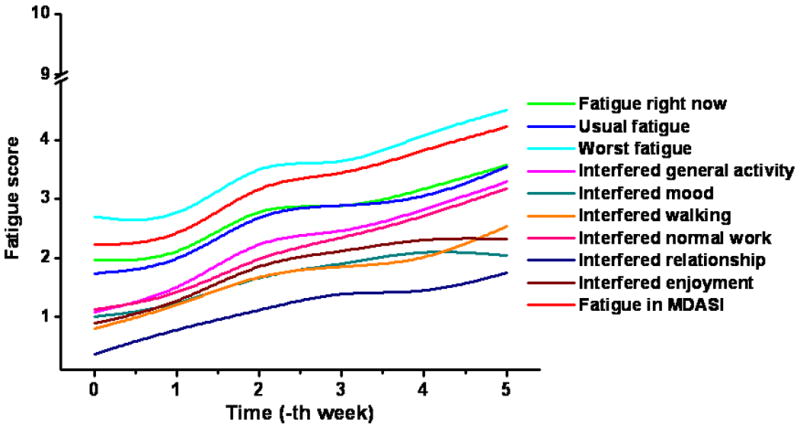
Table 4. Baseline BFI scores by tumor downstaging.
| BFI item | Score (mean ± SE) | p-value | |
|---|---|---|---|
| TDS – NO | TDS – YES | ||
| Fatigue right now | 2.25 ± 0.47 | 1.65 ± 0.44 | 0.36 |
| Usual level of fatigue (previous 24 hours) | 2.30 ± 0.39 | 1.13 ± 0.36 | 0.03 |
| Worst level of fatigue (previous 24 hours) | 3.35 ± 0.56 | 2.09 ± 0.52 | 0.11 |
| Interfered with general activity | 1.35 ± 0.31 | 0.70 ± 0.29 | 0.13 |
| Interfered with mood | 1.30 ± 0.34 | 0.70 ± 0.32 | 0.20 |
| Interfered with walking ability | 1.00 ± 0.28 | 0.43 ± 0.26 | 0.15 |
| Interfered with normal work | 1.20 ± 0.35 | 0.70 ± 0.33 | 0.30 |
| Interfered with interpersonal relation | 0.50 ± 0.16 | 0.26 ± 0.15 | 0.29 |
| Interfered with enjoyment of life | 1.20 ± 0.30 | 0.43 ± 0.28 | 0.07 |
Abbreviations: BFI = Brief Fatigue Inventory; TDS = Tumor downstaging.
Figure 5. Mean value of weekly fatigue score according to tumor downstaging.
Evaluation of the potential effect of symptom burden on pCR, LC, DFS, or OS was not possible because of the low number of events during the 2-year median follow-up period.
Discussion
Unlike studies that have documented improved quality of life among responders (7-10), our study demonstrates the converse, i.e. fatigue, a measure of symptom burden, independently predicted for tumor response. In the current study, lower levels of MDASI fatigue at baseline and at completion (week 5) of CRT were significant predictors of pathological response, as gauged by TDS (Table 3). Additionally, patients with TDS had lower levels of BFI ‘usual fatigue’ at baseline compared to those without TDS (Table 4). BFI ‘usual fatigue’ at completion of CRT showed marginal significance as a predictor of TDS (p = 0.09). Serial MDASI measurements confirmed that fatigue, pain, and drowsiness were the most common symptoms and that all symptoms increased progressively during treatment.
There are some similar reports that baseline symptom burden predicted for subsequent clinical outcomes such as overall survival and disease control (11-17). However, these studies were performed usually in patients with advanced disease, which is heterogeneous in its extent of systemic involvement, prior treatment variables, concurrent patient co-morbidities and overall performance status of the patient. Usually with early-stage disease, where symptoms are less evident and possibly less confounded by overall performance status, clinical objective data are more likely to override patients' symptoms in predicting survival (19). Recognizing these complexities in the interaction of symptom burden and overall clinical outcomes, our examination of the relationship between patients' symptom burden and the objective response of their tumors was confined to patients with locally advanced non-metastatic rectal cancer with minimal heterogeneity with respect to stage of disease, treatment and performance status. In this prospective study of a group of uniformly treated patients with rectal cancer, we correlated pre-treatment and during-treatment symptoms with pathological treatment outcomes at the time of surgery. Our results point to fatigue as a significant and independent predictor of pathological treatment outcomes and the suggestion that symptom burden could be a surrogate for tumor burden.
