Abstract
Obesity increases colorectal cancer (CRC) risk and progression. However, the impact of obesity on CRC in women is dependent on ovarian hormone status. The purpose of this study was to determine the interactive roles of obesity and ovarian hormones on serum markers of inflammation, cell signaling and transplanted colon tumor growth. Female C57BL/6 mice (6 weeks) were either ovariectomized (OVX) or ovaries left intact (NOVX) and randomized to receive a 1) control, 2) 30% calorie-restricted (CR), or 3) diet-induced obese (DIO) diet regimen for 20 weeks to induce differing levels of adiposity. Serum was collected and inflammatory and metabolic markers were measured using an antibody array (62 proteins) and ELISAs. Mice were subcutaneously injected with syngeneic MC38 colon cancer cells after 20 weeks and sacrificed 4 weeks later. CR mice had the smallest tumors irrespective of hormone status, whereas the largest tumors were observed in DIO-OVX mice. Glucose tolerance was impaired in ovariectomized mice, being most severe in the DIO-OVX group. Cytokine arrays suggested that in CR animals, inhibition of tumor growth paralleled insulin sensitivity and associated changes in leptin, adiponectin, and IGF-BPs. Conversely, in DIO-OVX animals, tumor growth was associated with insulin and leptin resistance as well as higher levels of pro-inflammatory proteins. In vitro, leptin and adiponectin had no effect, whereas insulin induced MC38 cell proliferation and MAPK activation. Co-treatment with estrogen blocked the stimulatory effects of insulin. Thus, our in vitro and in vivo data indicate female reproductive hormones have a modulating effect on obesity-induced insulin resistance and inflammation, which may directly or indirectly influence CRC progression.
INTRODUCTION
Obesity has risen dramatically over the past 25 years in the United States and more recently in developing countries [1,2]. Excess adiposity, especially in the abdominal area is associated with a number of chronic diseases including certain cancers [3,4]. Among these, colorectal cancer (CRC) is the fourth most common cancer in the U.S. and second leading cause of cancer related deaths [5]. Several epidemiological studies have demonstrated that obesity increases the risk of and mortality from CRC in males [6-8]. The relationship in females is somewhat inconsistent, in part due to methods used to assess obesity as well as to the protective effect that reproductive hormones have on CRC [6,9-11]. More recent data suggests that excess abdominal adiposity is associated with elevated risk in women [11,12]. In postmenopausal women however, this effect may be limited to individuals not currently using hormone replacement therapy (HRT) [11]. These studies indicate that a women’s risk of colon cancer are affected by hormonal status, the location of excess adipose tissue, and/or a combination of the two factors.
The protective effect of HRT on colon cancer has been reported in several epidemiological studies [9,13,14]. Despite these findings, the mechanisms linking estrogen and/or progestins to reduced cancer risk have not been fully elucidated. It has been suggested that estrogen may exert anti-cancer effects by reducing secondary bile acid production [15], enhancing Vitamin D receptor expression [16] as well as through direct, receptor-mediated effects in the colon mucosa [17-19]. There are two types of estrogen receptors (ER), ERα and ERβ and both are expressed in normal colon [20,21] ERβ is more predominately expressed than ERα, and appears to have an important role in maintaining epithelial kinetics, suggesting this isoform may protect against CRC [19,22]. In support of this, ER-β receptor is down-regulated in colon tumors [20,21,23,24] and inversely related to tumor differentiation [19,25].
Hormone replacement therapy also has beneficial effects on glucose homeostasis and adiposity [26]. Estrogen influences adipose tissue deposition and improves insulin sensitivity, presumably through an ER-α dependent mechanism [26-28]. In humans, the decline in circulating sex hormones during menopause is associated with an increase in visceral fat and a higher prevalence of insulin resistance and type 2 diabetes [29,30]. Hyperinsulinemia is an important metabolic abnormality linking obesity to CRC [31]. Colon epithelial cells possess insulin, insulin like growth factor (IGF)-1 and IGF-2 receptors [32,33], which are present at greater levels in tumors compared to normal colonic epithelium [34]. Insulin and IGF-1 are mitogenic to colon cancer cells in vitro [35,36], and case-control and cohort studies consistently demonstrate a positive association between colon cancer and/or colonic polyps with elevated levels of insulin [37-40].
Adipose tissue is a key regulator of insulin resistance [41] and contributes to systemic inflammation through production of a variety of proteins, hormones and cytokines referred to collectively as “adipokines”. These adipokines possess broad biological activities, including homeostatic and pathologic functions. Many secretory products of adipocytes, including tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), C-reactive protein, adiponectin, complement factors, and leptin, all serve dual roles in energy homeostasis and the immune response [42]. IL-6 signaling, in particular, supports numerous specific local functions [43-45]. An increase in visceral adiposity is associated with increased release of several pro-inflammatory adipokines [41], whereas adiponectin levels decline. Adipokines are thought to contribute to peripheral insulin resistance [46-48] and some have been associated with an increased risk of CRC [49-51], suggesting that they may be involved directly, through receptor mediated signaling, or indirectly through effects on glucose homeostasis, to one or more stages in the carcinogenic process.
In a previous study, Yakar et al. [52] demonstrated enhanced colon tumor growth in female ovariectomized mice fed a high fat diet. The purpose of this study was to further differentiate the interactive roles of obesity and ovarian hormone status on serum markers of inflammation in a mouse xenograph model of colon tumor growth. Female C57BL/6 mice were either ovariectomized (OVX) or had their ovaries left intact (NOVX) and fed one of three diets to induce varying levels of adiposity. We found that the DIO-OVX mice had the largest tumors and CR mice the smallest tumors compared to mice on the control diet independent of ovarian hormone status. Data from cytokine arrays, ELISAs, and glucose tolerance tests suggested that obesity-associated levels of metabolic hormones as well as pro-inflammatory mediators in the serum may be modulating effects of transplanted MC38 tumor growth in vivo. In vitro, insulin stimulated whereas estrogen inhibited MC38 proliferation. These changes were associated with activation of the MAPK p42/44 and MEK. These data indicate that estrogen modulates the growth stimulatory effects of insulin and imply that insulin resistance associated with obesity may adversely affect one or more processes involved in CRC, especially in post-menopausal women.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted. Recombinant murine proteins were purchased from R&D Systems unless otherwise noted (Minneapolis, MN). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Animals and diets
Female C57/BL6 mice, 6-weeks-old were purchased from Charles River Laboratories (National Cancer Institute, Frederick, MD) and diets from Research Diets, Inc. (New Brunswick, NJ). To determine the effects of sex steroids on obesity and tumor growth, both non-ovariectomized (NOVX) as well as ovariectomized (OVX) animals were used. For practical issues of space, manpower, and sufficient tissue availability, two identical blocks of mice (first block, n=10; second block; n=15) were included for NOVX mice. Only one block of 15 was included for OVX mice. Beginning at 6 weeks of age, mice were randomized to receive one of three diets: 1) a control diet (#D12450B: 29% protein, 57% carbohydrate and 14% fat) fed ad libitum, to generate overweight phenotype; 2) a calorie restricted diet (70% kilocalories of control group; CR; #D0302702: 20% protein, 70% carbohydrate and 10% fat) administered as a daily aliquot that results in a lean phenotype; and 3) a high fat diet, (#D12492: 20% protein, 20% carbohydrate and 60% fat) to generate an obese phenotype. The CR diet is supplemented to achieve 100% of essential nutrients necessary for normal growth and development (vitamins, minerals, essential fatty acids, and amino acids). Diets were purchased from Research Diets, Inc. (New Brunswick, NJ, USA) and the diet composition was previously published [53]. All diets were designed to provide similar amounts of micronutrients but variable amounts of calories. Animals were singly housed in temperature and humidity controlled rooms and administered diet for a total of 24 weeks. During that time, animals were weighed weekly and food consumption was recorded throughout the study. All procedures were conducted in accordance with the guidelines of the National Cancer Institute Animal Care and Use Committee.
