Abstract
Previously, we showed that ascorbate (Asc), by donating electrons to photosystem II (PSII), supports a sustained electron transport activity in leaves in which the oxygen-evolving complexes were inactivated with a heat pulse (49°C, 40 s). Here, by using wild-type, Asc-overproducing, and -deficient Arabidopsis (Arabidopsis thaliana) mutants (miox4 and vtc2-3, respectively), we investigated the physiological role of Asc as PSII electron donor in heat-stressed leaves (40°C, 15 min), lacking active oxygen-evolving complexes. Chlorophyll-a fluorescence transients show that in leaves excited with trains of saturating single-turnover flashes spaced 200 ms apart, allowing continual electron donation from Asc to PSII, the reaction centers remained functional even after thousands of turnovers. Higher flash frequencies or continuous illumination (300 μmol photons m−2 s−1) gradually inactivated them, a process that appeared to be initiated by a dramatic deceleration of the electron transfer from TyrZ to P680+, followed by the complete loss of charge separation activity. These processes occurred with half-times of 1.2 and 10 min, 2.8 and 23 min, and 4.1 and 51 min in vtc2-3, the wild type, and miox4, respectively, indicating that the rate of inactivation strongly depended on the Asc content of the leaves. The recovery of PSII activity, following the degradation of PSII proteins (D1, CP43, and PsbO), in moderate light (100 μmol photons m−2 s−1, comparable to growth light), was also retarded in the Asc-deficient mutant. These data show that high Asc content of leaves contributes significantly to the ability of plants to withstand heat-stress conditions.
Heat stress is a serious threat to plants, which can lead to a drastic reduction in crop yield (e.g. Wahid et al., 2007; Allakhverdiev et al., 2008). Exposure of plants to elevated temperatures results in the inactivation of Rubisco activase (Barta et al., 2010) and the oxygen-evolving complex (OEC) of PSII, including the removal of the extrinsic proteins as well as the release of calcium and manganese ions from their binding sites (Nash et al., 1985; Enami et al., 1994; Yamane et al., 1998; Barra et al., 2005). Heat stress can also damage the D1 and D2 proteins (De Las Rivas and Barber, 1997; Yoshioka et al., 2006).
Under natural conditions heat stress mostly occurs together with light stress. Though the effects of both stresses have been studied extensively, there are only a few studies where the mechanism of damage caused by combination of these two stress factors was investigated. It has been shown that in the absence of heat-adaptation processes, with yet an unknown mechanism(s), the two stress factors act in strong synergy to inactivate PSII in plants (Havaux, 1994), and in corals they lead to photobleaching (Abrego et al., 2008).
Inactivation of PSII by excess light and damage to the D1 protein are most probably induced by singlet oxygen, which is produced via interaction with the triplet reaction center chlorophyll (chl; 3P680) arising from the recombination of the charge-separated state between P680 and the pheophytin electron acceptor (3[P680+Phe]; Aro et al., 1993; Vass and Cser, 2009). This acceptor-side damage, as it has recently been shown by point mutation at the quinone-binding pocket of PSII in Synechocystis sp. PCC 6803 (Larom et al., 2010), can be alleviated by efficiently draining the electrons from the primary quinone acceptor QA to an exogenous electron acceptor, cytochrome c. In other terms it appears that photoinhibition can be prevented by warranting a rapid electron transfer from the acceptor side of PSII. A priori, it cannot be ruled out that photoinhibition under heat-stress conditions occurs with the same mechanism since heat stress can lead to a deceleration of the electron transfer rate between QA and QB, the primary and secondary quinone acceptors (Ducruet and Lemoine, 1985). However, elevated temperatures affect mainly the donor side of PSII, in particular the OEC. Experiments on PSII preparations and on leaves with chemically inactivated OECs indicate that the very high sensitivity of PSII to light appears to be caused by the impaired electron donation from the OEC. This, in turn, results in strong accumulation of highly oxidizing radicals, P680+, TyrZ+, and superoxide (Chen et al., 1995) or hydroxyl radicals (Spetea et al., 1997), and leads to a rapid inactivation and degradation of PSII reaction centers (Callahan et al., 1986; Blubaugh and Cheniae, 1990; Jegerschöld and Styring, 1996). This type of photodamage is called weak light or donor-side-induced photoinhibition. In analogy with the acceptor-side photoinhibition, it can be assumed, in fact in vitro data strongly suggest, that a continuous electron flow to PSII from alternative donors, such as diphenylcarbazide (DPC) or ascorbate (Asc), can alleviate the photoinactivation of the reaction centers (Mano et al., 1997).
In this article we provide experimental evidence that Asc plays a protective role in photoinhibition in heat-stressed leaves. Asc is present in the lumen probably at millimolar concentration (Foyer and Lelandais, 1996), and in the absence of active OEC serves as a relatively rapid (t1/2 approximately 25 ms) electron donor to PSII (Tóth et al., 2009), and thus might be capable of protecting PSII by supplying electrons to the reaction center. To test this hypothesis, we subjected intact leaves of wild-type, Asc-overproducing (miox4; Lorence et al., 2004), and -deficient mutant (vtc2-3; Conklin et al., 2000) Arabidopsis (Arabidopsis thaliana) plants to heat stress (40°C, 15 min) and investigated the time course and mechanism of photoinactivation of PSII.
RESULTS
Electron Donation Rates from Asc to PSII in Arabidopsis Genotypes with Different Asc Contents
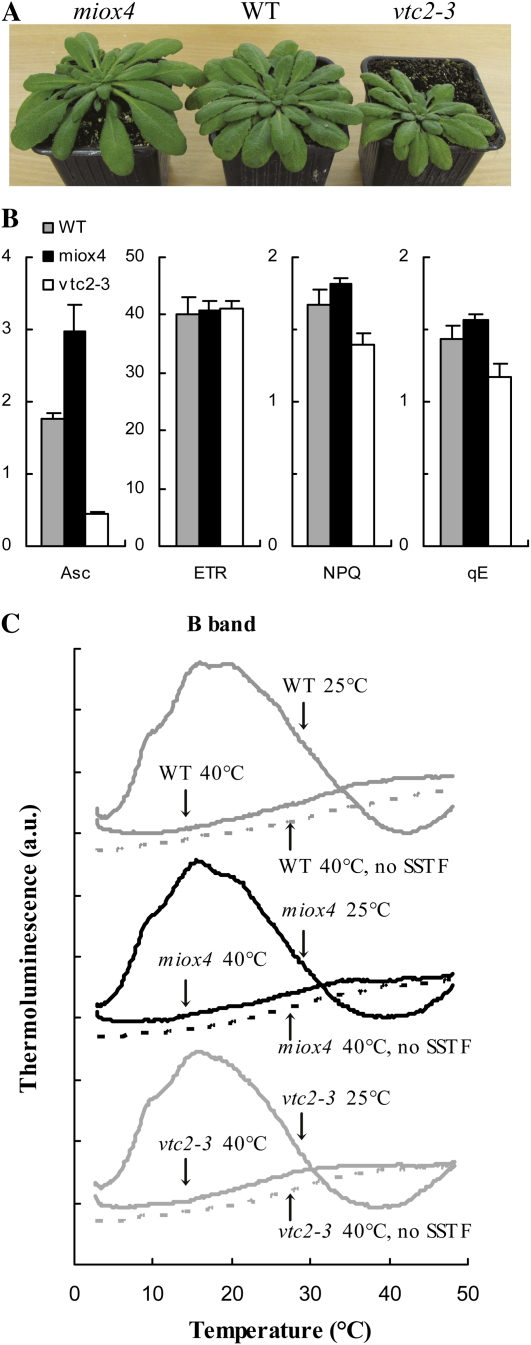
To study the physiological role of Asc as a PSII donor, we compared wild-type Arabidopsis, Asc-overproducing (miox4; Lorence et al., 2004), and Asc-deficient mutants (vtc2-3; Conklin et al., 2000) grown under identical conditions, at approximately 150 μmol photons m−2 s−1, 8-h light/16-h dark, the temperature was kept between 20°C and 24°C, and the plants were used when they were 2 to 3 months old. The Asc-deficient mutants were about 30% smaller than the wild-type plants (as reported by Müller-Moulé et al., 2004 as well) and the miox4 plants were somewhat larger (Fig. 1A). The Asc content of the vtc2-3 mutant was about 25% of the wild type and the Asc content of the miox4 mutant was about 70% higher than that of the wild type determined on chl basis (Fig. 1B; there were only minor differences between the chl contents of the three genotypes; data not shown). We also determined the nonphotochemical quenching (NPQ) and its energy-dependent quenching (qE) component, and the rate of electron transport (ETR) in the leaves. NPQ and qE were highest in the Asc-overproducing mutant (miox4) and lowest in the Asc-deficient mutant (vtc2-3) and there were no significant differences between the three genotypes in terms of ETR values (Fig. 1B). The results obtained on the vtc2-3 mutant are in agreement with the results of Müller-Moulé et al. (2002, 2003), who showed that the lower NPQ values are caused by the lower violaxanthin deepoxidase activity due to limitation in the available Asc in the lumen. The higher NPQ and qE values obtained on the miox4 mutant indicate that there is more Asc available in the lumen of the Asc-overproducing miox4 mutant than in the wild type. Determination of the lumenal Asc content does not seem to be feasible since upon chloroplast isolation Asc is mostly lost (Ivanov and Edwards, 2000).
