Abstract
Ethylene influences many processes in Arabidopsis (Arabidopsis thaliana) through the action of five receptor isoforms. We used high-resolution, time-lapse imaging of dark-grown Arabidopsis seedlings to better understand the roles of each isoform in the regulation of growth in air, ethylene-stimulated nutations, and growth recovery after ethylene removal. We found that ETHYLENE RECEPTOR1 (ETR1) is both necessary and sufficient for nutations. Transgene constructs in which the ETR1 promoter was used to drive expression of cDNAs for each of the five receptor isoforms were transferred into etr1-6;etr2-3;ein4-4 triple loss-of-function mutants that have constitutive growth inhibition in air, fail to nutate in ethylene, and take longer to recover a normal growth rate when ethylene is removed. The patterns of rescue show that ETR1, ETR2, and ETHYLENE INSENSITIVE4 (EIN4) have the prominent roles in rapid growth recovery after removal of ethylene whereas ETR1 was the sole isoform that rescued nutations. ETR1 histidine kinase activity and phosphotransfer through the receiver domain are not required to rescue nutations. However, REVERSION TO SENSITIVITY1 modulates ethylene-stimulated nutations but does not modulate the rate of growth recovery after ethylene removal. Several chimeric receptor transgene constructs where domains of EIN4 were swapped into ETR1 were also introduced into the triple mutant. The pattern of phenotype rescue by the chimeric receptors used in this study supports a model where a receptor with a receiver domain is required for normal growth recovery and that nutations specifically require the full-length ETR1 receptor.
Ethylene is a gaseous plant hormone that influences a number of processes in plants such as seed germination, abscission, senescence, fruit ripening, response to stress, and growth. In etiolated Arabidopsis (Arabidopsis thaliana) seedlings, ethylene causes a number of changes including reduced growth of the hypocotyl and root, increased radial expansion of the hypocotyl, increased tightening of the apical hook, and an increase in root hair formation (Abeles et al., 1992). Various studies have identified a number of components in the ethylene signaling pathway and led to increasingly refined models for signal transduction (Hall et al., 2007; Li and Guo, 2007).
Responses to ethylene in Arabidopsis are mediated by a family of five receptors called ETHYLENE RECEPTOR1 (ETR1), ETR2, ETHYLENE RESPONSE SENSOR1 (ERS1), ERS2, and ETHYLENE INSENSITIVE4 (EIN4; Chang et al., 1993; Hua and Meyerowitz, 1998; Hua et al., 1998; Sakai et al., 1998). The receptors are primarily localized to the endoplasmic reticulum (Chen et al., 2002, 2007; Ma et al., 2006; Dong et al., 2008; Grefen et al., 2008; Zhong et al., 2008) and have homology to bacterial two-component receptors that transduce signal via the autophosphorylation of a His residue in the kinase domain, followed by the transfer of phosphate to a conserved Asp residue in the receiver domain of a response regulator protein (Chang et al., 1993; West and Stock, 2001). The ethylene receptors in plants can be divided into two subfamilies. Subfamily I consists of ETR1 and ERS1 that are capable of His kinase activity whereas subfamily II includes ETR2, EIN4, and ERS2 that have Ser/Thr kinase activity in vitro (Gamble et al., 1998; Moussatche and Klee, 2004). ERS1 appears to be bifunctional and is capable of both His and Ser/Thr kinase activities depending on the assay conditions used (Moussatche and Klee, 2004). The kinase domain but not receiver domain of ETR1 is required for signaling (Qu and Schaller, 2004). However, while ethylene may inhibit His kinase activity in ETR1 (Voet-van-Vormizeele and Groth, 2008), His kinase activity is not needed for signaling (Wang et al., 2003; Binder et al., 2004b; Qu and Schaller, 2004) but does have a role in growth recovery after ethylene removal (Binder et al., 2004b) and in regulation of growth (Qu and Schaller, 2004; Cho and Yoo, 2007). The exact output of the receptors is still obscure, but genetic studies show that in the absence of ethylene, the receptors positively regulate CONSTITUTIVE TRIPLE RESPONSE1 (CTR1). CTR1 acts as a negative regulator of the pathway that in turn inhibits downstream components of the pathway, preventing ethylene responses (Kieber et al., 1993). When ethylene is added, the receptors become inactivated, which via an unknown mechanism inactivates CTR1 and releases downstream components from the inhibition by CTR1.