Body Composition
Mice were scanned using a GE Lunar Piximus II dual-energy X-ray absorptometer to determine body fat and lean muscle mass.
Glucose and Insulin Tolerance Test
A glucose tolerance test (GTT) was conducted after 19 weeks to measure glucose regulation in the lean, overweight, and obese animals. Animals (n=20 for NOXV groups; n=15 for OVX) were fasted overnight (12 hours) and the GTT was performed by intraperitoneal (i.p.) injection of 20% glucose (2g/kg) to mice. Blood was sampled from the tail vein and glucose was measured over a 2-hour time course using a Glucometer Elite (Bayer, Elkhart, IN).
Cytokine Antibody Array and ELISAs
After 10 weeks on dietary treatment, blood samples were drawn from the retroorbital venous plexus of anaesthetized mice. Serum from three mice in each treatment group was pooled into one sample due to sample volume limitations (n=4 for each treatment group). Serum was then diluted 1:10 and probed for cytokine profile using the RayBio® Mouse Cytokine Antibody Array 3.1 kit according to the manufacturer’s instructions (RayBiotech®; Norcross, GA). Briefly, membranes were blocked with a blocking buffer, and then 2 ml of pooled serum sample was individually added and incubated at 4°C overnight. Membranes were washed; primary biotin-conjugated antibody was added and incubated at room temperature for 2 hr. The membranes were then incubated with horseradish peroxidase-conjugated streptavidin at room temperature and cytokine presence was detected by chemiluminescence. Films of array dots were scanned with a densitometer and converted to densitometric units using Quantity One® software (Bio-Rad Laboratories; Hercules, CA) per the manufacturer’s instruction. Data were analyzed according to recommendations from RayBiotech. Briefly, autoradiography films were digitized and circles were measured using QuantityOne® software. Data were imported into an Excel® spreadsheet and normalized against a control across membranes, and final values were calculated using the RayBio® Murine Cytokine 3.1 Analysis Tool.
Serum adiponectin, leptin, and insulin were also measured at 10 weeks (n=15/group for NOVX; n=8/group for OVX animals). Adiponectin was measured using ELISA (R&D Systems; Minneapolis, MN) with serum diluted 1:7000 according to manufacturers’ instructions. The plate was read at 450 nm wavelength using a Synergy HT plate reader (Bio-Tek; Winooski, VT). Serum leptin and insulin were assayed using Multiplex Assays according to the manufacturer’s instructions and analyzed on a Bioplex 200 using Bioplex Manager 4.1 software.
Cells and Cell Culture Conditions
The murine carcinoma-38 (MC38) colon cancer cell line was derived from a murine colon tumor, grade III carcinoma, which was chemically induced in the C57Bl/6 female mouse [54]. This cell line was cultured in DMEM (Gibco; Rockville, MD) supplemented with 10% fetal bovine serum (Gibco; Rockville, MD) and 1% penicillin/streptomycin at 37°C with 5% CO2 [55].
Tumor Cell Injection and Measurement
After 20 weeks of dietary treatment (26 weeks of age), mice were injected subcutaneously on the flank with 5 × 104 mouse colon 38 (MC38) cells (n=25/group for NOVX; n=15/group for OVX mice). Mice were palpated 3 times a week and tumor size was measured with Vermeer™ calipers. All mice were euthanized after 4 weeks, when detectable tumors from animals reached approximately 2.0 cm in diameter.
Cell Proliferation Assay
MC38 cells were grown in 96-well plates as described above. Briefly, approximately 1,500 cells/well were seeded in 96-well plates (Corning Costar; City, State). Cells were treated (eight wells per treatment) with leptin (0.0, 0.1, 1 or 50 ng/mL; R & D Systems), insulin (0.001, 0.01, 1, 10 or 100 μg/mL; Sigma), full length adiponectin (1 0.001, 0.01, 0.1, 1, or 10μg/mL; Bio Vendor), and estrogen (0.01, 0.05, 0.1, 10, 50 or 100 μM; Sigma). Cell proliferation was measured after 24 hr of treatment using the commercial CelTiter96 Aqueous kit according to manufacturer’s instructions (Promega; Madison, WI). Briefly, 20 μl/well of CellTiter96 Aqueous One solution reagent was added to the 96-well plate containing the cells in 100 μl of culture media and incubated for 1 hr at 37°C in 5% CO2. Upon completion of the assay procedure the plate was read at 490 nm using the Synergy HT plate reader (Bio-Tek, Winooski, VT).
Western Blotting
Briefly, cells were washed twice with cold PBS and total cell lysate was harvested by scraping cells into 1 ml of cold lysis buffer (30 mM Tris pH 7.2, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanidate, 1% NP-40, and 10% glycerol) per flask. The cell suspension was then sonicated to insure cell lysis and centrifuged at 4°C for 15 min at 14,000 rpm. Nuclear and cytoplasmic fractions were collected using the NE-PER® kit according to the manufacturer’s instructions (Pierce Biotechnology Inc.; Rockford, IL). Protein content of the samples was determined by BCA assay (Bio-Rad Laboratories, Hercules, CA), and samples were loaded on an equal protein basis of approximately 20 μg/lane. Samples were subjected to SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with primary antibodies against insulin receptor-α, insulin receptor-β, leptin receptor (Ob-R), estrogen receptor-α, estrogen receptor-β (Santa Cruz Biotechnology Inc., Santa Cruz; CA) or phospho-specific pairs p-AMPK, p-ERK, p-MEK, p-insulin receptor, p-Akt (Cell Signaling Technology; Beverly, MA) with shaking overnight at 4°C. Incubation with the primary antibody was followed by appropriate infrared-labeled second antibodies and detected using the Odyssey Infrared Imaging System (LI-COR Biosciences; Lincoln NE).
