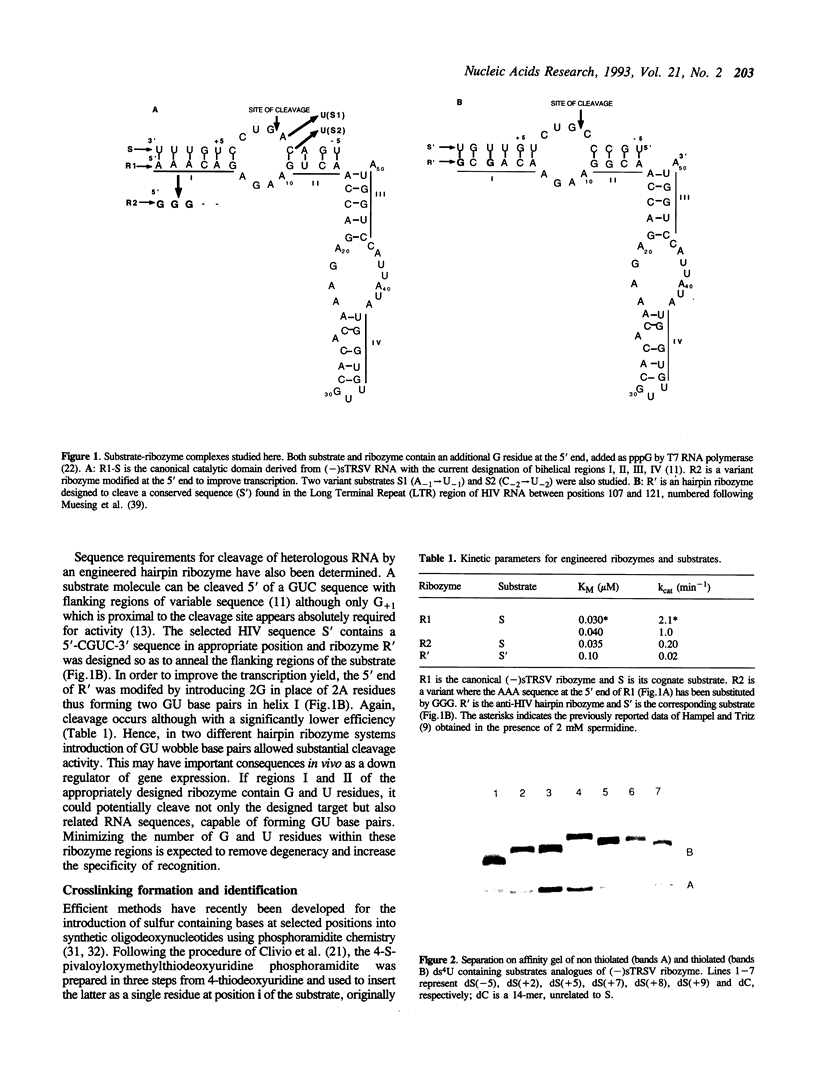
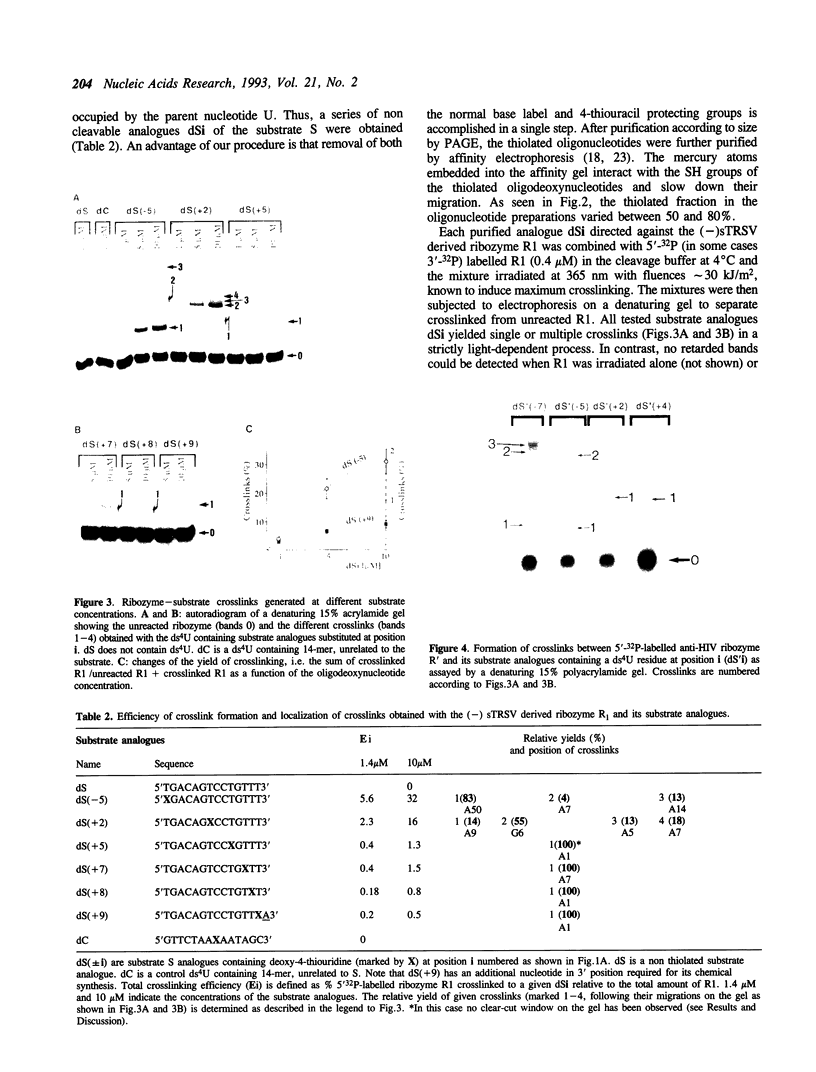
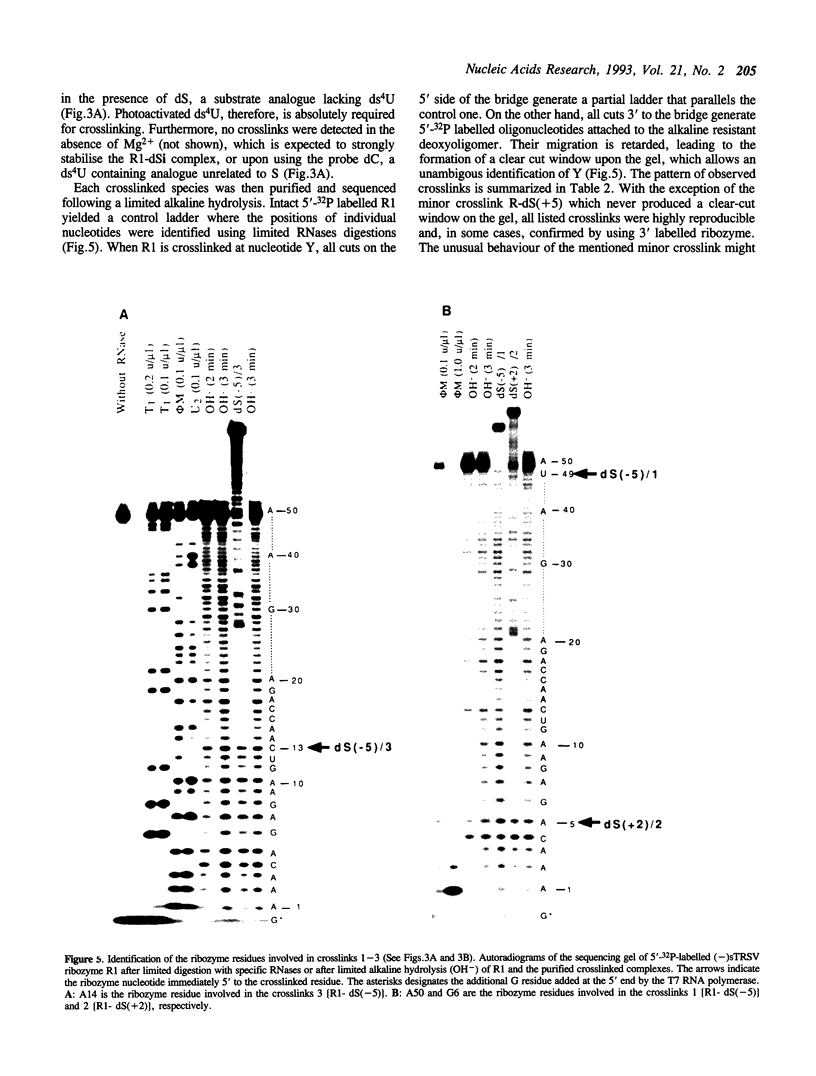
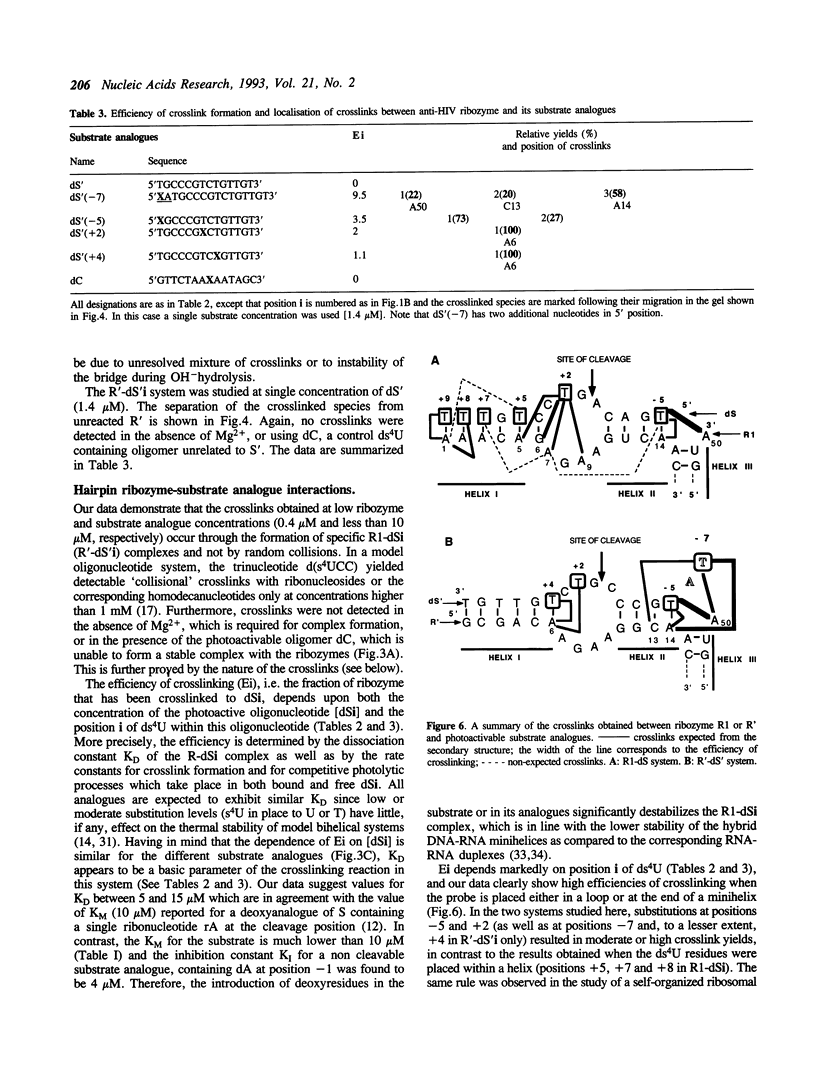
Abstract
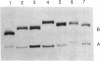
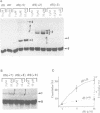
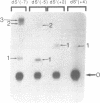
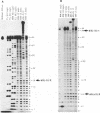
Hairpin ribozymes derived from (-)sTRSV RNA exhibit substantial cleavage activity when wobble GU base pairs are introduced in place of the AU pairs normally involved in helices I and II between substrate and ribozyme. This finding prompted us to synthesize by in vitro transcription a new hairpin ribozyme, active against a 14-mer substrate derived from a conserved HIV sequence. Interactions of the canonical and anti-HIV hairpin ribozymes with non cleavable DNA substrate analogues containing the photoaffinity probe deoxy-4-thiouridine (ds4U) at a single site were investigated. Upon near-UV light irradiation (365 nm), all these substrate analogues were covalently attached to ribozyme via single or multiple crosslinks. In contrast, no crosslinks were detected using either a DNA substrate analogue lacking ds4U or a ds4U containing oligomer unrelated to the substrate sequence. As expected, if the dissociation constant is in the range of 5-15 microM, the yield of crosslinked ribozyme increased markedly with increasing the substrate analogue concentration. The ribozyme residues involved in the crosslinks were determined by