Abstract
Kidney transplantation from deceased donors classified as increased risk for viral infection by the Centers for Disease Control (CDC) is controversial. Analyses of Organ Procurement and Transplantation Network (OPTN) data from 7/1/2004 to 7/1/2006 were performed. The primary cohort included 48 054 adults added to the kidney transplant wait list. Compared to receiving a standard criteria donor (SCD) kidney or remaining wait-listed, CDC recipients (HR 0.80, p = 0.18) had no significant difference in mortality. In a secondary cohort of 19 872 kidney recipients at 180 centers, SCD (reference) and CDC (HR 0.91, p = 0.16) recipients had no difference in the combined endpoint of allograft failure or death. Among centers performing >10 kidney transplants during the study period, the median proportion of CDC transplants/total transplants was 7.2% (range 1.1–35.6%). Higher volume transplant centers were more likely to use CDC kidneys compared to low and intermediate volume centers (p <0.01). An analysis of procured kidneys revealed that 6.8% of SCD versus 7.8% of CDC (p = 0.13) kidneys were discarded. In summary, center use of CDC kidneys varied widely, and recipients had good short-term outcomes. OPTN should collect detailed data about long-term outcomes and recipient viral testing so the potential risks of CDC kidneys can be fully evaluated.
Keywords: Centers for Disease Control (CDC), kidney transplantation, viral infection
Introduction
The increasing waiting time for a deceased donor kidney transplant and the high mortality associated with end-stage renal disease (ESRD) have driven efforts to increase the pool of donor kidneys. The number of kidney transplants has grown modestly through the use of extended criteria donors (ECD) and donors after cardiac death (DCD), although ECD kidneys have an increased rate of allograft failure and DCD kidneys have increased likelihood of delayed graft function compared to kidneys from standard criteria donors (SCD) (1–5). A recent decision analysis suggested the benefits of using kidneys from another group of donors—those designated by the United States Centers for Disease Control (CDC) as being at increased risk of infection with human immunodeficiency virus (HIV), hepatitis C and hepatitis B (6). However, quantifying the value of accepting CDC donor kidneys will require empiric evidence of actual clinical outcomes among transplant recipients. Additionally, patterns of usage of CDC kidneys have not been fully described, which may hamper efforts to develop effective policies governing the use of these organs.
All deceased donors undergo serologic testing for hepatitis B, hepatitis C and HIV. In 1994, the CDC published guidelines with a list of behavioral and clinical characteristics (such as intravenous drug use) identifying donors thought to be at increased risk for recent blood-borne viral infection (see Table 1). These donors might have negative viral serologies during the ‘window period’ when such tests have low sensitivity (7). The guidelines recommended discarding organs from these CDC donors unless the advantage of transplantation to the recipient exceeded the risk of infection (7).
Table 1.
Criteria for a seronegative Centers for Disease Control high behavioral risk adult organ donor
|
Since 1994, the wait list for kidney transplantation has risen rapidly and the technology for testing donors for blood-borne viral pathogens has improved (8). Unfortunately, primary data on the actual risk of infection for CDC organ recipients are lacking (9). The general risk of HIV infection among all donors to United States tissue banks was reported to be 1 in 55 000 (10). A decision analysis by Schweitzer et al. estimated that the risk of HIV infection from CDC donors ranges from 1/323 for commercial sex workers to 1/10 000 for incarcerated donors, although this study employed data from nonorgan transplant populations (6). The transmission of HIV and hepatitis C to four solid organ transplant recipients in 2007 from a single CDC donor drew public attention to these risks (11). On the other hand, these estimates of viral infection risk are much smaller than the observed annual mortality on dialysis of approximately 20% (12).
A detailed examination of the potential benefits and pitfalls of increasing CDC organ use would require comparison of outcomes for CDC kidney recipients to those kidney transplant candidates who remain on the wait list or receive an SCD kidney. We hypothesized that CDC kidney recipients would have no significant difference in mortality compared to ‘standard therapy’ defined as receiving an SCD kidney or remaining on the wait list. We also hypothesized that recipients of CDC kidneys would have no difference in the rate of allograft failure compared to SCD recipients.
The possible opportunities presented by CDC kidneys also depend on the willingness of organ procurement organizations (OPOs) to procure these organs and of transplant centers to accept them. We hypothesized that discard rates for CDC kidneys would be higher than those for SCD kidneys, and that the use of CDC kidneys would be greater at centers with the highest volume of kidney transplants.