Figure 1.
Comparison of wild-type, Asc-overproducing (miox4), and Asc-deficient (vtc2-3) Arabidopsis plants grown under identical conditions, at approximately 150 μmol photons m−2 s−1, 8-h light/16-h dark, the temperature was between 20°C and 24°C, and the plants were 2 to 3 months old. A, Photograph of the three genotypes. B, Asc contents (in nmol/μg chl), ETRs, NPQ, and qE values determined at 430 μmol photons m−2 s−1; ses are indicated. C, TL glow curves in leaves of untreated and heat-stressed (40°C, 15 min) plants. TL glow curves were detected after a SSTF or without preillumination. The curves are averages of three measurements and for clarity, they are shifted relative to each other. [See online article for color version of this figure.]
Detached leaves of wild-type, Asc-overproducing (miox4), and Asc-deficient (vtc2-3) Arabidopsis plants were subjected to heat stress at 40°C for 15 min in a water bath in the dark. This treatment was chosen to obtain a homogenous material with completely inactivated OECs and to separate the effects of heat and light stresses. The B thermoluminescence (TL) band (Vass, 2003) arising from recombination reactions between the S2 state of the OEC and QB−induced by a saturating single turnover flash (SSTF) was totally abolished by the heat treatment in all three genotypes, showing a total loss of oxygen-evolving activity (Fig. 1C). A 40°C heat treatment is physiologically relevant since leaf temperatures can easily reach 40°C in the field, especially under drought-stress conditions (Burghardt et al., 2008; Shahenshah and Isoda, 2010). This treatment leads to a moderate, less than 2-fold acceleration of the decay kinetics of the flash-induced electrochromic absorbance transient (Δ515), an indicator of the dissipation of the transmembrane electrical field (Junge, 1977): The t1/2 of the decay decreased from 55 to 34 ms, with similar behavior in the three genotypes. These data show that the permeability of the thylakoid membranes did not increase drastically as a result of the 40°C, 15-min heat treatment (in contrast to a 50°C heat treatment applied earlier that caused dissipation of the electric field within a few ms; Tóth et al., 2005); these data agree well with the results obtained on heat-treated Arabidopsis leaves (Krumova et al., 2010). Decrease in the initial amplitude of the flash-induced electrochromic absorbance transient was also observed, suggesting a partial inactivation of PSII reaction centers (data not shown; see below).
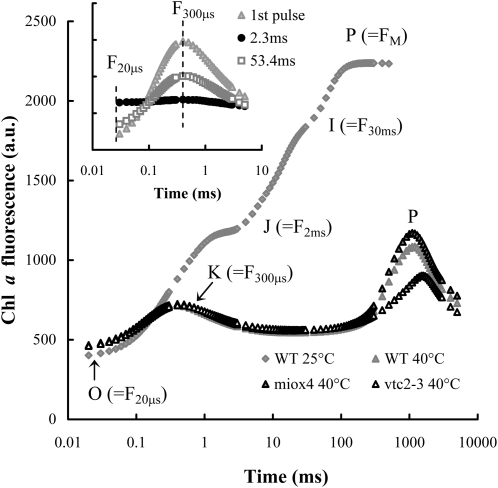
The fast chl a fluorescence (OJIP) transients (Govindjee, 2004; Lazár and Schansker, 2009) of untreated Asc-overproducing, Asc-deficient, and wild-type Arabidopsis plants were essentially the same, with identical Fv/Fm values (data not shown). The OJIP transient reflects the reduction of the electron transport chain including PSI (Schansker et al., 2005). In leaves containing PSII reaction centers with inactive OEC the K peak appears at around 300 μs with a concomitant disappearance of the J and I steps (Fig. 2). The K peak represents approximately one stable charge separation, with TyrZ as the electron donor (Srivastava et al., 1997; Tóth et al., 2007). After the K peak fluorescence intensity decreases to a level approaching F0 in a few ms due to the reoxidation of QA− by QB. In leaves a second peak appears at around 1 s (Fig. 2). We have shown previously that this latter phase is due to electron donation by Asc to PSII, leading to a partial reduction of the electron transport chain as shown by P700 measurements as well (Tóth et al., 2009). In heat-stressed (40°C, 15 min) Asc-deficient plants this second rise phase was considerably smaller than in the wild type and it was somewhat retarded, whereas in the Asc-overproducing mutant its intensity was somewhat higher than in the wild type (Fig. 2). This is in agreement with our previous finding that the intensity of this peak depends on the Asc content of the leaves (Tóth et al., 2009).
Figure 2.
OJIP transients in untreated wild type (WT) and heat-stressed (40°C, 15 min) wild-type, Asc-overproducing (miox4), and -deficient (vtc2-3) Arabidopsis mutants. The approximate positions of the different steps of the OJIP transient and of the K peak are indicated in parentheses. Inset: chl a fluorescence transients recorded on heat-stressed wild-type leaves during two 5-ms pulses that were spaced 2.3 and 53.4 ms apart. The excitation light (3,500 μmol photons m−2 s−1) was produced by three 650-nm LEDs and the chl a fluorescence was measured at wavelengths above 700 nm. The transients are averages of six kinetic traces.
