Abstract
Rice (Oryza sativa) endosperm has two isoamylase (ISA) oligomers, ISA1 homo-oligomer and ISA1-ISA2 hetero-oligomer. To examine their contribution to starch synthesis, expression of the ISA1 or ISA2 gene was differently regulated in various transgenic plants. Although suppression of ISA2 gene expression caused the endosperm to have only the homo-oligomer, no significant effects were detected on the starch phenotypes. In contrast, ISA2 overexpression led to endosperm having only the hetero-oligomer, and starch synthesis in the endosperm was drastically impaired, both quantitatively and qualitatively, because the starch was devoid of typical starch features, such as thermal and x-ray diffraction properties, and water-soluble highly branched maltodextrins were accumulated. In the ISA2 overexpressed line, about 60% to 70% of the ISA1-ISA2 hetero-oligomer was bound to starch, while the ISA homo- and hetero-oligomers from the wild type were mostly present in the soluble form at the early milking stage of the endosperm. Detailed analysis of the relative amounts of homo- and hetero-oligomers in various lines also led us to the conclusion that the ISA1 homo-oligomer is essential, but not the ISA1-ISA2 oligomer, for starch production in rice endosperm. The relative amounts of ISA1 and ISA2 proteins were shown to determine the ratio of both oligomers and the stoichiometry of both ISAs in the hetero-oligomer. It was noted when compared with the homo-oligomer that all the hetero-oligomers from rice endosperm and leaf and potato (Solanum tuberosum) tuber were much more stable at 40°C. This study provides substantial data on the structural and functional diversity of ISA oligomers between plant tissues and species.
Starch comprises linear and/or slightly branched amylose and highly branched amylopectin. Amylopectin has a distinct highly ordered structure called a “tandem-cluster structure,” in which most of the side chains are arranged in parallel and neighboring chains form double helices (Kainuma and French, 1972). Starch-debranching enzyme (DBE) has been considered to play an essential role in the synthesis of amylopectin, possibly by trimming the shape of the cluster (Ball et al., 1996). Higher plants and green algae have two classes of DBEs, namely isoamylase (ISA) and pullulanase (PUL). These enzymes can hydrolyze α-1,6 glucosidic linkages in branched glucan, although ISA cannot attack pullulan, while PUL can hardly degrade glycogen. Plants generally have three types of ISA isozymes (ISA1, ISA2, and ISA3) but have only one PUL form. Numerous investigations on the biochemical and genetic analyses of DBE mutants and the molecular analysis of transgenic plants with modified DBE gene expression have been performed. The loss of ISA1 protein in these plants results in a tremendous obstacle in the formation of starch granules in cereal endosperms such as maize (Zea mays; James et al., 1995), rice (Oryza sativa; Kubo et al., 1999), and barley (Hordeum vulgare; Burton et al., 2002), Arabidopsis (Arabidopsis thaliana) leaves (Delatte et al., 2005; Wattebled et al., 2005), potato (Solanum tuberosum) tuber (Hussain et al., 2003), and Chamydomonas cells (Mouille et al., 1996). In these tissues or cells, starch was observed to be replaced by a sort of water-soluble highly and randomly branched polysaccharide devoid of the cluster structure called phytoglycogen.
ISA2 by itself has no catalytic activity, owing to the absence of the consensus peptide region essential for enzymatic activity, but it contributes to the ISA activity through association with the ISA1 protein to form the ISA1-ISA2 protein complex (Hussain et al., 2003). However, it is unlikely that ISA3 forms a multimer with ISA1 or ISA2, but it exists as a monomer (Hishinuma et al., 2004; Takashima et al., 2007).
ISA is involved in starch biosynthesis as the ISA1-ISA2 hetero-oligomer in potato tuber (Hussain et al., 2003) and Arabidopsis leaves (Delatte et al., 2005; Wattebled et al., 2005), where both the proteins exist only as the hetero-oligomer. On the contrary, the endosperm of rice and maize contains both the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer (Utsumi and Nakamura, 2006; Kubo et al., 2010). Although the mechanism by which DBEs are involved in the synthesis of amylopectin is still debatable, it is thought that these enzymes play a crucial role in amylopectin biosynthesis by removing excess branches (Ball et al., 1996) or improper branches (Nakamura, 2002) that interfere with the formation of double helices of the cluster chains of amylopectin (Nakamura, 2002) and crystallization of the starch (Myers et al., 2000).
Considering the fact that the stoichiometry of ISA1 and ISA2 in the ISA1-ISA2 hetero-oligomer of potato and Arabidopsis is different from that of rice endosperm (Ishizaki et al., 1983; Hussain et al., 2003; Utsumi and Nakamura, 2006), studies on the impact of a reduction or overexpression of the ISA1 or ISA2 gene in rice endosperm will provide us with important insights into the contribution of ISA1 and ISA2 to starch synthesis of cereal endosperm and, hence, into the regulatory mechanism for the involvement of ISA isozymes in starch biosynthesis. In this study, we have mainly characterized the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer not only from the endosperm but also from the leaf of rice. The loss of ISA2 was found to have no effects on the starch phenotype of rice kernels. In contrast, overproduction of ISA2 caused shriveled kernels. The results show that the ISA1 homo-oligomer is indispensable for amylopectin biosynthesis in rice endosperm, while the ISA1-ISA2 hetero-oligomer seems to be essential for starch synthesis in leaf, although it is insufficient for normal starch accumulation in the endosperm. These phenomena indicate a case for the diversity of metabolic regulation among plant species and/or tissues.
Recently, Kubo et al. (2010) claimed that either the homo-oligomer or the hetero-oligomer is competent for starch biosynthesis in maize endosperm, based on the results obtained using maize ISA1 and ISA2 mutants. However, the phenotypes caused by the lesion of the ISA1 or ISA2 gene and the hypothesized functions of both the ISA proteins in starch synthesis differ between maize and rice endosperm. The comprehensive studies here with protein-based analyses of the ISA oligomers from a variety of rice transgenic lines could provide further insights into the functions and structure of both oligomers in plant tissues.
RESULTS
Construction of Binary Vectors for Transformation by RNA Interference or Overexpression and Generation of Transgenic Lines of Rice
To distinguish the functions of the homo- and hetero-oligomers of ISA in rice plants, four kinds of DNA constructs were designed in this study. The first construct was synthesized so that the ISA1 gene expression was suppressed by the RNA interference (RNAi) method (Supplemental Fig. S1A). The second construct was prepared to express the ISA1 gene in the ISA1-deficient sugary-1 mutant line EM914 (Supplemental Fig. S1C). In addition, to investigate the contribution of ISA2 to the function of ISA for the synthetic rate and structure of starch, vectors in which the expression of the ISA2 gene was suppressed or overexpressed were constructed (Supplemental Fig. S1, B and D) and introduced into the callus from the wild-type japonica rice cv Kinmaze or a pullulanase (pul)-null mutant generated from the wild-type japonica rice cv Nipponbare (Fujita et al., 2009).
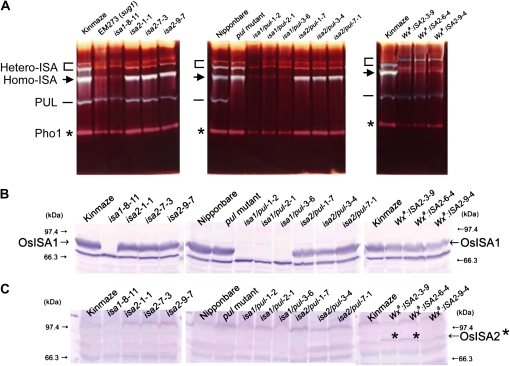
It is known that ISA from rice endosperm yielded at least three activity bands detected as blue bands on native polyacrylamide gels containing amylopectin (Fujita et al., 1999; Kubo et al., 1999). The bottom band and both the middle and top bands were recently assessed to correspond to the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer, respectively (Utsumi and Nakamura, 2006). The use of an ISA1 suppression vector resulted in a complete loss of ISA activity (Fig. 1A) and ISA1 protein (Fig. 1B). The amounts of ISA1 protein in isa2 and isa2/pul lines were at the same level as those in the wild type or the pul mutant (Fig. 1B). However, RNAi-mediated ISA2 gene suppression caused the transformants to have the ISA1 homo-oligomer activity but to lose the hetero-oligomer activity completely in endosperm (Fig. 1A). When the ISA2 gene was overexpressed, the ISA2 protein level was elevated (Fig. 1C) and the ISA activity was detected only in the hetero-oligomer, not in the ISA1 homo-oligomer (Fig. 1A), although the ISA1 protein level was significantly reduced in the transformed lines (Fig. 1B). The sugary-1 mutant line EM914 restored its capacity to produce starch in the endosperm by incorporating the normal ISA1 gene (Fig. 2A). These results indicate that all four transformation vectors functioned in the endosperm of the transgenic lines as planned.
Figure 1.
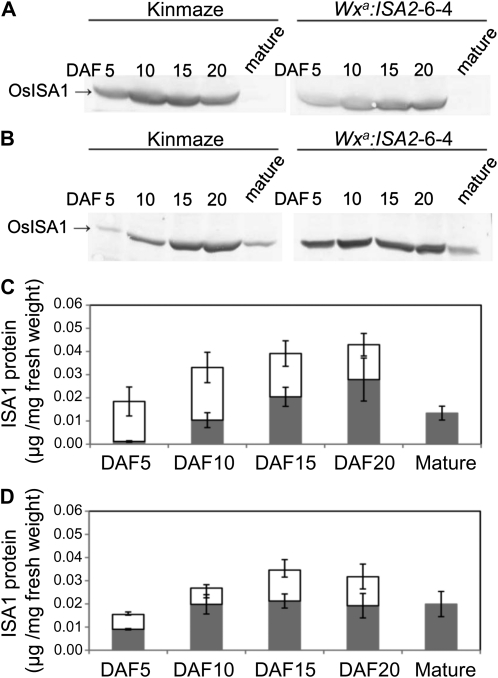
The activity of ISA and protein levels of ISA1 and ISA2 in the endosperm of transgenic lines and their wild-type host rice cv Kinmaze and Nipponbare. A, Aliquots (14 μL) of each of the crude enzyme extracts (total of 30 μL) prepared from a maturing endosperm at DAF15 were loaded onto a native polyacrylamide (5%) gel containing 0.4% (w/v) potato tuber amylopectin. After electrophoresis, the gel was incubated in 50 mm citrate-phosphate buffer (pH 6.0) containing 50 mm 2-mercaptoethanol for 1 h at 30°C and stained with iodine solution (1% I2/0.1% KI). The position of each activity band by the hetero-oligomer (Hetero-ISA), the homo-oligomer (Homo-ISA), pullulanase (PUL), or plastidial phosphorylase (Pho1) is shown. B and C, Aliquots (14 μL) of each of the crude enzyme extracts (total of 30 μL) prepared from a maturing endosperm were subjected to SDS-PAGE, and the protein amount of ISA1 (B) or ISA2 (C) was detected by immunoblot analysis with polyclonal antibody raised against ISA1 or ISA2, respectively, purified from rice maturing seed (Utsumi and Nakamura, 2006). Note that in B, the ISA1 protein could not be detected in the crude extract from the ISA1 suppressed lines by this method, whereas in the crude extract from Kinmaze (the wild type), ISA2 suppressed lines and ISA2 overexpressed lines (Wxa:ISA2) could be detected. In C, the ISA2 protein was detected only in the ISA2 overexpressed lines, as shown by asterisks on that band.