RNA sequencing. The pattern of crosslinks obtained with the two ribozyme systems provides additional evidence in support of the consensus secondary structure proposed for the hairpin domain. Minor alternative conformations were detected in the case of the (-)sTRSV system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzal-Herranz A., Joseph S., Burke J. M. In vitro selection of active hairpin ribozymes by sequential RNA-catalyzed cleavage and ligation reactions. Genes Dev. 1992 Jan;6(1):129–134. doi: 10.1101/gad.6.1.129. [DOI] [PubMed] [Google Scholar]
- Bevilacqua P. C., Turner D. H. Comparison of binding of mixed ribose-deoxyribose analogues of CUCU to a ribozyme and to GGAGAA by equilibrium dialysis: evidence for ribozyme specific interactions with 2' OH groups. Biochemistry. 1991 Nov 5;30(44):10632–10640. doi: 10.1021/bi00108a005. [DOI] [PubMed] [Google Scholar]
- Bezerra R., Favre A. In vivo incorporation of the intrinsic photolabel 4-thiouridine into Escherichia coli RNAs. Biochem Biophys Res Commun. 1990 Jan 15;166(1):29–37. doi: 10.1016/0006-291x(90)91907-a. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Hampel A., Bruening G. Nucleotide sequence and newly formed phosphodiester bond of spontaneously ligated satellite tobacco ringspot virus RNA. Nucleic Acids Res. 1986 Dec 22;14(24):9729–9743. doi: 10.1093/nar/14.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowrira B. M., Berzal-Herranz A., Burke J. M. Novel guanosine requirement for catalysis by the hairpin ribozyme. Nature. 1991 Nov 28;354(6351):320–322. doi: 10.1038/354320a0. [DOI] [PubMed] [Google Scholar]
