Abstract
Resumption of development by infective larvae (L3i) of parasitic nematodes upon entering a host is a critical first step in establishing a parasitic relationship with a definitive host. It is also considered equivalent to exit from the dauer stage by the free-living nematode Caenorhabditis elegans. Initiation of feeding, an early event in this process, is induced in vitro in L3i of Strongyloides stercoralis, a parasite of humans, other primates and dogs, by culturing the larvae in DMEM with 10% canine serum and 5 mM glutathione at 37 °C with 5% CO2. Based on the developmental neurobiology of C. elegans, resumption of development by S. stercoralis L3i should be mediated, in part at least, by neurons homologous to the ASJ pair of C. elegans. To test this hypothesis, the ASJ neurons in S. stercoralis first-stage larvae (L1) were ablated with a laser microbeam. This resulted in a statistically significant (33%) reduction in the number of L3i that resumed feeding in culture. In a second expanded investigation, the thermosensitive ALD neurons, along with the ASJ neurons, were ablated, but there was no further decrease in the initiation of feeding by these worms compared to those in which only the ASJ pair was ablated.
Index Descriptors and Abbreviations: Strongyloides stercoralis, Ancylostoma caninum, Caenorhabditis elegans, Haemonchus contortus, Lamellar cell neuron (ALD), Laser microbeam ablation, Nematode, Parasite
1. Introduction
Although once considered a very close relative of the much-studied free-living nematode Caenorhabditis elegans, the parasitic threadworm Strongyloides stercoralis is no longer thus classified (Blaxter et al., 1998). The cell bodies of its amphidial neurons, located in the lateral ganglia, are, nevertheless, located in positions similar to those of the amphidial neurons in C. elegans (White et al., 1986; Ashton et al., 1995), allowing positional homologs to be identified, and, consequently, like functions to be hypothesized (Ashton et al., 1995; Ashton et al., 1998; Ashton et al., 1999). For example, in laser-microbeam ablation studies with hatchling L1 of S. stercoralis, the amphidial neuron pairs ASF and ASI were killed and thus found to control the decision whether to develop via the homogonic (direct) or heterogonic (indirect) pathway (Ashton et al., 1998). These neurons are the positional homologs of those in C. elegans (ADF and ASI) that control a similar developmental decision, whether or not to enter into the dauer stage (Bargmann and Horvitz, 1991). Likewise, the finger cell neurons (AFD) are the thermosensitive neurons in C. elegans and ablation studies reveal a similar function, namely control of thermotaxis and of thermosensitive aspects of development, in their putative counterparts (ALD) in S. stercoralis larvae (Lopez et al., 2000; Nolan et al., 2004). Thus, it appears that neuronal identity and function is largely, although not necessarily completely, conserved between these somewhat distantly related nematode species, C. elegans and S. stercoralis.
In the C. elegans dauer larva, an environmentally resistant, developmentally arrested stage, the ASJ neuron pair is of primary importance in detecting environmental change, and, if this is favorable, the worm will exit from this stage and resume development (Bargmann and Horvitz, 1991). This function is supported by the action of other neurons, ADF, ASG and ASI. Resumption of pharyngeal pumping in this free-living nematode, resulting in the oral intake of nutrients, is one of the first steps in this process. This feeding response has been proposed also as a critical early physiological step in the initiation of parasitic development by infective nematode larvae (Hawdon and Schad, 1990). The transition between free-living and parasitic life is triggered by host-given chemical and/or physical signals that are similar for related nematodes with the same portal of entry into the host, but species-specific differences exist (Hawdon et al., 1993). Thus, in some species the initiation of feeding is delayed until after the second parasitic ecdysis (Gamble and Mansfield, 1996).