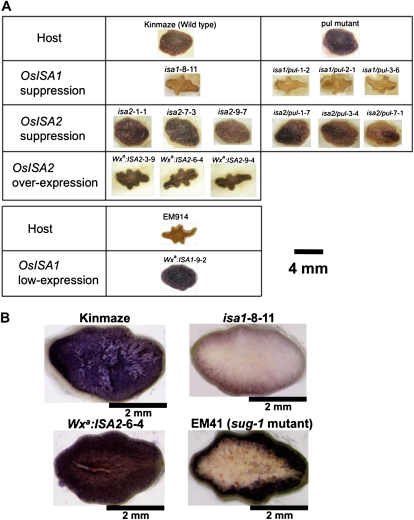
Figure 2.
Iodine staining of the cross-sections of kernels from Kinmaze (the wild type), EM41 (sug-1 mutant), the pul null mutant, and transgenic lines. A, Iodine-stained cross-sections of the dry kernels. B, Iodine-stained cross-sections of the maturing kernels harvested at DAF30.
Phenotypic Changes in Kernel Morphology and Production in Transgenic Lines
The ISA2 suppressed (isa2) lines had the same plumped seeds as the wild type, while the ISA1 suppressed (isa1) lines had shriveled kernels at maturity (Fig. 2A). The kernel morphology of the transformants was unaffected when ISA2 gene expression was inhibited. In contrast, overexpression of the ISA2 gene brought about a dramatic reduction in kernel size in the dry seed (Fig. 2A), and the transformants contained less than 50% of the starch in the kernel, while the content of soluble sugars, including maltodextrins (MD), malto-oligosaccharides (MOS), and simple sugars, increased by about 8-fold when compared with the host cv Kinmaze (Table I). The whole region of the developing endosperm of the ISA2 overexpressed line Wxa:ISA2-6-4 was stained with iodine solution, suggesting that both insoluble and soluble glucans were present in whole endosperm cells (Fig. 2B). We actually measured the amounts of insoluble glucans and soluble sugars in the different portions of the endosperm (Supplemental Fig. S2) and found that the above-mentioned idea was the case in the ISA2 overexpressed line. In contrast, phytoglycogen was localized in the inner region of the endosperm while starch was present in the outer region in the seed of a sugary-1 mutant line, EM41 (Fig. 2B). When the normal rice ISA1 gene was introduced into another sugary-1 mutant line, EM914, where starch is almost completely replaced by phytoglycogen in the endosperm, a transformed line, Wxa:ISA1-9-2, produced plenty of starch but not phytoglycogen with the plumped kernel (Fig. 2A, bottom column).
Table I. Carbohydrate contents in kernel and kernel weight of the wild type and transformants.
Means and sd were calculated from triplicate assays from three independent preparations.
| Line | Starch/Insoluble Glucans | Soluble Sugarsa | Kernel Weight |
| mg kernel−1 | |||
| Kinmaze | 15.8 ± 0.9 | 0.08 ± 0.01 | 20.1 ± 1.1 |
| isa1-8-11 | 2.4 ± 0.5 | 3.92 ± 0.72 | 10.7 ± 1.0 |
| isa2-1-1 | 15.6 ± 1.3 | 0.05 ± 0.00 | 20.2 ± 2.8 |
| isa2-7-3 | 14.4 ± 0.9 | 0.05 ± 0.01 | 20.0 ± 1.0 |
| isa2-9-7 | 15.0 ± 1.0 | 0.04 ± 0.00 | 20.7 ± 1.8 |
| Wxa:ISA2-3-9b | 7.1 ± 0.7 | 0.58 ± 0.04 | 10.2 ± 1.2 |
| Wxa:ISA2-6-4b | 7.3 ± 0.9 | 0.62 ± 0.05 | 10.4 ± 0.9 |
| Wxa:ISA2-9-4b | 6.9 ± 0.7 | 0.65 ± 0.06 | 10.5 ± 1.1 |
| Nipponbare | 16.5 ± 0.7 | 0.03 ± 0.00 | 21.7 ± 1.5 |
| pul mutant | 14.7 ± 0.9 | 0.03 ± 0.01 | 21.5 ± 1.2 |
| isa1/pul-1-2 | 1.5 ± 0.3 | 3.91 ± 0.71 | 9.6 ± 1.1 |
| isa1/pul-2-1 | 1.4 ± 0.5 | 3.78 ± 0.28 | 9.7 ± 0.8 |
| isa1/pul-3-6 | 1.3 ± 0.4 | 4.09 ± 0.28 | 10.3 ± 0.9 |
| isa2/pul-1-7 | 13.5 ± 2.1 | 0.07 ± 0.02 | 19.3 ± 1.1 |
| isa2/pul-3-4 | 10.7 ± 0.5 | 0.09 ± 0.02 | 17.2 ± 1.2 |
| isa2/pul-7-1 | 16.2 ± 0.3 | 0.07 ± 0.01 | 20.1 ± 3.0 |
| Taichung 65 | 15.3 ± 0.4 | 0.25 ± 0.01 | 21.0 ± 1.3 |
| EM914 | 0.6 ± 0.1 | 6.68 ± 0.13 | 12.5 ± 0.6 |
| Wxa:ISA1-9-2 | 12.8 ± 1.3 | 0.35 ± 0.02 | 18.9 ± 0.9 |
Soluble sugars contained MD, MOS, and simple sugars.
The contents of starch and soluble sugars of Wxa:ISA2-3-9, Wxa:ISA2-6-4, and Wxa:ISA2-9-4 lines were measured using kernels at DAF40.
Effects of Changes in ISA1 and ISA2 Gene Expression on the Composition of ISA Oligomers
To evaluate the functions of the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer in starch synthesis of rice endosperm, we examined in detail the relative amounts of these oligomers using various transformants having different levels of ISA1 and ISA2 proteins. In addition to the ISA2 overexpressed line (Wxa:ISA2-5-4) and the ISA1 low-expressed line (Wxa:ISA1-9-2), an ISA1 antisense line (G5a-1) was used because the ISA1 protein amount in endosperm of the line was reduced to about 6% of the wild type, but it could produce a great amount of starch-like glucans in its seed (Fujita et al., 2003), while the ISA1 protein amount in Wxa:ISA1-9-2 was about 56% of the wild-type level (data not shown).
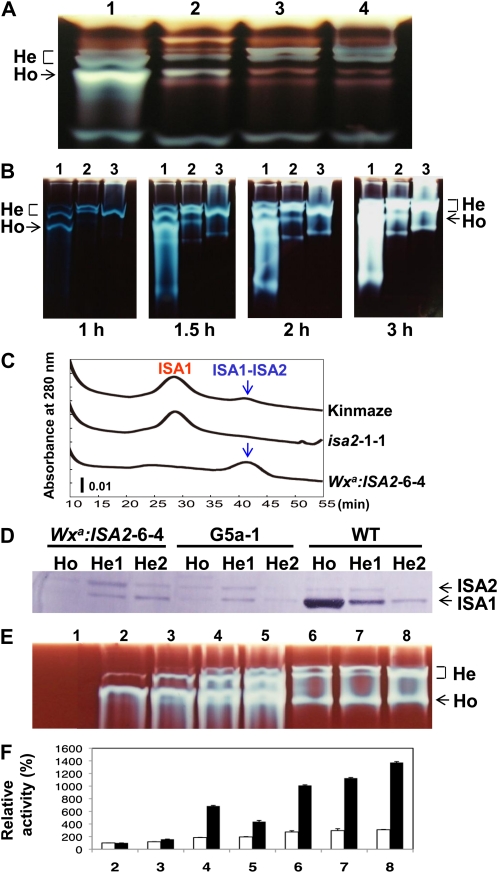
Figure 3A shows the relative activities of three major ISA bands in the zymogram. Owing to the low ISA1 protein level, the activity of the homo-oligomer was markedly lower in Wxa:ISA1-9-2 than in the wild type. The normal phenotype in the seed of the Wxa:ISA1-9-2 line (Fig. 2A) suggests that the ISA activity level of the wild type is much higher than the amount required for starch production in the rice endosperm and that the excess of ISA activity does not inhibit the starch synthesis in rice endosperm. Most of the ISA activity was accounted for by two hetero-oligomer bands in both ISA1 antisense and ISA2 overexpressed lines (Fig. 3A). To measure the homo-oligomer activity, if any, the incubation time was prolonged up to 3 h at 30°C in both lines. It was noted that with the increase in the enzymatic reaction time, the ISA1 homo-oligomer band was apparent in the ISA1 antisense line, while no activity band was detected in the ISA2 overexpressed line even after the 3-h incubation (Fig. 3B), indicating that the antisense line, but not the overexpressed line, had a small amount of the homo-oligomer. As shown in Figure 3B, trace levels of activity bands were sometimes visible at the extreme top of the zymogram, although these bands were observed especially when the incubation time was prolonged and a large amount of the purified ISA protein was applied to the native gel. We presume that these bands occurred due to the aggregation of the protein complex, because the ISA protein complex is very hydrophobic in nature. It is possible that this phenomenon was a kind of artifact, because this band was not detected when the freshly prepared crude enzyme extract was used for the zymogram analysis.
Figure 3.