Samples, for both cell types, for either the receptor or signaling experiments were loaded on the same gel. They were processed as a whole for all subsequent steps for optimal comparison. Densitometric analysis represents the signal mean ± SE for the two repetitions. Blots shown are from one experiment representative of the two.
Statistical Analysis
Data for body weight and composition, tumor size, and serum glucose, insulin, adiponectin, and leptin levels were analyzed with analysis of variance (ANOVA) using Prism software (Graph Pad; San Diego, CA). Prior to analyses, normal distribution of the data was tested and when appropriate, data were transformed prior to statistical analysis. When statistical differences were detected, individual comparisons were made using Bonferroni’s multiple comparison test. For glucose tolerance tests, the incremental area under the curve for glucose (AUC) was calculated and treatment differences in areas were analyzed using ANOVA.
Response value of cytokine array were analyzed using a linear model where the mean expression level of each gene was independently modeled as a function of a combination of ovary status (OVX or NOVX) and dietary status (control, DIO, CR). The models and permutation analysis used were analogous to those previously fit to log-intensity data in microarray experiments [56]. A set of linear contrast were used to test several hypothesis of interest. For example simple effects of dietary status were tested within each ovary status. Multiple test correction was applied using the false discovery rate and q-value <0.05 was used as significance criteria [57]. Additionally, hierarchical cluster analysis of samples and genes were performed and represented using a heatmap plot. All computations were performed in R through the bioconductor suite [58].
Cell proliferation and cell proliferation inhibition data were assessed statistically by comparing treated cell proliferation to control cell proliferation within each cell type. The experiments were repeated three times and data shown are from one of the experiments representative of all three. The data shown is the mean ± SEM within one representative experiment. Differences in proliferation were compared using ANOVA. Pair-wise differences were compared using Tukey’s multiple comparisons test. The Prism software package (Graph Pad; San Diego, CA) was utilized for this analysis.
RESULTS
The effect of diet on body weight in non-ovariectomized and ovariectomized C57BL/6 mice
The average body weight and body composition of NOVX and OVX mice are shown in Table 1. Dietary treatment effectively generated three phenotypes, differing primarily in the proportion of weight as adipose tissue. In both NOVX and OVX mice, CR animals gained the least whereas DIO-fed animals gained the most body fat after 20 weeks when compared to controls. Ovariectomy significantly influenced body weight and adipose tissue accumulation, with DIO-OVX mice weighing significantly more than their NOVX counterparts and having significantly more adipose tissue (P<0.05). Comparably, body weights of CR animals were less affected by ovarian hormone status. The difference in body weight between NOVX and OVX animals was not likely due to energy consumption, as total energy intake did not differ between animals on the same dietary regime (Table 1).
Table 1.
Chatics of non-ovariectomizmized (NOVX) and ovariectomized (OVX) mice after 20 weeks on treatment diets (mean ± SEM).
| Dieta | Hormone Statusa | Average kcal/dayb | Body weight (g)b | Body Composition (% Fat)b | Tumor Size (mm2)bc |
|---|---|---|---|---|---|
| Control | NOVX | 11.8 ± 0.248 | 27.1 ± 0.593 | 33.5 ± 2.11 | 220 ± 21.1 |
| CR | NOVX | 8.22 ± 0.179* | 20.9 ± 0.268* | 24.4 ± 1.18* | 92.8 ± 11.5* |
| DIO | NOVX | 13.8 ± 0.171* | 36.4 ± 1.12* | 54.5 ± 3.72* | 180 ± 16.5 |
| Control | OVX | 11.3 ± 0.191 | 31.0 ± 0.677+ | 45.6 ± 1.60+ | 252 ± 23.0 |
| CR | OVX | 7.89 ± 0.128* | 21.5 ± 0.334* | 28.9 ± 1.94* | 143 ± 25.0* |
| DIO | OVX | 13.6 ± 0.173* | 45.4 ± 1.22*+ | 62.2 ± 1.69* | 292 ± 37.2+ |
Abbreviations: CR, calorie restriction; DIO, diet-induced obesity; NOVX, non-ovariectomized; OVX, ovariectomized
Animals per group: Food intake (n=13); body weight (NOVX n=25; OVX n=15); body composition (NOVX n=25; OVX n=15); tumor size (NOVX n=25; OVX n=15).
Tumor volume measured 4 weeks post-inoculation with MC38 murine colon carcinoma cells (24 weeks on diet).
Indicates significant difference of diet compared to either NOVX or OVX controls (P<0.05);
indicates significant difference between ovariectomized compared to non-ovariectomized animals within the specific dietary treatment.
Diet-induced adiposity and hormone status differentially effect tumor growth
Results of dietary treatment and ovarian hormone status on MC38 tumor growth in vivo is presented in Table 1. Calorie restriction demonstrated an inhibitory effect on tumor growth compared to control and DIO mice independent of ovarian hormone status (P<0.05). In NOVX mice, there was no further difference in tumor size among dietary treatments. In ovariectomized animals, there was an increase tumor size with higher adiposity, in DIO-OVX mice (P<0.05).
The effect of diet on insulin resistance in non-ovariectomized and ovariectomized mice
Glucose metabolism was affected by dietary treatment as well as hormone status (Figure 1A,B). Non-ovariectomized, obese mice demonstrated impaired glucose tolerance beginning at 30 min post ip injection (Figure 1A). Effects on glucose tolerance between CR, control, and DIO mice were more pronounced in the OVX group (Figure 1B). Differences between all three groups peaked at 60 minutes and remained present at 120 min post injection.
Figure 1.
