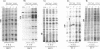Abstract
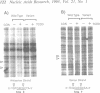
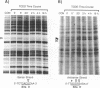
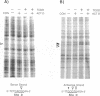
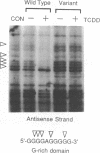
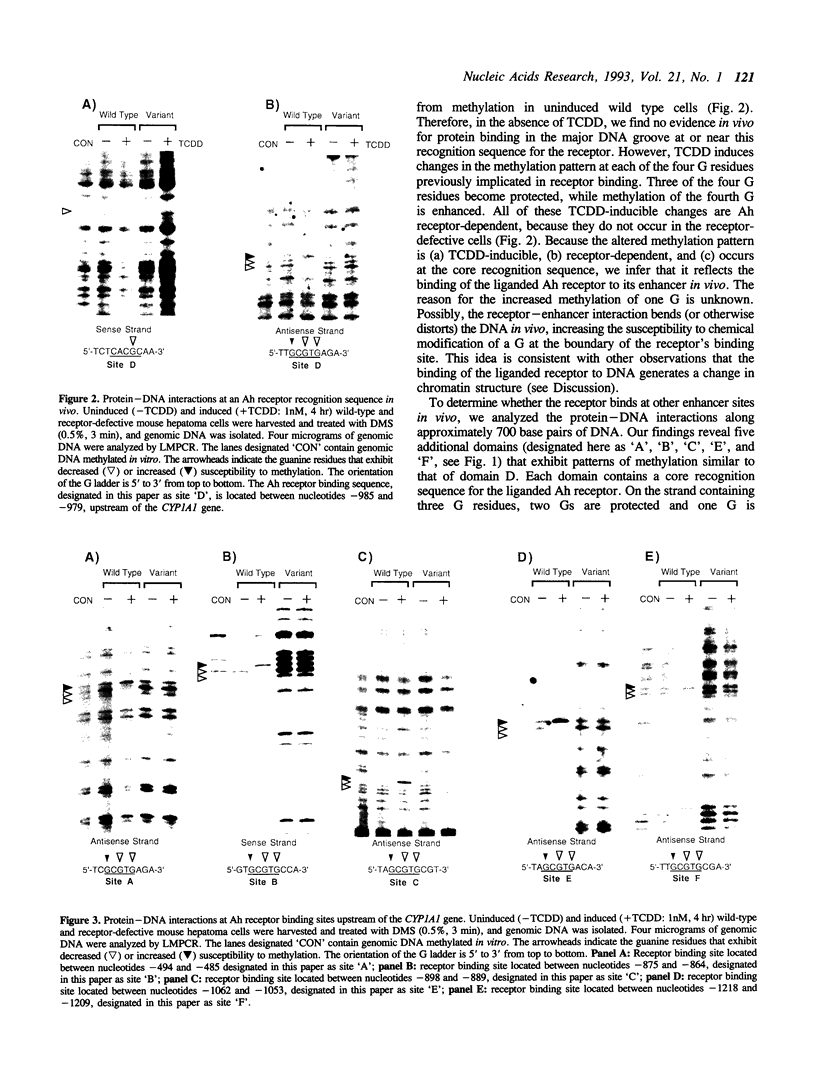
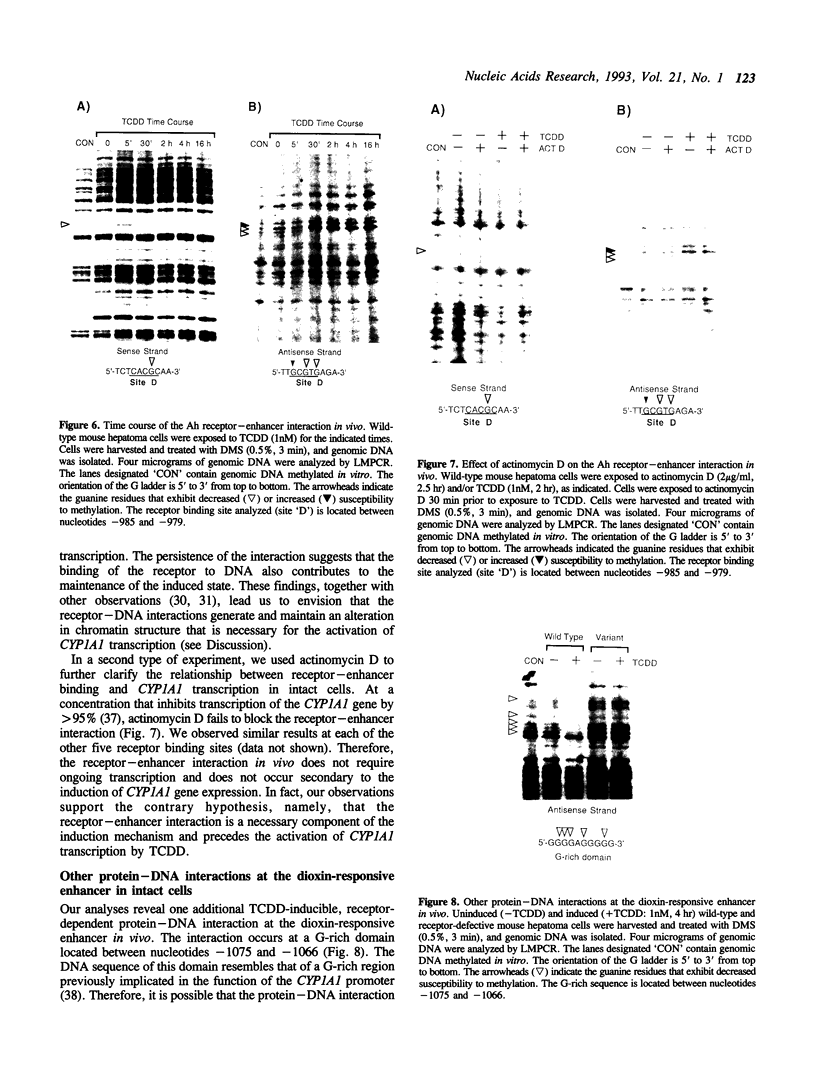
We have used a ligation-mediated polymerase chain reaction technique to analyze protein-DNA interactions at a dioxin-responsive enhancer upstream of the CYP1A1 gene in intact mouse hepatoma cells. In its inactive state, the enhancer binds few, if any, proteins within the major DNA groove in vivo. Thus, the inactive enhancer is relatively inaccessible to DNA-binding proteins. Exposure of cells to 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to the binding of the liganded Ah receptor at six sites within the major DNA groove of the enhancer. The receptor-enhancer interactions occur rapidly and do not require ongoing transcription, consistent with their role in regulating CYP1A1 gene expression. The liganded receptor, which is a heteromer composed of at least two basic helix-loop-helix proteins, is probably the only DNA-binding transcription factor necessary to activate the enhancer in vivo. The small size and irregular distribution of receptor binding sites suggest that chromatin structure imposes substantial steric constraints upon the function of the receptor-enhancer system in intact cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailar J. C., 3rd How dangerous is dioxin? N Engl J Med. 1991 Jan 24;324(4):260–262. doi: 10.1056/NEJM199101243240409. [DOI] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible aryl hydrocarbon receptor-mediated change in CYP1A1 chromatin structure occurs independently of transcription. Mol Cell Biol. 1989 Dec;9(12):5733–5737. doi: 10.1128/mcb.9.12.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink C. J., Gasiewicz T. A., Whitlock J. P., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Evidence that the transformed Ah receptor is heteromeric. J Biol Chem. 1990 Nov 25;265(33):20708–20712. [PubMed] [Google Scholar]