Methods
A retrospective cohort study was performed using data from the Organ Procurement and Transplantation Network (OPTN). OPTN registry data include detailed records of all solid-organ transplants performed across the United States. A CDC donor was defined as a deceased donor with (1) classification as having a risk factor for HIV, hepatitis C and/or hepatitis B infection on the basis of social and medical history data provided to OPTN by OPOs (see Table 1) and (2) negative serologic testing for HIV, hepatitis C and hepatitis B. Individuals with positive serological tests for HIV are legally prohibited from donating kidneys and were, therefore, absent from the dataset (7). A positive test for hepatitis C was defined as a positive antibody titer. A positive test for hepatitis B consisted of positive hepatitis B surface antigen without a positive hepatitis core antibody.
An ECD was defined as a deceased donor ≥ 60 years of age, or a donor ≥ 50 years with two of the following characteristics: history of hypertension, last serum creatinine > 1.5 mg/dL or cerebrovascular accident as cause of death (1). A DCD was defined as a donor for whom circulatory determination of death was confirmed prior to organ procurement.
Analyses were performed using Stata software (Stata 10.0, Stata Corporation, College Station, TX). For univariate comparisons of recipient, donor and allograft characteristics to donor type, ANOVA was used to compare means. Chi-square was used to compare categorical variables. For multi-variable Cox regression analysis, log–log plots were examined to assess model selection.
Primary cohort
To determine the relative benefits of the decision to accept a CDC kidney versus remaining on the wait list in the hope of receiving an SCD kidney, we assembled a cohort that included all adults initially wait-listed for a kidney transplant between 7/1/2004 and 7/1/2006. The initial date was chosen because the OPTN dataset reliably collected CDC donor status starting 7/1/2004. The end date was chosen so that members of the cohort would have the opportunity for at least 24 months of follow-up. The outcome for this analysis was mortality. Individuals who were wait-listed for multi-organ transplants were excluded. Patients with missing data on survival and recipients of prior kidney transplants were also excluded.
We employed a time-dependent Cox regression model that compared ‘standard therapy’ to receiving a CDC kidney, an ECD kidney or a DCD kidney. Standard therapy consisted of remaining on the wait list or undergoing kidney transplantation with an SCD kidney. All patients contributed data for time at risk to the standard therapy group starting at the time of registration for the kidney transplant wait list until death, removal from the wait list, undergoing a transplant or the end of follow-up (1,13). Patients undergoing SCD kidney transplantation continued to contribute time to the standard therapy group, whereas patients undergoing transplantation with a non-SCD kidney ‘switched’ therapies and after transplantation contributed time to the CDC group, the ECD group or the DCD group. Individuals who underwent transplantation with kidneys from live donors, hepatitis C seropositive donors or hepatitis B seropositive donors contributed time to the standard therapy group and were censored at the time of transplantation. For simplicity, we also censored recipients of kidneys from donors that met criteria for multiple categories of organ type (ECD-CDC, DCD-CDC and CDC-ECD-DCD, who comprised 0.5% of total cohort) at the time of transplantation. We adjusted for the following independent variables that relate to the transplant candidate: age at wait-list registration, black race, gender, dialysis while on the wait list, diabetes as cause of ESRD, blood type and elevated peak panel reactive antibody (PRA). Elevated PRA was defined as a peak PRA > 20 (a binary variable defined as high PRA/not) (14).
Secondary cohort
The secondary cohort included adult recipients of kidney transplants from deceased donors during the period from 7/1/2004 through 7/1/2006, regardless of when the recipients were added to the wait list. The outcomes were death-censored allograft failure, mortality and a combined endpoint of allograft failure or mortality. The secondary cohort was assembled in order to have the opportunity for longer follow-up time after transplantation than was possible in the primary cohort. Exclusion criteria included missing data on allograft or patient survival, and kidney transplantation using a hepatitis C and/or hepatitis B infected donor. We also did not analyze outcomes for the small proportion of recipients (1.8% of total) who underwent transplantation with kidneys that met criteria for multiple categories of organ type (such as CDC-ECD).
Multivariable Cox regression analysis was used to fit the models for the three outcomes. On the basis of published literature and clinical plausibility, we identified independent variables that would require adjustment in examining the relationship of kidney transplant donor category to allograft failure or death (1,15,16). Recipient variables included age, gender, race, diabetes, history of dialysis, prior kidney transplant, hepatitis C serostatus, multi-organ transplant (a binary variable defined as multi-organ/not), days on the wait list, antibody induction therapy (a binary variable defined as any induction/no induction), high peak PRA and initial immunosuppression regimen (regimen was analyzed as an indicator variable with three classifications: calcineurin inhibitor free regimen, regimen with tacrolimus or regimen with cyclosporine). Allograft variables included cold ischemia time, antigen mismatch (a binary variable: zero mismatch/not) and share type. Share type refers to the geographical relationship between the OPO that provides the organ and the center that accepts it. Share type was categorized as local/nonlocal. Donor variables included gender and race. Donor characteristics used to define ECD status—age, cause of death, history of hypertension and serum creatinine—were not included in the multivariable analysis.