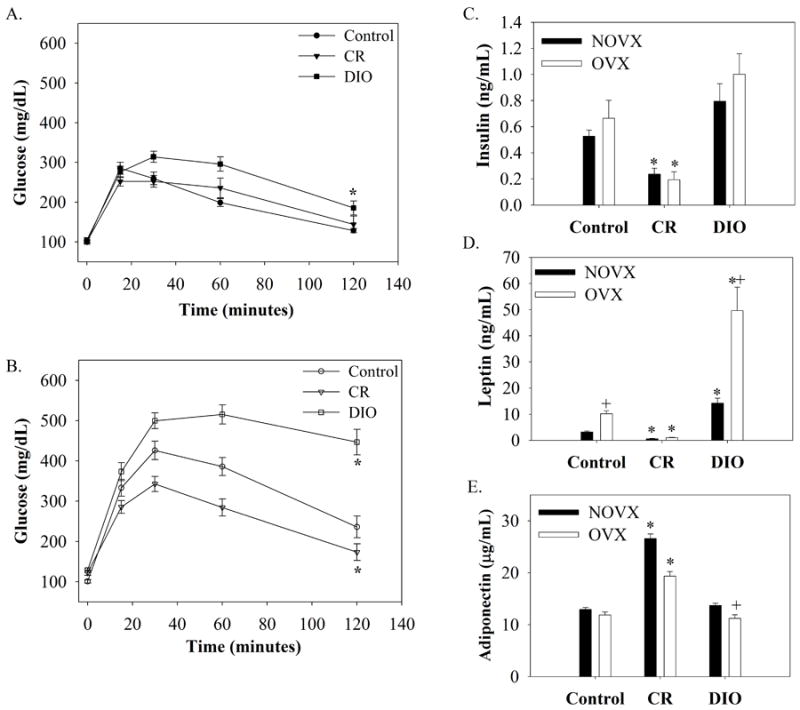
Glucose tolerance test in (A) non-ovariectomized (NOVX) and (B) ovariectomized (OVX) female mice fed a control, 30% calorie restricted (CR), or high fat (DIO) diet for 19 weeks (n=20/group for NOVX animals; n=15/group for OVX animals). Animals were fasted overnight and then given an i.p injection of 20% glucose (2g/kg). Blood samples were taken from the tail vein, and glucose was measured over a 2 hour period using a glucometer. The incremental area under the curves (AUC) for glucose were calculated, and asterisks (*) represent significant differences in mean AUC between diets (P<0.05). The AUC means ± SEM are as follows: CON-NOVX, 33427±2061; CR-NOVX, 18783±1755; DIO-NOVX, 24516±1402; CON-OVX, 33427±2061; CR-OVX, 24539±1694; DIO-OVX, 48038±2239). Changes in metabolic hormones in NOVX and OVX mice after 10 weeks on dietary treatments as detected by ELISA (n=15/group for NOVX; n=8/group for OVX animals). (C) Insulin, (D) Leptin, and (E) Adiponectin. *, Significant dietary differences compared to OVX or NOVX control; +, Significant estrogen*diet interaction (P<0.05).
The effect of diet on metabolic hormones after 20 weeks on diet
Differences in metabolic hormones were also assessed by ELISAs and results are presented in Figure 1. Fasting insulin levels were higher in DIO and lower in CR mice compared to controls irrespective of estrogen status (Fig 1C). Leptin levels followed a similar trend, however DIO-OVX mice had levels ~4 fold higher than in DIO-NOVX mice, suggesting leptin resistance (Fig. 1D). Adiponectin concentration significantly increased with caloric restriction (Fig. 1E).
Diet- and ovarian hormone-induced changes in serum inflammatory markers using cytokine arrays and ELISAs
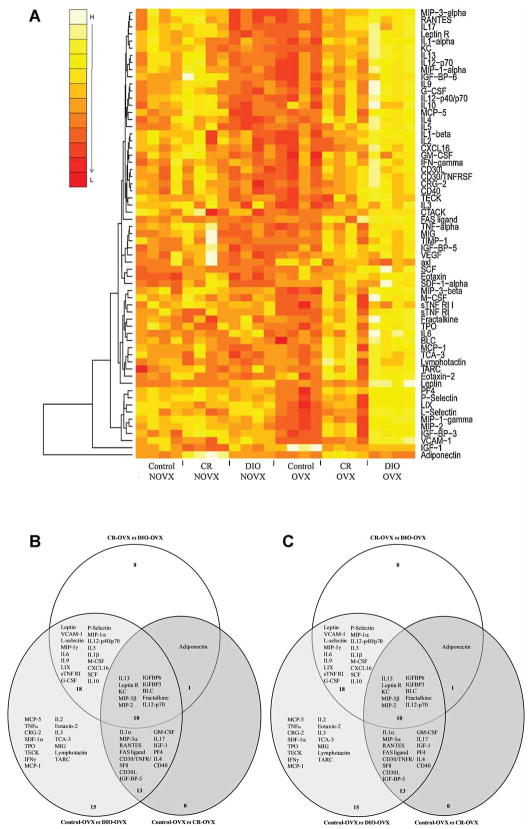
Serum from mice in each treatment group was exposed to cytokine antibody arrays. Of the 62 proteins present on the arrays, 61 were detectable in serum samples. A heat map image displaying changes in intensity values between groups relative to NOVX or OVX controls is shown in Figure 2. The hierarchical clustering method used to order samples (columns) of the heatmap was blind to treatment. Nevertheless, samples were ordered perfectly according to the estrogen-dietary group to which they belonged (Fig. 2A). This suggests that within group variability was considerable smaller than between group variability and indicates the existence of a group-specific expression patterns as confirmed by subsequent ANOVA. In general, dietary differences were more pronounced in the OVX group, with obese, ovariectomized animals exhibiting higher expression of a variety of proteins compared to controls (Fig. 2). In OVX mice, 57 proteins were significantly altered between diets, 39 of which were unique to loss of estrogen (data not shown). In comparison, few changes were observed among NOVX mice, with only 19 proteins altered between dietary treatments and 1 of them (eotaxin) specific to the presence of estrogen (data not shown).
Figure 2.
(A) Heat map plots of diet- and estrogen-dependent cytokine levels in the serum of mice after 10 weeks on dietary treatment constructed from Raybiotech cytokine antibody arrays (n=4 per group). (B-C) Venn diagrams showing those proteins significantly altered by estrogen/diet interaction were constructed. Briefly, statistical analyses to identify proteins altered by diet in the NON-OVX group were performed comparing CRvsDIO, CONvsCR and CONvsDIO (B). In addition, statistical analyses to identify proteins altered by diet in the OXV were performed comparing OVXCRvsOVXDIO, OVXCONvsOVXCR and OVXCONvsOVXDIO (C). Venn diagrams were constructed to visually depict those proteins that were significantly different in each individual comparison and then shared across the three comparisons (P<0.05). Each large circle represents the proteins significantly altered in the individual comparison. The overlapping regions are those proteins that were commonly altered in the other comparison(s). Note that directionality of the change cannot be depicted in this comparison. Please refer to the heatmap for the direction of the change.