Fatigue is the most frequently reported symptom of cancer patients (3). Because of its prevalence, it is often reported as the symptom that is most distressing and causes the greatest amount of interference with daily life. Fatigue-related items were rated as the most severe in the original validation study of MDASI and BFI (3, 4). The fatigue score was also the item with the greatest severity and widest variance in the current study (Figure 1). Logistic regression predicted better TDS in patients who had lower MDASI fatigue scores at baseline and during the 5th week of treatment. However, while BFI ‘usual fatigue’ at baseline was predictive of TDS, it was only marginally significant at the 5th week of treatment. This discrepancy in the logistic regression results between the MDASI fatigue and BFI fatigue at the 5th week of treatment is probably a reflection of the limited patient numbers in this study. Similarly, the lack of a significant correlation between fatigue and TDS during the middle of treatment may be a reflection of the limitations imposed by small sample sizes where larger standard deviations mask small differences.
Among the more common causes for fatigue in cancer patients is anemia. Although most patients in our study were not anemic, anemia is increasingly recognized as a common manifestation of cancer and its treatment (20). Indeed, among the multiple factors contributing to fatigue, anemia represents a readily treatable entity which can profoundly influence the patient's quality of life, including physical, psychosocial, and economic/occupations aspects (20, 21). Like fatigue, anemia in cancer patients in under-reported, under-diagnosed and under-treated. The etiology of anemia in cancer patients may be related to tumor factors (primary tumor type, stage, associated blood loss or hypersplenism or marrow involvement), patient/host factors (age, gender, nutritional status), and treatment factors (type of treatment, duration, intensity, combinations of treatments). Irrespective of etiology, low levels of hemoglobin not only lead to reduced quality of life but also correlate with poorer local control and overall survival in cancer patients (22-31). Available data support the concept that anemia adversely impacts treatment outcomes by reducing tumor oxygenation which, in turn, compounds the leads to tumor growth, angiogenesis, mutations, resistance to apoptosis and resistance to therapy (30, 32). While correction of anemia reduces associated symptoms and improves quality of life, it remains unclear whether the detrimental effect of anemia on cancer outcomes can be reversed. Packed red blood cell transfusion promptly increases hemoglobin levels and may improve treatment outcomes, but is associated with transfusion-related reactions, potential contamination with infectious agents and iron overload. In contrast, although erythropoietin reduces transfusion requirements, recent evidence suggests that it increases the risk of tumor progression and reduces overall survival in patients with advanced breast, head and neck, cervical, lymphoid and non-small cell lung cancers (33-38). The current National Comprehensive Cancer Network guidelines recommend the use of transfusion for treatment of tumor-related anemia, management of treatment-related anemia requiring rapid correction and management of symptomatic treatment-related anemia. Erythropoietin is only recommended for prevention of transfusion in adequately counseled patients undergoing non-curative intent therapy who have symptomatic treatment-related anemia and/or are at high risk for symptomatic treatment-related anemia requiring transfusion.(20)
Similar to anemia, fatigue in cancer patients may be caused by the disease itself or it may be caused by various treatments, such as chemotherapy, radiotherapy, or surgery. The baseline score is more reflective of tumor-related fatigue while the temporal pattern of fatigue over the course of treatment is more reflective of the composite of tumor-related fatigue and that caused by treatment(s) and/or intervention(s). In the current study, among all fatigue parameters evaluated (baseline fatigue, end-of-treatment fatigue, and temporal pattern of increase in fatigue during treatment), baseline fatigue was the most consistent (across MDASI and BFI) and strongest predictor of TDS. This suggests that tumor-related fatigue is probably a greater contributor to the observed correlation between fatigue and TDS than treatment-related fatigue. The first question that arises in the setting of tumor-related baseline fatigue (a measure of symptom burden) being a reflection of tumor burden at the start of treatment and an independent predictor of TDS (a measure of treatment efficacy) is whether this can be used to stratify patients into distinct prognostic groups. While this possibility is suggested by the current study, these results require prospective validation in a separate study. The more provocative question that this raises is whether this symptom burden is associated with specific pathophysiologic changes that are amenable to modification in the poor prognostic group.