Missing data
Some patients had missing data on cold ischemia time (n = 2561 or 12.7% of the secondary cohort). For these individuals, we imputed the mean value of cold ischemia time in the secondary cohort. Some patients also had missing data on peak PRA (n = 872 or 4.3% of the secondary cohort). Peak PRA was defined as a binary variable (high PRA/not); we analyzed these individuals as if they did not have a high peak PRA. Results were similar when the analyses were repeated without imputation of missing data.
Analysis of procured kidneys
We also obtained data on deceased donor kidneys procured in the United States. We compared the proportion of SCD, CDC, DCD and ECD kidneys that were discarded between 7/1/2004 and 7/1/2006 using the chi-square test. We excluded kidneys from donors who had positive serologic testing for hepatitis C or hepatitis B, as well as kidneys from donors who met criteria for multiple categories of organ type (such as ECD-CDC).
To assess whether the widely publicized transmission of HIV and HCV to donors in 2007 had an effect on discard rates, we also compared the discard rate of procured CDC kidneys during the period from 7/1/2004 to 7/1/2006 to CDC kidney discard rates in 2008.
Results
Primary cohort
The primary cohort included 48 054 adults added to the wait list for a kidney transplant. In this cohort, 29 214 (60.8%) individuals remained on the wait list until the end of the study, while 7016 (14.6%) received SCD transplants, 671 (1.4%) received CDC transplants, 1936 (4.0%) received ECD transplants and 794 (1.7%) received DCD transplants. The following groups were censored at the time of transplantation: 7779 (16.2%) live donor transplant recipients, 372 (0.8%) recipients of kidneys from HCV positive donors, 12 (0.03%) recipients of kidneys from HBV positive donors, 114 (0.2%) recipients of ECD-DCD kidneys, 69 (0.1%) recipients of ECD-CDC kidneys, 75 (0.2%) recipients of DCD-CDC kidneys and 2 (0.004%) recipients of ECD-DCD-CDC kidneys. Median follow-up time after addition to the wait list in this cohort was 920 days and median follow-up time after deceased donor kidney transplantation was 392 days.
Individuals who remained on the wait list were less likely to be white (42.7% of wait-listed patients versus 53.7% of SCD recipients, 50.8% of CDC recipients, 53.7% of ECD recipients and 54.9% of DCD recipients, p < 0.01). Individuals remaining on the wait list were more likely to have an elevated peak PRA (28.9% of wait-listed patients, versus 18.5% of SCD recipients, 18.0% of CDC recipients, 11.5% of ECD recipients and 15.2% of DCD recipients, p < 0.01.) Additionally, individuals remaining on the wait list were more likely to be blood group O (52.6% of waitlisted patients, versus 38.8% of SCD recipients, 42.5% of CDC recipients, 40.4% of ECD recipients and 34.3% of DCD recipients (Table 2).
Table 2.
Clinical and demographic characteristics of adults newly added to the wait list for kidney transplantation (primary cohort)1
| Wait list, no transplant2 (n = 29214) | SCD2 kidney recipient (n = 7016) | CDC2 kidney recipient (n = 671) | ECD2 kidney recipient (n = 1936) | DCD2 kidney recipient (n = 794) | p-Value | |
|---|---|---|---|---|---|---|
| Mean age in yrs (s.e) | 50.6 (0.1) | 50.6 (0.2) | 50.8 (0.5) | 59.6 (0.2) | 50.8 (0.5) | <0.01 |
| Male gender (%) | 17 380 (59.5) | 4226 (60.2) | 431 (64.2) | 1206 (62.3) | 500 (63.0) | <0.01 |
| Race (%) | <0.01 | |||||
| White | 12 475 (42.7) | 3769 (53.7) | 341 (50.8) | 1039 (53.7) | 436 (54.9) | |
| Black | 9439 (32.3) | 1769 (25.2) | 175 (26.1) | 502 (25.9) | 255 (32.1) | |
| Hispanic | 4804 (16.4) | 1031 (14.7) | 93 (13.9) | 221 (11.4) | 52 (6.6) | |
| Asian | 1856 (6.4) | 319 (4.6) | 47 (7.0) | 145 (7.5) | 39 (4.9) | |