All five receptor isoforms in Arabidopsis are involved in ethylene signaling and have overlapping roles in regulating various phenotypes such as growth (Hua et al., 1995, 1998; Hall and Bleecker, 2003). However, it is now emerging that the five receptor isoforms are not entirely redundant in their roles (Hall and Bleecker, 2003; Binder et al., 2004b, 2006; Seifert et al., 2004; O’Malley et al., 2005; Xie et al., 2006; Qu et al., 2007; Plett et al., 2009a, 2009b; Liu et al., 2010) and in some cases a particular isoform has a unique role in controlling a trait (Binder et al., 2006; Plett et al., 2009b). This appears to be a general feature of ethylene signaling since certain receptors have a prominent role in controlling fruit ripening in tomato (Solanum lycopersicum) whereas other isoforms have little or no role in this (Kevany et al., 2007). The basis for these nonoverlapping roles is unknown. We have previously used high-resolution, time-lapse imaging of seedlings growing in darkness to examine the role of each receptor isoform (Binder et al., 2004b, 2006). Using this system we obtained evidence that ETR1, ETR2, and EIN4 are important for rapid growth recovery after ethylene removal while ERS1 and ERS2 have little or no role in this phenotype (Binder et al., 2004b). More recently we showed that ethylene stimulates nutational bending of Arabidopsis hypocotyls and root tips through the known ethylene signaling pathway (Binder et al., 2006). Interestingly, this phenotype requires the ETR1 receptor, but not the other receptor isoforms. Loss-of-function etr1 mutations eliminated nutations while single loss-of-function mutants for the other receptor isoforms had no measurable effect on nutations. However, combinatorial loss-of-function mutations in the other receptor isoforms led to constitutive nutations in air. Thus, ETR1 appears to have a role in regulating nutations that is opposite to the other receptor isoforms (Binder et al., 2006). In this study we characterized the receptor and receptor domain requirements underlying growth in air, ethylene-stimulated nutations, and recovery of growth after removal of ethylene and found that these phenotypes have different receptor and receptor domain requirements.
RESULTS
ETR1 Is Both Required and Sufficient for Ethylene-Stimulated Nutational Bending
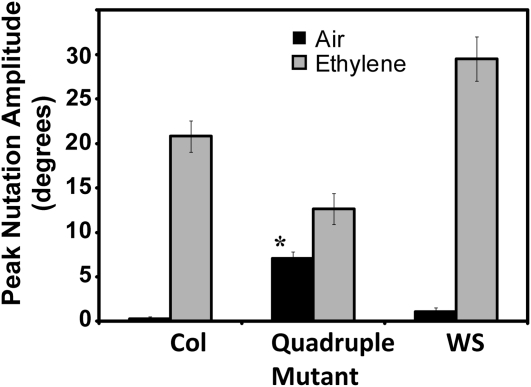
We have previously shown (Binder et al., 2006) that etr1 loss-of-function mutants, either alone or in combination with other receptor isoforms, are incapable of ethylene-stimulated nutations. This phenotype was rescued when these mutants were transformed with a full-length genomic ETR1 transgene. In contrast, single loss-of-function mutations in the other receptor isoforms have no measurable effect on ethylene-stimulated nutations whereas loss of these other isoforms in combination leads to constitutive nutations in air; wild-type seedlings show no measurable nutations in air under these conditions (Binder et al., 2006). Thus, unlike most phenotypes related to ethylene, a single receptor isoform is required. To determine if ETR1 alone is also sufficient for this phenotype, we examined quadruple ers1-3 etr2-3 ein4-4 ers2-3 loss-of-function mutants (Liu et al., 2010). These mutants were very stunted in growth and produced very few seeds (data not shown). As predicted from our previous results, we found that the quadruple mutant seedlings nutated constitutively in air (Fig. 1). Additionally, they nutated with slightly larger amplitude oscillations when treated with 1 μL L−1 ethylene for 23 h (Fig. 1). This indicates that ETR1 is not only necessary, but sufficient for ethylene-stimulated nutational bending in Arabidopsis hypocotyls.
Figure 1.