Several reports have correlated cancer-related fatigue with various biological factors (39-45). Significant correlations have been documented between tumor-related symptoms and serum levels of proinflammatory factors such as TGF-α and IL-6 in patients with metastatic colorectal cancer (44). In a mouse model of colitis-associated colorectal cancer, pro-inflammatory cytokines were noted to activate nuclear factor kappa-B (NFκB) during early tumor promotion and progression (46). Some studies have suggested that the levels of constitutive NFκB activity may predict radiosensitivity of colorectal cancer cells (47-49). In an effort to understand whether pro-inflammatory cytokines can explain some of the observed correlation between fatigue and treatment response, an analysis of serum cytokine profiles before and during CRT is on-going. Although other symptoms failed to correlate significantly with treatment response, this lack of correlation may be due to the limited sample size of this study population. In particular, the observation that the three predominant symptoms (fatigue, pain, and drowsiness) demonstrated co-occurrence (clustering) across time in the current study speaks to the possibility of a shared common pathophysiologic basis. This pattern of clustering of fatigue and drowsiness has been previously described (5). The shared pathophysiology may involve some of these pro-inflammatory cytokines under investigation.
In conclusion, unlike studies in other cancers that have documented improved quality of life among responders, we demonstrate that baseline and during-treatment fatigue independently predict for tumor response. Patients with lower baseline and during-treatment fatigue had better tumor response than those with greater and clinically relevant levels of fatigue. That symptom burden can serve as a surrogate for the effectiveness of treatment is provocative finding since this can be used to stratify patients into prognostic groups and possibly for making treatment decisions or adaptive modification of treatment. Correlation with on-going evaluation of changes in cytokine profile during CRT will hopefully shed light on the molecular underpinnings of this phenomenon.
Footnotes
Presented in part at the 2007 Gastrointestinal Cancer Symposium, Orlando, FL.
Authors' disclosures of potential conflicts of interest: None
References
- 1.Fallowfield L. Quality of life: a new perspective for cancer patients. Nat Rev Cancer. 2002;2:873–879. doi: 10.1038/nrc930. [DOI] [PubMed] [Google Scholar]
- 2.Paice JA. Assessment of symptom clusters in people with cancer. J Natl Cancer Inst Monogr. 2004:98–102. doi: 10.1093/jncimonographs/lgh009. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24:4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 6.Efficace F, Bottomley A, Osoba D, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials--does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21:3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 7.Geels P, Eisenhauer E, Bezjak A, et al. Palliative effect of chemotherapy: objective tumor response is associated with symptom improvement in patients with metastatic breast cancer. J Clin Oncol. 2000;18:2395–2405. doi: 10.1200/JCO.2000.18.12.2395. [DOI] [PubMed] [Google Scholar]
- 8.Langendijk JA, Aaronson NK, de Jong JM, et al. Prospective study on quality of life before and after radical radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2001;19:2123–2133. doi: 10.1200/JCO.2001.19.8.2123. [DOI] [PubMed] [Google Scholar]
- 9.Modi S, Panageas KS, Duck ET, et al. Prospective exploratory analysis of the association between tumor response, quality of life, and expenditures among patients receiving paclitaxel monotherapy for refractory metastatic breast cancer. J Clin Oncol. 2002;20:3665–3673. doi: 10.1200/JCO.2002.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Eton DT, Fairclough DL, Cella D, et al. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–1543. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 11.Earlam S, Glover C, Fordy C, et al. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14:171–175. doi: 10.1200/JCO.1996.14.1.171. [DOI] [PubMed] [Google Scholar]
- 12.Maisey NR, Norman A, Watson M, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38:1351–1357. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 13.Roychowdhury DF, Hayden A, Liepa AM. Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. J Clin Oncol. 2003;21:673–678. doi: 10.1200/JCO.2003.04.166. [DOI] [PubMed] [Google Scholar]