| Other | 640 (2.2) | 128 (1.8) | 15 (2.2) | 29 (1.5) | 12 (1.5) | |
| Dialysis (%) | 22 766 (77.9) | 5429 (77.4) | 479 (71.4) | 1518 (78.4) | 638 (80.4) | 0.01 |
| Hepatitis C seropositive (%)3 | N/A | 396 (5.7) | 48 (7.2) | 81 (4.2) | 33 (4.2) | <0.01 |
| Median days on the wait list | 889 | 419 | 383 | 453 | 545 | <0.01 |
| Diabetes | 13 063 (44.7) | 2453 (35.0) | 241 (35.9) | 872 (45.0) | 298 (37.5) | <0.01 |
| Median percent peak PRA | 2 | 0 | 0 | 0 | 0 | <0.01 |
| High PRA (%)4 | 8453 (28.9) | 1299 (18.5) | 121 (18.0) | 222 (11.5) | 121 (15.2) | <0.01 |
| Blood group (%) | <0.01 | |||||
| A | 8431 (28.9) | 2976 (42.4) | 246 (36.7) | 834 (43.1) | 382 (48.1) | |
| B | 4617 (15.8) | 783 (11.2) | 99 (14.8) | 200 (10.3) | 85 (10.7) | |
| AB | 808 (2.8) | 538 (7.7) | 41 (6.1) | 119 (6.2) | 55 (6.9) | |
| O | 15 358 (52.6) | 2719 (38.8) | 285 (42.5) | 783 (40.4) | 272 (34.3) |
Wait list: These individuals were added to the wait list but did not receive a transplant by the end of follow-up; SCD = standard criteria donor; CDC = Centers for Disease Control designation of donor as increased risk for viral infection; ECD = extended criteria donor; DCD = donation after cardiac death.
Recipients of kidneys from live donors, recipients of kidneys from deceased donors seropositive for hepatitis C and recipients of kidneys from deceased donors that met criteria for ECD-DCD, ECD-CDC, DCD-CDC or ECD-DCD-CDC were not included in the table; these recipients were censored at the time of transplantation.
OPTN does not collect data on hepatitis C status of individuals on the wait list.
Defined as peak PRA >20%.
CDC kidneys were more likely to be from male donors (75.1% of CDC allografts, compared to 61.0% of SCDs, 48.0% of ECDs and 67.5% of DCD donors, p < 0.01) and black donors (16.2% of CDC allografts, compared to 12.6% of SCDs, 10.6% of ECDs and 3.3% of DCD donors, p < 0.01) (Table 3).
Table 3.
Characteristics of deceased donor allografts classified as standard criteria, Centers for Disease Control increased risk, extended criteria and donation after cardiac death1
| SCD2 (n = 7016) | CDC2 (n = 671) | ECD2 (n = 1936) | DCD2 (n = 794) | p-Value | |
|---|---|---|---|---|---|
| Donor age (s.e.) | 33.9 (0.2) | 32.2 (0.4) | 60.5 (0.2) | 36.7 (0.5) | <0.01 |
| Donor male gender (%) | 4283 (61.0) | 504 (75.1) | 930 (48.0) | 536 (67.5) | <0.01 |
| Donor race (%) | <0.01 | ||||
| White | 4869 (69.4) | 461 (68.7) | 1478 (76.3) | 725 (91.3) | |
| Black | 887 (12.6) | 109 (16.2) | 205 (10.6) | 26 (3.3) | |
| Hispanic | 1035 (14.8) | 91 (13.6) | 184 (9.5) | 38 (4.8) | |
| Asian | 132 (1.9) | 5 (0.8) | 60 (3.1) | 5 (0.6) | |
| Other | 93 (1.3) | 5 (0.8) | 9 (0.5) | 0 (0.0) | |
| Cold ischemia (s.e.) | 18.1 (0.1) | 17.7 (0.4) | 19.1 (0.2) | 18.9 (0.3) | <0.01 |
This group of allografts relates to the primary cohort of patients added to the kidney transplant wait list from 7/1/2004 through 7/1/2006.
SCD = standard criteria donor; CDC = Centers for Disease Control designation of donor as increased risk for viral infection; ECD = extended criteria donor; DCD = donation after cardiac death.
Primary cohort outcomes
A total of 4846 (16.6%) individuals who remained on the wait list died, compared to 393 (5.6%) SCD kidney recipients, 199 (10.3%) ECD recipients, 37 (5.5%) CDC recipients and 49 (6.2%) DCD recipients.
Compared to ‘standard therapy’ of remaining on the wait list unless or until an SCD kidney became available, recipients of CDC kidneys had no significant difference in mortality rate (HR 0.80, CI 0.58 –1.11, p = 0.18) using multivariable Cox regression. DCD recipients also had no significant difference in mortality compared with standard therapy (HR 1.02, CI 0.77–1.36, p = 0.88), whereas ECD kidney recipients had increased mortality (HR 1.31, CI 1.13–1.51, p < 0.01) (Table 4).