The t1/2 of electron donation from Asc to TyrZ+ can be determined in samples with fully inactivated oxygen evolution by using two short (5-ms) light pulses and by varying the dark interval between them (Tóth et al., 2007, 2009). During the 5-ms light pulse one charge separation and the reoxidation of QA− by QB takes place. The inset of Figure 2 shows that after a 2.3-ms dark interval following the first light pulse there is no variable fluorescence, which is due to the full inactivation of oxygen evolution. However, with longer dark intervals, the K peak recovers following single exponential kinetics and the t1/2 of the regeneration of the K peak can be used as the half-time of the rereduction of TyrZ+ by Asc (Tóth et al., 2009). The t1/2 was approximately 30 ms in wild-type and miox4 leaves, whereas in the Asc-deficient mutant the electron donation was much slower, the t1/2 was approximately 50 ms (Table I). These results on heat-stressed leaves (40°C, 15 min) are in a good agreement with our previous findings where plants were subjected to a relatively harsh heat pulse of 49°C, 40 s (Tóth et al., 2009).
Table I. Half-times of electron donation to PSII from Asc (in heat-stressed wild-type and vtc2-3 Arabidopsis plants) and from Asc + DPC (in DPC-treated and heat-stressed vtc2-3 Arabidopsis mutant) and the half-time of the decrease of the amplitude of the K peak (as determined in Fig. 3B) in heat- + light-treated wild-type and vtc2-3 Arabidopsis plants.
| Parameters | Wild Type | vtc2-3 | vtc2-3 + 1 mm DPC |
| Half-time of electron donation to PSII | 30.5 ± 1.3 ms | 49.7 ± 3.6 ms | 30.4 ± 2.5 ms |
| Half-time of the decrease of the K peak in the light | 2.8 ± 0.5 min | 1.4 ± 0.1 min | 2.5 ± 0.3 min |
Asc-Dependent Multiple Turnover of PSII
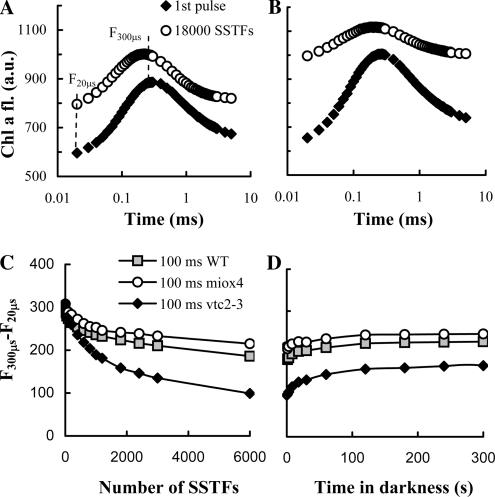
To ascertain the notion that in heat-stressed leaves Asc can continually support the electron transport through PSII, i.e. it sustains multiple turnovers of the reaction centers, without damage, we carried out experiments with trains of SSTFs. Figure 3A shows that when wild-type leaves were subjected to SSTFs that were spaced 200 ms apart, the K peak remained essentially unaffected even after 18,000 turnovers (flashing the leaves for 1 h). Similar data were obtained in vtc2-3 with 500-ms spacing between the flashes (data not shown). This shows that if the dark interval between the flashes is large enough to allow a continual electron donation from Asc to PSII (t1/2 approximately 30 and 50 ms in the wild type and the Asc-deficient mutant, respectively), there is no apparent limitation for the turnover of the reaction centers. In other terms, the pool of Asc available to PSII reduction is evidently very large; also, under these conditions, no significant damage appears to occur to PSII. A closer spacing of the SSTFs (e.g. Δt = 100 ms), which did not allow full regeneration of the K peak between flashes, led to the diminishment of the amplitude of the K peak (Fig. 3B). The decrease was strongest in vtc2-3 (the amplitude decreased by about 70% after 6,000 SSTFs) and weakest (30%) in miox4 (Fig. 3C). The decrease of the K peak progressed gradually. In the first phase, after about five to 10 flashes, the amplitude of the K peak decreased due to the approximately 9% increase in the F20μs value. This was followed by a much slower decrease in the amplitude of the K peak that strongly depended on the Asc content. The recovery of the K peak in the dark was also biphasic (Fig. 3D). The fast recovery of the K peak, occurring with t1/2 of about 4 s, can be accounted for by the recovery of non-QB-reducing centers (Chylla et al., 1987). The large, hardly recovering phase indicates that SSTFs in long train cause irreversible damage to PSII.
Figure 3.
Chl a fluorescence transients recorded on dark-adapted heat-stressed wild-type Arabidopsis leaves (first pulse) and after 18,000 SSTFs with 200-ms (A) or 100-ms (B) dark intervals between the consecutive flashes. C and D, Dependence of the amplitude of the K peak (F300μs − F20μs) as a function of SSTFs (C) spaced at 100 ms on wild-type (WT), Asc-overproducing (miox4), and -deficient (vtc2-3) Arabidopsis leaves, and its regeneration in the dark (D). The fluorescence transients after different numbers of SSTFs were recorded within 1 s after the last flash (A–C).
Inactivation of PSII Reaction Centers in Heat-Stressed Leaves Exposed to Continuous Illumination
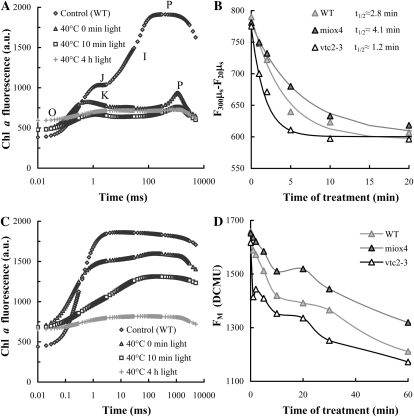
To investigate in more detail the photoinactivation of PSII and the influence of the Asc content, heat-stressed leaves (40°C, 15 min) were subjected to relatively strong continuous illumination (300 μmol photons m−2 s−1). This treatment resulted in relatively fast and gradual diminishment of the K peak as well as of the second peak at around 1 s (Fig. 4A). The rate of the diminishment of the K peak followed exponential kinetics and depended strongly on the Asc content of leaves: In the wild type it exhibited a t1/2 of 2.8 min, whereas the vtc2-3 and miox4 t1/2 values were 1.2 and 4.1 min, respectively (Fig. 4B).
Figure 4.
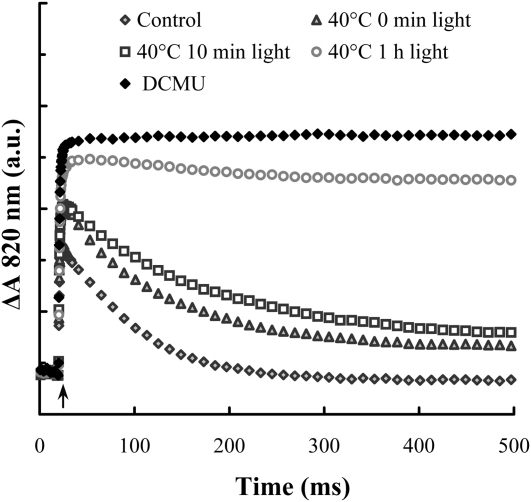
Chl a fluorescence transients (A and C) and the time course of the decrease of the K peak (B) and of the Fm value in the presence of DCMU (D) on heat-stressed wild-type (WT), Asc-overproducing (miox4), and -deficient (vtc2-3) Arabidopsis leaves exposed to continuous white light of 300 μmol photons m−2 s−1. The amplitude of the K peak on heat-stressed samples was calculated as F300μs − F20μs from traces shown in A, and data points were fitted with single exponentials, yielding t1/2 values as indicated in the figure. DCMU treatments were performed after the light treatments of heat-stressed leaves (C and D).