- Chowrira B. M., Burke J. M. Binding and cleavage of nucleic acids by the "hairpin" ribozyme. Biochemistry. 1991 Sep 3;30(35):8518–8522. doi: 10.1021/bi00099a003. [DOI] [PubMed] [Google Scholar]
- Dontsova O., Kopylov A., Brimacombe R. The location of mRNA in the ribosomal 30S initiation complex; site-directed cross-linking of mRNA analogues carrying several photo-reactive labels simultaneously on either side of the AUG start codon. EMBO J. 1991 Sep;10(9):2613–2620. doi: 10.1002/j.1460-2075.1991.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil Y. L., Expert-Bezançon A., Favre A. Conformation and structural fluctuations of a 218 nucleotides long rRNA fragment: 4-thiouridine as an intrinsic photolabelling probe. Nucleic Acids Res. 1991 Jul 11;19(13):3653–3660. doi: 10.1093/nar/19.13.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Epstein L. M., Gall J. G. Self-cleaving transcripts of satellite DNA from the newt. Cell. 1987 Feb 13;48(3):535–543. doi: 10.1016/0092-8674(87)90204-2. [DOI] [PubMed] [Google Scholar]
- Favre A., Bezerra R., Hajnsdorf E., Lemaigre Dubreuil Y., Expert-Bezançon A. Substitution of uridine in vivo by the intrinsic photoactivable probe 4-thiouridine in Escherichia coli RNA. Its use for E. coli ribosome structural analysis. Eur J Biochem. 1986 Nov 3;160(3):441–449. doi: 10.1111/j.1432-1033.1986.tb10060.x. [DOI] [PubMed] [Google Scholar]
