Summary
Specific sites of histone tail methylation are associated with transcriptional activity at gene loci. These methyl-marks are interpreted by effector molecules, which harbor protein domains that bind the methylated motifs and facilitate either active or inactive states of transcription. CARM1 and PRMT1 are transcriptional coactivators that deposit H3R17me2a and H4R3me2a marks, respectively. We used a protein domain microarray approach to identify the tudor domain-containing protein TDRD3 as a “reader” of these marks. Importantly, TDRD3 itself is a transcriptional coactivator. This coactivator activity requires an intact tudor domain. TDRD3 is recruited to an estrogen responsive element in a CARM1-dependent manner. Furthermore, ChIP-seq analysis of TDRD3 reveals that it is predominantly localized to transcriptional start sites. Thus, TDRD3 is an effector molecule that promotes transcription by binding methylarginine marks on histone tails.
Introduction
Protein arginine methyltransferases (PRMTs) modify a multitude of proteins, both in the nucleus and in the cytoplasm of the cell (Bedford and Clarke, 2009). These enzymes have been implicated in signal transduction processes, translation, DNA repair and epigenetic regulation. Three distinct types of methylarginine residues are found in mammalian cells: asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and monomethylarginine (MMA). All three of these modifications are present on the N-terminal histone tails of H3 and H4. The coactivator-associated arginine methyltransferase (CARM1/PRMT4) deposits ADMA marks at the H3R17 & H3R26 sites (Schurter et al., 2001). PRMT1 also functions as a transcriptional coactivator and methylates H4R3me2a. PRMT5 and PRMT6 are associated with transcriptional repression, and they deposit the H4R3me2s and H3R2me2a marks, respectively (Guccione et al., 2007; Hyllus et al., 2007; Iberg et al., 2008; Pal et al., 2004). Recently, PRMT6 was also demonstrated to possess transcriptional coactivator activity at estrogen-regulated loci (Harrison et al., 2010).
How these different methyl-marks are used for the interpretation of active and inactive chromatin states is a subject of great interest. In the case of PRMT6 and the H3R2me2a mark, effector molecules that are involved in transcriptional activation, like WDR5, BPTF and JMJD2a, are blocked from binding the H3K4me3 mark (Hyllus et al., 2007; Iberg et al., 2008). Recently, it was found that the PHD domain of DNMT3a binds the H4R3me2s repressive mark, which is laid down by PRMT5 (Zhao et al., 2009). This provides a very elegant link between a histone modification and de novo DNA methylation targeting. These two mechanisms of action – one that blocks the recruitment of transcriptional activators, and the other that facilitates the recruitment of a transcriptional repressor – help explain how certain genomic loci are transcriptionally silenced by the methylarginine marks. However, it remains unclear how the marks deposited by PRMT1 and CARM1 (H4R3me2a, H3R17me2a & H3R26me2a) help support active transcription.
In a non-chromatin context, there is precedent for both ADMA and SDMA motifs binding to tudor domain-containing proteins. The tudor domain of SMN, SPF30 and TDRD3 interacts with SDMA motifs present in splicing factors like SmB (Cote and Richard, 2005; Friesen et al., 2001) and with ADMA motifs present in CA150, a transcription elongation factor (Cheng et al., 2007). All these described methylarginine-regulated protein-protein interactions are between tudor domains and glycine/arginine-rich (GAR) motifs, which harbor numerous methylated arginine residues in a patch. However, no methylarginine binding protein has yet been identified that can dock onto the isolated methyl-marks found on histone tails, with the exception of the recently described interaction between the PHD domain of DNMT3a and the H4R3me2s repressive mark (Zhao et al., 2009).
In order to identify effector molecules that can read methylarginine marks associated with transcriptional activation, we screen a protein domain microarray (CADOR array) with a peptide corresponding to the primary histone methylation site of CARM1, H3R17me2a. This screen identified TDRD3 as a H3R17me2a binder. TDRD3 can also bind the H4R3me2a mark, which is catalyzed by PRMT1. Importantly, in transcriptional reporter assays, TDRD3 functions as a coactivator, whereas other known methylarginine-binding proteins, SMN and SPF30, do not. This finding is consistent with the idea that TDRD3 binds marks deposited by the coactivators, CARM1 and PRMT1, and interprets them. In this regard, we show that TDRD3 is associated with the pS2 promoter in a CARM1 dependent manner. Using an unbiased approach (ChIP-Seq), we further find that TDRD3 is generally associated with transcriptional start sites. This study describes an effector molecule for methylarginine marks that are associated with transcriptional activation.