Activities, protein amounts, and some properties of ISA1 homo-oligomers (Ho) and ISA1-ISA2 hetero-oligomers (He) from the endosperm of transgenic lines and their wild-type host cv Kinmaze or Nipponbare and from recombinant ISA1 and ISA2 proteins. A, ISA activity detection on the native polyacrylamide gel (containing 0.4% potato tuber amylopectin) of the crude enzyme extract prepared with 75 μL of solution A containing 50 mm imidazole-HCl (pH 7.4), 8 mm MgCl2, 50 mm 2-mercaptoethanol, and 12.5% (v/v) glycerol from three maturing kernels at the mid milking stage of the ISA1 low-expressed line (Wxa:ISA1-9-2), the ISA1 antisense line (G5a-1), the ISA2 overexpressed line (Wxa:ISA2-6-4), and wild-type cv Nipponbare. The same aliquot (2.5 μL) of the sample was applied onto each lane. The enzymatic reaction was run at 30°C for 1.5 h, and the activity was detected as described in Figure 2. Lane 1, Nipponbare; lane 2, Wxa:ISA1-9-2; lane 3, G5a-1; lane 4, Wxa:ISA2-6-4. B, Activities of ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer in maturing endosperm from G5a-1, Wxa:ISA2-6-4, and Nipponbare. The ISA preparation including both homo- and hetero-oligomers was partially purified from 5 g of maturing kernels using anion-exchange column chromatography and concentrated to 400 μL. The same aliquot (3 μL) of the sample was applied into each lane. The enzymatic reactions were run for 1, 1.5, 2, and 3 h as shown. The other conditions were the same as in Figure 2. Lane 1, Nipponbare; lane 2, G5a-1; lane 3, Wxa:ISA2-6-4. C, The protein amounts of the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer in the fractions of hydrophobic chromatography. The ISA preparations prepared from 20 g of maturing kernels from Kinmaze, isa2-1-1 (the ISA2 suppressed line), and Wxa:ISA2-6-4 by anion-exchange chromatography were subjected to TSKgel Ether-5PW chromatography, as described in “Materials and Methods.” The horizontal axis indicates the elution time (min). The two peaks eluted from 25 to 35 min and from 37.5 to 45 min in the chromatogram corresponded to the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer, respectively (Utsumi and Nakamura, 2006). D, The detection of ISA1 and ISA2 protein amounts by immunoblot analysis. Proteins in 5 g of maturing kernels from Wxa:ISA2-6-4, G5a-1, and wild-type (WT) cv Nipponbare were fractionated into the homo-oligomer fraction, the first hetero-oligomer fraction (He1), and the second hetero-oligomer fraction (He2) using Ether-5PW chromatography and concentrated to 250 μL. The same aliquot (8 μL) of the sample was applied onto each lane of the SDS-PAGE gel. Immunoblot analysis was performed using both the antibodies raised against purified rice ISA1 and ISA2, as described in “Materials and Methods.” E, Native PAGE/activity staining of the recombinant OsISA mixture containing different amounts of purified OsISA1 and OsISA2 proteins. Two micrograms of each of the purified OsISA1 proteins was added with various amounts (0, 0.2, 1.0, 2.0, 4.0, 10, and 20 μg of protein; corresponding to lanes 2–8, respectively) of purified OsISA2 protein in the buffer solution consisting of 50 mm imidazol-HCl (pH 7.4), 10 mm dithiothreitol, 12.5% glycerol, and 0.1 m NaCl in a total volume of 100 μL, whereas only the purified OsISA2 protein (4.0 μg) for lane 1 was suspended in the same buffer solution. The solutions were incubated at 0°C overnight, and a portion (18 μL) of each was loaded onto the native gel containing 0.4% potato tuber amylopectin. After chromatography, ISA activities were detected as blue bands by staining the gel with iodine solution. F, ISA activities toward amylopectin and phytoglycogen of the mixture containing different amounts of purified OsISA1 and OsISA2 proteins. The mixtures including different amounts of purified OsISA1 and/or OsISA2 proteins as above (E) were incubated in 100 μL of the buffer solution containing 50 mm MES-NaOH (pH 6.0) and 0.5 mg of rice amylopectin or rice phytoglycogen at 30°C for 10 min. DBE activity was determined by measuring the amount of reducing ends increased after the DBE reaction according to the method described by Utsumi et al. (2009). The numbers on the horizontal axis correspond to the lanes in E. The activity was expressed as relative values when the activities of OsISA1 (2 μg) only toward amylopectin or phytoglycogen (lane 2) were estimated to be 100%.
The ISA proteins were purified using anion-exchange and hydrophobic chromatography. The ISA oligomers were almost purified, and the A280 roughly corresponded to the ISA protein amount (Fig. 3C). Although the wild-type endosperm had both the homo- and hetero-oligomers, the ISA2 suppressed line (isa2-1-1) contained only the homo-oligomer. However, the ISA2 overexpressed line (Wxa:ISA2-6-4) possessed the only hetero-oligomer. These results suggest that the relative amounts of the ISA1 and ISA2 proteins determine the ratio of the homo-oligomer to the hetero-oligomer, consistent with the in vitro experiment in which the purified recombinant ISA1 and ISA2 proteins were mixed with various ratios, as described below (Fig. 3E).
To determine the protein ratio of ISA1 to ISA2 in the ISA oligomers, both proteins were measured by immunoblot analysis using antibodies raised against ISA1 and ISA2 purified from rice endosperm (Utsumi and Nakamura, 2006). In this experiment, the ISA oligomers were fractionated into the homo fraction, the first hetero fraction, and the second hetero fraction separated by Ether-5PW chromatography. Figure 3D shows that in the wild type, the homo fraction included almost only ISA1 protein, while the first hetero fraction had much more ISA1 protein when compared with ISA2 protein. In contrast, the ISA2 amount was similar to the ISA1 amount in the first hetero fraction from the ISA1 antisense line (Fig. 3D). It was noted that in the ISA2 overexpressed line, the amount of ISA2 protein was rather higher than that of ISA1 protein in the first hetero fraction, whereas the reverse was true in the second hetero fraction (Fig. 3D). The ratios of the protein amount of ISA2 to that of ISA1 in the first hetero fractions of the wild type, the antisense line, and the overexpressed line were calculated to be about 0.22, 0.77, and 1.58, respectively, while that in the second hetero fraction of the overexpressed line was found to be approximately 0.84, by comparing the relative intensities of both the protein bands detected with Coomassie Brilliant Blue staining with those with immunoblot detection (Supplemental Fig. S3).
To reveal the relationship between the relative amounts of ISA1 and ISA2 proteins and the activity of the hetero-oligomer, in vitro experiments were carried out by incubating different amounts of recombinant ISA2 protein with the fixed amount of recombinant ISA1 protein. With the increase in the ISA2 protein, activities in the bottom hetero-oligomer band increased, followed by the top hetero-oligomer band (Fig. 3E), and the activities toward amylopectin slightly increased while those toward phytoglycogen dramatically increased (Fig. 3F), indicating that the catalytic properties of the ISA complex vary by the stoichiometry of ISA1 and ISA2 proteins.
Effects of Changes in ISA2 Gene Expression on the Levels of Other Starch Synthetic Enzymes
We examined whether the overexpression of the ISA2 gene affected the levels of enzymes involved in starch biosynthesis. The transcript levels of starch synthase (SS) isozymes (SSI, SSIIIa, and SSIVb), the protein amounts of starch-branching enzyme (BE) isozymes, BEI and BEIIb, and the activities of BEIIa as well as BEI and BEIIb in the ISA2 overexpressed line were similar to those in the wild type (Supplemental Fig. S4). The activities of PUL and plastidial phosphorylase were decreased to some extent by the transformation (Fig. 1A). In summary, it is unlikely that other starch synthetic enzymes could markedly influence the phenotypic changes, if any, in the ISA2 transformants.
Scanning Electron Microscopy Observations of Starch Granules or Insoluble Glucan Granules
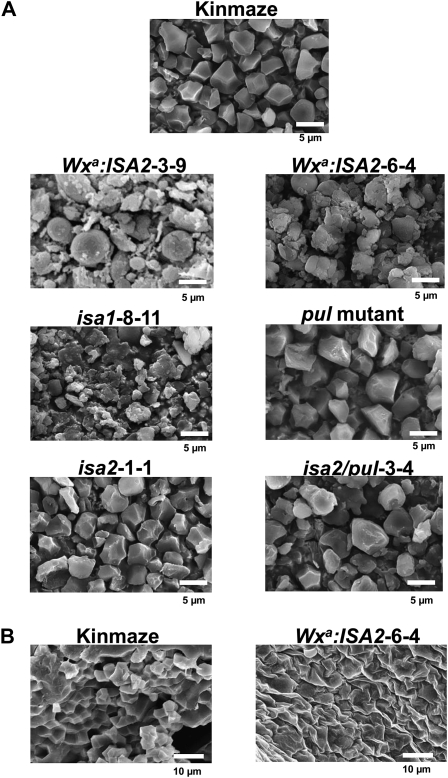
The scanning electron microscopy (SEM) images showed that Kinmaze formed similar sizes of polygonal starch granules with sharp edges and a smooth surface (Fig. 4A). The ISA2 suppressed lines (isa2-1-1 and isa2/pul-3-4) and the pul mutant had a morphology of starch granules similar to that of Kinmaze. On the contrary, the ISA2 overexpressed lines (Wxa:ISA2-3-9 and Wxa:ISA2-6-4) had various sizes of insoluble glucan granules with irregular shapes and a rough surface in the endosperm, containing many more small granules. A marked change in the granular structure in the ISA2 overexpressed line was also observed in the micrograph of the surface of the cross-section of the kernel (Fig. 4B). The dramatic changes in starch granule morphology were also found in an ISA1 suppressed line (isa1-8-11; Fig. 4A), as seen in the sugary-1 mutant (Wong et al., 2003).
Figure 4.
SEM of starch and insoluble glucan granules in the endosperm of transgenic lines and their wild-type host rice cv Kinmaze. A, SEM of starch and insoluble glucan granules purified from mature kernels of Kinmaze, the pul mutant, isa1-8-11, isa2-1-1, Wxa:ISA2-6-4, and isa2/pul-3-4. Bars = 5 μm. B, SEM of cross-sections of maturing kernels of Kinmaze and Wxa:ISA2-6-4 harvested at DAF30. Bars = 10 μm.
Thermal Properties and X-Ray Diffraction Patterns of Starch Granules
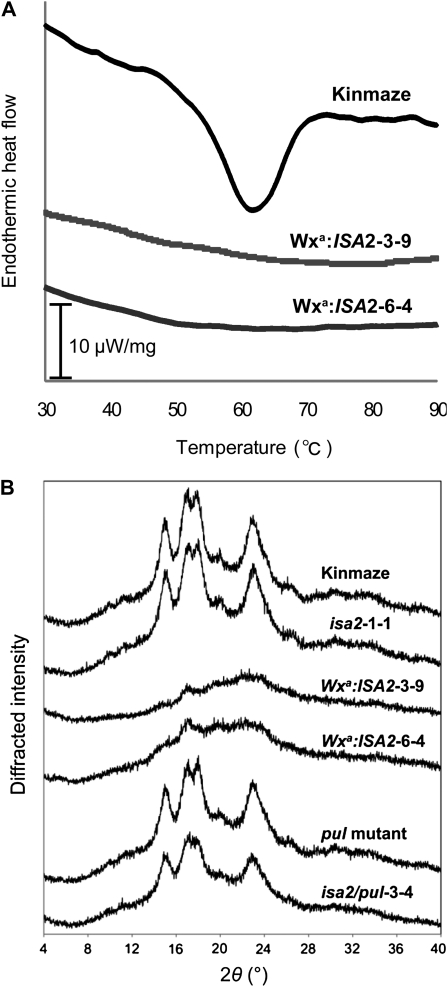
Thermal properties of the starch granules analyzed by using differential scanning calorimetry showed that gelatinization onset temperature was 53.3°C for Kinmaze but slightly lower (46.0°C–50.2°C) for ISA2 suppressed lines (Supplemental Table S2). However, it is striking that the parameters of thermal properties were not determined for the ISA2 overexpressed lines (Fig. 5A; Supplemental Table S2), indicating a drastic change in the physicochemical properties in the insoluble glucans.
Figure 5.
Physicochemical properties of insoluble glucans in ISA2 overexpressed lines. A, Differential scanning calorimetric curve of purified starch or insoluble glucans in endosperm of ISA2 overexpressed lines (Wxa:ISA2-6-4 and Wxa:ISA2-3-9) and its wild-type host cv Kinmaze. B, X-ray diffraction pattern of purified starch or insoluble glucans in the endosperm of Wxa:ISA2-6-4, Wxa:ISA2-3-9, and Kinmaze.