Venn diagrams depicting specific dietary changes within NOVX and OVX mice are presented in Figure 2. Proteins of particular interest were those that displayed differential expression between dietary treatments and that paralleled tumor data. In non-ovariectomized mice, adiponectin, MCP-5, and IGFBPs 3, 5, and 6 were generally higher in CR mice than control or DIO groups; whereas leptin and IGF-1 were lower in CR mice and increased with increasing adiposity (Fig. 2A,B). In ovariectomized animals, adiponectin was the lowest and leptin the highest in DIO mice, whereas the opposite trend was observed for CR mice (Fig. 2A,C). The only other proteins that followed a specific pattern corresponding to tumorigenesis were those elevated in DIO mice compared to either control-fed or CR animals. Some of these proteins include adhesion molecules (VCAM-1, P-Selectin, L-selectin), chemokines (MCP-5; MIP-1α; CXCL16), and cytokines (IL-1β, IL-2,3,9, TNFα).
In vitro proliferation studies using MC38 tumor cells
MC38 colon tumor cells were treated with leptin, insulin, or adiponectin to understand which hormones altered in the serum elicited tumor proliferation in vitro. First we verified that the receptor protein was present by western blot for the leptin receptor (Ob-R), insulin receptor, adiponectin receptor 1 and 2 and ERα and β (data not shown). ObR was not confirmed by western blot but the other receptors were (data not shown). Consistent with lack of detectable levels of ObR, treatment of cells with leptin treatment did not influence cell number at any dose tested (Fig. 3A). Insulin induced cell proliferation at 1, 10 and 100 μg/ml (P≤0.01, Fig. 3B). Adiponectin had no effect on cell number at any dose tested (Fig. 3C). We also tested the hypothesis that adiponectin may reduce cell number in response to insulin. However, co-treatment of insulin at 1 or 10 μg/ml with 1 or 10 μg/ml adiponectin had no effect on the insulin induced cell proliferation (Fig. 3D).
Figure 3.
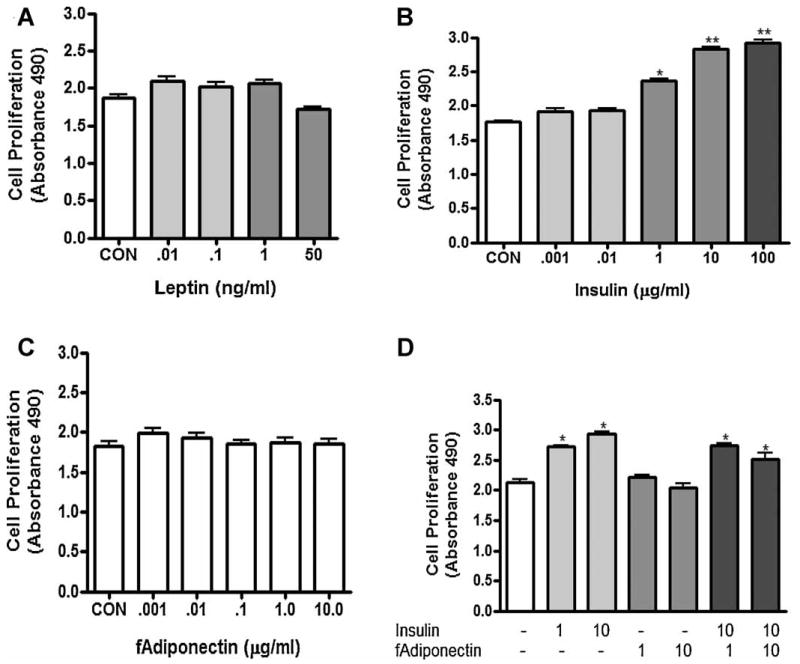
(A) The effect of leptin on MC38 cell proliferation. Cells were treated with leptin from 0.01 to 50 ng/ml for 48 hr. (B) The effect of insulin on MC38 cell proliferation. Cells were treated with insulin from 0.001 to 100 μg/ml for 48 hr. (C) The effect of full length adiponectin (f adipo) on MC38 cell proliferation. Cells were treated with fadipo from 0.001 to 10 ng/ml for 48 hr. (D) The effect of co-treatment of insulin and full length adiponectin (10 μg/ml) on MC38 cell proliferation. Cells were treated with 1 or 10 μg/ml insulin and/or 1 or 10 μg/ml full length adiponectin (fadipo). Con, Serum Free Control. * = P<0.01 (compared to control); ** = P<0.001 (compared to control).
Then we hypothesized that estrogen treatment may mediate the proliferative response to insulin. Estrogen treatment alone reduced cell number at 10, 50, and 100 μM (P≤0.05) (Fig. 4A). Estrogen co-treatment with insulin reduced insulin-induced (1μg/ml) cell proliferation at 50 and 100 μM estrogen (P≤0.05) (Fig. 4B). We hypothesized that the co-treatment of insulin, estrogen, and adiponectin would further suppress the insulin induced cell proliferation. However, the co-treatment of insulin, estrogen and adiponectin to mimic the CR NOVX mouse did not further decrease cell proliferation induced by insulin (Fig. 4C).
Figure 4.

(A) The effect of estrogen on MC38 cell proliferation. Cells were treated with estrogen from 0.01 to 100 μM for 48 hr. (B) The effect of cotreatment with estrogen (0.1, 10, 50 or 100 μM) and insulin (1 μg/ml) on MC38 cell proliferation for 48 hr. (C) The effect of cotreatment with insulin/estrogen/fadiponectin (fadipo). Cells were treated with insulin (1 μg/ml) insulin and/or fadipo (1 μg/ml) and/or estrogen (100 μM). SF, serum free control; CM, media with 10% serum; INS, insulin at 1 μg/ml.* = P<0.01 (compared to control); ** = P<0.001 (compared to control).
Insulin and estrogen modulate ERK and MEK phosphorylation in MC38 cells
We next queried which cell signaling pathways might be responsible for the increased cell proliferation induced by insulin and then attenuated by estrogen. We treated cells with insulin at 1 μg/ml and the insulin + estrogen at 50 μM. Insulin induced a peak increase in phosphorylation of ERK1/2 and MEK1/2 at 5 minutes post treatment that was reduced by co-treatment with estrogen by 50% (Fig. 5A-B). Insulin did not induce phosphorylation of AMPK or p38, while Akt and STAT3 phosphorylation was induced by insulin, however there was no time dependence or alteration with estrogen treatment (Figure 5A).
Figure 5.