To examine the relative amount of active PSII reaction centers, leaves were incubated in the dark for 2 h in 3-(3′,4’-dichlorophenyl)-1,1-dimethylurea (DCMU) solution after the heat + light treatment (Fig. 4C) to ensure that all PSII reaction centers are closed during the fluorescence measurement. It can be seen that the heat stress per se induced about 15% decrease in the Fm value. Upon light treatment of heat-stressed samples, the fluorescence intensity gradually decreased and after 4 h the Fm value almost equaled F0. Again, the decrease in the amplitude was significantly faster in the Asc-deficient mutant than in the wild type (apparent t1/2 10 and 23 min, respectively). This difference was even more pronounced when comparing it with the Asc-overproducing mutant (t1/2 approximately 51 min; Fig. 4D). Very similar rates were obtained in the presence of the protein synthesis inhibitor lincomycin, showing that in 4 h, no significant recovery occurred (data not shown). It is also interesting to note that in the light-treated leaves the fluorescence rise was strongly decelerated: In the wild type, the t1/2 increased from 0.3 to 2.3 ms in 1 h; deceleration was even stronger in vtc2-3 (from 0.3–3 ms).
The fast decrease in the amplitude of the K peak and the slowdown of the fluorescence rise determined in the presence of DCMU suggest that the first step of photoinactivation is the slowdown of the TyrZ-P680+ (as suggested by Blubaugh et al., 1991) and this is followed by a complete inactivation of the charge separation activity of PSII (see “Discussion” for more details). Our data also show that the rate of PSII inactivation depends strongly on the Asc content of the leaves. Asc is not only an alternative electron donor to PSII but also a direct scavenger of reactive oxygen species (ROS) produced during donor-side-induced photoinhibition (e.g. Chen et al., 1995; Spetea et al., 1997). To ascertain that the Asc dependence correlates with the ability of Asc to act as a PSII electron donor, we incubated intact vtc2-3 leaves in DPC solution (1 mm DPC, an artificial electron donor of PSII, with no ROS-scavenging properties) before the heat treatment. In DPC-treated vtc2-3 leaves the t1/2 of electron donation to PSII became similar to that in the wild type (about 30 ms; Table I) and the fluorescence intensity at around 1 s also increased. In DPC-incubated leaves exposed to light, the K peak diminished more slowly than in untreated leaves (t1/2s of 2.5 and 1.4 min, respectively), at a rate similar to that found in wild-type leaves (2.8 min; see also Fig. 4B). This is a strong indication that Asc slows down the inactivation of the PSII reaction centers by acting as PSII electron donor and not only as a scavenger of ROS.
It is also known that Asc is required for the formation of NPQ (more specifically, qE), because it is a substrate for violaxanthin deepoxidase (Hager, 1969). qE plays a photoprotective role and therefore we have to consider if the faster rate of PSII inactivation observed in the vtc2-3 mutant might be caused by smaller qE. Determination of NPQ and qE in heat-treated samples is not possible because Fm can only be reached when DCMU is present (Fig. 4; see also Tóth et al., 2007). However, the data show that the t1/2 of electron donation from Asc to PSII in heat-treated samples is slow compared to electron donation by active OECs (30–50 ms versus 0.1–1 ms; Babcock et al., 1976) and therefore, significant and persistent reduction of the electron transport chain, a prerequisite for the formation of qE, is very unlikely: Illumination of samples with completely inactivated OECs at 300 μmol photons m−2 s−1 would result in electron transport comparable to that occurring at around 10 μmol photons m−2 s−1 in the case of non-heat-treated samples. Light intensities below 40 μmol photons m−2 s−1 are insufficient for the formation of qE in any of the three genotypes studied (data not presented; see Müller-Moulé et al., 2002), therefore photoprotection by qE formation during our photoinactivation experiments is unlikely to occur.
As shown in Figure 5, in untreated wild-type leaves, fast oxidation of P700 was followed by rereduction in about 200 ms due to electrons arriving from PSII. In heat-treated leaves, in which the electrons were donated by Asc, the rereduction was slower, in good accordance with our earlier data obtained on leaves exposed to heat pulses (49°C, 40 s; Tóth et al., 2009). Light treatment of heat-stressed leaves caused further, gradual deceleration of the rereduction and after about 1 h only a very limited rereduction could be observed. These data show that upon light treatment of heat-stressed leaves, electron transport to PSI was strongly decelerated or even stopped and this might lead to some loss in the amount of P700, as indicated by the slight decrease in the amplitude after 1 and 4 h of illumination, compared to DCMU-treated leaves.
Figure 5.
Light-induced 820-nm absorbance transients in untreated (control), heat-stressed (40°C, 15 min), and heat- + light-treated wild-type Arabidopsis plants using white light of 300 μmol photons m−2 s−1 for different time intervals as indicated in the figure; for comparison, a transient obtained on DCMU-treated leaves is also plotted. The measurements were performed on dark-adapted samples using red light of 5,000 μmol photons m−2 s−1. The traces are averages of four to five measurements.
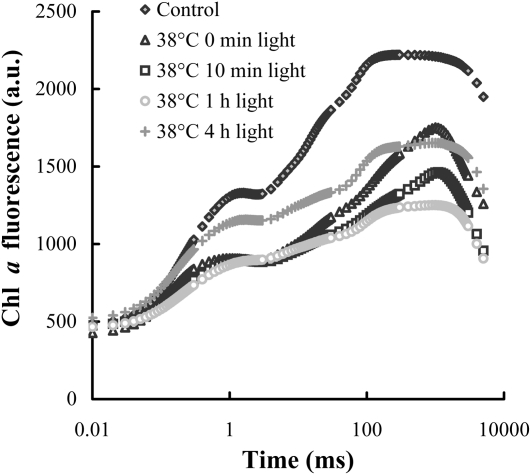
Photoinactivation was studied in leaves with partially inhibited OEC activity as well. To this end, wild-type leaves were subjected to a 15-min heat stress at 38°C, inactivating about 40% of oxygen evolution, as estimated on the basis of TL measurements (data not shown). Accordingly, the fluorescence transient (Fig. 6) carries the characteristics of both the untreated leaves and those that contain no active OECs (compare with Fig. 4A): The variable fluorescence decreased, albeit less than after the 40°C treatment, and Fm peaked at around 1 s, similarly to the sample with fully inactivated OEC, but also exhibited a shoulder at 200 ms, characteristic of the untreated leaves. Illumination (300 μmol photons m−2 s−1) caused a further decrease of the variable fluorescence that was mostly due to the disappearance of the peak around 1 s, showing that the contribution of reaction centers with inactive OECs gradually decreased during the light treatment, i.e. they became inactivated by light as in leaves with fully inhibited oxygen evolution (Fig. 4A). Indeed, after 1 h illumination the OJIP transient exhibited the typical steps, but with lower amplitudes. After 4 h, a significant part of this loss was recovered in the light.
Figure 6.
OJIP transients of untreated and heat-treated (38°C, 15 min) wild-type Arabidopsis plants, measured after various times in the light (300 μmol photons m−2 s−1), as indicated in the figure.