Table 4.
Multivariable Cox regression analysis of mortality for adults newly added to the wait list for kidney transplantation (primary cohort)1
| Characteristic | Hazard ratio | Confidence interval | p-Value |
|---|---|---|---|
| Standard therapy2 | Reference | Reference | Reference |
| CDC kidney recipient2 | 0.80 | 0.58–1.11 | 0.18 |
| ECD kidney recipient2 | 1.31 | 1.13–1.51 | <0.01 |
| DCD kidney recipient2 | 1.02 | 0.77–1.36 | 0.88 |
The analysis was adjusted for these independent variables (all relate to the transplant candidate): age at wait-list registration, black race, gender, dialysis while on the wait list, diabetes as cause of ESRD, peak PRA and blood type.
Standard therapy represents receiving a standard criteria kidney or remaining on the wait list. ECD = extended criteria donor; CDC = Centers for Disease Control designation of donor as increased risk for viral infection; DCD = donation after cardiac death.
Secondary cohort
A total of 19 872 adult kidney transplant recipients were included in the secondary analysis. Among these recipients, SCD kidney recipients comprised 14 175 (71.3%), CDC recipients comprised 1447 (7.3%), ECD recipients comprised 2971 (15.0%) and DCD recipients comprised 1279 (6.4%). We excluded from the secondary analysis the additional 143 individuals who received kidneys from ECD-DCD donors, the 113 individuals who received kidneys from ECD-CDC donors, 108 who received kidneys from DCD-CDC donors and 7 who received kidneys from ECD-CDC-DCD donors.
Outcomes in the secondary cohort
Median follow-up time after kidney transplantation in the secondary cohort was 1066 days. In unadjusted analysis, death-censored allograft failure occurred among 8.3% of SCD recipients (reference), compared with 7.5% of CDC kidney recipients (p = 0.32), 13.3% of ECD recipients (p < 0.01) and 10.4% of DCD recipients (p = 0.01). In unadjusted analysis, 10.1% of SCD recipients died (reference), compared with 9.0% of CDC recipients (0.19), 18.2% of ECD recipients (p < 0.01) and 10.6% of DCD recipients (p = 0.52). Figure 1 displays the combined endpoint of death and/or allograft failure by deceased donor type using the Kaplan–Meier estimation method.
Figure 1. Unadjusted allograft failure or death after kidney transplantation, by donor type.
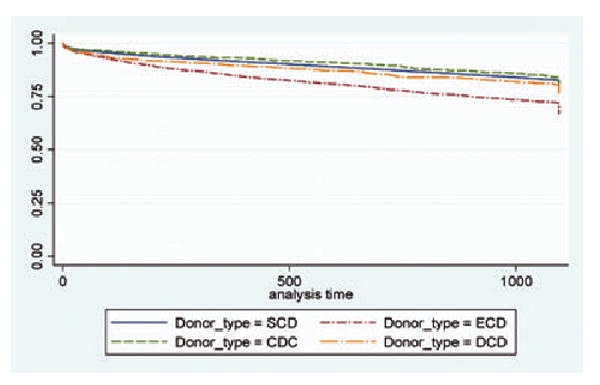
Secondary cohort of kidney transplant recipients from 7/1/2004 to 7/1/2006.
In multivariable Cox regression, compared to SCD kidney recipients, CDC recipients had no significant difference in the rate of death (HR 0.86, CI 0.72–1.03, p = 0.10), death-censored allograft failure (HR 0.91, CI 0.74–1.11, p = 0.36) or a combined endpoint of death and/or allograft failure (HR 0.91, CI 0.80–1.04, p = 0.16) (Table 5).
Table 5.
Multivariable Cox regression analyses of death, allograft failure, or both after kidney transplantation (secondary cohort)1
| Recipient type | Death | Death-censored allograft failure | Allograft failure or death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | HR | CI | p | |
| SCD kidney recipient2 (n = 14 175) | Reference | Reference | Reference | ||||||
| CDC kidney recipient2 (n = 1447) | 0.86 | 0.72–1.03 | 0.10 | 0.91 | 0.74–1.11 | 0.36 | 0.91 | 0.80–1.04 | 0.16 |
| ECD kidney recipient2(n = 2971) | 1.34 | 1.24–1.54 | <0.01 | 1.76 | 1.54–2.00 | <0.01 | 1.48 | 1.36–1.61 | <0.01 |
| DCD kidney recipient2(n = 1279) | 1.10 | 0.92–1.32 | 0.28 | 1.27 | 1.05–1.54 | 0.01 | 1.14 | 1.00– 1.30 | 0.05 |
Independent variables included recipient age, gender, race, diabetes, history of dialysis, prior kidney transplant, multi-organ transplant, days on the wait list, antibody induction therapy, peak PRA and immunosuppression regimen. Allograft variables included cold ischemia, antigen mismatch and share type. Donor variables included gender and race. Patients in the secondary cohort were kidney transplant recipients between 7/1/2004 and 7/1/2006.