If the environmentally resistant infective stage (L3i) of S. stercoralis is the life-stage equivalent of the C. elegans dauer larva, as has been proposed (Hotez et al., 1993) and is increasingly accepted, then resumption of development on finding and entering an appropriate host—the equivalent of exit from the dauer stage—should be controlled, at least in part, by neurons functionally homologous with the ASJ pair of C. elegans. Furthermore, if such neurons are ablated, then significant numbers of infective larvae should fail to resume development, either upon entering a host, or on being placed in host-mimicking culture conditions. To test this hypothesis, we ablated neuron pair ASJ (Ashton et al., 1995) in hatchling first-stage larvae (L1) of S. stercoralis harvested from coprocultures, raised the operated larvae to the L3i stage, and examined their ability to initiate feeding in a host-mimicking in vitro system (see below).
On entering a homeothermic host from the external environment, an infective larva encounters an entirely new, very different and complex milieu. There is a change in temperature, as well as in osmotic, ionic, and other physico-chemical conditions. Thus, it is probable that additional neurons may detect this complex of changes and, consequently, have a role along with ASJ-class neurons in mediating resumption of development. Therefore, in addition to studies in which only the ASJ neuron pair was ablated, we also ablated the ALD thermosensory neuron pair, the so-called lamellar cells, along with the ASJ neurons. These neurons had been shown to influence developmental switching in the free-living larvae of S. stercoralis (Nolan et al., 2004). Laser-operated larvae, and appropriate controls, were then raised to the L3i stage and tested in the in vitro host-mimicking system.
In the research reported here, resumption of feeding was used as an indicator of reactivated larval development, i.e., in vitro-simulated parasitic development. In vivo, infective larvae of S. stercoralis are stimulated to feed after entering into mammalian skin. Host-specificity is not marked, the potential to feed being triggered in vivo during a brief period of adaptation in either gerbil or canine skin (Schad et al., unpublished). Feeding can be demonstrated by incubating larvae recovered from skin in standard tissue culture media, containing fluorescein isothiocyanate (FITC) as an ingestible marker, under an atmosphere of 5% CO2 in air at 37 °C. Additionally, tissue culture media and supplements to these media (canine serum, glutathione, and both of these supplements combined) were found to activate feeding in vitro in infective larvae taken directly from coprocultures. In a system similar to that used previously to stimulate feeding in hookworm L3i (Hawdon and Schad, 1990), DMEM supplemented with 10% dog serum and 5 mM glutathione was found to be the optimal in vitro system for triggering and sustaining feeding by S. stercoralis L3i. Ingestion of FITC-labeled culture medium indicates resumption of development in L3i. The lumen of both the pharynx and the intestine fluoresce (Fig. 1). Unstimulated control larvae (not shown) do not, there being no autofluorescence in S. stercoralis L3i.
Fig. 1.
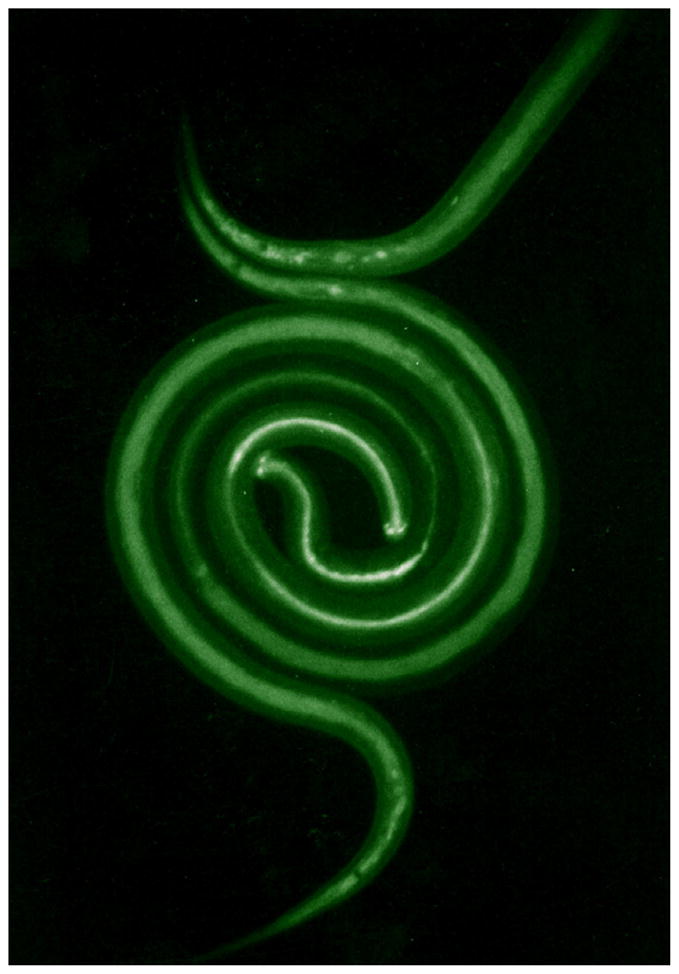
Fluorescence micrograph of S. stercoralis L3i which have ingested FITC-containing culture medium. The pharyngi of the two worms, which are coiled about each other, fluoresce strongly, while their intestines also contain fluorescent material (non-feeding, control larvae show almost no structure under these conditions).