Results
Protein domain microarrays identify TDRD3 as a reader for CARM1/PRMT1 marks
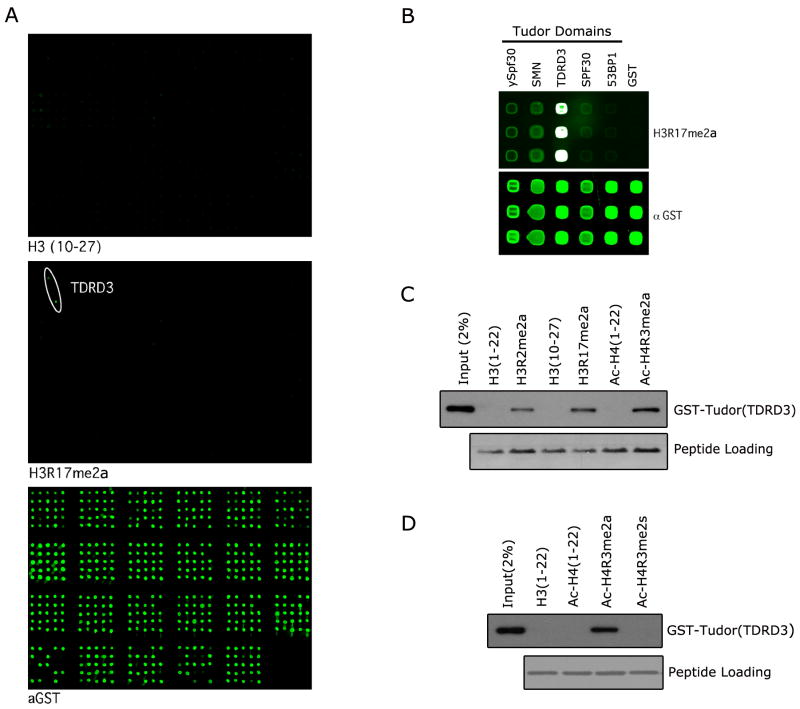
The CADOR microarray has been used to identify novel methyllysine binding domains that interact with histone tail peptides (Kim et al., 2006), methylated nucleosomes (Schotta et al., 2008) and non-histone proteins (Huang et al., 2007). Here, for the first time we used this approach to screen for protein domains that can bind methylarginine marks on histone tails. A biotinylated H3R17me2a peptide was pre-conjugated to Cy3 and used as a probe on the CADOR microarray. A specific interaction was detected with the recombinant tudor domain of TDRD3 (Figure 1A). We then generated a focused array of selected human tudor domains from TDRD3, SMN, SPF30, 53BP1, and ySpf30 from S. pombe. Again, we observed strong binding of the H3R17me2a peptide to TDRD3, and weaker binding to the SMN, SPF30 and ySpf30 (Figure 1B). The array approach is qualitative, but not quantitative, and thus not suitable to establish which methylarginine mark binds the tudor domain of TDRD3 the strongest. Peptide pull-down experiments were thus performed with a set of three known methylarginine histone marks, demonstrating that the tudor domain of TDRD3 binds strongest to H4R3me2a and H3R17me2a marks (Figure 1C). Next, we investigated whether the tudor domain of TDRD3 could distinguish between the H4R3me2a activation mark deposited by PRMT1 and the H4R3me2s mark generated by the transcriptional repressor PRMT5. Peptide pull-down experiments demonstrated that TDRD3 preferentially binds the PRMT1 generated H4R3me2a mark associated with transcriptional activation (Figure 1D). The tudor domain of TDRD3 is thus the first reported domain that selectively binds an ADMA motif and cannot bind the same sequence when it is symmetrically methylated.
Figure 1.
The tudor domain of TDRD3 binds arginine methylation marks on histone tails. (A) The CADOR array was probed with a peptide harboring the CARM1 methyl-mark H3R17me2a. Binding was observed on two spots representing the tudor domain of TDRD3 (middle panel). The unmodified peptide displays no binding under the same conditions (upper panel). The array complexity is revealed by probing with an anti-GST antibody (bottom panel). The array is composed of GST fusion proteins that carry protein domains usually found in chromatin associated proteins (for key see Figure S1). Proteins are arrayed in duplicate. (B) A focused array was probed using tudor domains that have been reported to bind methylarginine motifs (ySPF30, SMN, TDRD3 & SPF30) and the methyllysine binding tudor domain of 53BP1. The H3R17me2a peptide binds most strongly to the tudor domain of TDRD3. (C) The tudor domain of TDRD3 binds a number of methylarginine marks found on histone tails. Pull-down experiments were performed with H3R2me2a, H3R17me2a and Ac-H4R3me2a peptides. The observed pull-down efficiency is as follows: Ac-H4R3me2a > H3R17me2a > H3R2me2a. (D) TDRD3 tudor binds the asymmetric, but not the symmetric, dimethylarginine mark on H4R3. Pull-down experiments were performed with Ac-H4R3me2s and Ac-H4R3me2a peptides. A specificity switch is observed with the tudor domain of TDRD3 which binds more strongly to the Ac-H4R3me2a active mark (see also Figure S1).