- Feldstein P. A., Buzayan J. M., Bruening G. Two sequences participating in the autolytic processing of satellite tobacco ringspot virus complementary RNA. Gene. 1989 Oct 15;82(1):53–61. doi: 10.1016/0378-1119(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Thermodynamic and structural properties of pentamer DNA.DNA, RNA.RNA, and DNA.RNA duplexes of identical sequence. Biochemistry. 1991 Nov 5;30(44):10606–10613. doi: 10.1021/bi00108a002. [DOI] [PubMed] [Google Scholar]
- Hampel A., Tritz R., Hicks M., Cruz P. 'Hairpin' catalytic RNA model: evidence for helices and sequence requirement for substrate RNA. Nucleic Acids Res. 1990 Jan 25;18(2):299–304. doi: 10.1093/nar/18.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel A., Tritz R. RNA catalytic properties of the minimum (-)sTRSV sequence. Biochemistry. 1989 Jun 13;28(12):4929–4933. doi: 10.1021/bi00438a002. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Sequences required for self-catalysed cleavage of the satellite RNA of tobacco ringspot virus. Gene. 1989 Oct 15;82(1):43–52. doi: 10.1016/0378-1119(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Heus H. A., Pardi A. Nuclear magnetic resonance studies of the hammerhead ribozyme domain. Secondary structure formation and magnesium ion dependence. J Mol Biol. 1991 Jan 5;217(1):113–124. doi: 10.1016/0022-2836(91)90615-d. [DOI] [PubMed] [Google Scholar]