ETR1 is sufficient for ethylene-stimulated nutations. Peak hypocotyl nutation amplitude for seedlings treated with air or 1 μL L−1 ethylene were measured as described in the “Materials and Methods.” Responses of ers1-3 etr2-3 ein4-4 ers2-3 quadruple mutants are compared to responses of Col and WS wild-type seedlings. Average peak nutation amplitude ± sem is plotted. The nutation amplitudes in air were analyzed with t tests and the amplitude of the quadruple mutant showed a significant (P < 0.0001) increase in air over wild-type controls (*).
ETR1 Promoter-Driven Expression of Receptors and the Rescue of Seedling Phenotypes
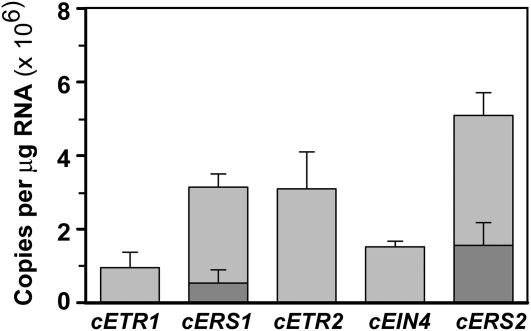
Earlier studies support a model where all five receptor isoforms regulate growth, while ETR1, ETR2, and EIN4 play the predominant roles in the rate of growth recovery after ethylene removal and only ETR1 is required for ethylene-stimulated nutations (Hua et al., 1995, 1998; Hua and Meyerowitz, 1998; Sakai et al., 1998; Hall and Bleecker, 2003; Binder et al., 2004b, 2006). To further delineate the roles of the various receptor isoforms in ethylene responses, we transformed etr1-6 etr2-3 ein4-4 mutants with cDNA constructs for each of the receptor isoforms. This triple mutant was chosen because it has reduced growth in air (Hua and Meyerowitz, 1998; Binder et al., 2004b; Qu and Schaller, 2004; Liu et al., 2010), exhibits very slow growth rate recovery after the removal of ethylene (Binder et al., 2004b), and fails to nutate in the presence of ethylene (Binder et al., 2006). To investigate the possibility that differences in expression level or pattern could account for the different roles in ethylene signaling, cDNAs of all five receptor genes were placed under the control of the ETR1 promoter. This approach has previously been used to investigate the roles of subfamily I and II receptors in growth (Wang et al., 2003). We quantitated transcript levels for each receptor transgene using reverse transcription (RT)-PCR (Wang et al., 2003; O’Malley et al., 2005). This revealed that all receptor transgenes were measurably expressed with cETR1 showing the lowest expression levels (Fig. 2).
Figure 2.
Transcript level of transgenic and endogenous genes in etr1-7 etr2-3 ein4-4 mutants. Triple etr1 etr2 ein4 mutants were transformed with cDNA for the indicated receptor. All transgenes were under the promoter control of ETR1. Transcript levels were quantified by RT-PCR as previously described (Wang et al., 2003; O’Malley et al., 2005). Dark gray indicates the expression of the endogenous genes and light gray the expression of transgenes. Only transcript levels of wild-type alleles are shown.
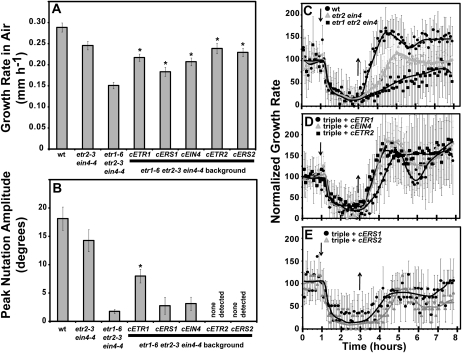
Consistent with prior research, the etr1-6 etr2-3 ein4-4 triple null mutants had a reduced hypocotyl growth rate in air (Hua and Meyerowitz, 1998; Binder et al., 2004b; Qu and Schaller, 2004; Liu et al., 2010). The growth rate of Columbia (Col) seedlings in air was approximately 0.29 mm h−1 whereas the etr1-6 etr2-3 ein4-4 triple mutants grew at rate of approximately 0.15 mm h−1 (Fig. 3A). ETR1-driven expression of all five receptor isoforms rescued the reduced growth rate in air of the triple mutant toward the growth rates seen with the etr2-3 ein4-4 double mutant, although, the cERS1 transgene rescued growth less than the other constructs (Fig. 3A). Thus, all five constructs produced functional protein. These results support a model where all five receptor isoforms are involved in the regulation of growth.
Figure 3.