TDRD3 is a transcriptional coactivator
The H3R17me2a and H4R3me2a marks which are deposited by the transcriptional coactivators CARM1 and PRMT1 respectively, are read by the TDRD3 tudor domain. We thus wanted to determine whether TDRD3 itself had transcriptional coactivator activity. ERE (estrogen response element) luciferase reporter assays were performed with the three full-length tudor domain-containing proteins that bound H3R17me2a marks – TDRD3, SPF30 and SMN (Figure 1B & S2B). Of the three, only TDRD3 possessed coactivator activity (Figure 2A & S2D). Furthermore, shRNA mediated knockdown of endogenous TDRD3 caused a reduction of luciferase activity, when using the same assay (Figure 2B & S2C). TDRD3 also functioned as a transcriptional coactivator for androgen receptor mediated transcription (Figure S2E & F).
Figure 2.
TDRD3 is a transcriptional coactivator that is recruited to the pS2 promoter in a CARM1 dependent manner. (A) An ERE-Luc reporter assay was performed in MCF7 cells by co-transfection of GFP-SPF30, GFP-SMN and GFP-TDRD3 expression vectors. (B) ERE-Luc reporter activity is reduced in MCF7 cells with shRNA-mediated knockdown of endogenous TDRD3. Error bars represent standard deviation calculated from triplicate luciferase assays. (C) and (D) The recruitment of TDRD3 to the promoter of the estrogen-responsive pS2 promoter was evaluated by ChIP/ReChIP. MCF7 cells were cultured in charcoal-striped serum for three days and then treated with estradiol (E2+) for 45 min before ChIP/ReChIP experiments were performed with indicated antibodies. Immunoprecipitated DNA was analyzed in triplicates by qPCR with primers flanking the pS2 promoter region. Mean values were expressed as the fold change of E2+ versus E2-. E2- was represented as 1. (E) TDRD3 knockdown results in a reduction of the pS2 expression level. Hormone starved MCF7 cells were transfacted with shRNA against either GFP or TDRD3, respectively. Cells were either untreated or treated with E2 for 30 min. Total RNA was analyzed by RT-qPCR for pS2 expression and normalized for GAPDH. Error bars represent standard deviation calculated from triplicate qPCR reactions (see also Figure S2).
It has previously been shown that CARM1 plays a central role in regulating estrogen receptor-mediated gene expression (Chen et al., 1999; Frietze et al., 2008; Lupien et al., 2009; Yadav et al., 2003). CARM1 knockdown reduces expression of the pS2 (also called TFF1) gene by 50% (Frietze et al., 2008). We thus focused on this well characterized estrogen regulated locus to evaluate the role that TDRD3 plays in CARM1-mediated gene expression. TDRD3 antibodies were raised to facilitate these studies (Figure S2, G-J). We performed ChIP experiments in MCF7 cells before and after estrogen treatment to investigate the recruitment of TDRD3 to the pS2 promoter. After estrogen treatment, TDRD3 was enriched along with ERα CARM1, CARM1 activity (αH3R17me2a), PRMT1 and PRMT1 activity (αH4R3me2a) (Figure 2C). TDRD3 is not enriched at the GAPDH locus after estrogen treatment (Figure S2K). Thus, providing evidence that the recruitment of CARM1/PRMT1 activities and TDRD3 are concomitant. CARM1 (and its mark) is the predominant PRMT recruited to the pS2 promoter after E2-treatment – raising the possibility that TDRD3 was recruited to this promoter in a CARM1-dependent manner. To strengthen this supposition, we performed the same set of ChIP experiments in a MCF7 cell line harboring a Dox inducible CARM1 shRNA. When CARM1 protein levels are reduced, we still observed the recruitment of ERα to the pS2 promoter. However, as we would expect CARM1 activity is reduced at this site, but more importantly, so is TDRD3 recruitment (Figure S2L). Furthermore, reChIP experiments show that TDRD3 coexists with both activator-associated methylarginine marks (Figure 2D). We also showed that TDRD3 knockdown reduces the expression of the pS2 gene (Figure 2E). Thus, the local enrichment of the H3R17me2a/H4R3me2a marks is associated with TDRD3 recruitment, as would be expected for a CARM1/PRMT1 effector molecule. Furthermore, adequate TDRD3 levels are required for normal pS2 gene expression.