X-ray diffraction pattern, which is one of the characteristics of normal starch granules as seen in Kinmaze, almost disappeared in the insoluble glucans from the ISA2 overexpressed lines (Fig. 5B).
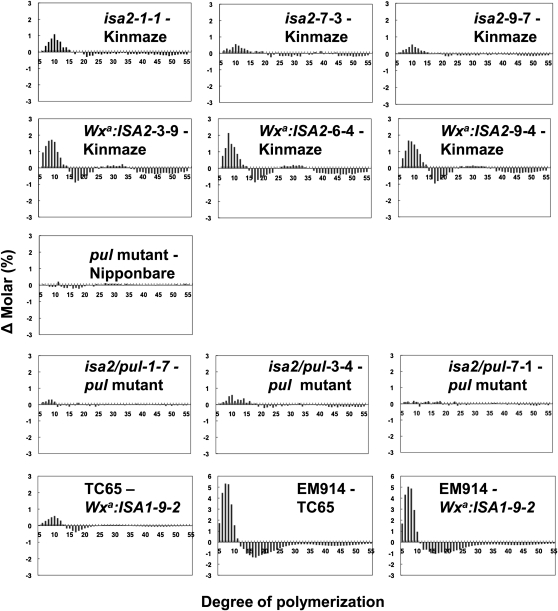
Analysis of Amylopectin and Insoluble Glucan Structure by the Fluorophore-Assisted Carbohydrate Electrophoresis Method
The fine structure of amylopectin in endosperm starch or glucan granules was examined by determining the chain-length distribution of amylopectin using the fluorophore (8-amino-1,3,6-pyrenetrisulfonic acid)-assisted carbohydrate electrophoresis method (Morell et al., 1998). The insoluble glucans of the ISA2 suppressed lines having wild-type Kinmaze and the pul mutant as backgrounds showed a similar chain profile as wild-type amylopectin (Fig. 6). In contrast, a marked change in the fine structure occurred in the insoluble glucans of the ISA2 overexpressed lines. The chain profiles showed a higher proportion of short chains of degree of polymerization (DP) 6 to 14 and a lower proportion of intermediate chains of DP 15 to 23 and long chains of DP ≥ 37 (Fig. 6). Since the extent of the decrease in the long chains of DP ≥ 37 was pronounced, the peak (DP 35–50) found in the normal amylopectin was missing in the insoluble glucans (Supplemental Fig. S5).
Figure 6.
Changes in the chain-length distribution of amylopectin or insoluble glucans purified from mature kernels of Kinmaze, the pul null mutant, and transgenic lines. Differential plots are shown by subtracting the molar percentages of MOS with DP6 to DP55 from amylopectin or insoluble glucans of the individual lines from those of the corresponding transformed line. Values are means from the measurement of triplicate samples.
Time-Dependent Changes in the Content of Insoluble Glucans and Soluble Sugars in the ISA2 Overexpressed Transformant during the Course of Endosperm Development
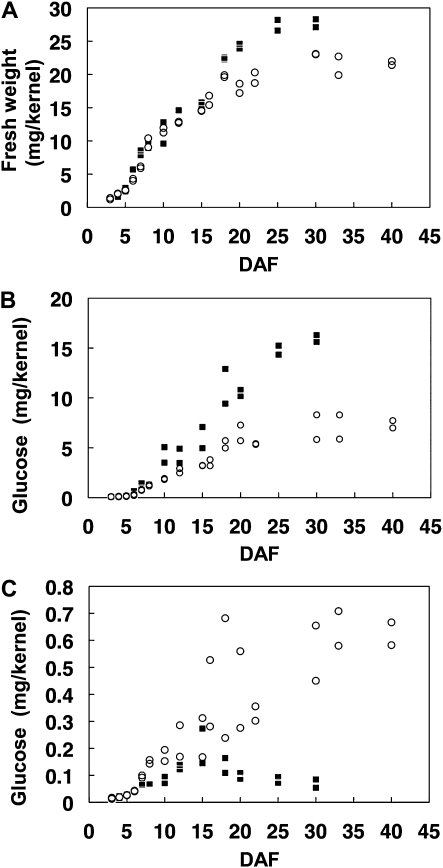
To characterize the effects of ISA2 gene overexpression on starch and the related sugar metabolism, contents and structures of the insoluble glucan (starch) and soluble sugars were measured during the course of endosperm development. Figure 7A shows that the fresh weights of kernels from the wild type and the ISA2 overexpressed line started to increase at 3 d after flowering (DAF3) of the seed and then were saturated at about DAF30, although the weight of the overexpressed line kernel was the same until DAF15 but then dropped to be about 80% of that of the wild type. Although the insoluble glucan content per kernel of the transformant increased during the early to mid developmental stages up to about DAF20, it did not increase thereafter, and it was less than 50% of that of the former (wild type) at the mature stage (Fig. 7B). On the contrary, the content of the soluble sugar fraction in the transformant was markedly higher than that of the wild type (Fig. 7C). It is noted that in the wild type, the soluble fraction increased until DAF12 to DAF15 but gradually decreased after DAF15, while in the transformant, the soluble fraction continued to increase until DAF30 and then was saturated.
Figure 7.
Changes in the fresh weight of the kernel and amounts of insoluble glucans and soluble sugars in the endosperm during the course of endosperm development of the ISA2 overexpressed transgenic line and its wild-type host cv Kinmaze. White circles, Wxa:ISA2-6-4; black squares, Kinmaze. A, Fresh weight of the kernel. B, The amount of insoluble glucans or starch in the kernel. C, The amount of soluble sugars including MD, MOS, and Glc in the kernel of Kinmaze and Wxa:ISA2-6-4. The starch or insoluble glucans and soluble sugars in the kernel were separated by centrifugation (3,000g, 4°C, 10 min), and the carbohydrates were quantified by measuring the released Glc after digestion with amyloglucosidase and α-amylase. The carbohydrate contents were calculated as Glc equivalents from triplicate assays of two independent preparations.
To examine the phenomenon in more detail, we focused on an analysis of the events that occurred at the early stage of endosperm development. First, the soluble fraction prepared from the kernels harvested at DAF15 was further fractionated into MD, MOS, and simple sugars. The simple sugars were found to be mainly composed of Suc, Glc, and Fru in all the preparations examined. The amounts of Suc and Glc were higher by approximately two and five times, respectively, in the transformant kernel than those in the wild type, whereas the Fru content was unchanged between the two sources (Table II).
Table II. Carbohydrate contents in maturing kernels harvested at DAF15.
Means and sd were calculated from triplicate assays from two independent preparations.
| Line | Starch/Insoluble Glucans | MD + MOS | Glc | Fru | Suc |
| mg kernel−1 | |||||
| Kinmaze | 8.0 ± 1.1 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.26 ± 0.04 |
| isa1-8-11 | 1.0 ± 0.2 | 2.27 ± 0.49 | 0.10 ± 0.01 | 0.02 ± 0.01 | 0.46 ± 0.08 |
| isa2-1-1 | 7.0 ± 3.0 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.01 ± 0.01 | 0.20 ± 0.03 |
| isa2-7-3 | 6.7 ± 2.6 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.23 ± 0.01 |
| Wxa:ISA2-3-9 | 4.4 ± 0.5 | 0.35 ± 0.03 | 0.14 ± 0.02 | 0.02 ± 0.00 | 0.55 ± 0.05 |
| Wxa:ISA2-6-4 | 6.5 ± 0.5 | 0.27 ± 0.01 | 0.15 ± 0.03 | 0.01 ± 0.01 | 0.51 ± 0.10 |
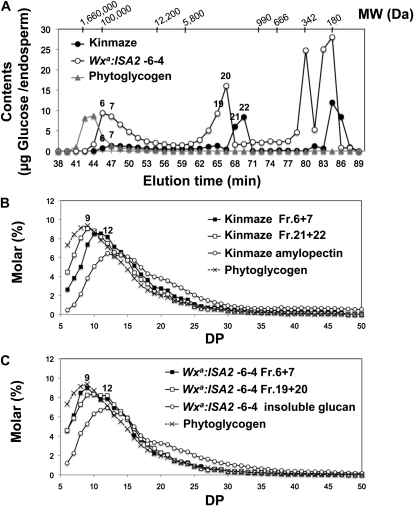
Second, water-soluble MD, MOS, and simple sugars were fractionated by using size-permeation HPLC columns, as shown in Figure 8. The MD in the wild type showed two major peaks, with the molar sizes of about 98 (fraction 7) and 2.3 (fraction 22) kD, while that in the ISA2 overexpressed line contained two major peak fractions of approximately 107 (fraction 6) and 3.4 (fraction 20) kD (Fig. 8A). The amount of MD was greatly higher in the transformant than in the wild type (Fig. 8A; Table II).
Figure 8.
Gel filtration HPLC and structural analyses of soluble MD, MOS, and simple sugars in developing endosperm harvested at DAF10 of ISA2 overexpressed line, ISA1 suppressed line, and their wild-type host cv Kinmaze. MD, MOS, and simple sugars from rice kernels were prepared as described in “Materials and Methods.” A, Gel filtration chromatographs of the soluble sugar fractions including MD, MOS, and simple sugars in the kernels of Wxa:ISA2-6-4 and Kinmaze. Phytoglycogen purified from the rice sugary-1 mutant line EM914 was also applied as a control. Their Glc contents were measured by the enzymatic assay method after treatment with glucoamylase. Numbers in the figure indicate fractions of the chromatographs. B, Chain-length distribution of MD, MOS, and amylopectin from Kinmaze and phytoglycogen after treatment with Pseudomonas ISA. The fractions used were obtained from the chromatograph shown in A. C, Chain-length distribution of the fractions of MD, MOS, and insoluble glucans from Wxa:ISA2-6-4 and phytoglycogen after treatment with Pseudomonas ISA. The fractions used were obtained from the chromatograph shown in A.
The structural feature of MD was examined in more detail. When the molar amount of each MOS labeled with the fluorescent probe 8-amino-1,3,6-pyrenetrisulfonic acid at its reducing end was analyzed by capillary electrophoresis before and after treatment of the MD fraction with a bacterial ISA, the amount of MOS was greatly accelerated after the treatment in all the MD fractions analyzed (Supplemental Fig. S6), indicating that branched dextrins accounted mostly for the MD fraction. In the wild type, the high-molecular-mass MD fraction (fractions 6 and 7) had fewer short chains and more long chains when compared with the low-molecular-mass MD fractions (fractions 21 and 22), while in the transformant, the chain-length distribution pattern for the high-molecular-mass MD fraction (fractions 6 and 7) was similar to that for the low-molecular-mass MD fraction (fractions 21 and 22; Fig. 8, B and C). It was noted that the MD in the ISA2 overexpressed line had a different chain profile from that from insoluble glucan in the transformant or the wild type (amylopectin) or phytoglycogen from the sugary-1 mutant (Fig. 8C).