Rescue of growth, nutations, and growth recovery by various ethylene receptor isoforms. Triple etr1-6 etr2-3 ein4-4 mutants were transformed with cDNA for ETR1, ERS1, ETR2, EIN4, and ERS2. All constructs were under the promoter control of ETR1. For comparison, data from Col (wt), etr2-4 ein4-4 double mutant, and etr1-6 etr2-3 ein4-4 triple mutant seedlings are shown. A, The growth rates in air of triple mutants transformed with the indicated receptor isoform are shown. Basal growth rate in air was determined from the first hour of growth kinetic measurements prior to the introduction of ethylene. Data represents the average growth rate in air ± sem. B, Nutations in response to 1 μL L−1 ethylene were measured in etr1-6 etr2-3 ein4-4 triple mutants transformed with the indicated receptor isoform and amplitudes calculated as described in the “Materials and Methods.” The average peak nutation amplitude ± sem is plotted. In A and B data were analyzed with t tests and increases over the etr1-6 etr2-3 ein4-4 triple mutants were considered statistically significant for P < 0.05 (*). C, Growth kinetic profiles for wt, etr2-3 ein4-4 double mutants, and etr1-6 etr2-3 ein4-4 triple mutants are plotted. D to E, Growth kinetic profiles for triple mutants transformed with the indicated receptor isoform are plotted. For C to E, seedlings were allowed to grow for 1 h in air, followed by the addition of 1 μL L−1 ethylene (down arrow). Two hours later ethylene was removed (up arrow) and seedlings grown in air for an additional 5 h. Growth rates were normalized to the growth rate during the air pretreatment and represents the average ± sd for at least four seedlings total in at least three separate experiments. Lines were fitted by hand.
We observed a very different pattern of rescue when we examined hypocotyl nutations. Wild-type (Col) seedlings nutated with an average peak amplitude of approximately 18° when treated with 1 μL L−1 ethylene (Fig. 3B). The etr2-3 ein4-4 double mutants had slightly smaller nutations while the etr1-6 etr2-3 ein4-4 triple mutants had very small oscillations (less than 2°). The triple mutant had similar amplitude oscillations whether ethylene was present (data not shown) and it was difficult to determine whether these small oscillations were true nutational bending, bending due to movement over the agar surface, or due to some other factor. Only the cETR1 transgene rescued nutations yielding seedlings that nutated with an amplitude approximately 50% that of the wild-type or etr2-3 ein4-4 controls (Fig. 3B). None of the other transgenes rescued nutations and cETR2 and cERS2 might have inhibited nutational bending. These results support the idea that ETR1 has a unique role in controlling nutational bending and suggest that differences in expression patterns do not underlie ETR1’s unique role.
Earlier studies on Arabidopsis seedlings uncovered two phases of hypocotyl growth inhibition when 10 μL L−1 ethylene was applied (Binder et al., 2004a, 2004b, 2007). The first phase of inhibition starts about 10 min after ethylene is added and lasts approximately 15 min when it reaches a new steady state. This new steady state lasts approximately 20 min when the second phase of growth inhibition occurs that further inhibits growth. A new steady-state growth rate is reached after approximately 20 min and lasts for as long as a saturating concentration of ethylene is present. When ethylene is removed, hypocotyl growth recovers to pretreatment rates in approximately 85 min. The time for growth recovery is increased in etr1, etr2, and ein4 loss-of-function mutants (either singly or in combination) while ers1 and ers2 mutants have wild-type recovery rates (Binder et al., 2004b). In this study, none of the receptor loss-of-function mutants or receptor isoform transformants had measurably altered growth inhibition kinetics when ethylene was added (Fig. 3, C–E). Consistent with our earlier work, the rate of growth recovery in the etr1-6 etr2-3 ein4-4 triple mutant seedlings after treatment with 1 μL L−1 ethylene was very slow (Fig. 3C). The growth rate of these mutants failed to completely recover to pretreatment levels in the 5 h after the removal of ethylene. The etr2-3 ein4-4 double mutants recovered approximately 2 h after removal of ethylene whereas wild-type seedlings recovered after approximately 75 to 80 min. Wild-type seedlings recovered slightly faster than recovery observed in prior studies (Binder et al., 2004b, 2007). This likely reflects the lower concentration of ethylene used in this study. Interestingly, the cETR1, cETR2, and cEIN4 transgenes rescued rapid growth recovery (Fig. 3D) while the cERS1 and cERS2 transgenes failed to rescue rapid growth recovery or did so very poorly (Fig. 3E).