SCD = standard criteria donor; CDC = Centers for Disease Control donor; ECD = extended criteria donor; DCD = donation after cardiac death.
Center use of CDC kidneys
In the secondary cohort, CDC donor kidneys were used by 180 renal transplant centers (75% of 240 centers that performed adult renal transplantation during the study period). Among centers that used any CDC donor kidneys, the median number of kidney transplants from a CDC donor was 8 (range 1–105). Among centers performing >10 kidney transplants during the study period, the median proportion of CDC transplants/total transplants was 7.2% (range 1.1– 35.6%).
We divided centers into three tertiles of kidney transplant volume. We created the center volume tertiles by dividing the number of patients undergoing kidney transplantation during the study period into three approximately equal groups. Centers in the lowest volume tertile performed <82 mean kidney transplants per year, while centers in the intermediate volume tertile performed ≥ 83 and <147 mean kidney transplants per year, and the highest volume centers performed >147 mean kidney transplants per year.
Centers in the highest volume tertile used a significantly greater proportion of CDC donor kidneys (9.3% of all kidneys), compared to intermediate (6.2%) and lowest volume centers (6.0%) (p < 0.01).
Analysis of kidney discards by donor type
During the study period, 25 406 kidneys were procured that met inclusion criteria and 3645 (14.3%) were discarded. The difference in the proportion of discards between SCD donor kidneys (6.8%) and CDC donor kidneys (7.8%) did not reach statistical significance (p = 0.13). Compared to SCD donor kidneys, ECD kidneys (39.8%, p < 0.01) and DCD kidneys (14.7%, p < 0.01) were much more likely to be discarded (Table 6).
Table 6.
Discards of procured deceased donor kidneys, by organ type1
| Kidney type | Number discarded | % | p-Value |
|---|---|---|---|
| SCD kidney2 (n = 16 782) | 1147 | 6.8 | Reference |
| CDC kidney2 (n = 1686) | 132 | 7.8 | 0.13 |
| ECD kidney2 (n = 5374) | 2136 | 39.8 | <0.01 |
| DCD kidney2 (n = 1564) | 230 | 14.7 | <0.01 |
Discards are counted per kidney procured from 7/1/2004 through 7/1/2006.
SCD = standard criteria donor; CDC = Centers for Disease Control designation of donor as increased risk for viral infection; ECD = extended criteria donor; DCD = donation after cardiac death.
CDC kidney discards in two time periods
The CDC kidney discard rate in 2008 (6.7%) was not significantly different than in the earlier period from 7/1/2004 to 7/1/2006 (7.8%) (p = 0.35).
Discussion
The high mortality for ESRD patients and the long wait list for a kidney transplant have created an imperative to make optimal use of good quality organs. Although transplanting organs from CDC donors has raised controversy (11,17), this study demonstrates that CDC kidneys already represent a substantial proportion of the donor pool, and that recipients of these organs have similar short-term allograft survival compared to SCD recipients. Additionally, short-term mortality for CDC recipients was comparable to standard therapy of receiving an SCD kidney or remaining on the wait list. We found that CDC donors provided a similar proportion of kidneys as DCD donors, and CDC kidneys were accepted by the majority of transplant centers. Taken together, these data suggest that centers are using CDC donor kidneys as an important source of kidney allografts. Because our study lacks information about viral transmission outcomes for recipients and long-term mortality data, further studies will be necessary to fully characterize the possible risks and benefits of these kidneys.
The finding that patient mortality from the time of registration for the kidney transplant wait list was not statistically different for recipients of CDC kidneys compared to standard therapy suggests the value of considering CDC organs for kidney transplant candidates. For a wait-listed individual who considers an offer of any non-SCD kidney, the decision involves accepting an organ now versus the uncertainty of waiting for an offer of a different kidney with unknown characteristics (or dying before an acceptable offer comes). As others have noted, all transplants involve risks of different kinds and remaining on the wait list is associated with a high mortality rate (18). Our analysis suggests that in the short term, transplant candidates willing to consider a CDC organ will not have increased mortality if a CDC organ is offered. Choosing to accept CDC kidneys also provides transplant candidates with a way to shorten their time on the wait list.