Light-Induced Degradation of PSII Proteins in Heat-Stressed Leaves
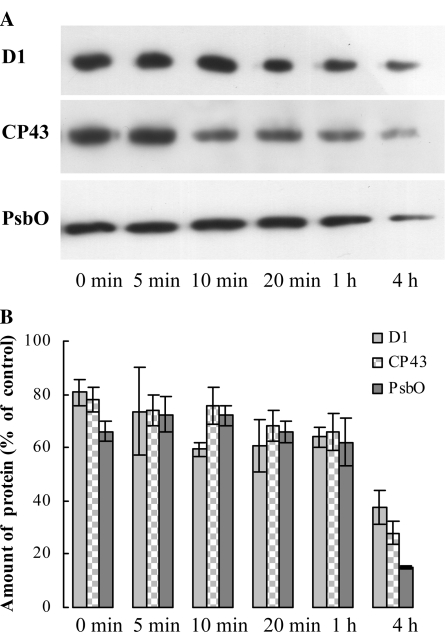
As shown above, after 4 h of illumination of heat-stressed leaves (40°C, 15 min; Fig. 4C), there was a complete loss of charge separation activity of PSII reaction centers. During acceptor-side photoinhibition, the inactivation of the reaction centers is accompanied by protein degradation, which is confined to the D1 protein (Schuster et al., 1988; Aro et al., 1993; van Wijk et al., 1994). To investigate if PSII reaction centers in heat-stressed leaves suffer the same or different light-induced damage, western-blot analyses were performed, using wild-type leaves.
Data in Figure 7 show that upon the heat stress about 20% of D1 protein was lost, which is in agreement with earlier results (Yoshioka et al., 2006; Yamashita et al., 2008). Light treatment on heat-stressed leaves resulted in further, gradual degradation of D1: In 1 h D1 content decreased by about 40% relative to the untreated control and after 4 h approximately 65% of the D1 protein was lost. These data are in good agreement with data obtained with chl a fluorescence measurements in the presence of DCMU (Fig. 4C). In contrast to acceptor-side photoinhibition, we also observed significant losses in the amount of CP43 and the PsbO protein; by the 4th h of the light treatment of heat-treated leaves, 70% and 80% of CP43 and PsbO were lost, respectively. These data suggest that there was a complete disassembly of PSII reaction centers upon the light treatment of heat-stressed leaves.
Figure 7.
Dependence of the amounts of D1, CP43, and PsbO, determined by western-blot analyses in heat (40°C, 15 min) and heat- + light-treated wild-type Arabidopsis plants as a function of illumination time (white light of 300 photons μmol m−2 s−1). Typical blots (A) and averages (B) determined by densitometry from four to five blots as expressed in percentage of the untreated (no heat-, no light-treated) control.
Time Course of PSII Inactivation and Recovery
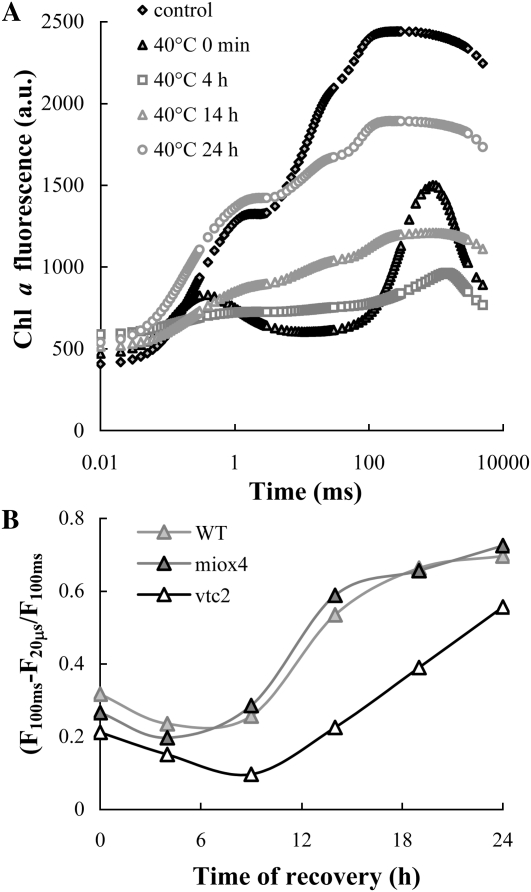
We investigated the effect of Asc also during the light-dependent recovery of PSII activity following photoinactivation in heat-stressed leaves. The experiments were carried out at moderate light intensity, 100 μmol photons m−2 s−1 that is comparable to the growth light. In the dark neither significant damage nor recovery occurs (Tóth et al., 2005). On the other hand, at 300 μmol photons m−2 s−1 recovery was very slow and some photobleaching occurred (data not shown). It can be seen that at 100 μmol photons m−2 s−1 the K peak substantially diminished in a few hours (Fig. 8A), indicating a significant perturbation of the charge separation activity of PSII reaction centers, similar to the case at the higher light intensity i.e. at 300 μmol photons m−2 s−1. This was followed by a gradual recovery of the OJIP transient, which was completed in about 24 h. To monitor the recovery of PSII activity in the vtc2-3, miox4, and wild-type plants, we used the (F100ms − F20μs)/F100ms parameter introduced earlier (Tóth et al., 2005), rather than the Fv/Fm ratio, which in the presence of inactive OEC also depends on the Asc content of the leaves (compare with Fig. 2). Our data show that the rate of recovery was significantly faster in the wild type and in miox4 than in vtc2-3, which appeared to suffer more substantial damage (Fig. 8B).
Figure 8.
Recovery of the photosynthetic activity in the light (at 100 μmol photons m−2 s−1, at 24°C), as monitored by OJIP transients, in heat- + light-treated Arabidopsis plants. A, OJIP transients measured 0, 4, 14, and 24 h after the heat treatments on wild-type Arabidopsis. B, Time course of the recovery of photosynthetic activity, as reflected by the (F100ms − F20μs)/F100ms parameter in the wild-type, Asc-overproducing (miox4), and -deficient (vtc2-3) Arabidopsis mutants.
DISCUSSION
Asc as a PSII Donor
In this work, by using Asc-overproducing (miox4), -deficient (vtc2-3), and wild-type plants, we show that Asc retards the photoinactivation of PSII in heat-stressed leaves with inactive OECs by acting as a PSII donor. We also provide evidence that photoinhibition in heat-stressed leaves occurs with a similar mechanism and time course as described for samples with chemically inactivated OECs (Blubaugh et al., 1991; Jegerschöld and Styring, 1996).
Our experiments on heat-stressed leaves subjected to trains of SSTFs show that the reaction centers are capable of tens of thousands of turnovers, without noticeable damage—provided that the electron donation rate by Asc warrants full rereduction of TyrZ+ between flashes. This depends on the flash frequency and the Asc content of the leaves (Fig. 3). These data show that the Asc pool was capable to support tens of thousands of stable charge separations in PSII. Indeed, it has been estimated that the Asc content of chloroplasts is about 25 to 50 mm (Eskling and Åkerlund, 1998; Smirnoff, 2000) and at least 4 mm in the thylakoid lumen (Foyer and Lelandais, 1996). The transport through the chloroplast envelope membranes is relatively fast and is rapidly equilibrated (Foyer and Lelandais, 1996); it is probably equally fast through the thylakoid membrane (Mano et al., 2004). Oxidized Asc regenerates in the Halliwell-Asada cycle and probably by other means as well (Potters et al., 2002).