2. Materials and methods
2.1. Parasite
A canine strain of S. stercoralis of local origin is maintained in dogs at the University of Pennsylvania School of Veterinary Medicine. Feces containing larvae were collected from an infected donor animal and used to make charcoal cultures in 100 mm Petri dishes, which were incubated at 26 °C. First-stage larvae were obtained from 2-day-old cultures, using a modified Baermann technique.
2.2. Laser microbeam ablation
First-stage larvae of S. stercoralis (5–8 per slide) were mounted on agarose pads. These were prepared from 2 ml of 1.5% (w/v) low gelling point agarose (Type 1-A, Sigma) containing 6.5–7.0 μl of 1-phenoxy-2-propanol (Janssen Chimica, Geel, Belgium) as an anesthetic (Ashton et al., 1998). The cell bodies of the neurons selected for ablation were visualized by differential interference contrast microscopy and identified using a map that was generated from a SYNU three-dimensional reconstruction (Hessler et al., 1992) of the lateral ganglia in S. stercoralis L1 larvae (Ashton et al., 1998).
In the first series of three experiments, we ablated the ASJ neuron pair in hatchling L1 larvae. For an ablation control, the ASK neuron pair was chosen, as these neurons have no known developmental function in C. elegans (Bargmann and Mori, 1997), and presumably do not have a developmental function in S. stercoralis. As a second control, larvae were exposed to the anesthetic alone, with no further treatment, for a period of time equivalent to that used to anesthetize the ablated larvae.
In a second series of 11 experiments, the thermosensitive lamellar cells, ALD were ablated along with the ASJ neuron pair. As suggested previously, infective larvae entering a host are confronted with numerous environmental changes, which, in a homeothermic host, will frequently include a change in temperature. The former neuron pair, ALD, known to be thermosensitive in S. stercoralis (Lopez et al., 2000), are also known to play a roll in the control of larval development in S. stercoralis (Nolan et al., 2004). Therefore, ALD, was chosen to complement the ASJ neurons as an ablation target in our experiments. The ASK and ALD neurons were chosen as the ablation control combination, along with anesthetic controls as before.
Laser microsurgery (Bargmann and Avery, 1995) was done either with a Micropoint Coumarin-440 dye laser (Photonic Instruments, Inc., St. Charles, Ill 60175) that was pumped with a Model 377 nitrogen laser (Laser Science, Inc. Franklin, MA 02038) or with the system provided by Laser Science, Inc, consisting of the Model 377 nitrogen laser fitted with a DLM-110 dye laser module. Both systems direct the laser microbeam through the epifluorescence port of the microscope. The nucleus of each cell body was exposed to sufficient laser pulses to produce a “welt” or other clearly visible changes in nuclear structure, indicating that enough damage was done to disrupt cell function (Bargmann and Avery, 1995; Li et al., 2000). In S. stercoralis, individual worms differ markedly in the clarity with which target cells can be visualized. Therefore, worms in which all target nuclei could not be identified were destroyed with a high-power pulse from the laser and discarded. Subsequent to ablation of the cell bodies, the successfully operated, anesthetized L1 were revived from anesthesia in BU, our standard physiological saline, (Hawdon and Schad, 1991) for 3 to 5 min. Upon full recovery, as judged by resumed motility, the L1 were placed in the center-wells of 15 × 60-mm organ culture dishes, each containing 1 ml of 1.0% agarose (Type 1-A, Sigma) that was seeded with approximately 0.05 g of helminth-free gerbil feces to provide food for developing larvae. The outer well of each dish contained BU to provide moisture and to trap any L3i that might escape from the center well. The cultures were incubated in the dark at 26 °C. By the third day in culture the larvae had developed to the L3i. Additional L1, which were subjected to the anesthetic but no further treatment, were similarly cultured to the infective stage. These unoperated control L3i, along with the operated larvae, were harvested from the organ culture plates and kept briefly in BU until tested in the assay system.