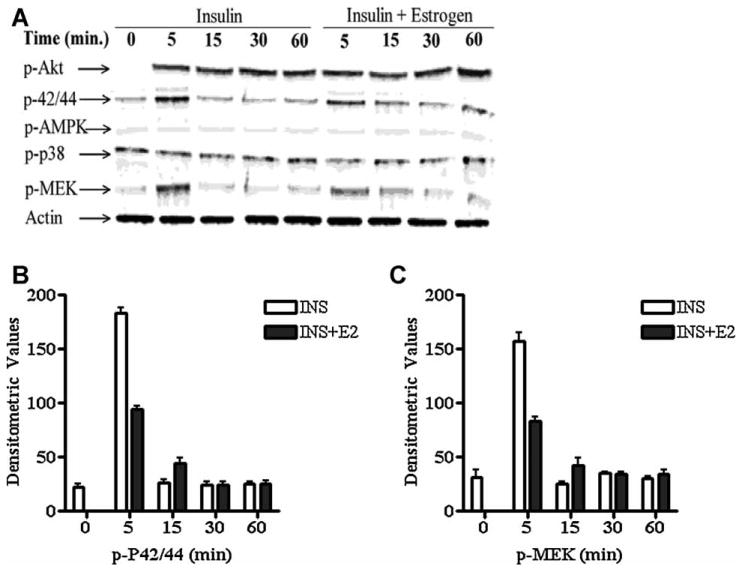
(A) Insulin treatment (1μg/ml) or co-treatment with estrogen (100 μM) of MC38 cells and phosphorylation (activation) of ERK, MEK 1/2, p38, Akt, and AMPK and Actin loading control. (B) Total protein control for pair matched ERK, MEK 1/2, p38, Akt and AMPK. Cells were incubated 6 hr in serum-free medium prior to exposure. At the indicated times post treatment, total cell lysates were collected and western-blot analysis was performed. Blots shown are from one representative experiment of two. (C) Densitometric analysis of p-ERK and (D) p-MEK.
DISCUSSION
The purpose of this study was to determine the interactive roles of obesity and female hormone status on serum markers of inflammation and colon tumor growth. Mice were ovariectomized or ovaries left intact to model pre- and postmenopausal status. Mice were then placed on one of three diets to induce differing levels of adiposity. Serum was utilized from and proteins examined at a single time point with antibody arrays and ELISAs. Using this mouse model, we sought to further differentiate how obesity and endogenous and/or exogenous sex hormones may interact to influence colon cancer cell growth in vivo.
We found that both diet as well as ovariectomy influenced adipose tissue deposition. CR mice had the least and DIO mice had the most adipose tissue compared to controls. Loss of ovarian hormones resulted in a further increase in body fat in control-fed and DIO mice compared to non-ovariectomized animals despite similar energy intakes. This finding is consistent with previous studies using ERα knockout [27,28], aromatase deficient [59,60], and ovariectomized animals [61,62]. Xenograph tumor growth in vivo was also influenced by dietary treatment and hormone status. Calorie restricted animals, irrespective of hormone status had the smallest overall tumor growth compared to controls whereas obese, ovariectomized mice had the largest tumors. Comparably, there was no further increase in tumor size in NOVX-DIO mice, indicating that ovariectomy potentiates tumorigenesis in obese animals.
We next assessed changes in metabolic and inflammatory parameters in the serum from mice. Glucose tolerance was impaired in all mice consuming the DIO diet. However, ovariectomized animals fed either a control or DIO diet had even further impairments in glucose homeostasis. Data from cytokine arrays and ELISAs indicated unique patterns associated with diet and hormone status. In CR-NOVX animals, adiponectin, MCP-5, and IGFBPs 3, 5, and 6 were generally higher than in control or DIO groups; whereas leptin, insulin, and IGF-1 were lower. Ovariectomized, obese animals exhibited higher levels of insulin and leptin, chemokines (MIP-1α, CXCL16), cytokines (IL-1β, IL-2, IL-3, IL-9), and adhesion proteins (P-selectin, L-selectin), in addition to lower serum adiponectin. Based on these observations, we hypothesized that tumor growth was inhibited by adiponectin and stimulated during leptin and/or insulin resistance.
The association of adiponectin, leptin, and/or insulin to CRC has been evaluated in several studies. Adiponectin may influence cancer risk through its well-recognized effects on insulin sensitivity [63]. However, adiponectin may also act on tumor cells directly. Low serum adiponectin is associated with several cancers including colon [49-51], prostate [64], breast [65], endometrial [66] and gastric cancer [67]. In addition, serum adiponectin levels are negatively associated with histological grade and disease stage [64,65]. Serum leptin levels correlate with body fat indices in humans [68], however no consistent association with leptin has been observed in individuals with CRC [69,70]. Leptin receptors are present in normal and colon cancer tissue [71] and treatment of rodents with leptin in vivo or with colon cancer cells in vitro stimulates cell proliferation [71]. Aside from direct receptor-mediated effects, a more recent study indicates that leptin may influence carcinogenesis by stimulating cells to secrete growth factors that induce angiogenesis [72]. The association of insulin with CRC has been documented in several studies [73]. Epidemiology consistently demonstrates a positive association between colon cancer and/or colonic polyps with elevated levels of insulin. Additionally, hyperinsulinemia has been associated with aggressiveness of tumors as diabetics have a higher mortality from CRC [74,75] as well as risk of more advanced colon tumors compared to non-diabetics [75].
Because levels of these adipokines were differentially expressed in our study in vivo, we examined whether treatment of MC38 tumor cells would influence cell proliferation and cell signaling pathways of MC38 tumor cells in vitro. MC38 tumor cells were first cultured with leptin, insulin or adiponectin to determine the effect of each of these adipokines on MC38 cell proliferation. Insulin significantly concentrations increased, whereas leptin did not influence cell proliferation of MC38 cells. Adiponectin also did not influence cell proliferation at any dose tested. We had expected a significant decrease in cell proliferation based on observations in the literature.
We then hypothesized that in the face of a stimulus (insulin) that adiponectin would reduce cell proliferation. However, adiponectin had no effect on insulin-induced cell proliferation directly. This ruled out a direct role for adiponectin on MC38 cell proliferation in vitro. These data indicated that hyperinsulinemia was likely the primary influence on MC38 cell proliferation in our in vivo tumor model. Next we wanted to mimic the influence of estrogen on the tumor cells. We first treated the cells with estrogen and found that treatment reduced cell proliferation of MC38 cells at 10-100 μM. These data were consistent from a previous study using the same cell line [76]. MC38 cells were then stimulated with insulin were then co-treated with estrogen to determine if estrogen can reduce insulin induced cell proliferation. Estrogen was able to reduce insulin-stimulated cell proliferation by approximately 50%. We further examined downstream signaling pathways influenced by insulin in this study and found that insulin treatment activated both pAKT as well as the MEK-MAP kinase pathway. Although pAKT regulates diverse cellular functions (survival, cell cycle, and metabolism) and is often activated in a number of cancers [77], estrogen treatment did not influence this pathway at the time points examined. However, insulin-induced phosphorylation of both ERK and MEK were attenuated by estrogen, which likely mitigated some of the growth-stimulatory pathways induced by insulin.