- 14.Fang FM, Tsai WL, Chiu HC, et al. Quality of life as a survival predictor for esophageal squamous cell carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:1394–1404. doi: 10.1016/j.ijrobp.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 15.Efficace F, Bottomley A, Coens C, et al. Does a patient's self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42:42–49. doi: 10.1016/j.ejca.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 16.McCarter H, Furlong W, Whitton AC, et al. Health status measurements at diagnosis as predictors of survival among adults with brain tumors. J Clin Oncol. 2006;24:3636–3643. doi: 10.1200/JCO.2006.06.0137. [DOI] [PubMed] [Google Scholar]
- 17.Groenvold M, Petersen MA, Idler E, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan S, Janjan NA, Skibber JM, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 19.Efficace F, Bottomley A. Toward a clearer understanding of the prognostic value of health-related quality-of-life parameters in breast cancer. J Clin Oncol. 2005;23:1335–1336. doi: 10.1200/JCO.2005.05.349. author reply 1336. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers GM, 3rd, Cella D, Chanan-Khan A, et al. Cancer- and treatment-related anemia. J Natl Compr Canc Netw. 2005;3:772–789. doi: 10.6004/jnccn.2005.0046. [DOI] [PubMed] [Google Scholar]
- 21.Mock V, Atkinson A, Barsevick A, et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Williston Park) 2000;14:151–161. [PubMed] [Google Scholar]
- 22.Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan S, Rana V, Janjan NA, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107:2589–2596. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 24.Lee WR, Berkey B, Marcial V, et al. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85-27. Int J Radiat Oncol Biol Phys. 1998;42:1069–1075. doi: 10.1016/s0360-3016(98)00348-4. [DOI] [PubMed] [Google Scholar]
- 25.Dubray B, Mosseri V, Brunin F, et al. Anemia is associated with lower local-regional control and survival after radiation therapy for head and neck cancer: a prospective study. Radiology. 1996;201:553–558. doi: 10.1148/radiology.201.2.8888257. [DOI] [PubMed] [Google Scholar]
- 26.Prosnitz RG, Yao B, Farrell CL, et al. Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:1087–1095. doi: 10.1016/j.ijrobp.2004.07.710. [DOI] [PubMed] [Google Scholar]
- 27.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Laurie SA, Ding K, Whitehead M, et al. The impact of anemia on outcome of chemoradiation for limited small-cell lung cancer: a combined analysis of studies of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2007;18:1051–1055. doi: 10.1093/annonc/mdm077. [DOI] [PubMed] [Google Scholar]
- 29.Henke M, Sindlinger F, Ikenberg H, et al. Blood hemoglobin level and treatment outcome of early breast cancer. Strahlenther Onkol. 2004;180:45–51. doi: 10.1007/s00066-004-1123-7. [DOI] [PubMed] [Google Scholar]
- 30.Fyles AW, Milosevic M, Pintilie M, et al. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57:13–19. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 31.Rades D, Lang S, Schild SE, et al. Prognostic value of haemoglobin levels during concurrent radio-chemotherapy in the treatment of oesophageal cancer. Clin Oncol (R Coll Radiol) 2006;18:139–144. doi: 10.1016/j.clon.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 34.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 35.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 37.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 38.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 39.Geinitz H, Zimmermann FB, Stoll P, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 40.Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Meyerhardt JA, Sloan JA, Sargent DJ, et al. Associations between plasma insulin-like growth factor proteins and C-peptide and quality of life in patients with metastatic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1402–1410. doi: 10.1158/1055-9965.EPI-04-0862. [DOI] [PubMed] [Google Scholar]
- 43.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 44.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 45.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 47.Mukogawa T, Koyama F, Tachibana M, et al. Adenovirus-mediated gene transduction of truncated I kappa B alpha enhances radiosensitivity in human colon cancer cells. Cancer Sci. 2003;94:745–750. doi: 10.1111/j.1349-7006.2003.tb01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voboril R, Weberova-Voborilova J. Constitutive NF-kappaB activity in colorectal cancer cells: impact on radiation-induced NF-kappaB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–523. [PubMed] [Google Scholar]
- 49.Voboril R, Weberova-Voborilova J. Sensitization of colorectal cancer cells to irradiation by IL-4 and IL-10 is associated with inhibition of NF-kappaB. Neoplasma. 2007;54:495–502. [PubMed] [Google Scholar]