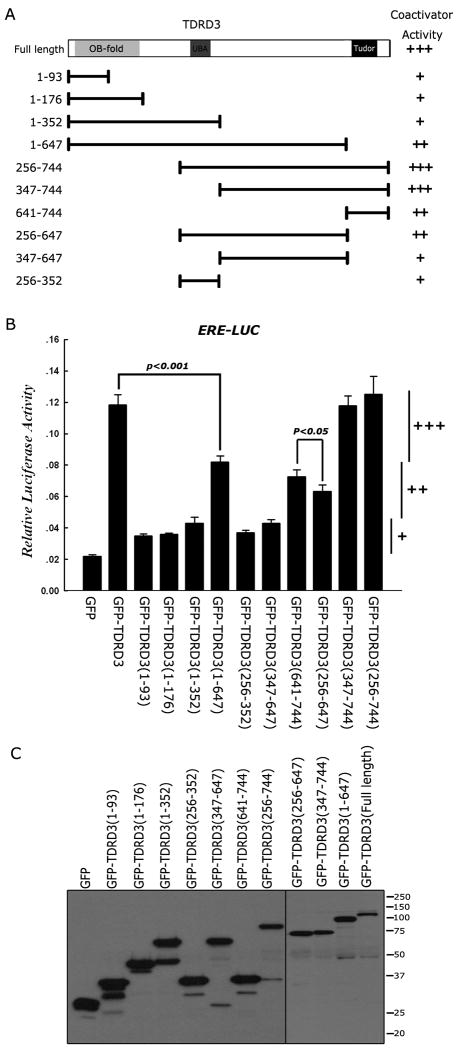
TDRD3 requires its Tudor domain for full coactivator activity
We have shown that CARM1 activity facilitates the recruitment of TDRD3 to an ERE. Next, we investigated the contribution of the different protein domains of TDRD3 to its transcriptional coactivator function. A series of GFP-fused deletion constructs was generated and tested in a ERE luciferase reporter assays (Figure 3A). Only constructs that harbored the tudor domain together with the full linker region that attaches it to the UBA domain displayed full coactivator activity (Figure 3B). The expression levels of the different deletion constructs were roughly equivalent in these transient transfection assays (Figure 3C). Thus, the ability of TDRD3 to dock with methylated substrates through its tudor domain plays a significant role in its coactivator function. It is important to note that the ERE reporter plasmid will only be partially chromatinized during these transient transfection assays, and the contributions of the N-terminal OB-fold and the UBA domain to promote transcription may not be fully appreciated under these conditions.
Figure 3.
TDRD3 requires its tudor domain for optimal coactivator activity. (A) A series of GFP-fusion deletion constructs was generated. The region fused to GFP is graphically depicted. (B) An ERE-Luc reporter assay was performed in MCF7 cells by cotransfection of GFP-fusion deletion expression vectors. Relative activity is scored with +, ++ or +++. These scores are also shown in (A) to facilitate the interpretation of the data. Student t-test was performed for the indicated pairs. (C) The deletion construct series is expressed at roughly the same level. Western analysis was performed with αGFP. Error bars represent standard deviation calculated from triplicate luciferase assays.