An emerging issue in the area of energy balance and cancer is the relative effects of nature versus nurture (ie, the contributions of systemic factors [78] [which have been the focus of this paper] in the context of cell autonomous effects). The recent observations by Kalaany et al that cancer cells with constitutively activated PI3K mutations are proliferative in vitro in the absence of insulin or IGF-1 and that they form calorie restriction-resistant tumors in vivo illustrate this issue. These findings suggest that cell autonomous alterations, such as certain types of activating PI3K mutations, may influence the response of cells to energy balance–related host factors, additionally illustrating the complexity of the relationships between energy balance, host factors, and cancer progression [79].
Results from our in vitro studies suggest that the late stage MC38 tumor cells are not responsive to growth directly by leptin or adiponectin. Although adiponectin receptors were detected in this cell line, leptin receptors were below detectable levels, consistent with lack of response. This indicated that tumor growth in our model may be primarily influenced by the insulin resistant state. Mice lacking estrogen (OVX) with high body fat (DIO) had increased available insulin and glucose likely contributing to tumor growth. Caloric restriction protected mice with and without estrogen from insulin resistance, suggesting there may be an indirect effect of adiponectin on tumor growth. Therefore, it is likely that minimal free insulin or glucose is available as a substrate for tumors consistent with the smallest size in these groups.
While the interaction of adiponectin, estrogen and insulin resistance is unclear, estrogen appears to improve insulin sensitivity either directly or through negative regulation of adipose tissue deposition. Adiponectin concentrations were not affected either by estrogen treatment or ovariectomy in women [80]. In addition, in human adipocytes, expression and secretion of adiponectin was unaffected by sex steroid treatment [81]. However, insulin resistance is higher in postmenopausal women than in premenopausal women [82]. Additionally HRT improves metabolic markers of insulin resistance and visceral adiposity in post-menopausal women [83]. In mice, estrogen treatment exerts anti-diabetic and anti-obesity effects by lowering lipogenic genes in white adipose tissue as well as by suppressing hepatic glucose output [84]. These observations consistent with our data indicate that estrogen and/or reproductive status may improve insulin sensitivity in a more indirect manner, potentially by regulating energy balance.
Findings from this study are consistent with a recent epidemiological study indicating that HRT may confer protection against CRC in obese, post-menopausal women [11]. Our in vitro and in vivo data indicate female reproductive hormones exert a modulating effect on the insulin resistant state associated with obesity in mice. In addition, evidence is provided for a direct effect of estrogen on tumor growth by dampening cell proliferation induced by insulin signaling. Estrogen mediated cell signaling events may provide cross-talk, directly or indirectly, to mitigate/modulate insulin-insulin receptor-initiated signal transduction cascades. Putative estrogen mediated signaling through plasma membrane and nuclear estrogen receptors may block insulin receptor-mediated kinases and activation of transcriptional targets. The finding of elevated levels of some pro-inflammatory proteins in DIO-OVX animals was not further evaluated in this study but is worth future consideration. Although we focused on overall metabolic patterns across different groups, specific elevation of one or more inflammatory proteins may also have influenced tumor growth in OVX-DIO mice either directly or through immunomodulatory mechanisms. Given the lack of data supporting the association of adipokine, cytokine and chemokine patterns with specific anthropomorphic patterns and associated cancer risk, this study provides valuable prospective evidence in female mice that specific adipokiness are associated with transplanted tumor growth.
Acknowledgments
Research supported in part by NIEHS Grant P30 ES007784 and the Michigan Agriculture Experiment Station.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Obesity and overweight fact sheet. World Health Organization; 2006. [Google Scholar]
- 3.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Facts and Figures. American Cancer Society; 2009. [Google Scholar]
- 6.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13(31):4199–4206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 9.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106(5):574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar C, Marjoribanks J, Lethaby A, Suckling JA, Lamberts Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2009;2:CD004143. doi: 10.1002/14651858.CD004143.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98(13):920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 12.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: A meta-analysis of cohort studies. World Journal of Gastroenterology. 2007;13(31):4199–4206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy - Scientific review. Jama-Journal of the American Medical Association. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 15.McMichael AJ, Potter JD. Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis. J Natl Cancer Inst. 1980;65(6):1201–1207. [PubMed] [Google Scholar]
- 16.Smirnoff P, Liel Y, Gnainsky J, Shany S, Schwartz B. The protective effect of estrogen against chemically induced murine colon carcinogenesis is associated with decreased CpG island methylation and increased mRNA and protein expression of the colonic vitamin D receptor. Oncol Res. 1999;11(6):255–264. [PubMed] [Google Scholar]
- 17.Wolf LA, Terry PD, Potter JD, Bostick RM. Do factors related to endogenous and exogenous estrogens modify the relationship between obesity and risk of colorectal adenomas in women? Cancer Epidemiol Biomarkers Prev. 2007;16(4):676–683. doi: 10.1158/1055-9965.EPI-06-0883. [DOI] [PubMed] [Google Scholar]
- 18.Kim SE, Shim KN, Jung SA, Yoo K, Moon IH. An association between obesity and the prevalence of colonic adenoma according to age and gender. J Gastroenterol. 2007;42(8):616–623. doi: 10.1007/s00535-007-2074-4. [DOI] [PubMed] [Google Scholar]
- 19.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9(4):385–391. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 20.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61(2):632–640. [PubMed] [Google Scholar]
- 21.Nussler NC, Reinbacher K, Shanny N, et al. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend Med. 2008;5(3):209–217. doi: 10.1016/j.genm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Wada-Hiraike O, Imamov O, Hiraike H, et al. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103(8):2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maseide K, Kandel RA, Bell RS, et al. Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin Cancer Res. 2004;10(13):4464–4471. doi: 10.1158/1078-0432.CCR-03-0541. [DOI] [PubMed] [Google Scholar]
- 24.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60(2):245–248. [PubMed] [Google Scholar]
- 25.Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ER beta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. European Journal of Cancer. 2003;39(9):1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 26.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens Protect against High-Fat Diet-Induced Insulin Resistance and Glucose Intolerance in Mice. Endocrinology. 2009;150(5):2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 27.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 29.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55(5):950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 30.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836–842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 32.Heinz-Erian P, Kessler U, Funk B, Gais P, Kiess W. Identification and in situ localization of the insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptor in the rat gastrointestinal tract: comparison with the IGF-I receptor. Endocrinology. 1991;129(4):1769–1778. doi: 10.1210/endo-129-4-1769. [DOI] [PubMed] [Google Scholar]