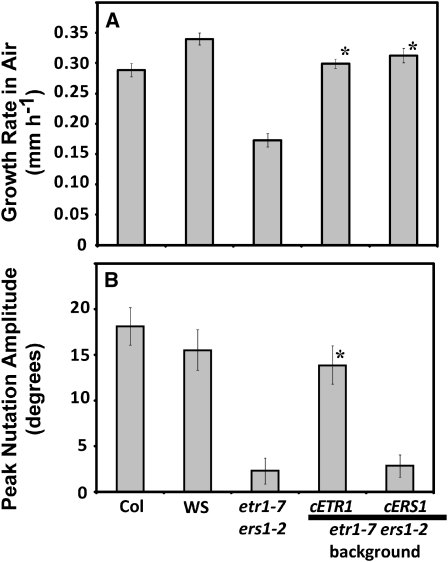
As noted above, the cERS1 transgene failed to rescue nutations and rescued growth in air less than the other transgenes. This failure of the cERS1 transgene to rescue all phenotypes does not appear to be caused by reduced levels of this transgene since it is actually expressed at higher levels than cETR1 (Fig. 2). To confirm that cERS1 cannot rescue nutations, we examined etr1-7 ers1-2 double mutants transformed with the cERS1 transgene. For comparison, we studied double mutants transformed with the cETR1 transgene. Prior research showed that both transgenes are expressed in this mutant background although cERS1 is expressed at approximately 5 times the levels of cETR1 (Wang et al., 2003). In agreement with this previous study, we found that both cETR1 and cERS1 fully rescue growth in air (Fig. 4A). Our previous study revealed that etr1-7 ers1-2 double mutants fail to nutate in the presence of 10 μL L−1 ethylene (Binder et al., 2006). We obtained similar results in this study using seedlings treated with 1 μL L−1 ethylene (Fig. 4B). Transformation of these double mutants with the cETR1 transgene rescued nutations while transformation with the cERS1 transgene did not (Fig. 4B). Thus, even though cERS1 is expressed at higher levels than cETR1 in these double mutants and can fully rescue growth in air, it fails to rescue nutations. In contrast, cETR1 rescues both phenotypes, indicating that there is a functional difference between the two receptor isoforms as suggested by a prior study (Liu et al., 2010).
Figure 4.
Rescue of growth and nutations in etr1 ers1 double mutant. Double etr1-7 ers1-2 mutants were transformed with cDNA for ETR1 or ERS1. Both constructs were under the promoter control of ETR1. For comparison, data from Col, WS, and double mutants are shown. A, The growth rates in air of the double mutants transformed with the indicated receptor are shown. Date represents the average growth rate in air ± sem. B, Peak hypocotyl nutation amplitude for seedlings treated with 1 μL L−1 ethylene were measured as described in the “Materials and Methods.” Average peak nutation amplitude ± sem is plotted. Data were analyzed with t tests and rescue over the etr1-7 ers1-2 double mutant considered statistically significant for P < 0.0001 (*).
These results support the idea that the receptors containing a receiver domain play the largest roles in the rate of growth recovery after removal of ethylene with perhaps a slight contribution from ERS1 and ERS2. They also show that ETR1 alone has the major role in regulating ethylene-stimulated nutations.