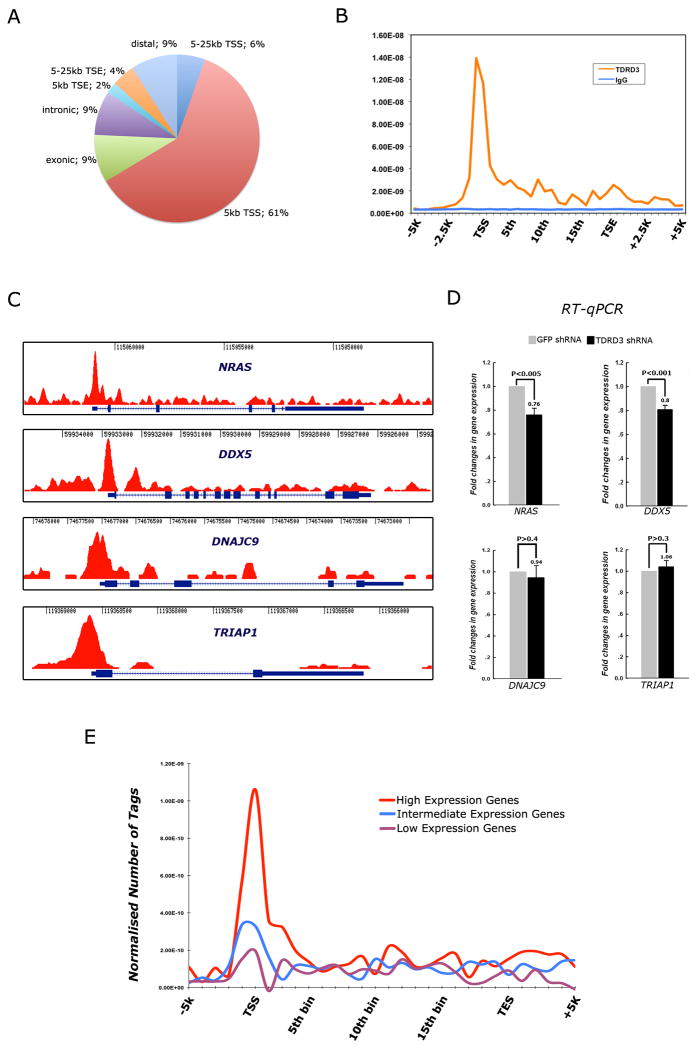
TDRD3 maps predominantly to regions upstream of transcriptional start sites
TDRD3 is recruited to the pS2 promoter in MCF7 cells (Figure 2). To determine how general the recruitment is, we performed an unbiased genome-wide analysis of TDRD3 in proliferating MCF7 cells. We then identified 822 peaks with significant TDRD3 ChIP-enrichment. Strikingly, the majority (61%) of chromatin bound TDRD3 associates with promoter regions. In addition, a significant amount (18%) of TDRD3 is also found in the body of genes (exons and introns) (Figure 4A). The distribution of TDRD3 can be presented in a different fashion over a normalized gene body (Figure 4B). To demonstrate the enrichment of TDRD3 in areas around the transcriptional start site (TSS), we depict the raw reads of the ChIP-seq tracings for four genes – DNAJC9, NRAS, DDX5 and TRIAP1 (Figure 4C). Tracings of pS2 and MYC (two genes regulated by ER) show similar TSS enrichment, although pS2 also has multiple peaks of TDRD3 within its gene body (Figure S2M & N).
Figure 4.
TDRD3 is predominantly recruited to transcriptional start sites (TSS) and promoters of genes. (A) TDRD3 ChIP-seq analysis was performed using MCF7 cells. A pie chart is used to illustrate the genomic regions that are enriched for TDRD3 association. TDRD3 is predominantly coupled to promoter regions, including the TSS. (B) The distribution of TDRD3 was normalized across all genes in which peaks were identified, and its localization spikes over promoters, and displays some enrichment over the gene body. The TSS and TSE (transcriptional end) are shown. (C) Raw reads of the ChIP-seq tracings for four genes – NRAS, DDX5, DNAJC9 and TRIAP1 – demonstrating the enrichment of TDRD3 at promoters. (D) MCF7 cells were transfected with shRNA against GFP or TDRD3. Total RNA was extracted and RT-qPCR was performed to detect the level of gene expression at these four loci. Error bars represent standard deviation calculated from triplicate qPCR reactions. (E) Correlations between the level of gene expression in MCF7 cells and the occurrence of TDRD3 peaks at promoters. Gene expression levels were gauged using Affymetrix arrays (see also Figure S3).
We further confirmed, using a traditional ChIP approach, that TDRD3, CARM1, PRMT1 and the two activator-associated methylarginine marks are indeed enriched at these TSSs and to a lesser extent, or not at all, in the gene body (Figure S3A). The recruitment of TDRD3 to promoters is consistent with the fact that CARM1 and PRMT1 activity have been generally associated with gene promoters by ChIP experiments, and often there is cooperation between PRMT1 and CARM1 (An et al., 2004; Kleinschmidt et al., 2008). Indeed, the ability of these two PRMTs to synergize in reporter assays was first reported some time ago (Koh et al., 2001). We did not detect an interaction between TDRD3 and PRMT1, CARM1, PRMT6 or ER (data not shown), strongly supporting the notion that TDRD3 is recruited to promoters by reading methylarginine marks.