Third, it was found that at DAF5, there were both types of ISA oligomers in the wild type, whereas only the ISA hetero-oligomer was detected in the ISA2 suppressed line (Supplemental Fig. S7A), indicating that the effects of ISA2 overexpression could be observed at the early developmental stage of endosperm.
Fourth, the chain profile for the insoluble glucans from endosperm at DAF5 was similar to that of the insoluble glucan prepared from mature seeds (Supplemental Fig. S7, B and C).
In summary, the phenotypic changes generated by overexpression of the ISA2 gene occurred from the very early stage of endosperm development, and these phenotypes were distinct when compared with any other rice mutants and transformants so far analyzed by our group.
The Association of ISA Oligomers with Starch Granules during Endosperm Development in ISA2 Overexpressed Transformants and the Wild Type
To elucidate how the presence of the only ISA1-ISA2 hetero-oligomer devoid of the ISA1 oligomer inhibits the efficient activity of starch synthesis in the endosperm, the protein was fractionated into the soluble fraction, the starch granule loosely bound fraction, and the starch granule tightly bound fraction according to the method described by Fujita et al. (2006), and the partitioning of the amount of ISA1 protein into the three fractions was determined by immunoblot analysis (Fig. 9, A and B). We found that the ISA1 protein was present in both the soluble and loosely bound fractions but not in the tightly bound fraction. The protein amount in the soluble fraction accounted for about 95% of the total protein amount in the endosperm of the wild type at DAF5 (Fig. 9C), while in the ISA2 overexpressed line, it was only 45% (Fig. 9D). These results suggest that the ISA1-ISA2 hetero-oligomer is easily bound to starch granules, resulting in low or no capacity of the protein for starch synthesis.
Figure 9.
Localization of ISA1 protein in the soluble fraction or the starch granule-bound fraction from the endosperm harvested at different developmental stages of the ISA2 overexpressed line and its host cv Kinmaze. The amount of ISA1 protein was measured by immunoblot analysis using antibodies raised against purified ISA1 from maturing endosperm of rice cv Kinmaze (Utsumi and Nakamura, 2006). A, The amount of ISA1 protein in the soluble fraction. B, The amount of ISA1 protein in the starch granule loosely bound protein fraction. C, The amounts of ISA1 protein in the soluble (white bars) and starch granule loosely bound (gray bars) fractions harvested from the endosperm of Kinmaze. The data were obtained by calculating the duplicates of individual measurements from two independent preparations. D, The amounts of ISA1 protein in the soluble (white bars) and starch granule loosely bound (gray bars) fractions harvested from the endosperm of Wxa:ISA2-6-4. The data were obtained by calculating the duplicates of individual measurements from two independent preparations.
We also examined the changes in the affinities of starch synthetic enzymes for glucan granules caused by transformation. Although the amount of granule-bound SSI in the loosely bound fraction of the ISA2 overexpressed line was higher than that of the wild type, the amounts of SSI, BEI, and BEIIb proteins in the loosely bound fraction as well as the tightly bound fraction were present at the same levels as those in the wild type (Supplemental Fig. S8).
The Composition of ISA Oligomers in Rice Leaf and Potato Tuber and the Heat Stability of ISA1 Homo-Oligomer and ISA1-ISA2 Hetero-Oligomer
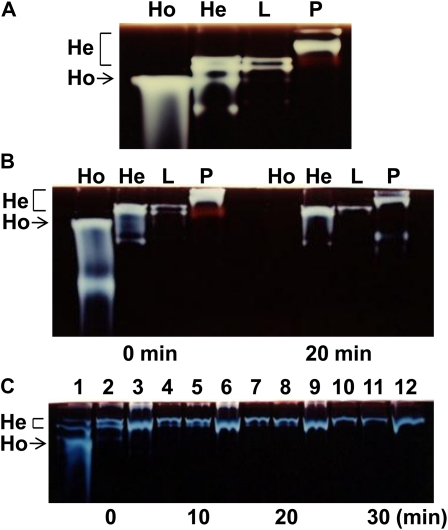
It is important to compare the composition and properties of ISA oligomers from various sources and to analyze whether some plant tissues such as rice leaf have both homo- and hetero-oligomers. First, in an attempt to examine the presence of homo- and/or hetero-oligomers, the partially purified ISA fraction was prepared from rice green leaf or potato tuber by using HPLC. During these HPLC procedures, the ISA activity was always eluted as a single peak. Figure 10A illustrates that rice leaf had only two ISA1-ISA2 hetero-oligomer bands, but not the homo-oligomer band, in the zymogram, with the two heteromer migrating bands agreeing with those of the rice endosperm in terms of their mobilities on the gel. Although potato tuber is known to have only hetero-oligomer (Hussain et al., 2003), two activity bands with much lower mobility were detected on the native gel when compared with the rice ISA hetero-oligomers (Fig. 10A).
Figure 10.
Composition and heat stability of the ISA homo-oligomer and hetero-oligomer in rice endosperm, rice leaf, and potato tuber. The rice endosperm ISA homo-oligomer and the ISA1-ISA2 hetero-oligomer were purified from 20 g of developing rice (cv Nipponbare) grains, as described in “Materials and Methods.” The total ISA activity preparations were obtained by partially purifying the rice green leaf (L) and potato tuber (P), as described in “Materials and Methods.” A, ISA activity detection on the native polyacrylamide gel (containing 0.4% potato tuber amylopectin) of the purified homo-oligomer and hetero-oligomer preparations from the developing rice endosperm, the green leaf ISA preparation, and the potato tuber ISA preparation. Volumes of samples applied were 0.75, 0.75, 8.5, and 0.75 μL, respectively. After electrophoresis, the enzymatic reaction was run at 30°C for 2.5 h, and the activity was detected as described in Figure 1. B, Effects of heat treatment on the activities of ISA oligomers from rice endosperm homo-oligomer, the rice endosperm hetero-oligomer, rice leaf, and potato tuber. Each preparation was incubated at 40°C for 20 min, and the treated sample as well as the nontreated sample (0 min) was applied onto native PAGE. Volumes of the samples applied were 1.5, 1.5, 8.5, and 1.5 μL, respectively. After electrophoresis, the enzymatic reaction was run at 30°C for 2.5 h, and the activity was detected as described in Figure 1. C, Effects of heat treatment on the activities of ISA oligomers in the partially purified ISA preparations from the maturing endosperm of Nipponbare, the ISA1 antisense line (G5a-1), and the ISA2 overexpressed line (Wxa:ISA2-6-4) of rice. The preparations used were the same as those presented in Figure 3B. All the preparation were incubated at 40°C for 10, 20, and 30 min, and the treated sample (0 min) as well as the nontreated sample was applied onto native PAGE. The volume of each sample applied was 3.3 μL. After electrophoresis, the enzymatic reaction was run at 30°C for 1.5 h, and the activity was detected as described in Figure 1. Lanes 1, 4, 7, and 10, Nipponbare; lanes 2, 5, 8, and 11, G5a-1; lanes 3, 6, 9, and 12, Wxa:ISA2-6-4.
Second, the heat stability of the ISA oligomer was tested by incubating the partially purified ISA preparations at 40°C. Incubation for 20 min caused a complete loss of the activity of rice endosperm ISA1 homo-oligomer, whereas all the hetero-oligomers from rice endosperm, rice leaf, and potato tuber demonstrated these activities to a large extent, although the hetero-oligomer top band was slightly more resistant to high temperature than the hetero-oligomer bottom band (Fig. 10B). Figure 10C shows that the rice endosperm homo-oligomer was inactivated when it was incubated at 40°C for 10 min, while the rice hetero-oligomers with different ISA1-ISA2 ratios from the ISA1 antisense line, the ISA2 overexpressed line, and the wild type were mostly stable even if incubated at 40°C for at least 30 min, indicating that all the hetero-oligomers were stable at 40°C.
DISCUSSION
Changes in ISA Gene Expression Affected Amylopectin Biosynthesis in Rice Endosperm
It is known that rice (Utsumi and Nakamura, 2006) and maize (Kubo et al., 2010) endosperms have two types of ISA oligomers, the ISA1 homo-oligomer and the ISA1-ISA2 hetero-oligomer, whereas there is only the hetero-oligomer in potato tuber (Hussain et al., 2003; Bustos et al., 2004) and Arabidopsis leaf (Delatte et al., 2005; Wattebled et al., 2005, 2008; Streb et al., 2008). The reason for this apparent discrepancy between plant sources and the molecular structures, such as the stoichiometry and functions and/or physiological significance of both oligomers, remains to be resolved. One of the limitations in such studies is the difficulties in purifying the ISA oligomers from plant tissues. In addition, the available plant materials having different amounts of the homo-oligomer and/or hetero-oligomer and different compositions of ISA1 and ISA2 in the hetero-oligomers were limited in the previous studies. Thus, to date, no comprehensive analyses on a protein basis have been performed. Several basic questions arise. First, which oligomers are essential for starch biosynthesis in rice endosperm? Second, what are the differences in the properties and structure between both oligomers? Third, what determines the relative amount of the homo-oligomer to the hetero-oligomer and the stoichiometry of both ISAs in the hetero-oligomer? Fourth, what is the relationship between the stoichiometry and the properties of each form of the hetero-oligomer if there are multiple forms in the hetero-oligomer? Fifth, what is the physiological significance of the fact that the cereal endosperm has both homo- and hetero-oligomers but the other tissues so far examined possess only the hetero-oligomer? To answer these questions, our investigations were performed using various rice transgenic lines in which expression of the ISA1 and ISA2 genes was differently regulated in the endosperm. In the isa1 and isa2 lines, expression of the ISA1 and ISA2 genes was almost completely inhibited, respectively (Fig. 1). In the endosperm of the isa1 lines including phytoglycogen instead of starch, all the ISA oligomers were lacking (Fig. 1), while the endosperm of the isa2 lines had only the homo-oligomers (Fig. 1) but could synthesize starch almost similarly to the wild type (Figs. 2A and 4A; Table I). It is striking to find that overexpression of the ISA2 gene caused transformants (Wxa:ISA2-6-4 and Wxa:ISA2-3-9) to have only hetero-oligomers (Figs. 1A and 3, A and B) and brought about a dramatic decrease in the rate of starch synthesis and abnormal starch structure (Figs. 2, 4, 5, and 6; Table I).
The Features of Phenotypes in the ISA2 Overexpressed Line
To our knowledge, the biochemical events and/or phenotype that occurred in the ISA2 overexpressed line are specific in many ways among the starch mutants and transformants of rice so far reported, even when compared with those found in sugary-1 mutants of rice (Nakamura et al., 1997; Kubo et al., 1999, 2005). For example, the insoluble glucans prepared from the ISA2 overexpressed line almost lost the thermal properties measured by differential scanning calorimetry (Fig. 5A; Supplemental Table S2) and showed no clear x-ray diffraction pattern (Fig. 5B), irrespective of the granular structure (Fig. 4). These results seem to be remarkable when compared with our previous reports showing that sugary-amylopectin found in the starch region of the sugary-1 mutants (Wong et al., 2003) and glucans from the ISA1 antisense line (Fujita et al., 2003) conserve the thermal properties.