- Igloi G. L. Interaction of tRNAs and of phosphorothioate-substituted nucleic acids with an organomercurial. Probing the chemical environment of thiolated residues by affinity electrophoresis. Biochemistry. 1988 May 17;27(10):3842–3849. doi: 10.1021/bi00410a048. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Nikiforov T. T., Connolly B. A. Oligodeoxynucleotides containing 4-thiothymidine and 6-thiodeoxyguanosine as affinity labels for the Eco RV restriction endonuclease and modification methylase. Nucleic Acids Res. 1992 Mar 25;20(6):1209–1214. doi: 10.1093/nar/20.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M. J., Midden W. R., Babasick D. M., Haugen D. A. Photochemistry of 4-thiouridine and thymine. Photochem Photobiol. 1988 Aug;48(2):229–232. doi: 10.1111/j.1751-1097.1988.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Rubino L., Tousignant M. E., Steger G., Kaper J. M. Nucleotide sequence and structural analysis of two satellite RNAs associated with chicory yellow mottle virus. J Gen Virol. 1990 Sep;71(Pt 9):1897–1903. doi: 10.1099/0022-1317-71-9-1897. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem Sci. 1989 Nov;14(11):445–450. doi: 10.1016/0968-0004(89)90103-5. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wollenzien P., Expert-Bezançon A., Favre A. Sites of contact of mRNA with 16S rRNA and 23S rRNA in the Escherichia coli ribosome. Biochemistry. 1991 Feb 19;30(7):1788–1795. doi: 10.1021/bi00221a009. [DOI] [PubMed] [Google Scholar]