TDRD3 knockdown reduces the expression of NRAS and DDX5 (Figure 4D), suggesting that its presence at promoters is often associated with actively transcribed gene. Both NRAS and DDX5 are highly expressed in MCF7 cells (Figure S3B). We thus investigated whether a correlation exists between the level of gene expression in MCF7 cells and the presence of TDRD3 peaks at promoters, and indeed there is (Figure 4E).
Discussion
TDRD3 has distinct nuclear and cytoplasmic roles
TDRD3 is localized to both the nuclear (30%) and cytosolic (70%) compartments of the cells (Goulet et al., 2008). TDRD3 accumulates in the nucleus when the nuclear export factor CRM1 is chemically inhibited (Figure S2A). In the nucleus, TDRD3 is an effector molecule for CARM1 and PRMT1 generated methyl-marks and, in this context, functions as a transcriptional coactivator. In the cytoplasm, TDRD3 interacts with the methylated GAR motif of the Fragile-X syndrome protein FMRP (Linder et al., 2008). In response to cellular stress, TDRD3 accumulates in stress granules (SGs) in HeLa cells, where it has been proposed to function as a translational repressor of key transcripts that will be used during the recovery of the cell (Goulet et al., 2008; Linder et al., 2008). SGs are cytosolic foci that are enriched for RNA binding protein and also contain stalled 48S pre-initiation complexes (Yamasaki and Anderson, 2008). Thus, under normal cell growth conditions TDRD3 functions as a transcriptional coactivator, and under suboptimal conditions (stress) it functions as a posttranscriptional regulator in SGs. This “split personality” is reminiscent of the master transcriptional coactivator SRC-3/AIB1, which is also sequestered in stress granules under adverse conditions (Yu et al., 2007). Elevated expression levels of SRC-3/AIB1 are associated with poor disease-free survival in breast cancer patients. Importantly, elevated levels of TDRD3 also form part of the expression profile signature that is used as a predictor of poor prognosis for breast cancer patients (Nagahata et al., 2004).
The TDRD3 tudor domain “reads” more than one methylarginine motif
The tudor domain of TDRD3 was demonstrated to bind SDMA GAR motifs using pull-down approaches (Cote and Richard, 2005) and a protein microarray approach (Kim et al., 2006). In this study, we show that this tudor domain binds strongly to the isolated methyl-marks of H3R17me2a and H4R3me2a, and more weakly to the H3R2me2a mark. Furthermore, the TDRD3 tudor domain can discriminate between H4R3me2a and H4R3me2s marks, and preferentially binds the ADMA version of this modification, which is associated with active chromatin. Thus, the tudor domain of TDRD3 can bind isolated ADMA and/or SDMA methyl-marks, and the docking site does not necessarily have to be in a arginine-rich GAR motif. In this respect, the tudor domain of TDRD3 is distinct from that of Tudor-SN, which preferentially recognizes only SDMA motifs (Friberg et al., 2009). The ability of the tudor domain of TDRD3 to recognize multiple methylated motifs is not unique for methyl effector molecules. Indeed, the canonical methyllysine-reader, HP1, binds H3K9me (Bannister et al., 2001), H1K26me (Daujat et al., 2005) and a number of non-histone proteins (Rathert et al., 2008). Thus, HP1 binds a number of methyllysine motifs that are associated with transcription repression, and conversely, TDRD3 binds a variety of methylarginine marks associated with transcription activation.
Other tudor domain containing proteins that bind SDMA motifs include SMN and SPF30 (Cote and Richard, 2005). The tudor of SMN also binds ADMA motifs (Cheng et al., 2007). Recently, the Piwi-family of proteins (Miwi and Mili), which interact with a sub-class of small noncoding RNAs (piRNAs) were shown to be arginine methylated (Chen et al., 2009; Vagin et al., 2009). A large class of tudor domain containing proteins are highly expressed in germ cells and play an important role in nuage/chromatoid body formation, one of which (TDRD1) has been shown to bind methylated Miwi and Mili (Vagin et al., 2009).