The Mechanism for Impaired Starch Synthesis in the ISA2 Overexpressed Line
Why did the ISA1-ISA2 hetero-oligomer only line have a severe phenotype with abnormal starch, modified amylopectin chain-length distribution, and low starch content? It is possible that the activity of the ISA1-ISA2 hetero-oligomer was in excess or too low to produce normal amylopectin during starch synthesis. In the ISA1 low-expressed line (Wxa:ISA1-9-2) and an ISA1 antisense line (G5a-1), the ISA1 protein amounts and/or activities were reduced to about 56% and 6% (Fujita et al., 2003), respectively, while both lines exhibited near-normal starch synthesis in the endosperm (Fig. 2A; Table I; Fujita et al., 2003). In contrast, the ISA activity in the ISA2 overexpressed line was significantly lower, but apparently higher than 6% (detected in G5a-1) when compared with that of the wild type (Figs. 1, 3, and 9). Overall, these results indicate that the possibility is unlikely.
The most striking difference between the ISA1 antisense and ISA2 overexpressed lines was that the antisense line had a small amount of homo-oligomer, while no homo-oligomer was found in the overexpressed line (Fig. 3B). All these results are consistent with the idea that the homo-oligomer plays an essential part in starch biosynthesis in rice endosperm, whereas the ISA1-ISA2 hetero-oligomer is insufficient for the debranching capacity needed for amylopectin synthesis in rice endosperm. What are the differences between the homo-oligomer and the hetero-oligomers? First, the molar size of the hetero-oligomer is larger than that of the homo-oligomer in rice (Utsumi and Nakamura, 2006) and maize (Kubo et al., 2010) ISA multimers. Second, the hetero-oligomer was more hydrophobic than the homo-oligomer (Fig. 3C; Utsumi and Nakamura, 2006). Third, the catalytic activity of the hetero-oligomer toward glucans, especially toward phytoglycogen, is significantly higher than that of homo-oligomer (Fig. 3F; Utsumi and Nakamura, 2006). Fourth, the hetero-oligomer was more easily bound to the glucan granules than the homo-oligomer (Fig. 9). Fifth, the hetero-oligomer was distinctly more stable to high temperatures than the homo-oligomer (Fig. 10, B and C). Thus, the most likely possibility is that the hetero-oligomer has different enzymatic properties from the homo-oligomer and is incapable of trimming properly the amylopectin structure in rice endosperm.
Figure 3D shows that the proportion of ISA1 and ISA2 proteins in the overexpressed line was higher than that in the wild type. These results led us to question whether the hetero-oligomers have different functional properties in starch biosynthesis when the proportion of both proteins in the hetero-oligomers is altered. With the increase in the relative amount of ISA2 versus ISA1, the catalytic activity (Fig. 3F) and the hydrophobic properties increased, since the relative amount of the ISA activity band with lower mobility to that with higher mobility in the native polyacrylamide gel tended to be higher (Fig. 3, A, D, and E). This study also revealed, to our knowledge for the first time, that there are a number of forms in the hetero-oligomers with different stoichiometry between ISA1 and ISA2 in the rice endosperm (Fig. 3D). At the same time, it is noted that the hetero-oligomer was found to be composed of a mixture of heteromers with different ISA1/ISA2 compositions, not only in transgenic lines but also in the wild type (Fig. 3D). The gross or average ratios were considered to be determined by the molar amounts of ISA1 and ISA2 proteins either under in vivo (Figs. 1 and 3D) or in vitro (Fig. 3E) conditions. This finding suggests that the structure and composition of the ISA hetero-oligomer vary depending on the physiological conditions or tissues. In fact, it is known that the transcript level of rice ISA1 is severalfold higher than that of ISA2 in endosperm, while in leaf, ISA2 has a slightly higher transcript level than ISA1 (Ohdan et al., 2005), which is in agreement with the result with respect to maize (Kubo et al., 2010) and the fact that the proportion of transcript levels of ISA1 and ISA2 changes during the developmental stage of rice (Ohdan et al., 2005) and maize (Kubo et al., 2010) endosperm. However, it should be pointed out that the mobilities of the two bands were unchanged (Fig. 3, A and E) and that the heat stability was consistent among the hetero-oligomers (Fig. 10C). We assume that the properties of ISA hetero-oligomers are not seriously influenced by the altered composition of ISA1-ISA2, although it is difficult to show whether the phenotype in the overexpressed line was caused by the altered composition of ISA1 and ISA2 proteins, whereas a normal hetero-oligomer found in wild-type endosperm can function to some extent in starch synthesis.
Physiological Role of ISA1 Homo-Oligomer in Amylopectin Biosynthesis in Endosperm
Why does cereal endosperm use the ISA1 homo-oligomer instead of the ISA1-ISA2 hetero-oligomer for amylopectin biosynthesis? Although the composition of the ISA homo-oligomer and hetero-oligomer has been examined only with a limited number of plant tissues and species, we speculate that the ISA1 homo-oligomer might be evolved to be specified for starch synthesis in the endosperm of monocots, where the rate of starch synthesis, the structure of starch granules, and the composition and expression patterns of isozymes such as BEIIb are different from those in assimilatory tissues and dicots. In addition, recent studies indicated that BEIIb plays a specific role in the association of SS-BE isoforms in the endosperms of wheat (Triticum aestivum) and maize (Hennen-Bierwagen et al., 2008; Tetlow et al., 2008). These results strongly suggest that branching reactions responsible for starch biosynthesis in cereal endosperm are regulated in a different manner than in leaves; therefore, the capacity of ISA required for normal starch synthesis might be different between the two tissues.
These results markedly differ from the recent results with maize stating that either the homo- or hetero-oligomer is sufficient for near-normal starch synthesis in endosperm (Kubo et al., 2010). In addition, maize hetero-oligomer is considered to have a distinct role in starch granule initiation or growth, because the ISA2-null mutant line has smaller, numerous starch granules in endosperm despite the presence of the homo-oligomer (Kubo et al., 2010). However, it is unlikely that the rice endosperm hetero-oligomer has such a function, because no phenotypic changes occurred in the isa2 transformants (Figs. 2 and 4). It is believed that the role of ISA is to clear the ill-positioned α-1,6 branches to trim the shape of the amylopectin cluster (Ball et al., 1996; Myers et al., 2000; Nakamura, 2002), and therefore, the trimming process is closely related to the position, frequency, and length of chains that are newly formed by BE. We speculate that the characteristics, relative activities of many enzymes involved in amylopectin biosynthesis, especially BE isozymes, and hence the structures of the substrates for ISA are different in endosperm between rice and maize. BEIIb is specifically expressed in cereal endosperm, playing a distinct role in the formation of short chains in the crystal line lamellae of the cluster (Nishi et al., 2001; Nakamura, 2002; Tanaka et al., 2004). Our recent in vitro study established that rice BEIIb almost exclusively transfers chains of DP7 and DP6 whereas BEIIa forms a wide range of short chains (Nakamura et al., 2010), suggesting strongly that the rice ISA1 homo-oligomer is specified to remove short branches of DP7 and DP6 formed by BEIIb in the endosperm. However, it is possible that maize BEIIb has no such specific properties like rice BEIIb, although no such information on maize BEIIb is available. In fact, it is known that phenotypes such as the amylopectin structure found in the BEIIb-deficient mutants are dramatically different between maize (Baba and Arai, 1984) and rice (Nishi et al., 2001) endosperm.
Diversity of Structure and Function of ISA Oligomers and Their Functions
It should be stressed that rice leaf had only the ISA hetero-oligomers, not the homo-oligomer (Fig. 10A). We assume that the regulatory mechanism by ISA for starch biosynthesis in rice differs between endosperm and leaf, and this might be reasonable based on the following differences between the two tissues. First, the rate of starch biosynthesis and the activity levels of starch biosynthetic enzymes in the endosperm are extremely higher than those in the leaf (Nakamura et al., 1989). Second, in leaves, starch is deposited during the light period, but it must be degraded in the subsequent dark period. On the contrary, such mobility is not required in the endosperm, but it might be rather inhibited until the seed germinates. The fine structure of amylopectin, physicochemical and crystalline properties, and the way of packing of amylopectin molecules into the starch granules must differ between assimilatory starch in the leaf and reserve starch in the endosperm. These differences can be achieved by different expression patterns of a set of genes for starch synthetic enzymes including ISAs between two tissues (Ohdan et al., 2005). Third, since the leaf is exposed to more severe environmental conditions during the longer growth period than the endosperm, the stable nature of the hetero-oligomer must be particularly important.
What is the physiological reason for the rice and maize endosperms to have two types of ISA oligomers and that only the ISA hetero-oligomer plays an essential role in starch biosynthesis in the leaf? We found that the hetero-oligomer was resistant to high temperature (40°C), whereas the homo-oligomer completely lost its activity under that temperature within 10 min. The significance of the hetero-oligomer might be to support the principal role of the homo-oligomer in starch biosynthesis in the endosperm under certain physiological and/or growth conditions (e.g. when the plants are grown at high temperature of around 40°C). The investigations here clearly show that the structure of the functional ISA oligomer is markedly different between the plant species and tissues, but the molecular basis for the precise mechanism by which ISA is involved in amylopectin synthesis needs to be elucidated.
MATERIALS AND METHODS
Gene Construction of the RNAi Vector
The specific region of the rice (Oryza sativa) OsISA1 gene covering 141 bp (474–614) or the OsISA2 gene covering 162 bp (641–802) was amplified using a full-length OsISA1 or OsISA2 cDNA (Fujita et al., 1999; Utsumi and Nakamura, 2006) as a template using PCR of KOD-Plus DNA polymerase (Toyobo) and the specific primers shown in Supplemental Table S1. Both fragments were subcloned into the pDONR/Zeo cloning vector (Invitrogen) to yield entry vectors. RNAi constructs carrying these fragments were made using the pESWA vector (Miki and Shimamoto, 2004). The transgene in pESWA is known to be expressed in the endosperm and pollen by the Wxa promoter from the indica rice cv Labell (Itoh et al., 2003).
Construction of a Wxa Promoter
pESWA was digested with SacI and EcoI, and then the SacI-EcoI fragment (3,067 bp) of the Wxa promoter was inserted into the SacI-EcoI of pBluescript SK− (pB-preWxa). The DNA fragments (460 or 698 bp) containing exon 2 or exon 2 and the sequence encoding amyloplast-transit peptide of a Wx gene were amplified from the genomic DNA from cv Nipponbare as a template using PCR of KOD-Plus DNA polymerase and the specific primers shown in Supplemental Table S1, and then the NcoI and XhoI sites were added at the 3′ terminus. The two DNA fragments were digested with EcoRI and XhoI and then were inserted into the EcoRI-XhoI sites of pB-preWxa.