Donor-Side-Induced Photoinhibition and Dependence on the Asc Content
When the dark intervals between SSTFs were not sufficiently long (in wild type and vtc2-3, shorter than 100 and 200 ms) and appeared not to allow full regeneration of the K peak between flashes the amplitude of the K peak gradually diminished (Fig. 3). The extent of this diminishment, which was largely irreversible, depended strongly on the Asc content of the leaves and occurred in several minutes (Fig. 3C). In continuous light of 300 μmol photons m−2 s−1, the amplitude of the K peak decreased with 1.2, 2.8, and 4.1 min t1/2s in vtc2-3, the wild type, and miox4, respectively. This diminishment of the K peak was irreversible, indicating damage to the reaction center. The fact that Asc in vtc2-3 could be replaced with DPC (Table I) points to the role of this endogenous alternative electron donor in photoprotection, and shows that the disappearance of the K peak is indeed correlated with limitation in electron donation. In the presence of DCMU variable fluorescence was significantly higher than in its absence (up to about 1 h of illumination), indicating that charge separation activity of PSII is yet retained, albeit the fluorescence rise gradually slowed down during the illumination period. Similar phenomenon was observed by Callahan and Cheniae (1985) in hydroxylamine-treated leaves. The deceleration of the fluorescence rise originates from slowing down of the electron transfer between TyrZ and P680+, as suggested earlier by Blubaugh et al. (1991) based on electron paramagnetic resonance measurements. The disappearance of the K peak, together with the relatively high Fm value detected in the presence of DCMU, shows that in the heat- + light-treated samples the rate of TyrZ-P680+ electron transfer becomes comparable to the rate of QA to QB electron transfer, i.e. there is a deceleration of several orders of magnitude, from about 100 ns to at least hundreds of μs.
It is known that heat stress might lead to a slowdown of electron transport between QA and QB (Ducruet and Lemoine, 1985). However, OEC is more sensitive to high temperature than the acceptor side of PSII and slowdown of electron transfer from QA− to QB can be observed only above 42°C (Pospíšil and Tyystjärvi, 1999). Thus, the occurrence of acceptor-side photoinhibition is quite unlikely. Also, our data showing that in the presence of DCMU much higher Fm values could be obtained than in its absence points to a much stronger limitation at the donor side of PSII as well. Further, in acceptor-side photoinhibition ROS are produced (e.g. Hideg et al., 1998), which would not be possible to slow down by DPC, an artificial PSII donor with no ROS-scavenging properties.
Gradual inactivation of charge separation activity of PSII in continuous light occurred on a time scale of tens of minutes, as reflected by the decrease of the Fm value in the presence of DCMU as well as by the redox changes of P700 (Figs. 4C and 5). Based on our data showing the parallel loss of CP43 and PsbO protein and on earlier experiments on isolated PSII and thylakoid membranes (Mori and Yamamoto, 1992; Shing and Shinghal, 1999), we suggest that donor-side-induced photoinhibition is accompanied by extensive protein degradation, during which probably the whole PSII reaction center disassembled. This is in contrast to acceptor-side photoinhibition where protein degradation is mostly confined to the D1 protein (Schuster et al., 1988; van Wijk et al., 1994). Again, the fluorescence transients in presence of DCMU clearly show that the rate of full inactivation of PSII reaction centers strongly depends on the Asc content of the leaves (Fig. 4D).
The rates of inactivation of PSII were very similar in the presence and absence of the protein synthesis inhibitor lincomycin (data not shown). This indicates that the damage and degradation of the D1 protein is the primary cause of photoinactivation and Asc may primarily prevent damage to the D1 protein by donating electron to TyrZ+ and then to P680+.
Physiological Significance
In nature heat stress is usually accompanied by high light, and therefore, photoinactivation of PSII is very likely to occur at high temperatures. As concerns the physiological significance of the Asc-dependent retardation of photoinactivation of PSII, we must take into account that full protection could only be achieved at low frequencies of the exciting flashes, corresponding to very low light intensities, in the range of several μmol photons m−2 s−1. Higher flash frequencies or continuous illumination (100 and 300 μmol photons m−2 s−1) lead to losses in PSII activity (Figs. 4 and 8). Photoinactivation occurred in samples with partial OEC inhibition as well (38°C; Fig. 6) and even in the case of a very mild heat treatment (36°C, 1 min) PSII reaction centers did not recover on the time scale of tens of minutes (J. Frolec, personal communication), suggesting that the same mechanism is taking place as in the case of heat stresses abolishing all OEC activity.
Our data showing complete inactivation of PSII (Figs. 4 and 6) and protein degradation (Fig. 7) suggest that recovery is preceded by a complete inactivation of PSII reaction centers and does not appear to occur directly from the OEC-inactivated state. When investigating the rate of recovery from the photoinactivated state, large differences were found between the Asc-deficient and the wild-type and Asc-overproducing plants (Fig. 8). This might indicate that since the rate of photoinactivation is faster in the Asc-deficient plants, during photoinactivation more ROS (hydroxyl radical or superoxide) can be produced, which in turn cause more severe damage, not only to the reaction centers but also other compounds of the thylakoid membranes; in addition, the repair processes might also be affected, which might also depend on the rate of ROS production (Nishiyama et al., 2006), and it is also conceivable that Asc acts an electron donor during the synthesis of PSII units, before the extrinsic proteins are attached to the reaction center. In the light of these data and considerations, we propose that in heat-stressed leaves under natural conditions the role of Asc as alternative PSII electron donor is to slow down the photoinactivation processes and by this means minimize the generation of ROS in the thylakoid membranes, and thus alleviate the damage to the entire photosynthetic machinery.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana Columbia-0 [wild-type Arabidopsis]), Asc-deficient (vtc2-3; Conklin et al., 2000), and Asc-overproducing mutant (miox4; Lorence et al., 2004) plants were grown in a growth chamber under short-day conditions (8-h light, 16-h dark), at approximately 150 μmol photons m−2 s−1 in the light period. The temperature was kept between 20°C and 24°C.
The vtc2 mutants are affected in the Gal pathway of Asc biosynthesis, more specifically in GDP-l-Gal phosphorylase, an enzyme that is at a branch point of between Asc synthesis and incorporation of l-Gal into cell wall polysaccharides (Asc biosynthesis was reviewed by Linster and Clarke, 2008). The miox4 mutant was created by constitutive expression of miox4, a gene encoding myoinositol oxygenase in Arabidopsis plants (Lorence et al., 2004).
Heat Treatments and Chemical Treatments
Heat treatment: Whole leaves were submerged in a water bath in darkness for 15 min. Complete inactivation of oxygen evolution was achieved by a treatment at 40°C or sometimes 41°C, depending on the age of the plants; inactivation of OEC was tested with the aid of TL measurements. To obtain partial inactivation of OEC, the heat treatment was performed at 38°C.
Light treatments: Trains of SSTFs from a xenon lamp were given with dark intervals of 100, 200, and 500 ms for different time periods (10 min–1 h). Continuous light treatments of heat-treated leaves were carried out at 300 μmol photons m−2 s−1 and the treatment lasted for up to 4 h. For recovery experiments, heat-treated leaves were illuminated for 24 h at 100 μmol photons m−2 s−1, at 24°C.
DPC treatment: vtc2-3 leaves were incubated in 1 mm DPC. Leaves were placed in petri dishes and covered with one layer of filter paper for 2 h; by this means, the leaves were exposed to white light of approximately 50 μmol photons m−2 s−1.
DCMU treatment: Whole leaves were incubated in 0.2 mm DCMU solution for 2 h in complete darkness after the heat + light treatment. The solution contained 0.2% dimethyl sulfoxide to dissolve the DCMU.