In considering the use of CDC organs, it is also useful to draw attention to published studies about ECD kidney transplantation. Prior analyses have identified specific groups, such as elderly and diabetic individuals, who are particularly likely to derive benefit from accepting ECD kidneys due to increased mortality rates while on the wait list (1,2). Patients with a prolonged anticipated wait time due to region, blood type and/or sensitization may also benefit from accepting a non-SCD kidney (19). Our results suggest that transplant professionals caring for these patients may want to consider CDC organs as well as ECD organs for patients likely to suffer poor health outcomes while on the wait list. Notably, the increased mortality for ECD recipients in our study is likely related to the short duration of follow-up (approximately 1 year) for transplant recipients in the primary cohort. A prior study of ECD kidney transplantation by Merion et al., for instance, reported that the time to equal cumulative mortality for ECD recipients versus standard therapy was 3.5 years after transplantation (1). We were not able to analyze a longer duration of follow-up from wait list using this dataset because OPTN began collecting data on CDC donor designation in July 2004.
The analysis of our secondary cohort demonstrated that CDC designation was not associated with an increased likelihood of short-term allograft failure or death. Given that the CDC designation was developed to predict a small increased risk of viral infection among donors, the finding that CDC donor status was not associated with worse allograft survival may not be surprising. On the other hand, the CDC health behaviors could have been associated with other health conditions, with implications for allograft outcomes. For instance, intravenous drug users might be exposed to bacteremia or other systemic toxins that could affect renal parenchyma. Prisoners or sex workers might have undiagnosed or untreated health conditions such as hypertension that could also affect organ quality (20–22). Our results should allay concerns that risky health behaviors or characteristics that may be more common among CDC donors would impact allograft survival among recipients of these organs.
We also found that use of CDC kidneys was common, but varied widely across centers. In our secondary cohort, CDC donors provided 7.3% of all transplanted kidneys, and these kidneys were used by 75% of renal transplant centers in the United States. The center-specific proportion of kidneys from CDC donors ranged from 0.8 to 34.2%. The causes of this variation are uncertain and deserve further examination. This variation could be explained by differential willingness of OPOs to pursue CDC donors, or by differential willingness of transplant centers or transplant candidates to accept CDC donor organs. We found that higher volume transplant centers used a higher proportion of CDC donor kidneys compared to other centers. We hypothesize that higher center volume is associated with more aggressive use of non-SCD organs, including CDC kidneys.
Notably, Kurcika et al. recently demonstrated wide variation in the proportion of OPO donor volume from CDC donors (23). Their study showed that variation in OPO volume of CDC donors appeared to have little correlation with the prevalence of acquired immunodeficiency syndrome (AIDS) in the donor service area. These findings suggest the possibility that some OPOs and transplant centers could increase deceased donor transplantation overall through greater efforts to procure CDC organs. The use of nucleic acid testing for viral detection, which has greater sensitivity for diagnosis during the window period after infection, might also provide reassurance to centers and transplant candidates about the use of CDC organs (23).
The possible opportunity presented by CDC donor kidneys depends on the number of potential CDC donors whose kidneys are not currently procured as well as kidney discard rates. We found that the CDC discard rate was not significantly greater than the SCD discard rate, suggesting that CDC donor status is not currently a major barrier to kidney use. Given negative publicity and lawsuits following the HIV and hepatitis C transmission from an organ donor to four recipients in 2007 (11,17), it is important to note that the discard rate of CDC donor kidneys did not rise in 2008. These findings suggest that efforts to decrease CDC kidney discard rates are unlikely to meaningfully increase the organ supply. It is possible that transplantation using CDC kidneys could grow if OPOs developed a greater willingness to procure CDC kidneys, but the size of the pool of nonprocured CDC organs is unknown.
Our analysis has substantial limitations that must be acknowledged. Recipients of kidneys from CDC and other donors may have acquired HIV, hepatitis C or hepatitis B infections through transplantation that were not diagnosed and/or reported to OPTN. Studies that focus specifically on recipient viral outcomes will be essential to illuminate the relative risks associated with these organs. Another limitation is that our dataset lacks specification about which health behavior(s) for any donor led to CDC designation. For instance, the risk of HIV infection for an active intravenous drug user is likely higher than the risk for a prisoner (6), but our dataset cannot distinguish outcomes between groups.
Our analyses are also limited by short-term outcomes. Longer-term follow-up studies are needed to determine whether CDC and SCD kidney recipients continue to have similar mortality and allograft survival. Lastly, analyses of registry data are susceptible to residual confounding despite multivariable adjustment. For example, important recipient health metrics such as functional status and demographic attributes such as socioeconomic status are not adequately captured in the OPTN dataset.