Construction of OsISA1 and OsISA2 Overexpression Vectors
The fragments of the Wxa promoter and the Wxa promoter containing transit peptide were removed from the pBluescript SK− using SacI and NcoI. The coding regions for OsISA1 and OsISA2 were digested with NcoI and SalI. The pCAMBIA1300 containing ribulose-1,5-bisphosphate carboxylase small subunit-3A poly(A) signal from pea (Pisum sativum; pC-tRbc) was digested using SacI and NcoI. The entry vector for overexpression of the OsISA2 gene was produced by a triple ligation reaction among the SacI-NcoI fragment of the Wxa promoter, the NcoI-SalI fragment of the OsISA2 gene, and the SacI-SalI fragment of pC-tRbc.
Transformation
The vectors used for the suppression of ISA1 or ISA2 gene expression by the RNAi method and for the expression of the ISA1 or ISA2 gene were introduced into Agrobacterium tumefaciens EHA105 (Hood et al., 1993). The RNAi and ISA2 overexpression vectors were transformed to calli of cv Kinmaze and the pul null mutant (Fujita et al., 2009) according to the method described by Toki (1997). The ISA1 expression vector was transformed to calli of EM914. For selection, the transformed calli were cultured on medium containing 50 mg L−1 hygromycin and 500 mg L−1 carbenicillin.
Selection of Homozygous Lines
About 15 plantlets of the ISA1 suppressed, ISA2 suppressed, ISA1 expressed, or ISA2 overexpressed lines were regenerated from different callus backgrounds. For selection of the ISA1 suppressed lines and the ISA2 overexpressed lines, three T0 progeny lines were chosen on the basis that about 50% of the kernels had shriveled morphology. The 15 plantlets of the ISA1 expressed line were selected by kernel morphology. About 20 developing T2 seeds at DAF15 were randomly chosen from a total of 60 T1 plants. The OsISA1 suppressed lines were screened by the phenotype showing no ISA activity bands. The OsISA2 overexpressed lines were screened by judging that the ISA1 homo-oligomer specific activity band was deleted. All of these lines were considered to be homozygous, because the phenotypes of all the T3 seeds derived from these T2 plants were the same. The T3 seeds from these homozygous lines were used for further analysis and stored in the Biotechnology Center at Akita Prefectural University.
Construction of an Expression Vector in Escherichia coli
The DNA region encoding mature ISA1 (corresponding to its cDNA; 163–2,436) or ISA2 (corresponding to its cDNA; 109–2,403) was amplified using the primers shown in Supplemental Table S1 as described above. The pGEM-T Easy vectors including the PCR products for OsISA1 or OsISA2 cDNA were digested using KpnI and SalI. The entry vectors were produced by ligation reaction between the KpnI-SalI fragment of pCold I (Takara) and the KpnI-SalI fragment for ISA1 or ISA2.
Isolation of Total RNA from Rice Endosperm, cDNA Synthesis, and Quantitative Real-Time PCR Assays of SS Genes
The total RNA was purified from rice endosperm of two kernels that were removed by hulls and pericarp using the RNeasy Plant Mini Kit (Qiagen). Quantitative PCR was performed by the method described by Ohdan et al. (2005).
Expression of Recombinant Proteins of Rice ISA1 and ISA2
All of these plasmids were incorporated into E. coli BL21(DE3) star strain (Invitrogen). The recombinant rice ISA (OsISA) proteins in the transformed cells were induced and prepared as described previously (Utsumi and Nakamura, 2006).
Purification of OsISA1 and OsISA2
The OsISA1 or OsISA2 protein was purified using a HisTrapQ HP column (5 mL; GE Healthcare) as described previously (Utsumi and Nakamura, 2006). The partially purified preparation was applied to the TSKgel DEAE-5PW column (7.5 mm diameter × 75 mm length; Tosoh Corp.) equilibrated with solution B including 50 mm imidazol-HCl (pH 7.4), 8 mm MgCl2, and 50 mm 2-mercaptoethanol. Then, OsISA1 or OsISA2 was eluted with a linear gradient of 0 to 0.5 m NaCl in solution B at a flow rate of 1 mL min−1. The OsISA1 or OsISA2 protein was collected and concentrated using a Centricon 50 centrifugal concentrator (Millipore), and then the buffer solution was substituted for 50 mm imidazol-HCl (pH 7.4), 8 mm MgCl2, 10 mm dithiothreitol, 0.5 m NaCl, and 12.5% glycerol. The purified OsISA1 or OsISA2 preparation was stored at −80°C until further use.
Preparation of the Crude Enzyme Extract from Rice Endosperm
Three to five hulled maturing rice kernels at the mid to late milking stage were homogenized using a plastic pestle in a microtube on ice in 25 to 50 μL per kernel of solution A consisting of 50 mm imidazole-HCl (pH 7.4), 8 mm MgCl2, 50 mm 2-mercaptoethanol, and 12.5% (v/v) glycerol. The homogenate was centrifuged at 10,000g for 20 min at 4°C, and the supernatant was used as the crude enzyme extract.
Purification of ISA Homo- and Hetero-Oligomers from the Developing Endosperm of Rice
The ISA1 homo-oligomer and ISA1-ISA2 hetero-oligomer were purified to near homogeneity from developing rice kernels (20 g fresh weight) at mid milking stage by using the HitrapQ HP column (5 mL) followed by the TSKgel Ether-5PW column (7.5 × 7.5 mm; Tosoh Corp.) according to the method described by Utsumi and Nakamura (2006).
In some experiments shown in Figure 3B, both ISA homo- and hetero-oligomers were partially purified. Five grams each of the developing grains from Wxa:ISA2-6-4, G5a-1, and the wild type (cv Nipponbare) were homogenized at 0°C with 20 mL of solution A, and the crude enzyme extract was prepared as described above. The enzyme extract was applied onto the HitrapQ HP column (5 mL). The proteins were eluted with a linear gradient of 0 to 0.7 m NaCl in solution B for 45 min at 1.0 mL min−1. The ISA activity fractions were pooled and concentrated to 400 μL of solution A.
For the measurement of ISA1 and ISA2 protein amounts in both oligomers (Fig. 3D), the crude extract prepared with 10 mL of solution A from 2 g (fresh weight) each of the developing grains from Wxa:ISA2-6-4, G5a-1, and Nipponbare was applied onto the TSKgel Ether-5PW column. The proteins were eluted with a descending linear gradient of 0.8 to 0.24 m ammonium sulfate in solution B for 45 min at 1.0 mL min−1. The ISA1 homo-oligomer fraction (29–34 min), the first ISA1-ISA2 hetero-oligomer fraction (35.5–40 min), and the second hetero-oligomer fraction (40–43 min) were individually pooled and concentrated to 250 μL of solution A.
Purification of ISA Oligomers from Rice Green Leaf and Potato Tuber
Twenty grams (fresh weight) of rice green leaves grown in a greenhouse at 30°C under natural light conditions for about 3 weeks after germination was ground with a mortar and pestle in liquid nitrogen, suspended in 80 mL of solution A on ice, and filtered through four layers of gauze. The filtrate was centrifuged twice at 10,000g at 4°C for 30 min. The supernatant was applied onto the HitrapQ HP column (5 mL). The proteins were eluted with a linear gradient of 0 to 0.7 m NaCl in solution B for 45 min at a flow rate of 1.0 mL min−1. The ISA activity fractions were combined and concentrated to 100 μL and then applied onto a TSKgel G3000SWXL column (7.5 × 300 mm; Tosoh Corp.), and the proteins were eluted with the same solution at a flow rate of 1.0 mL min−1. The ISA eluate was concentrated to 160 μL. The ISA fraction was applied onto the TSKgel DEAE-5PW column, and the proteins were eluted with a linear gradient of 0 to 0.7 m NaCl in solution B for 45 min at 1.0 mL min−1. The ISA activity fractions were combined and concentrated to 100 μL of solution A. The partially purified potato (Solanum tuberosum) tuber ISA preparation was stored at −80°C until further use.
Fresh tuber (500 g fresh weight) of potato (cv Dansyaku) purchased from a local market was grated with 600 mL of solution A on ice and filtered through four layers of gauze. The filtrate was centrifuged twice at 10,000g at 4°C for 30 min. The proteins in the supernatant were precipitated by 42.5% saturation of ammonium sulfate at 0°C and suspended with 10 mL of solution A. The suspension was dialyzed three times against 500 mL of solution A. The dialyzed solution was applied onto the HitrapQ HP column (10 mL). The proteins were eluted with a linear gradient of 0 to 0.7 m NaCl in solution B for 45 min at a flow rate of 1.0 mL min−1. The ISA activity fractions were combined and concentrated to 2 mL of solution A. The concentrated ISA preparation was applied onto the TSKgel G3000SWXL column, and the proteins were eluted with solution B at a flow rate of 1.0 mL min−1. The ISA eluate was concentrated to 400 μL. The ISA fraction was applied onto the TSKgel DEAE-5PW column, and proteins were eluted with a linear gradient of 0 to 0.7 m NaCl in solution B for 45 min at a flow rate of 1.0 mL min−1. The ISA activity fractions were combined and concentrated to 150 μL of solution A. The partially purified potato tuber ISA preparation was stored at −80°C until further use.
The Proportion of Soluble and Starch-Bound ISA1 Protein
The soluble protein, loosely bound protein, and tightly bound protein were prepared by the method described by Fujita et al. (2006). Randomly chosen two to 10 maturing (DAF5, -10, -15, and -20) or mature kernels of the ISA2 overexpressed line (Wxa:ISA2-6-4) and its host cv Kinmaze, respectively, were homogenized in 3 volumes of solution C containing 50 mm imidazol-HCl (pH 7.4), 8 mm MgCl2, and 12.5% glycerol using a pestle in an Eppendorf tube, and the homogenate was centrifuged at 10,000g for 20 min at 4°C. The supernatant was used as the soluble protein fraction. The precipitate was washed three times using 100 μL of solution C. The resulting precipitate was suspended in 10 volumes of SDS solution (55 mm Tris-HCl [pH 6.8], 2.3% SDS, 5% 2-mercaptoethanol, and 10% glycerol) and centrifuged at 10,000g for 20 min at 4°C. The supernatant was used as the loosely bound protein fraction. The precipitate was washed twice with 2 volumes of SDS solution. The washed precipitate was added by 10 volumes of the SDS solution and heated for 10 min at 100°C. After centrifuging at 10,000g for 10 min at 4°C, the supernatant obtained was used as the tightly bound protein fraction.
Gel Electrophoresis and Immunoblot Analysis
The native PAGE/activity staining of DBE, SS, or BE was performed as described previously (Fujita et al., 2003). Furthermore, SDS-PAGE and immunoblot analysis were performed as described previously (Kubo et al., 1999).
Carbohydrate Extraction and Quantification
One to five kernels of the maturing seeds were homogenized with a pestle in 750 μL of 100% ethanol. After the homogenate was boiled for 10 min, it was dried in a vacuum. The samples were suspended in 1.0 mL of distilled water containing 0.1% (w/v) NaN3, and were centrifuged at 3,000g for 10 min. These procedures were repeated four times. All the supernatants were combined, and the amounts of Glc, Fru, Suc, and Glc equivalents of soluble sugars were measured by the enzymatic method.
