Abstract
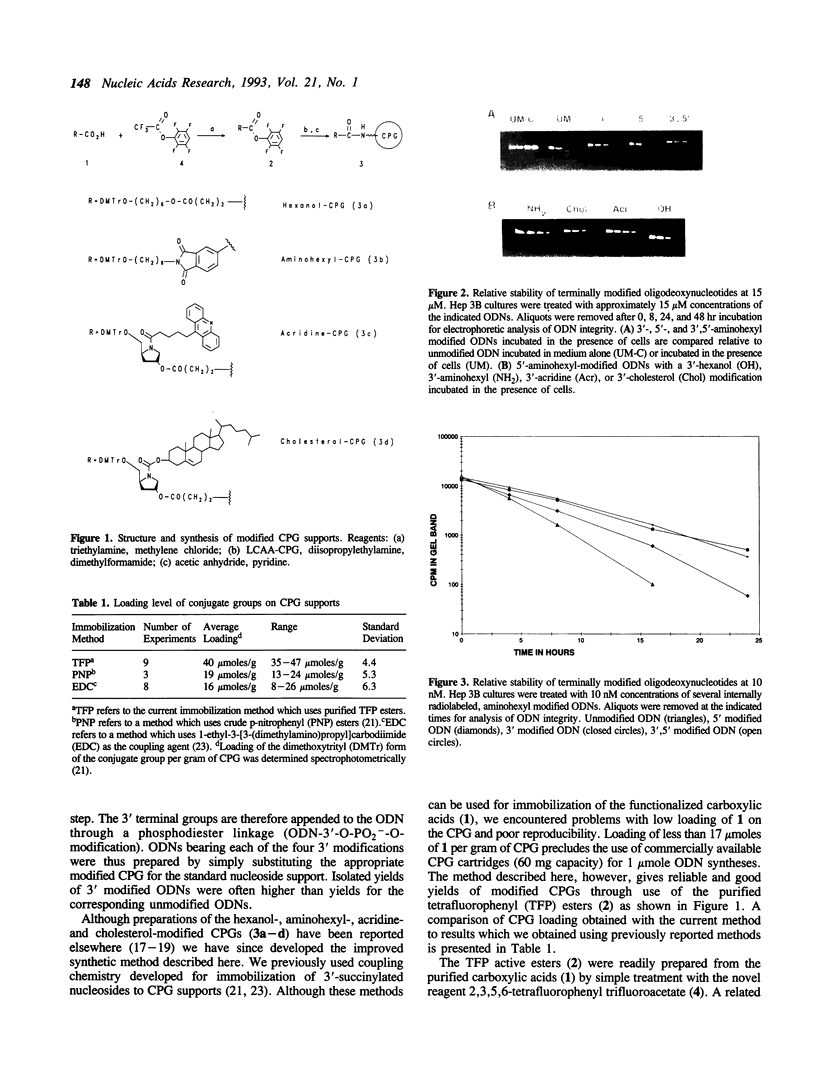
An efficient chemical procedure for the immobilization of carboxylate containing conjugate groups onto controlled pore glass (CPG) is described. The derivatized supports were used in the automated synthesis of an oligodeoxynucleotide (20-mer ODN) containing a 3' phosphodiester linked hexanol, aminohexyl, acridine, or cholesterol group. The stability of the oligomer in a hepatoma cell culture was found to be prolonged two to three fold by the presence of any of the 3' tails. By contrast, an aminohexyl group appended to the 5' terminus of the ODN only marginally improved its nuclease resistance. These data support the notion that antisense ODNs are primarily degraded by 3' exonucleases. Introduction of simple 3' tails which incorporate a normal phosphodiester linkage can increase ODN stability by interfering with these enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar S., Kole R., Juliano R. L. Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci. 1991;49(24):1793–1801. doi: 10.1016/0024-3205(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Weeks D. L., Walder J. A. Pathways of degradation and mechanism of action of antisense oligonucleotides in Xenopus laevis embryos. Antisense Res Dev. 1991 Spring;1(1):11–20. doi: 10.1089/ard.1991.1.11. [DOI] [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Fraga D., Reed M. W. 3'-modified antisense oligodeoxyribonucleotides complementary to calmodulin mRNA alter behavioral responses in Paramecium. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8601–8605. doi: 10.1073/pnas.89.18.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie C. R., Reed M. W., Adams A. D., Meyer R. B., Jr An improved CPG support for the synthesis of 3'-amine-tailed oligonucleotides. Bioconjug Chem. 1992 Jan-Feb;3(1):85–87. doi: 10.1021/bc00013a014. [DOI] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]
- Reed M. W., Adams A. D., Nelson J. S., Meyer R. B., Jr Acridine- and cholesterol-derivatized solid supports for improved synthesis of 3'-modified oligonucleotides. Bioconjug Chem. 1991 Jul-Aug;2(4):217–225. doi: 10.1021/bc00010a005. [DOI] [PubMed] [Google Scholar]
- Saison-Behmoaras T., Tocqué B., Rey I., Chassignol M., Thuong N. T., Hélène C. Short modified antisense oligonucleotides directed against Ha-ras point mutation induce selective cleavage of the mRNA and inhibit T24 cells proliferation. EMBO J. 1991 May;10(5):1111–1118. doi: 10.1002/j.1460-2075.1991.tb08051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Kaiser K. Oligodeoxyribonucleotides containing 1,3-propanediol as nucleoside substitute. Nucleic Acids Res. 1987 Apr 10;15(7):3113–3129. doi: 10.1093/nar/15.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Mori K., Loke S. L., Subasinghe C., Shinozuka K., Cohen J. S., Neckers L. M. Phosphorothioate and normal oligodeoxyribonucleotides with 5'-linked acridine: characterization and preliminary kinetics of cellular uptake. Gene. 1988 Dec 10;72(1-2):333–341. doi: 10.1016/0378-1119(88)90160-6. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Zendegui J. G., Vasquez K. M., Tinsley J. H., Kessler D. J., Hogan M. E. In vivo stability and kinetics of absorption and disposition of 3' phosphopropyl amine oligonucleotides. Nucleic Acids Res. 1992 Jan 25;20(2):307–314. doi: 10.1093/nar/20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]




