Abstract
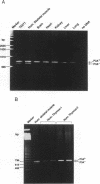
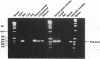
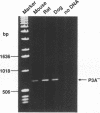
The nicotinic acetylcholine receptor (nAChR) is an oligomeric transmembrane glycoprotein consisting of four homologous subunits in stoichiometry of alpha 2, beta (gamma or epsilon). Recently the presence of a novel exon (P3A) in human alpha AChR gene has been reported. Two variants of the human alpha subunit arise from alternate RNA splicing, one with and one without the P3A exon. However, the evolutionary origin of the P3A exon and the regulation of the expression of the two variants in human muscle and non-human tissues is currently unknown. Examination of genomic DNA from various species shows that the P3A exon sequence is present only in hominoids, old world and new world primates species and is absent in the muscle cDNA or genomic DNA from rat, mouse or dog, indicating that P3A exon is evolutionary conserved for at least 50 million years. The P3A+ variant of alpha subunit was found to be constitutively expressed in skeletal muscle, brain, heart, kidney, liver, lung and thymus, while P3A-variant was differentially expressed only in skeletal muscle. Thus it appears that the P3A+ variant is generated by 'default' selection by the splicing machinery, while expression of the P3A- variant is regulated by tissue-specific factors in the skeletal muscle. Mechanisms regulating differential expression of the alpha subunit variants may be pertinent to the pathophysiology of myasthenia gravis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeson D., Morris A., Vincent A., Newsom-Davis J. The human muscle nicotinic acetylcholine receptor alpha-subunit exist as two isoforms: a novel exon. EMBO J. 1990 Jul;9(7):2101–2106. doi: 10.1002/j.1460-2075.1990.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Frail D. E., Musil L. S., Buonanno A., Merlie J. P. Expression of RAPsyn (43K protein) and nicotinic acetylcholine receptor genes is not coordinately regulated in mouse muscle. Neuron. 1989 Jan;2(1):1077–1086. doi: 10.1016/0896-6273(89)90232-8. [DOI] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hugnot J. P., Gilgenkrantz H., Vincent N., Chafey P., Morris G. E., Monaco A. P., Berwald-Netter Y., Koulakoff A., Kaplan J. C., Kahn A. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7506–7510. doi: 10.1073/pnas.89.16.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg K. E., Mudd J., Shah V., Merlie J. P. Nucleotide sequence of the mouse muscle nicotinic acetylcholine receptor alpha subunit. Nucleic Acids Res. 1986 Jun 25;14(12):5111–5111. doi: 10.1093/nar/14.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Lennon V. A., Griesmann G. E. Evidence against acetylcholine receptor having a main immunogenic region as target for autoantibodies in myasthenia gravis. Neurology. 1989 Aug;39(8):1069–1076. doi: 10.1212/wnl.39.8.1069. [DOI] [PubMed] [Google Scholar]
- Luther M. A., Schoepfer R., Whiting P., Casey B., Blatt Y., Montal M. S., Montal M., Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989 Mar;9(3):1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nishio H., Nakajima T., Kitoh Y., Takumi T., Koga J., Nakamura H. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991 Jun;87(6):2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Kaufman D. L., Berson E. L., Tobin A. J., Shih V. E., Gusella J. F., Ramesh V. Splicing defect at the ornithine aminotransferase (OAT) locus in gyrate atrophy. Am J Hum Genet. 1990 Nov;47(5):790–794. [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Morris A., Beeson D., Jacobson L., Baggi F., Vincent A., Newsom-Davis J. Two isoforms of the muscle acetylcholine receptor alpha-subunit are translated in the human cell line TE671. FEBS Lett. 1991 Dec 16;295(1-3):116–118. doi: 10.1016/0014-5793(91)81399-s. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Axel R., Shneider N. A. Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Piette J., Klarsfeld A., Changeux J. P. Interaction of nuclear factors with the upstream region of the alpha-subunit gene of chicken muscle acetylcholine receptor: variations with muscle differentiation and denervation. EMBO J. 1989 Mar;8(3):687–694. doi: 10.1002/j.1460-2075.1989.tb03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Merlie J. P. A developmental and tissue-specific enhancer in the mouse skeletal muscle acetylcholine receptor alpha-subunit gene regulated by myogenic factors. J Biol Chem. 1991 Nov 25;266(33):22588–22596. [PubMed] [Google Scholar]
- Prody C. A., Merlie J. P. The 5'-flanking region of the mouse muscle nicotinic acetylcholine receptor beta subunit gene promotes expression in cultured muscle cells and is activated by MRF4, myogenin and myoD. Nucleic Acids Res. 1992 May 11;20(9):2367–2372. doi: 10.1093/nar/20.9.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. CD4+ T cell response to the human acetylcholine receptor alpha subunit in myasthenia gravis. A study with synthetic peptides. J Immunol. 1990 Feb 15;144(4):1276–1281. [PubMed] [Google Scholar]
- Satokata I., Tanaka K., Miura N., Miyamoto I., Satoh Y., Kondo S., Okada Y. Characterization of a splicing mutation in group A xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9908–9912. doi: 10.1073/pnas.87.24.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluep M., Willcox N., Vincent A., Dhoot G. K., Newsom-Davis J. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol. 1987 Aug;22(2):212–222. doi: 10.1002/ana.410220205. [DOI] [PubMed] [Google Scholar]
- Schönbeck S., Padberg F., Hohlfeld R., Wekerle H. Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J Clin Invest. 1992 Jul;90(1):245–250. doi: 10.1172/JCI115843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R. M., Finer-Moore J. Acetylcholine receptor structure, function, and evolution. Annu Rev Cell Biol. 1985;1:317–351. doi: 10.1146/annurev.cb.01.110185.001533. [DOI] [PubMed] [Google Scholar]
- Sun J. B., Harcourt G., Wang Z. Y., Hawke S., Olsson T., Fredrikson S., Link H. T cell responses to human recombinant acetylcholine receptor-alpha subunit in myasthenia gravis and controls. Eur J Immunol. 1992 Jun;22(6):1553–1559. doi: 10.1002/eji.1830220631. [DOI] [PubMed] [Google Scholar]
- Tsay H. J., Schmidt J. Skeletal muscle denervation activates acetylcholine receptor genes. J Cell Biol. 1989 Apr;108(4):1523–1526. doi: 10.1083/jcb.108.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Tsay H. J., Schmidt J. Expression of the acetylcholine receptor delta-subunit gene in differentiating chick muscle cells is activated by an element that contains two 16 bp copies of a segment of the alpha-subunit enhancer. EMBO J. 1990 Mar;9(3):783–790. doi: 10.1002/j.1460-2075.1990.tb08174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu H. P., Wang X. M., Ballivet M., Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988 Aug;1(6):527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P., Zurn A. D., Fulpius B. W. Intrathymic pathogenesis of myasthenia gravis: transient expression of acetylcholine receptors on thymus-derived myogenic cells. Eur J Immunol. 1978 Aug;8(8):579–582. doi: 10.1002/eji.1830080808. [DOI] [PubMed] [Google Scholar]
- Wheatley L. M., Urso D., Tumas K., Maltzman J., Loh E., Levinson A. I. Molecular evidence for the expression of nicotinic acetylcholine receptor alpha-chain in mouse thymus. J Immunol. 1992 May 15;148(10):3105–3109. [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]