2.3. Feeding assay
The larvae were first axenized for 3 h at room temperature in 10 ml of sterile BU containing 100 U/ml penicillin, 100 μg/ml streptomycin and 1 mg/ml tetracycline, pH 7.0. After washing in sterile BU, the antibiotic-treated larvae were considered ready for use in the feeding assay. Operated and control L3i were incubated in 1 ml of the DMEM-based culture medium described above for 21 h at 37 °C in a 5% CO2 atmosphere in the wells of a 48-well plate. Subsequently, 25 μl of the FITC marker (20 mg of FITC dissolved in 1 ml dimethyl formamide) were added to each of the wells. After a 3 h incubation with FITC the larvae from each of the wells were washed 5–6 times in BU. The washed larvae were then transferred to microscope slides and screened for ingestion of FITC using epifluorescence microscopy. Living larvae with dye-filled pharynges (esophagi) and intestines were counted as feeding, and thus considered transformed from the non-feeding free-living to “parasitic” third-stage larvae (Fig. 1). Larvae that did not contain the dye-filled pharynges and intestines, in contrast to those shown in the figure, were counted as having failed to transform and, in the experimental groups, as having been successfully operated.
2.4. Statistical analysis
The data were analyzed using logistic regression, and Poisson regression methods, although only the results of the logistic regression analysis are reported. The Poisson regression analysis was used to confirm the robustness of findings from the logistic analysis, and also to test the sustainability of the assumptions (e.g. independence of observations) implicit in the logistic approach. All results from the logistic regression analysis are reported as odds ratios, as well as P-values for significance against the appropriate alternative hypothesis, and confidence intervals for the odds ratios. A P-value of 0.05 or less was taken as reflecting the likely rule-out of chance alone as a cause for the pattern of outcomes analyzed. Stata 8.2® (StataCorp LP, College Station, TX, USA) was used for all analyses reported.
3. Results
When, in preliminary experiments, larvae were incubated in the in vitro feeding assay system (Hawdon and Schad, 1990) 80–90% of normal unoperated S. stercoralis L3i harvested from coprocultures had ingested the FITC fluorescent marker, indicating initiation of “parasitic” development. In this connection, it is relevant that the infective larvae of S. stercoralis, unlike other skin-penetrating nematode larvae, do not have a cuticular sheath that must be cast before feeding can commence.
Consistent with feeding among normal larvae in preliminary experiments, 54 of 66 anesthetic-control larvae (81.8%) and 45 of 51 ablation-control larvae (88.2%), in which the ASK neurons were targeted for ablation, initiated feeding as indicated by ingestion of the FITC marker. In marked contrast, only 26 of 44 L3i (59.1%) of the principal experimental group, in which the ASJ neurons were targeted for ablation, initiated feeding (Fig. 2). The odds of initiating feeding for the ASK-ablated worms were ≈5 times that for the ASJ-ablated larvae (Odds Ratio ASK-ablated vs. ASJ-ablated worms: 5.19, P = 0.002, 99% confidence interval: 1.82–14.77). Likewise, the odds of feeding for the worms exposed to anesthetic alone were ≈3 times that for the ASJ-ablated larvae (Odds Ratio anesthetic-control worms vs. ASJ-ablated worms: 3.11, P =0.01, 99% confidence interval: 1.30–7.43). On the other hand, the odds of initiating feeding for ASK-ablated larvae were not different from the odds of that for the anesthetic-control larvae (P = 0.35).