- Elferink C. J., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible, Ah receptor-mediated bending of enhancer DNA. J Biol Chem. 1990 Apr 5;265(10):5718–5721. [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Wu L., Denison M. S., Whitlock J. P., Jr Organization and function of a dioxin-responsive enhancer. J Biol Chem. 1990 Jun 15;265(17):9676–9681. [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz T. A., Elferink C. J., Henry E. C. Characterization of multiple forms of the Ah receptor: recognition of a dioxin-responsive enhancer involves heteromer formation. Biochemistry. 1991 Mar 19;30(11):2909–2916. doi: 10.1021/bi00225a026. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone function in transcription. Annu Rev Cell Biol. 1990;6:643–678. doi: 10.1146/annurev.cb.06.110190.003235. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Dominant and recessive aryl hydrocarbon hydroxylase-deficient mutants of mouse hepatoma line, Hepa-1, and assignment of recessive mutants to three complementation groups. Somatic Cell Genet. 1983 Jul;9(4):497–514. doi: 10.1007/BF01543050. [DOI] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Söderkvist P., Wilhelmsson A., Pongratz I., Tukey R. H., Johnson E. F., Gustafsson J. A., Poellinger L. Liver cells contain constitutive DNase I-hypersensitive sites at the xenobiotic response elements 1 and 2 (XRE1 and -2) of the rat cytochrome P-450IA1 gene and a constitutive, nuclear XRE-binding factor that is distinct from the dioxin receptor. Mol Cell Biol. 1991 Sep;11(9):4314–4323. doi: 10.1128/mcb.11.9.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Whitlock J. P., Jr Functional analysis of the transcriptional promoter for the CYP1A1 gene. Mol Cell Biol. 1990 Oct;10(10):5098–5105. doi: 10.1128/mcb.10.10.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. Transcriptional regulation by dimerization: two sides to an incestuous relationship. Cell. 1990 Apr 6;61(1):9–11. doi: 10.1016/0092-8674(90)90207-u. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Durrin L. K., Galeazzi D. R., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci U S A. 1986 May;83(9):2802–2806. doi: 10.1073/pnas.83.9.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Irresistible force meets immovable object: transcription and the nucleosome. Cell. 1991 Nov 29;67(5):833–836. doi: 10.1016/0092-8674(91)90354-2. [DOI] [PubMed] [Google Scholar]
- Miller A. G., Israel D., Whitlock J. P., Jr Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J Biol Chem. 1983 Mar 25;258(6):3523–3527. [PubMed] [Google Scholar]