ETR1 His Kinase Activity and Phosphotransfer through the Receiver Domain Are Not Required for Ethylene-Stimulated Nutations
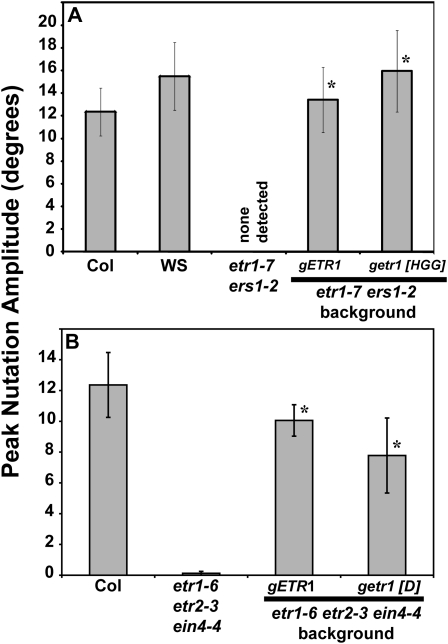
The basis for this unique role of ETR1 in nutations remains to be determined. One possible mechanism underlying ETR1’s unique role in nutations is suggested by the fact that only ETR1 has both a functional His kinase and receiver domain (Chang et al., 1993; Gamble et al., 1998; Moussatche and Klee, 2004). Prior studies showed that ETR1 His kinase activity is not required for growth inhibition in the presence of ethylene (Wang et al., 2003; Binder et al., 2004b). Rather, ETR1 His kinase activity appears to have a role in growth recovery after removal of ethylene (Binder et al., 2004b) and in regulation of growth (Qu and Schaller, 2004; Cho and Yoo, 2007). To determine the role of ETR1 His kinase activity in ethylene-stimulated nutations, we examined ers1-2 etr1-7 double mutants and double mutants transformed with either a wild-type genomic ETR1 transgene (gETR1) or a kinase-inactivated ETR1 genomic clone (getr1-[HGG]). Col seedlings treated with 10 μL L−1 ethylene nutated with an average peak amplitude of approximately 12° while Wassileweskija (WS) nutated with an average peak amplitude of approximately 16°; the double mutant failed to nutate (Fig. 5A). Transformation of the double mutant with gETR1 rescued nutations as previously noted (Binder et al., 2006). Interestingly, transformation of the double mutant with the kinase-inactivated getr1-[HGG] transgene also rescued nutations (Fig. 5A). This shows that ETR1 His kinase activity is not required for ethylene-stimulated nutations. It is possible that there is residual His kinase activity present because the ers1-2 allele is a knockdown rather than a knockout mutation. However, the ers1-3 mutant is a knockout (Xie et al., 2006; Qu et al., 2007) that nutates in the presence of ethylene. This is true both in the single ers1-3 mutant (Binder et al., 2006) as well as in the quadruple ers1-3 etr2-3 ein4-4 ers2-3 mutants (Fig. 1).
Figure 5.
ETR1 His-kinase activity and phosphotransfer through the receiver domain are not required for ethylene-stimulated nutations. The peak hypocotyl nutation amplitude for seedlings treated with 10 μL L−1 ethylene for 22 h is shown. Nutation amplitude was determined as described in “Materials and Methods.” Average peak nutation amplitudes ± sem are plotted from at least six seedlings total in three separate experiments. A, A wild-type genomic ETR1 (gETR1) and a kinase inactivated ETR1 construct (getr1 [HGG]) were transformed into etr1-7 ers1-2 double mutant seedlings. Col and WS are shown as wild-type controls. B, A wild-type gETR1 and mutant ETR1 construct lacking the conserved Asp-659 needed for phosphotransfer (getr1 [D]) were transformed into etr1-6 etr2-3 ein4-4 triple mutant seedlings. Col responses are shown as a wild-type control. Data were analyzed using t tests and rescue over the mutant background was considered statistically significant for P < 0.0001 (*).
Phosphotransfer through the conserved Asp659 in the receiver domain has no obvious role in ethylene-induced inhibition of growth but does play a role in recovery of growth after the removal of ethylene (Binder et al., 2004b). To study the role of phosphotransfer through the ETR1 receiver domain in ethylene-stimulated nutations, we examined etr1-6 etr2-3 ein4-4 plants lacking the receptor isoforms containing receiver domains. These were then transformed with either gETR1 or a mutant transgene (getr1-[D]) lacking the conserved Asp659 required for phosphotransfer. The etr1-6 etr2-3 ein4-4 triple loss-of-function mutants failed to nutate while transformation with gETR1 rescued the nutation phenotype (Fig. 5B) consistent with earlier work (Binder et al., 2006). This phenotype was also rescued by the getr1-[D] transgene, indicating that phosphotransfer is also not required for ethylene-stimulated nutations.