Determination of Asc Content
The Asc contents of wild-type Arabidopsis, vtc2-3, and miox4 mutants were determined by a spectroscopic method using the absorption at 265 nm of Asc (Takahama and Oniki, 1992).
TL Measurements
TL was measured using a custom-made TL apparatus described by Wiessner and Demeter (1988). Leaf discs were placed on a copper sample holder, connected to a cold finger immersed in liquid nitrogen. A heater coil, placed under the sample holder, ensured the desired temperature of the sample during the measurements. Thoroughly dark-adapted samples were illuminated at 1°C by a SSTF and TL was measured while heating the sample to 70°C in darkness with a heating rate of 20°C min−1. The emitted TL was measured with a Hamamatsu end-window photomultiplier.
Measurement of Flash-Induced Electrochromic Absorbance Transients (ΔA515)
Electrochromic absorbance changes, induced by single turnover flashes, were measured at 515 nm, the maximum of the electrochromic transients, in a set up described by Büchel and Garab (1995). The time constant was set to 100 μs; 32 kinetic traces were collected with a repetition rate of 2 s−1 and averaged. The transients were recorded at room temperature on detached Arabidopsis leaves.
Determination of ETR, NPQ, and qE; Measurement of the Oxidation-Reduction Kinetics of P700
The ETR, NPQ, and qE were determined by a Dual-PAM-100 instrument (Heinz Walz GmbH), on overnight dark-adapted leaves, with saturating pulses of 5,000 μmol photons m−2 s−1 during illumination at 430 μmol photons m−2 s−1 for 8 min (ETR, NPQ) and a subsequent dark adaptation for 2 min (qE). Redox changes of P700 were measured with the same instrument. The absorbance changes in continuous red light (5,000 μmol photons m−2 s−1) were measured at 830/870 nm with a time resolution of 60 μs.
OJIP Measurements
Fluorescence measurements were carried out at room temperature with a special version of the Handy-PEA instrument (Hansatech Instruments Ltd.) that allows reducing the length of the measurement to 300 μs. Leaf samples were illuminated with continuous red light emitted by three LEDs (3,500 μmol photons m−2 s−1, 650 nm peak wavelength; the spectral half-width was 22 nm; the light is cut off at 700 nm by a near-infrared short-pass filter). The first reliably measured point of the fluorescence transient is at 20 μs, which was taken as F0. The length of the measurements was 5 s or 5 ms. In the case of the double 5-ms pulses, the dark intervals between the light pulses were 2.3, 9.6, 16.9, 31.5, 38.8, 53.4, 75.3, 100, 200, or 500 ms.
Western-Blot Analysis
Leaf discs equivalent to a total area of 3.1 cm2 cut from Arabidopsis leaves were frozen in liquid nitrogen and ground to a fine powder and then homogenized in 500 μL Laemmli buffer. The homogenates were incubated at 90°C for 5 min followed by a 20-min incubation at 37°C, and then proteins were separated by 15% denaturing SDS-PAGE. The proteins were blotted on nitrocellulose membranes using a semidry blotting system with methanol-containing buffer. The nitrocellulose membranes were blocked using 5% skim milk powder in Tris-buffered saline plus Tween 20 (TBST) buffer (10 mm TRIS pH 8.0, 0.15 m NaCl, 0.1% Tween 20) for 2 h and incubated with primary antibodies raised against PsbA (D1), PsbC (CP43), and PsbO (33 kD OEC protein; Agrisera AB) for 2 h in TBST buffer with 5% milk powder. The membranes were washed three times for 5 min in TBST buffer and incubated with goat anti-rabbit IgG horseradish peroxidase conjugate (Millipore) at a 1:5,000 dilution in TBST buffer with 5% milk powder for 2 h. Immunoblotted membranes were incubated for 5 min in ECL plus horseradish peroxidase substrate (GE Healthcare Bio-Sciences) and chemiluminescence was detected on Hyperfilm ECL photographic film (GE Healthcare Bio-Sciences). The developed film was digitalized and analyzed by 1D Scan software package.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_118819.2 (vtc2) and NM_118759.4 (miox4).
Acknowledgments
We thank Dr. Argelia Lorence and Prof. Craig L. Nessler (Arkansas State University) for the miox4 mutant and Prof. Patricia Conklin (State University of New York College at Cortland) for providing the vtc2 mutants. We also thank Dr. Gert Schansker (Biological Research Center Szeged) for critical reading of the manuscript and helpful suggestions.
References
- Abrego D, Ulstrup KE, Willis BL, van Oppen MJH. (2008) Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc Biol Sci 275: 2273–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98: 541–550 [DOI] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B. (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Babcock GT, Blankenship RE, Sauer K. (1976) Reaction kinetics for positive charge accumulation on the water side of chloroplast photosystem II. FEBS Lett 61: 286–289 [DOI] [PubMed] [Google Scholar]
- Barra M, Haumann M, Dau H. (2005) Specific loss of the extrinsic 18 KDa protein from photosystem II upon heating to 47°C causes inactivation of oxygen evolution likely due to Ca release from the Mn-complex. Photosynth Res 84: 231–237 [DOI] [PubMed] [Google Scholar]
- Barta C, Dunkle AM, Wachter RM, Salvucci ME. (2010) Structural changes associated with the acute thermal instability of Rubisco activase. Arch Biochem Biophys 499: 17–25 [DOI] [PubMed] [Google Scholar]
- Blubaugh DJ, Atamian M, Babcock GT, Golbeck JH, Cheniae GM. (1991) Photoinhibition of hydroxylamine-extracted photosystem II membranes: identification of the sites of photodamage. Biochemistry 30: 7586–7597 [DOI] [PubMed] [Google Scholar]
- Blubaugh DJ, Cheniae GM. (1990) Kinetics of photoinhibition in hydroxylamine-extracted photosystem II membranes: relevance to photoactivation and sites of electron donation. Biochemistry 29: 5109–5118 [DOI] [PubMed] [Google Scholar]
- Büchel C, Garab G. (1995) Electrochromic absorbance changes in the chlorophyll-c-containing alga Pleurochloris meiringensis (Xanthophyceae). Photosynth Res 43: 49–56 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Burghardt A, Gall J, Rosenberger C, Riederer M. (2008) Ecophysiological adaptations of water relations of Teucrium chamaedrys L. to the hot and dry climate of xeric limestone sites in Franconia (Southern Germany). Flora 203: 3–13 [Google Scholar]
- Callahan FE, Becker DW, Cheniae GM. (1986) Studies on the photoactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery. Plant Physiol 82: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan FE, Cheniae GM. (1985) Studies on the photoactivation of the water-oxidizing enzyme. I. Processes limiting photoactivation in hydroxylamine-extracted leaf segments. Plant Physiol 79: 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GX, Blubaugh DJ, Homann PH, Golbeck JH, Cheniae GM. (1995) Superoxide contributes to the rapid inactivation of specific secondary donors of the photosystem II reaction center during photodamage of manganese-depleted photosystem II membranes. Biochemistry 34: 2317–2332 [DOI] [PubMed] [Google Scholar]
- Chylla RA, Garab G, Whitmarsh J. (1987) Evidence for slow turnover in a fraction of photosystem II complexes in thylakoid membranes. Biochim Biophys Acta 894: 562–571 [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Rivas J, Barber J. (1997) Structure and thermal stability of photosystem II reaction centers studied by infrared spectroscopy. Biochemistry 36: 8897–8903 [DOI] [PubMed] [Google Scholar]
- Ducruet JM, Lemoine Y. (1985) Increased heat sensitivity of the photosynthetic apparatus in triazine-resistant biotypes from different plant species. Plant Cell Physiol 26: 419–429 [Google Scholar]