- Morgan J. E., Whitlock J. P., Jr Transcription-dependent and transcription-independent nucleosome disruption induced by dioxin. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11622–11626. doi: 10.1073/pnas.89.23.11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Gonzalez F. J., Jaiswal A. K., Nebert D. W. Dioxin-inducible enhancer region upstream from the mouse P(1)450 gene and interaction with a heterologous SV40 promoter. DNA. 1986 Oct;5(5):403–411. doi: 10.1089/dna.1986.5.403. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Aryl hydrocarbon (Ah) receptor DNA-binding activity. Sequence specificity and Zn2+ requirement. J Biol Chem. 1990 Jun 5;265(16):9251–9258. [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Multiple DNA-binding factors interact with overlapping specificities at the aryl hydrocarbon response element of the cytochrome P450IA1 gene. Mol Cell Biol. 1990 Dec;10(12):6408–6416. doi: 10.1128/mcb.10.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992 Apr 5;267(10):6815–6819. [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr The potential role of DNA methylation in the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1989 Oct 25;264(30):17754–17758. [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr The potential role of DNA methylation in the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1989 Oct 25;264(30):17754–17758. [PubMed] [Google Scholar]
- Watson A. J., Hankinson O. Dioxin- and Ah receptor-dependent protein binding to xenobiotic responsive elements and G-rich DNA studied by in vivo footprinting. J Biol Chem. 1992 Apr 5;267(10):6874–6878. [PubMed] [Google Scholar]
- Watson A. J., Weir-Brown K. I., Bannister R. M., Chu F. F., Reisz-Porszasz S., Fujii-Kuriyama Y., Sogawa K., Hankinson O. Mechanism of action of a repressor of dioxin-dependent induction of Cyp1a1 gene transcription. Mol Cell Biol. 1992 May;12(5):2115–2123. doi: 10.1128/mcb.12.5.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Wu L., Whitlock J. P., Jr Mechanism of dioxin action: Ah receptor-mediated increase in promoter accessibility in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4811–4815. doi: 10.1073/pnas.89.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]