Fig. 2.
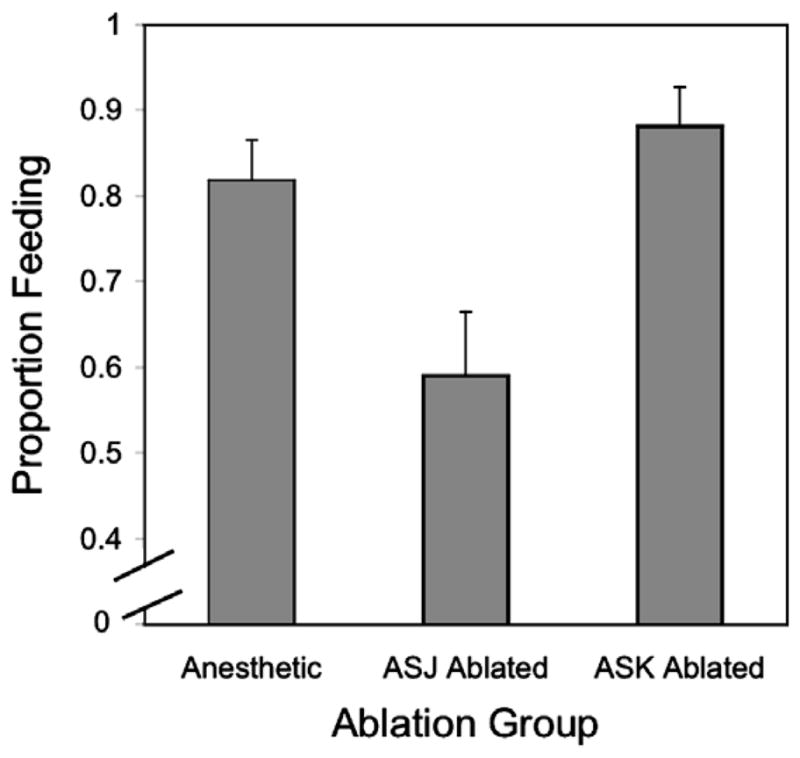
Percentage of S. stercoralis L3i that initiated feeding after: Exposure to the anesthetic, but no further treatment (see Section 2), laser microbeam ablation of the ASK neurons, or laser microbeam ablation of the ASJ neurons. Error bars indicate the Standard Error of the Mean.
In a second series of experiments, 162 (69.2%) of 234 anesthetic-control worms, and 101 (71.1%) of 142 ASK/ALD-ablated L3i initiated feeding, but only 70 (47.6%) of 147 ASJ/ALD ablated worms did so (Fig. 3). The odds of initiating feeding for the ASK/ALD-ablated larvae were ≈2.7 times that for the ASJ/ALD-ablated worms (Odds Ratio ASK/ALD-ablated worms vs. ASJ/ALD-ablated worms: 2.71, P < 0.001, 99% confidence interval: 1.66–4.41), while the odds of feeding for the worms exposed to the anesthetic alone were ≈2.5 times the odds of feeding for the ASJ/ALD-ablated worms (Odds Ratio anesthetic-control worms vs. ASJ/ALD-ablated larvae: 2.47, P < 0.001, confidence interval: 1.62–3.79). In contrast, the odds of feeding for the ASK/ALD-ablated worms were not different from that for the anesthetic-control larvae. When the two sets of experiments were compared by logistic regression with judicious selection of referent state, it became apparent that the probability of initiating feeding by the ASJ/ALD-ablated worms was not significantly different from that for the worms in which only the ASJ neurons were ablated (P = 0.18).
Fig. 3.