TDRD3 is found at gene promoters
Chromatin immunoprecipitations and massively parallel sequencing (ChIP-Seq) shows that TDRD3 is predominantly associated with the 5′-regions of genes. As demonstrated by numerous ChIP experiments, these are the same regions that are enriched for CARM1 and PRMT1. Although PRMT1 and CARM1 were initially identified as steroid receptor coactivators, they are now emerging as coregulators of a large number of transcription factors including p53, YY1, NF-κB, PPARγ, RUNX1, E2F1, c-Fos and FXR (Bedford and Clarke, 2009). Importantly, these two PRMTs function synergistically and their corresponding methyl-marks are enriched synchronously at the CITED2 promoter shortly after the stimulation of transcription (Kleinschmidt et al., 2008). The presence of at least two different tudor domain-binding motifs (H3R17me2a & H4R3me2a) at promoter regions may stabilize the recruitment of TDRD3. Although a number of traditional ChIP studies have established that CARM1 functions at promoters, recent ChIP/chip experiments found CARM1 activity peaked predominantly at regions far from ER-regulated promoters (Lupien et al., 2009). This study showed that functional enhancers are enriched for liganded ERα, the pioneer factor FoxA1 and CARM1 activity. It has been proposed by Lupien et al. that looping occurs between these cistromes and the promoters of genes harboring Pol II, and recent chromosome conformation capture experiments demonstrated that these long-range chromatin interactions do indeed occur (Fullwood et al., 2009). As TDRD3 is strongly associated with promoters, it may facilitate this looping process by facilitating the interaction between arginine methylated cistromes and promoters. Importantly, an ERE motif was not detected below the TDRD3 promoter-associated peaks. However, enrichment was observed for ATF3 and CREB binding motifs (Figure S3C & D).
TDRD3 as a scaffolding molecule
TDRD3 does not possess any enzymatic activity of its own, but instead likely functions as a scaffolding molecule onto which a protein complex is assembled. TDRD3 is composed of three different protein domains; an OB-fold, a UBA domain and a C-terminal tudor domain. The linker region between the tudor and UBA domains also contributes to coactivator activity. The N-terminal OB-fold contains substantial homology to a similar domain in Blap75/Rim1, which complexes with the BLM helicase (Yin et al., 2005). The role of this OB-fold domain is unclear at present. The UBA domain, which is sandwiched between the OB-fold and the tudor domain, has the ability to bind a Lys48 linked tetra-ubiquitin chain (Linder et al., 2008). We propose that the tudor domain of TDRD3 binds methyl-marks on the core histone tails that are associated with transcriptional activation (H3R17me2a and H4R3me2a). The UBA domain stabilizes this interaction by binding ubiquitinated proteins at the transcriptional start sites of genes, and the unique N-terminal domain and linker recruit as yet unidentified protein to promote transcription at these arginine methylated loci.
Experimental Procedures
Protein domain microarrays and GST pull-down
The generation of CADOR protein microarray and peptide probe preparation have been described (Kim et al, 2006). A list of the protein domains on this array is provided in the supplemental material. Peptide pull-downs were performed as described (Kim et al, 2006).
Chromatin immunoprecipitation
MCF7 cells were grown in DMEM medium supplemented with 10% charcoal-dextran-stripped FBS for 3 days before E2 treatment. After 10 nM E2 treatment for 45 min, cells were cross-linked with 1% formaldehyde at 37°C for 10 min. ChIP assay was performed using antibodies as indicated and followed the protocols as described (Iberg, et al 2008). The primers used for PCR were listed in the supplemental data.