- 33.Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL, Pommier GJ. Insulin-like growth factor-I protects colon cancer cells from death factor-induced apoptosis by potentiating tumor necrosis factor alpha-induced mitogen-activated protein kinase and nuclear factor kappaB signaling pathways. Cancer Res. 2000;60(7):2007–2017. [PubMed] [Google Scholar]
- 34.Hakam A, Yeatman TJ, Lu L, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30(10):1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 35.Fenton JI, Hord NG, Lavigne JA, Perkins SN, Hursting SD. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1646–1652. doi: 10.1158/1055-9965.EPI-04-0916. [DOI] [PubMed] [Google Scholar]
- 36.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80(1):51–58. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bird CL, Ingles SA, Frankl HD, Lee ER, Longnecker MP, Haile RW. Serum lipids and adenomas of the left colon and rectum. Cancer Epidemiol Biomarkers Prev. 1996;5(8):607–612. [PubMed] [Google Scholar]
- 38.Yamada K, Araki S, Tamura M, et al. Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol. 1998;27(5):794–798. doi: 10.1093/ije/27.5.794. [DOI] [PubMed] [Google Scholar]
- 39.Komninou D, Ayonote A, Richie JP, Jr, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003;228(4):396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 40.Pais R, Silaghi H, Silaghi AC, Rusu ML, Dumitrascu DL. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol. 2009;15(41):5141–5148. doi: 10.3748/wjg.15.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144(6):2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 42.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19(4):547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Wang XP, Schunck M, Kallen KJ, et al. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123(1):124–131. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- 44.Giraud AS, Jackson C, Menheniott TR, Judd L. Role of trefoil peptides and IL-6 cytokine family signaling in gastric homeostasis. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00382.2006. [DOI] [PubMed] [Google Scholar]
- 45.Steensberg A. The role of IL-6 in exercise-induced immune changes and metabolism. Exerc Immunol Rev. 2003;9:40–47. [PubMed] [Google Scholar]
- 46.Gnacinska M, Malgorzewicz S, Stojek M, Lysiak-Szydlowska W, Sworczak K. Role of adipokines in complications related to obesity: a review. Adv Med Sci. 2009;54(2):150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–633. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 48.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14(11-12):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otake S, Takeda H, Fujishima S, et al. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastroenterol. 2010;16(10):1252–1257. doi: 10.3748/wjg.v16.i10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11(10):3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 51.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97(22):1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 52.Yakar S, Nunez NP, Pennisi P, et al. Increased Tumor Growth in Mice with Diet-Induced Obesity: Impact of Ovarian Hormones. Endocrinology. 2006 doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 53.Nunez NP, Perkins SN, Smith NC, et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60(4):534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 54.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35(9):2434–2439. [PubMed] [Google Scholar]
- 55.Pajtasz-Piasecka E, Szyda A, Rossowska J, et al. Loss of tumorigenicity of murine colon carcinoma MC38/0 cell line after transduction with a retroviral vector carrying murine IL-12 genes. Folia Biol (Praha) 2004;50(1):7–14. doi: 10.14712/fb2004050010007. [DOI] [PubMed] [Google Scholar]
- 56.Cui XG, Hwang JTG, Qiu J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics. 2005;6(1):59–75. doi: 10.1093/biostatistics/kxh018. [DOI] [PubMed] [Google Scholar]
- 57.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 58.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5(10) doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144(4):1474–1480. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- 60.Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Mol Cell Endocrinol. 2002;193(1-2):7–12. doi: 10.1016/s0303-7207(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 61.Kamei Y, Suzuki M, Miyazaki H, et al. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol (Tokyo) 2005;51(2):110–117. doi: 10.3177/jnsv.51.110. [DOI] [PubMed] [Google Scholar]
- 62.Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91(1):258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65(6):1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 65.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 66.Dal Maso L, Augustin LS, Karalis A, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89(3):1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11(2 Pt 1):466–472. [PubMed] [Google Scholar]
- 68.Ruhl CE, Harris TB, Ding JZ, et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. American Journal of Clinical Nutrition. 2007;85(4):1121–1126. doi: 10.1093/ajcn/85.4.1121. [DOI] [PubMed] [Google Scholar]
- 69.Stattin P, Palmqvist R, Soderberg S, et al. Plasma leptin and colorectal cancer risk: A prospective study in Northern Sweden. Oncology Reports. 2003;10(6):2015–2021. [PubMed] [Google Scholar]
- 70.Cong JC, Dai XW, Shen MY, et al. Expression of obesity hormone leptin in human colorectal cancer. Chinese Journal of Cancer Research. 2009;21(2):142–146. [Google Scholar]
- 71.Hardwick JCH, Van den Brink GR, Offerhaus GJ, Van Deventer SJH, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121(1):79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 72.Birmingham JM, Busik JV, Hansen-Smith FM, Fenton JI. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis. 2009;30(4):690–697. doi: 10.1093/carcin/bgp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114(1):63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 74.Trevisan M, Liu J, Muti P, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiology Biomarkers & Prevention. 2001;10(9):937–941. [PubMed] [Google Scholar]
- 75.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: A case-control study. Digestive Diseases and Sciences. 2008;53(9):2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 76.Motylewska E, Lawnicka H, Melen-Mucha G. Oestradiol and tamoxifen inhibit murine Colon 38 cancer growth and increase the cytotoxic effect of fluorouracil. Endokrynol Pol. 2007;58(5):426–434. [PubMed] [Google Scholar]
- 77.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther. 2007;6(8):2139–2148. doi: 10.1158/1535-7163.MCT-07-0120. [DOI] [PubMed] [Google Scholar]
- 78.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chalvatzas N, Dafopoulos K, Kosmas G, Kallitsaris A, Pournaras S, Messinis IE. Effect of ovarian hormones on serum adiponectin and resistin concentrations. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 81.Horenburg S, Fischer-Posovszky P, Debatin KM, Wabitsch M. Influence of sex hormones on adiponectin expression in human adipocytes. Horm Metab Res. 2008;40(11):779–786. doi: 10.1055/s-0028-1083780. [DOI] [PubMed] [Google Scholar]
- 82.Leung KC, Xu A, Craig ME, Martin A, Lam KS, O’Sullivan AJ. Adiponectin isoform distribution in women--relationship to female sex steroids and insulin sensitivity. Metabolism. 2009;58(2):239–245. doi: 10.1016/j.metabol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 83.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 84.Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]