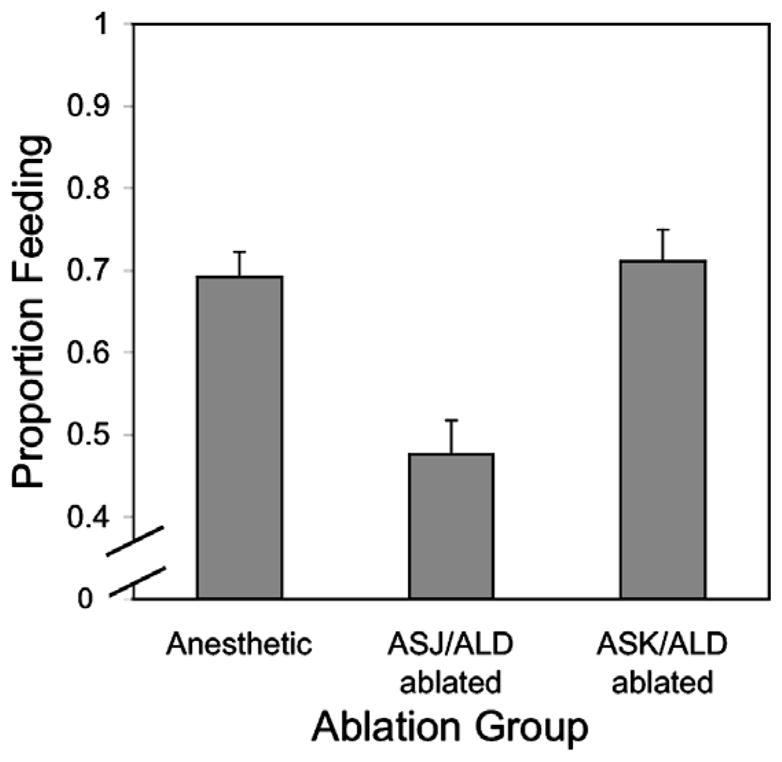
Percentage of S. stercoralis L3i that initiated feeding after: exposure to the anesthetic, but no further treatment (see Section 2), laser microbeam ablation of the ASK and ALD neurons, or laser microbeam ablation of the ASJ and ALD neurons. Error bars indicate the Standard Error of the Mean.
4. Discussion
Based upon studies of dauer recovery in C. elegans (Bargmann and Horvitz, 1991; Hotez et al., 1993), we hypothesized that in S. stercoralis, the neuron pair recognized as ASJ (Ashton et al., 1995) would play a major role in detecting host-given signals that stimulate resumption of development. Results of the studies reported here, in which ablation of ASJ by laser microsurgery resulted in a significant decrease in the proportion of L3i that resumed pharyngeal pumping under host-like in vitro culture conditions, support this hypothesis and further bolster the general identification of amphidial neurons in S. stercoralis by anatomical and functional comparison with C. elegans. While the effect of ASJ ablation on dauer recovery in C. elegans was profound (an 80% reduction in the frequency of recovery among control worms), it was not complete, and Bargmann and Horvitz (1991) hypothesized that input from additional sensory neurons was involved in triggering this developmental event. By systematic ablation of other amphidial cell bodies in combination with that of ASJ they were able to identify ADF, ASG and ASI as likely participants.
The magnitude of the effect of ASJ ablation on the proportion of S. stercoralis L3i resuming development under host-like culture conditions (33% reduction from controls), while statistically significant, was not as large as the reported effect of this operation on dauer recovery in C. elegans (Bargmann and Horvitz, 1991). Thus, it appears that the contribution of sensory neurons other than ASJ to resumption of development during the infective process in S. stercoralis is greater than in the analogous process of dauer recovery in C. elegans. Assuming that S. stercoralis L3i ascend a temperature gradient as they invade the host, we reasoned that the thermosensory neuron pair ALD might complement input from ASJ in stimulating resumption of development. However, the frequency of feeding responses among larvae having undergone ablation of ALD in combination with ASJ were not significantly different from responses in larvae in which ASJ alone was ablated. It is worth noting, however, that ALD neurons do regulate other behavioral and developmental functions associated with the infection process in S. stercoralis (Lopez et al., 2000; Nolan et al., 2004). We are currently in the process of assessing the extent to which amphidial neurons other than ASJ and ALD regulate resumption of development by S. stercoralis L3i. Identification of the precise set of neurons controlling this key step in initiation of infection is important as it could provide the basis for entirely new approaches to parasite control involving interference with development at the time and place of initial contact with a host.