Conclusion
Short-term follow-up revealed that CDC kidney recipients had similar allograft survival compared to SCD recipients. In addition, short-term mortality for CDC recipients was comparable to standard therapy of receiving an SCD kidney or remaining on the wait list. Given the high mortality associated with remaining on the wait list, kidney transplant candidates may benefit from considering the acceptance of CDC kidneys. Center use of CDC kidneys in the United States varied widely. This variation suggests that transplantation of CDC kidneys could be increased at centers with low usage. OPTN should collect detailed data about long-term outcomes and recipient viral testing so the potential risks and benefits of CDC kidneys can be fully evaluated.
Acknowledgments
The authors acknowledge Stacey Doll for her helpful suggestions for the study. Dr. Reese is supported by NIH grant K23 - DK078688-01. Dr. Feldman is supported by NIH grant K24 - DK002651. Dr. Halpern is supported by a Greenwall Foundation Faculty Scholar Award in Bioethics.
UNOS Disclaimer: This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
References
- 1.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol. 2006;1:532–538. doi: 10.2215/CJN.01130905. [DOI] [PubMed] [Google Scholar]
- 3.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: Implications for allocation and preservation. Am J Transplant. 2007;7:1797–1807. doi: 10.1111/j.1600-6143.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 4.Ho KJ, Owens CD, Johnson SR, et al. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation. 2008;85:1588–1594. doi: 10.1097/TP.0b013e318170b6bb. [DOI] [PubMed] [Google Scholar]
- 5.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am J Transplant. 2007;7:2769–2774. doi: 10.1111/j.1600-6143.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer EJ, Perencevich EN, Philosophe B, Bartlett ST. Estimated benefits of transplantation of kidneys from donors at increased risk for HIV or hepatitis C infection. Am J Transplant. 2007;7:1515–1525. doi: 10.1111/j.1600-6143.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for preventing transmission of human immunodeficiency virus through transplantation of human tissue and organs. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1994;43:1–17. [PubMed] [Google Scholar]
- 8.Fishman JA, Greenwald MA, Kuehnert MJ. Enhancing transplant safety: A new era in the microbiologic evaluation of organ donors? Am J Transplant. 2007;7:2652–2654. doi: 10.1111/j.1600-6143.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 9.Fishman JA. Informing candidates for transplantation about donor risk factors. N Engl J Med. 2008;359:1182. doi: 10.1056/NEJMc081540. author reply 1183. [DOI] [PubMed] [Google Scholar]
- 10.Zou S, Dodd RY, Stramer SL, Strong DM. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751–759. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 11.Grady D. Four transplant recipients contract HIV. New York Times; Nov 13, 2007. [Google Scholar]
- 12.USRDS. Reference table: Patient survival. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [updated 2008; cited]; Available from: http://www.usrds.org/adr.htm. [Google Scholar]
- 13.Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139–144. doi: 10.1111/j.1600-6143.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 14.Szczech LA, Berlin JA, Feldman HI. The effect of antilymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Anti-Lymphocyte Antibody Induction Therapy Study Group. Ann Intern Med. 1998;128:817–826. doi: 10.7326/0003-4819-128-10-199805150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 16.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589–597. doi: 10.1681/ASN.V123589. [DOI] [PubMed] [Google Scholar]
- 17.CBS. Chicago organ recipients infected with HIV. 2007 November 13; Published: http://wcco.com/national/HIV.organ.transplant.2.566338.html.
- 18.Halpern SD, Shaked A, Hasz RD, Caplan AL. Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med. 2008;358:2832–2837. doi: 10.1056/NEJMsb0800674. [DOI] [PubMed] [Google Scholar]
- 19.Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008;52:553–586. doi: 10.1053/j.ajkd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Baillargeon J, Black SA, Pulvino J, Dunn K. The disease profile of Texas prison inmates. Ann Epidemiol. 2000;10:74–80. doi: 10.1016/s1047-2797(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist CH, Lindquist CA. Health behind bars: Utilization and evaluation of medical care among jail inmates. J Community Health. 1999;24:285–303. doi: 10.1023/a:1018794305843. [DOI] [PubMed] [Google Scholar]
- 22.Martin RE, Gold F, Murphy W, Remple V, Berkowitz J, Money D. Drug use and risk of bloodborne infections: A survey of female prisoners in British Columbia. Can J Public Health. 2005;96:97–101. doi: 10.1007/BF03403669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucirka LM, Alexander C, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Viral nucleic acid testing (NAT) and OPO-level disposition of high-risk donor organs. Am J Transplant. 2009;9:620–628. doi: 10.1111/j.1600-6143.2008.02522.x. [DOI] [PubMed] [Google Scholar]