- Enami I, Kitamura M, Tomo T, Isokawa Y, Ohta H, Katoh S. (1994) Is the primary cause of thermal inactivation of oxygen evolution in spinach PSII membranes release of the extrinsic 33 kDa protein or of Mn? Biochim Biophys Acta 1186: 52–58 [Google Scholar]
- Eskling M, Åkerlund H-E. (1998) Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth Res 57: 41–50 [Google Scholar]
- Foyer CH, Lelandais MA. (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol 148: 391–398 [Google Scholar]
- Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. Papageorgiou GC, Govindjee, , Chlorophyll a Fluorescence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration, Vol 19. Springer, Dordrecht, The Netherlands, pp 1–42 [Google Scholar]
- Hager A. (1969) Lichtbedingte pH-erniedrigung in einem chloroplasten-kompartiment als ursache der enzymatischen violaxanthin-zeaxanthin-umwandlung; beziehungen zur photophosphorylierung. Planta 89: 224–243 [DOI] [PubMed] [Google Scholar]
- Havaux M. (1994) Temperature-dependent modulation of the photoinhibition-sensitivity of photosystem II in Solanum tuberosum leaves. Plant Cell Physiol 35: 757–766 [Google Scholar]
- Hideg É, Kálai T, Hideg K, Vass I. (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 37: 11405–11411 [DOI] [PubMed] [Google Scholar]
- Ivanov B, Edwards G. (2000) Influence of ascorbate and the Mehler peroxidase reaction on non-photochemical quenching of chlorophyll fluorescence in maize mesophyll chloroplasts. Planta 210: 765–774 [DOI] [PubMed] [Google Scholar]
- Jegerschöld C, Styring S. (1996) Spectroscopic characterization of intermediate steps involved in donor-side-induced photoinhibition of photosystem II. Biochemistry 35: 7794–7801 [DOI] [PubMed] [Google Scholar]
- Junge W. (1977) Membrane potentials in photosynthesis. Annu Rev Plant Physiol 28: 503–536 [Google Scholar]
- Krumova SB, Laptenok SP, Kovács L, Tóth T, van Hoek A, Garab G, van Amerongen H. (2010) Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth Res 105: 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larom S, Salama F, Schuster G, Adir N. (2010) Engineering of an alternative electron transfer path in photosystem II. Proc Natl Acad Sci USA 107: 9650–9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazár D, Schansker G. (2009) Models of Chlorophyll a fluorescence transients. Laisk A, Nedbal L, Govindjee, , Photosynthesis in Silico; Understanding Complexity from Molecules to Ecosystems, Advances in Photosynthesis and Respiration, Vol 29. Springer, Dordrecht, The Netherlands, pp 85–123 [Google Scholar]
- Linster CL, Clarke SG. (2008) L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci 13: 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL. (2004) Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134: 1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J, Hideg É, Asada K. (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429: 71–80 [DOI] [PubMed] [Google Scholar]
- Mano J, Ushimaru T, Asada K. (1997) Ascorbate in thylakoid lumen as an endogenous electron donor to photosystem II: protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase. Photosynth Res 53: 197–204 [Google Scholar]
- Mori H, Yamamoto Y. (1992) Deletion of antenna chlorophyll-a-binding proteins CP43 and CP47 by Tris-treatment of PSII membranes in weak light: evidence for a photo-degradative effect on the PSII components other than the reaction center-binding proteins. Biochim Biophys Acta 1100: 293–298 [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK. (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Golan T, Niyogi KK. (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Havaux M, Niyogi KK. (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133: 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D, Miyao M, Murata N. (1985) Heat inactivation of oxygen evolution in photosystem II particles and its acceleration by chloride depletion and exogenous manganese. Biochim Biophys Acta 807: 127–133 [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757: 742–749 [DOI] [PubMed] [Google Scholar]
- Pospíšil P, Tyystjärvi E. (1999) Molecular mechanism of high-temperature-induced inhibition of acceptor side of photosystem II. Photosynth Res 62: 55–66 [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40: 537–548 [Google Scholar]
- Schansker G, Tóth SZ, Strasser RJ. (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706: 250–261 [DOI] [PubMed] [Google Scholar]
- Schuster G, Timber R, Ohad I. (1988) Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur J Biochem 177: 403–410 [DOI] [PubMed] [Google Scholar]
- Shahenshah Isoda A. (2010) Effects of water stress on leaf temperature and chlorophyll fluorescence parameters in cotton and peanut. Plant Prod Sci 13: 269–278 [Google Scholar]
- Shing AK, Shinghal GS. (1999) Formation of cross-linking between photosystem 2 proteins during irradiation of thylakoid membranes at high temperature. Photosynthetica 36: 213–223 [Google Scholar]
- Smirnoff N. (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea C, Hideg E, Vass I. (1997) Low pH accelerates light-induced damage of photosystem II by enhancing the probability of the donor-side mechanism of photoinhibition. Biochim Biophys Acta 1318: 275–283 [Google Scholar]
- Srivastava A, Guissé B, Greppin H, Strasser RJ. (1997) Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll-a fluorescence transient: OKJIP. Biochim Biophys Acta 1320: 95–106 [Google Scholar]
- Takahama U, Oniki T. (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33: 379–387 [Google Scholar]
- Tóth SZ, Puthur JT, Nagy V, Garab G. (2009) Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol 149: 1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Garab G, Strasser RJ. (2007) Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem II. Biochim Biophys Acta 1767: 295–305 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Kissimon J, Kovács L, Garab G, Strasser RJ. (2005) Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). J Plant Physiol 162: 181–194 [DOI] [PubMed] [Google Scholar]
- van Wijk KJ, Nilsson LO, Styring S. (1994) Synthesis of reaction center proteins and reactivation of redox components during repair of photosystem II after light-induced inactivation. J Biol Chem 269: 28382–28392 [PubMed] [Google Scholar]
- Vass I. (2003) The history of photosynthetic thermoluminescence. Photosynth Res 76: 303–318 [DOI] [PubMed] [Google Scholar]
- Vass I, Cser K. (2009) Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci 14: 200–205 [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61: 199–223 [Google Scholar]
- Wiessner W, Demeter S. (1988) Comparative thermoluminescence study of autotrophically and photoheterotrophically cultivated Chlamydobotrys stellata. Photosynth Res 18: 345–356 [DOI] [PubMed] [Google Scholar]
- Yamane Y, Kashino Y, Koike H, Satoh K. (1998) Effects of high temperatures on the photosynthetic systems in spinach: oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth Res 57: 51–59 [Google Scholar]
- Yamashita A, Nijo N, Pospísil P, Morita N, Takenaka D, Aminaka R, Yamamoto Y, Yamamoto Y. (2008) Quality control of photosystem II: reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J Biol Chem 283: 28380–28391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Uchida S, Mori H, Komayama K, Ohira S, Morita N, Nakanishi T, Yamamoto Y. (2006) Quality control of photosystem II: cleavage of reaction center D1 protein in spinach thylakoids by FtsH protease under moderate heat stress. J Biol Chem 281: 21660–21669 [DOI] [PubMed] [Google Scholar]