Control of crucial developmental events by multiple sensory inputs may be highly adaptive for parasitic nematodes, as they appear to be for free-living ones. On entering a host, a free-living infective larva encounters a new environment in which there are profound physico-chemical differences. While the infective larva needs to detect such host-given signals, it should not resume development under conditions in the external environment that happen to be “somewhat host-like,” but where development would lead inevitably to the death of the worm. Single-neuron control would probably lead to such inappropriate execution of this developmental event. More precise control over resumption of development could be achieved by input from a complex of neurons, including the ASJ homologs. Suppression of the feeding response in about 33% of the population of worms following ablation of ASJ alone may indicate that required signals from these and participating neuron classes act in parallel and in additive fashion on a common intermediate target within the worm, perhaps an interneuron. The ASJ stimulus alone might be sufficient to trigger this target to execute further downstream effects in a minority of L3i while in the majority of individuals multiple inputs are required to reach this threshold. The basis for this heterogeneous response is unknown at this time.
Acknowledgments
This project was supported in part by NIH Grant R01 AI22662 to G.A. Schad, AI50668 to J.B. Lok and RR02512 to M. Haskins, and by a grant from the Research Foundation of the University of Pennsylvania. The 3-Dimensional reconstruction programs were provided by the National Center for Microscopy and Imaging Research supported by NIH Research Resource Grant P41 RR04050 to Dr. Mark Ellisman. We thank Dr. Veena Bhopale for the development of the culture medium used to initiate larval feeding, Andrea Ketschek for help in making the figures and Dr. Thomas Nolan for critical reading of the manuscript.
References
- Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuro-anatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. Journal of Comparative Neurology. 1995;357:281–295. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. Journal of Parasitology. 1998;84:691–695. [PubMed] [Google Scholar]
- Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Veterinary Parasitology. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press; San Diego, CA: 1995. pp. 225–250. [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Mori I. Chemotaxis and th thermotaxis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 717–738. [PubMed] [Google Scholar]
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Gamble HR, Mansfield LS. Characterization of excretory-secretory products from larval stages of Haemonchus contortus cultured in vitro. Veterinary Parasitology. 1996;62:291–305. doi: 10.1016/0304-4017(95)00871-3. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Serum-stimulated feeding in vitro by third-stage infective larvae of the canine hookworm Ancylostoma caninum. Journal of Parasitology. 1990;76:394–398. [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Long term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. Journal of the Helminthological Socociety of Washington. 1991;58:140–142. [Google Scholar]
- Hawdon JM, Volk SW, Rose R, Pritchard DI, Behnke JM, Schad GA. Observations on the feeding behaviour of parasitic third-stage hookworm larvae. Parasitology. 1993;106 (Pt 2):163–169. doi: 10.1017/s0031182000074953. [DOI] [PubMed] [Google Scholar]
- Hessler D, Young SJ, Carragher BO, Martone ME, Lamont S, Whittaker M, Milligan RA, Masliah E, Hinshaw JE, Ellisman MH. Programs for visualization in three-dimensional microscopy. Neuroimage. 1992;1:55–67. doi: 10.1016/1053-8119(92)90007-a. [DOI] [PubMed] [Google Scholar]
- Hotez P, Hawdon JM, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans daf-c paradigm. Parasitology Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu X, Boston R, Ashton FT, Gamble HR, Schad GA. Thermotaxis and thermosensory neurons in infective larvae of Haemonchus contortus, a passively ingested nematode parasite. Journal of Comparative Neurology. 2000;424:58–73. doi: 10.1002/1096-9861(20000814)424:1<58::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. International Journal for Parasitology. 2000;30:1115–1121. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Nolan TJ, Brenes M, Ashton FT, Zhu X, Forbes WM, Boston R, Schad GA. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology. 2004;129:753–759. doi: 10.1017/s0031182004006092. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]