ChIP-seq analysis
Deep sequencing was performed using the Illumina Solexa Genome Analyzer II. The IgG control had 5,350,756 mapped tags and TDRD3 had mapped tags 26,320,528. This data can be accessed at - GEO accession # GSE22612
Supplementary Material
Acknowledgments
M. Bedford is supported by NIH grant number DK62248 and, in part, by institutional grant NIEHS ES07784. Y. Yang is Epigenetics Scholar supported by “The Center for Cancer Epigenetics” at MDACC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. doi: 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, et al. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A. 2009;106:20336–20341. doi: 10.1073/pnas.0911640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- Cheng D, Cote J, Shaaban S, Bedford MT. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell. 2007;25:71–83. doi: 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Cote J, Richard S. Tudor domains bind symmetrical dimethylated arginines. J Biol Chem. 2005;280:28476–28483. doi: 10.1074/jbc.M414328200. [DOI] [PubMed] [Google Scholar]
- Daujat S, Zeissler U, Waldmann T, Happel N, Schneider R. HP1 binds specifically to Lys26-methylated histone H1.4, whereas simultaneous Ser27 phosphorylation blocks HP1 binding. J Biol Chem. 2005;280:38090–38095. doi: 10.1074/jbc.C500229200. [DOI] [PubMed] [Google Scholar]
- Friberg A, Corsini L, Mourao A, Sattler M. Structure and ligand binding of the extended Tudor domain of D. melanogaster Tudor-SN. J Mol Biol. 2009;387:921–934. doi: 10.1016/j.jmb.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Friesen WJ, Massenet S, Paushkin S, Wyce A, Dreyfuss G. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol Cell. 2001;7:1111–1117. doi: 10.1016/s1097-2765(01)00244-1. [DOI] [PubMed] [Google Scholar]
- Frietze S, Lupien M, Silver PA, Brown M. CARM1 regulates estrogen-stimulated breast cancer growth through up-regulation of E2F1. Cancer Res. 2008;68:301–306. doi: 10.1158/0008-5472.CAN-07-1983. [DOI] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet I, Boisvenue S, Mokas S, Mazroui R, Cote J. TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules. Hum Mol Genet. 2008;17:3055–3074. doi: 10.1093/hmg/ddn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E, Bassi C, Casadio F, Martinato F, Cesaroni M, Schuchlautz H, Luscher B, Amati B. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Tang YH, Dowhan DH. Protein arginine methyltransferase 6 regulates multiple aspects of gene expression. Nucleic Acids Res. 2010;38:2201–2216. doi: 10.1093/nar/gkp1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- Hyllus D, Stein C, Schnabel K, Schiltz E, Imhof A, Dou Y, Hsieh J, Bauer UM. PRMT6-mediated methylation of R2 in histone H3 antagonizes H3 K4 trimethylation. Genes Dev. 2007;21:3369–3380. doi: 10.1101/gad.447007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iberg AN, Espejo A, Cheng D, Kim D, Michaud-Levesque J, Richard S, Bedford MT. Arginine methylation of the histone h3 tail impedes effector binding. J Biol Chem. 2008;283:3006–3010. doi: 10.1074/jbc.C700192200. [DOI] [PubMed] [Google Scholar]
- Kim J, Daniel J, Espejo A, Lake A, Krishna M, Xia L, Zhang Y, Bedford MT. Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep. 2006;7:397–403. doi: 10.1038/sj.embor.7400625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt MA, Streubel G, Samans B, Krause M, Bauer UM. The protein arginine methyltransferases CARM1 and PRMT1 cooperate in gene regulation. Nucleic Acids Res. 2008;36:3202–3213. doi: 10.1093/nar/gkn166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001;276:1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- Linder B, Plottner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, Keidel E, Fischer U. Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP. Hum Mol Genet. 2008;17:3236–3246. doi: 10.1093/hmg/ddn219. [DOI] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Krum SA, Rhodes DR, Liu XS, Brown M. Coactivator function defines the active estrogen receptor alpha cistrome. Mol Cell Biol. 2009;29:3413–3423. doi: 10.1128/MCB.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahata T, Onda M, Emi M, Nagai H, Tsumagari K, Fujimoto T, Hirano A, Sato T, Nishikawa K, Akiyama F, et al. Expression profiling to predict postoperative prognosis for estrogen receptor-negative breast cancers by analysis of 25,344 genes on a cDNA microarray. Cancer Sci. 2004;95:218–225. doi: 10.1111/j.1349-7006.2004.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathert P, Dhayalan A, Murakami M, Zhang X, Tamas R, Jurkowska R, Komatsu Y, Shinkai Y, Cheng X, Jeltsch A. Protein lysine methyltransferase G9a acts on non-histone targets. Nat Chem Biol. 2008;4:344–346. doi: 10.1038/nchembio.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta G, Sengupta R, Kubicek S, Malin S, Kauer M, Callen E, Celeste A, Pagani M, Opravil S, De La Rosa-Velazquez IA, et al. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev. 2008;22:2048–2061. doi: 10.1101/gad.476008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurter BT, Koh SS, Chen D, Bunick GJ, Harp JM, Hanson BL, Henschen-Edman A, Mackay DR, Stallcup MR, Aswad DW. Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry. 2001;40:5747–5756. doi: 10.1021/bi002631b. [DOI] [PubMed] [Google Scholar]
- Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–1762. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N, Lee J, Kim J, Shen J, Hu MC, Aldaz CM, Bedford MT. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci U S A. 2003;100:6464–6468. doi: 10.1073/pnas.1232272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Curr Opin Cell Biol. 2008;20:222–226. doi: 10.1016/j.ceb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W. BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. EMBO J. 2005;24:1465–1476. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25:765–778. doi: 10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–311. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.