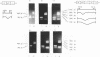Abstract
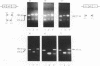
Sequence analysis of two human tenascin encoding cDNA clones from a cDNA library of the U251 glioblastoma cell line revealed the presence of a novel 276 bp tenascin type III fibronectin like repeat. This alternatively spliced type III repeat designated AD1 is located between the previously identified repeats 10 and 11 and has sequence homology with human, chicken and mouse tenascin type III repeats. These results show that tenascin has at least 16 consecutive fibronectin like type III repeats. PCR amplification of random primed mRNA with specific type III repeat primers revealed a pattern of multiple alternative splices of AD1 and flanking type III repeats. The alternative splice variants were confirmed by direct sequencing. Differences were observed in the expression of the various alternative splices of tenascin mRNA between tumor and normal cells and may thus indicate differences in tenascin isoform expression and function in normal and tumor cells. PCR and Southern analysis of genomic DNA indicate that AD1 is coded by a single exon present in both human and mouse genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufderheide E., Ekblom P. Tenascin during gut development: appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol. 1988 Dec;107(6 Pt 1):2341–2349. doi: 10.1083/jcb.107.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Matthews T. J., Pizzo S. V., Bigner D. D. Immunochemical and biochemical characterization of a glioma-associated extracellular matrix glycoprotein. J Cell Biochem. 1985;28(3):183–195. doi: 10.1002/jcb.240280302. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989 Mar;108(3):1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Wikstrand C. J., Furthmayr H., Matthews T. J., Bigner D. D. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res. 1983 Jun;43(6):2796–2805. [PubMed] [Google Scholar]
- Bronner-Fraser M. Distribution and function of tenascin during cranial neural crest development in the chick. J Neurosci Res. 1988 Oct-Dec;21(2-4):135–147. doi: 10.1002/jnr.490210206. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Chuong C. M., Chen H. M. Enhanced expression of neural cell adhesion molecules and tenascin (cytotactin) during wound healing. Am J Pathol. 1991 Feb;138(2):427–440. [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander D. R., Hoffman S., Edelman G. M. Functional mapping of cytotactin: proteolytic fragments active in cell-substrate adhesion. J Cell Biol. 1988 Dec;107(6 Pt 1):2329–2340. doi: 10.1083/jcb.107.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Alexakos M. J., Ravikant N. A., Sturgill M. E., Marton L. S., Stefansson K. Structure of the human hexabrachion (tenascin) gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9438–9442. doi: 10.1073/pnas.88.21.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986 Dec;103(6 Pt 2):2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Husmann K., Faissner A., Schachner M. Tenascin promotes cerebellar granule cell migration and neurite outgrowth by different domains in the fibronectin type III repeats. J Cell Biol. 1992 Mar;116(6):1475–1486. doi: 10.1083/jcb.116.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Indik Z., Yeh H., Ornstein-Goldstein N., Sheppard P., Anderson N., Rosenbloom J. C., Peltonen L., Rosenbloom J. Alternative splicing of human elastin mRNA indicated by sequence analysis of cloned genomic and complementary DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5680–5684. doi: 10.1073/pnas.84.16.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. E., Hemesath T. J., Kim J. H., Gulcher J. R., Stefansson K. The complete cDNA sequence of human hexabrachion (Tenascin). A multidomain protein containing unique epidermal growth factor repeats. J Biol Chem. 1991 Feb 15;266(5):2818–2823. [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H., Goldhamer D. J., Tassava R. A. An extracellular matrix molecule of newt and axolotl regenerating limb blastemas and embryonic limb buds: immunological relationship of MT1 antigen with tenascin. Development. 1990 Apr;108(4):657–668. doi: 10.1242/dev.108.4.657. [DOI] [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Qualitative and quantitative changes of human tenascin expression in transformed lung fibroblast and lung tumor tissues: comparison with fibronectin. Cancer Res. 1991 Sep 15;51(18):4876–4881. [PubMed] [Google Scholar]
- Pagani F., Zagato L., Vergani C., Casari G., Sidoli A., Baralle F. E. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol. 1991 Jun;113(5):1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. C., Sandell L. J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990 Jun 25;265(18):10334–10339. [PubMed] [Google Scholar]
- Rüegg C. R., Chiquet-Ehrismann R., Alkan S. S. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7437–7441. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tsukamoto T., Jing N., Kusakabe M., Sakakura T. Murine tenascin: cDNA cloning, structure and temporal expression of isoforms. Gene. 1991 Aug 15;104(2):177–185. doi: 10.1016/0378-1119(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991 Oct;13(10):527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Spencer C. S., Wilson C. L. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol. 1989 Dec;109(6 Pt 2):3445–3453. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Siri A., Carnemolla B., Saginati M., Leprini A., Casari G., Baralle F., Zardi L. Human tenascin: primary structure, pre-mRNA splicing patterns and localization of the epitopes recognized by two monoclonal antibodies. Nucleic Acids Res. 1991 Feb 11;19(3):525–531. doi: 10.1093/nar/19.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Svoboda K. K., Nishimura I., Sugrue S. P., Ninomiya Y., Olsen B. R. Embryonic chicken cornea and cartilage synthesize type IX collagen molecules with different amino-terminal domains. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7496–7500. doi: 10.1073/pnas.85.20.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka L., Roiko K., Soininen R., Prockop D. J., Tryggvason K. A HindIII polymorphism in the 3' end of the human alpha 1(IV) collagen gene. Nucleic Acids Res. 1987 Jul 10;15(13):5497–5497. doi: 10.1093/nar/15.13.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A., Beck S., Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991 Jan;112(2):355–362. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]