Analysis of Soluble MDs and MOSs in Rice Endosperm
To denature the enzymes attacking α-glucans and other carbohydrates, 20 frozen seeds were suspended in 3 mL of a mixture (ethanol:acetic acid, 3:1, v/v) at −80°C for 1 h. The treated seeds were removed by hulls and pericarp and then homogenized in ethanol using a mortar to a final volume of 5 mL. The homogenate was boiled for 10 min and dried in vacuum. The dried sample was mixed with 3 mL of distilled water at room temperature, and the supernatant (containing MD, MOS, and simple sugars) and precipitate (containing insoluble glucans or starch) were separated by centrifugation at 3,000g for 10 min. The precipitate was washed with 3 mL of distilled water and centrifuged. The combined soluble fractions were pooled and added to 3.75 mL of water-saturated chloroform. The solution was vigorously shaken and centrifuged at 7,000g for 10 min. The soluble fraction was withdrawn from the aqueous layer and concentrated to 0.5 mL. The concentrated sample was stored at −30°C until further use.
The separation of MD, MOS, and simple sugars in the soluble fraction was subjected to two pairs of the TSKgel G3000PWXL and a TSKgel Oligo-PW (7.5 × 75 mm; Tosoh Corp.) columns. These sugars were eluted with distilled water at 0.5 mL min−1 and kept at 60°C.
Analysis of Chain-Length Distribution of α-Glucans and MD
The chain-length distribution of glucan including amylopectin, MD, and MOS was determined by the method described by Wong et al. (2003) and Morell et al. (1998).
SEM of Starch or Insoluble Glucan Granules
Starch and glucan granules were prepared from the rice kernels from transgenic plants and their hosts, and the morphology was observed by SEM according to the methods described by Fujita et al. (2003).
Thermal Properties and X-Ray Diffraction Patterns of Starch and Insoluble Glucans
Glucan granules and starch granules were prepared as above from rice kernels from transgenic plants and its host cv Kinmaze, and their thermal properties and x-ray patterns were measured as described by Wong et al. (2003).
Sequence data from this article can be found in the GenBank/EMBL/DDBJ databases under accession numbers AB093426 (OsISA1) and Os05g0393700 (OsISA2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Schematic representation of constructs for regulating the expression of ISA1 and ISA2 genes used for the transformation of rice plants.
Supplemental Figure S2. Distribution of starch and soluble sugars in the dissected regions of the endosperm from the ISA2 gene overexpressed line, the ISA1 suppressed lines, the sugary-1 mutant line, and their host or parent cv Kinmaze.
Supplemental Figure S3. Comparison of the intensities of ISA1 and ISA2 protein bands detected by Coomassie Brilliant Blue staining with those by immunoblot detection.
Supplemental Figure S4. Effects of overexpression and suppression of the ISA2 gene on activity and expression levels of starch synthetic enzymes.
Supplemental Figure S5. The chain-length distribution of amylopectin or insoluble glucans purified from mature kernels of Kinmaze, the pul null mutant, and transgenic lines.
Supplemental Figure S6. Capillary electrophoresis analysis of MOS in the soluble sugars in endosperm of the ISA2 overexpressed line and its host cv Kinmaze.
Supplemental Figure S7. Comparison of the composition of ISA oligomers and the fine structure of glucans in the endosperm harvested at the early stage of development at DAF5 between the ISA2 overexpressed line and its host cv Kinmaze.
Supplemental Figure S8. Immunoreaction of BEI, BEIIb, SSI, and granule-bound SSI in loosely bound protein and tightly bound protein.
Supplemental Table S1. PCR primers used in this study.
Supplemental Table S2. Thermal properties of purified starch granules or insoluble glucans.
Acknowledgments
We thank Prof. Hikaru Satoh (Kyushu University) and Dr. Kimiko Ito (Niigata University) for providing the rice mutant lines and the rice Wxa promoter, respectively, and Mr. Satoshi Sasaki (Akita Prefectural University) for help in preparing the transgenic Wxa:ISA1 lines. We also thank the Biotechnology Center at Akita Prefectural University for providing technical assistance with DNA sequencing.
References
- Baba T, Arai Y. (1984) Structural characterization of amylopectin and intermediate material in amylomaize starch granules. Agric Biol Chem 48: 1763–1775 [Google Scholar]
- Ball S, Guan HP, James M, Myers A, Keeling P, Mouille G, Buléon A, Colonna P, Preiss J. (1996) From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell 86: 349–352 [DOI] [PubMed] [Google Scholar]
- Burton RA, Jenner H, Carrangis L, Fahy B, Fincher GB, Hylton C, Laurie DA, Parker M, Waite D, van Wegen S, et al. (2002) Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J 31: 97–112 [DOI] [PubMed] [Google Scholar]
- Bustos R, Fahy B, Hylton CM, Seale R, Nebane NM, Edwards A, Martin C, Smith AM. (2004) Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc Natl Acad Sci USA 101: 2215–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte T, Trevisan M, Parker ML, Zeeman SC. (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J 41: 815–830 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Francisco PB, Jr, Nakakita M, Harada K, Minaka N, Nakamura Y. (1999) Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice. Planta 208: 283–293 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Suh DS, Wong KS, Jane JL, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y. (2003) Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm. Plant Cell Physiol 44: 607–618 [DOI] [PubMed] [Google Scholar]
- Fujita N, Toyosawa Y, Utsumi Y, Higuchi T, Hanashiro I, Ikegami A, Akuzawa S, Yoshida M, Mori A, Inomata K, et al. (2009) Characterization of pullulanase (PUL)-deficient mutants of rice (Oryza sativa L.) and the function of PUL on starch biosynthesis in the developing rice endosperm. J Exp Bot 60: 1009–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y. (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140: 1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen TA, Liu F, Marsh RS, Kim S, Gan Q, Tetlow IJ, Emes MJ, James MG, Myers AM. (2008) Starch biosynthetic enzymes from developing maize endosperm associate in multisubunit complexes. Plant Physiol 146: 1892–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma R, Komatsu A, Ichinose Y, Iwahashi Y, Kato T, Komae K. (2004) Different expression of three HvISO genes and their subunits of barley isoamylase. Plant Cell Physiol 45: S179 [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Hussain H, Mant A, Seale R, Zeeman S, Hinchliffe E, Edwards A, Hylton C, Bornemann S, Smith AM, Martin C, et al. (2003) Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell 15: 133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki Y, Taniguchi H, Maruyama Y, Nakamura M. (1983) Debranching enzymes of potato tubers (Solanum tuberosum L.). I. Purification and some properties of potato isoamylase. Agric Biol Chem 47: 771–779 [Google Scholar]
- Itoh K, Ozaki H, Okada K, Hori H, Takeda Y, Mitsui T. (2003) Introduction of Wx transgene into rice wx mutants leads to both high- and low-amylose rice. Plant Cell Physiol 44: 473–480 [DOI] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM. (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainuma K, French D. (1972) Naegeli amylodextrin and its relationship to starch granule structure. II. Role of water in crystallization of B-starch. Biopolymers 11: 2241–2250 [Google Scholar]
- Kubo A, Colleoni C, Dinges JR, Lin Q, Lappe RR, Rivenbark JG, Meyer AJ, Ball SG, James MG, Hennen-Bierwagen TA, et al. (2010) Functions of heteromeric and homomeric isoamylase-type starch-debranching enzymes in developing maize endosperm. Plant Physiol 153: 956–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y. (1999) The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol 121: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Rahman S, Utsumi Y, Li Z, Mukai Y, Yamamoto M, Ugaki M, Harada K, Satoh H, Konik-Rose C, et al. (2005) Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 gene supports a direct role for isoamylase1 in amylopectin biosynthesis. Plant Physiol 137: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Shimamoto K. (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45: 490–495 [DOI] [PubMed] [Google Scholar]
- Morell MK, Samuel MS, O’Shea MG. (1998) Analysis of starch structure using fluorophore-assisted carbohydrate electrophoresis. Electrophoresis 19: 2603–2611 [DOI] [PubMed] [Google Scholar]
- Mouille G, Maddelein ML, Libessart N, Talaga P, Decq A, Delrue B, Ball SG. (1996) Preamylopectin processing: a mandatory step for starch biosynthesis in plants. Plant Cell 8: 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AM, Morell MK, James MG, Ball SG. (2000) Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol 122: 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43: 718–725 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kubo A, Shimamune T, Matsuda T, Harada K, Satoh H. (1997) Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J 12: 143–153 [Google Scholar]
- Nakamura Y, Utsumi Y, Sawada T, Aihara S, Utsumi C, Yoshida M, Kitamura S. (2010) Characterization of the reactions of starch branching enzymes from rice endosperm. Plant Cell Physiol 51: 776–794 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yuki K, Park SY, Ohya T. (1989) Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol 30: 833–839 [Google Scholar]
- Nishi A, Nakamura Y, Tanaka N, Satoh H. (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127: 459–472 [PMC free article] [PubMed] [Google Scholar]
- Ohdan T, Francisco PB, Jr, Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y. (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56: 3229–3244 [DOI] [PubMed] [Google Scholar]
- Streb S, Delatte T, Umhang M, Eicke S, Schorderet M, Reinhardt D, Zeeman SC. (2008) Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell 20: 3448–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Senoura T, Yoshizaki T, Hamada S, Ito H, Matsui H. (2007) Differential chain-length specificities of two isoamylase-type starch-debranching enzymes from developing seeds of kidney bean. Biosci Biotechnol Biochem 71: 2308–2312 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, Kawasaki S, Nakamura Y. (2004) The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotechnol J 2: 507–516 [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Beisel KG, Cameron S, Makhmoudova A, Liu F, Bresolin NS, Wait R, Morell MK, Emes MJ. (2008) Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol 146: 1878–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S. (1997) Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep 15: 16–21 [Google Scholar]
- Utsumi Y, Nakamura Y. (2006) Structural and enzymatic characterization of the isoamylase1 homo-oligomer and the isoamylase1-isoamylase2 hetero-oligomer from rice endosperm. Planta 225: 75–87 [DOI] [PubMed] [Google Scholar]
- Utsumi Y, Yoshida M, Francisco PB, Jr, Sawada T, Kitamura S, Yasunori N. (2009) Quantitative assay method for starch branching enzyme with bicinchoninic acid by measuring the reducing terminals of glucans. J Appl Glycosci 56: 215–222 [Google Scholar]
- Wattebled F, Dong Y, Dumez S, Delvallé D, Planchot V, Berbezy P, Vyas D, Colonna P, Chatterjee M, Ball S, et al. (2005) Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiol 138: 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattebled F, Planchot V, Dong Y, Szydlowski N, Pontoire B, Devin A, Ball S, D’Hulst C. (2008) Further evidence for the mandatory nature of polysaccharide debranching for the aggregation of semicrystalline starch and for overlapping functions of debranching enzymes in Arabidopsis leaves. Plant Physiol 148: 1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Kubo A, Jane J, Harada K, Satoh H, Nakamura Y. (2003) Structures and properties of amylopectin and phytoglycogen in the endosperm of sugary-1 mutants of rice. J Cereal Sci 37: 139–149 [Google Scholar]