REVERSION TO SENSITIVITY1 Modulates Ethylene-Stimulated Nutations
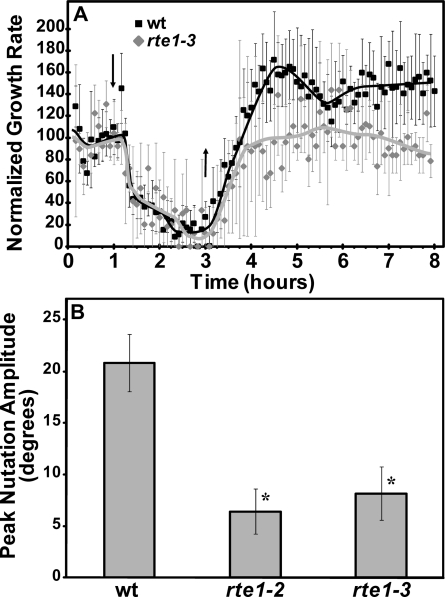
Recently it was found that REVERSION TO SENSITIVITY1 (RTE1) specifically interacts with ETR1 to modulate ethylene responses in Arabidopsis as a positive regulator of ETR1, but does not regulate the other receptor isoforms (Resnick et al., 2006; Zhou et al., 2007; Rivarola et al., 2009; Dong et al., 2010). Interestingly, rte1 null mutants display phenotypes similar to etr1 null mutants including reduced growth in air, ethylene hypersensitivity, and enhanced growth inhibition to application of ethylene (Resnick et al., 2006). Because of this, we examined rte1 null mutants for alterations in growth kinetics and ethylene-stimulated nutations to determine if RTE1 also modulates these phenotypes. rte1-3 seedlings had growth inhibition kinetics indistinguishable from wild-type seedlings (Fig. 6A). The rate of growth recovery was also unaltered in rte1-3 seedlings. However, a growth rate overshoot that normally occurs during recovery in wild-type seedlings was absent in rte1-3 seedlings (Fig. 6A). In contrast to growth recovery, both rte1-2 and rte1-3 mutants had measurably reduced nutation amplitudes in the presence of ethylene (Fig. 6B). rte1-2 seedlings had an average nutation amplitude approximately 30% of wild-type plants and rte1-3 seedlings an average nutation amplitude approximately 40% of wild-type plants. Thus, RTE1 modulates but is not absolutely required for ethylene-stimulated nutations.
Figure 6.
rte1 mutants affect growth kinetics and nutation amplitude. Growth kinetic and nutation responses are compared between rte1 mutants and Col (wt). A, To examine growth kinetics, seedlings were treated with air for 1 h, followed by treatment with 1 μL L−1 ethylene (down arrow). Two hours later, ethylene was removed (up arrow) and growth in air followed for an additional 5 h. Growth rates were normalized to the growth during the air pretreatment. Average normalized growth ± sd are plotted. Lines are drawn by hand. B, Seedlings were treated with 1 μL L−1 ethylene for 24 h and peak nutation amplitudes calculated as described in the “Materials and Methods.” Average peak nutation amplitude ± sem are plotted. Data were analyzed with t tests and a decrease in nutation amplitude from wt was considered statistically significant with a P < 0.001 (*).
ETR1 Domain Requirements for the Rescue of Seedling Phenotypes
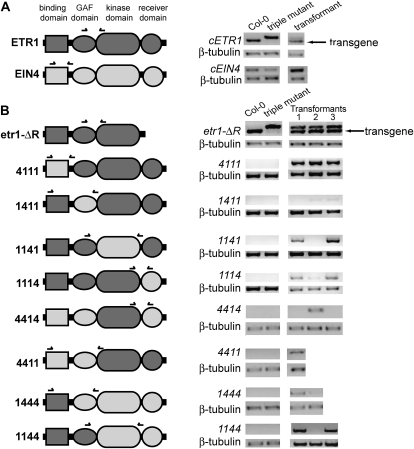
Our results point to a prominent role for ETR1 in both growth recovery and ethylene-stimulated nutations. To better understand the role of ETR1 in these phenotypes we created chimeric receptor cDNA constructs where domains from EIN4 were swapped for comparable domains in ETR1 (Fig. 7; Supplemental Fig. S1). Both single and multiple domains were swapped into ETR1. These constructs were all placed under the promoter control of ETR1. Since the receiver domains of ETR1, ETR2, and EIN4 may have a role in regulating certain responses, we also generated a truncated ETR1 transgene lacking the receiver domain (cetr1-ΔR). Figure 7 shows a schematic diagram for each construct used. Each chimeric transgene was transformed into the etr1-6 etr2-3 ein4-4 triple mutant background. In general, at least three independent transgenic lines were generated and the transcript levels assessed by RT-PCR (Fig. 7). The locations of primers used for each chimera are shown above each diagram and the sequences for these primers listed (Supplemental Table S1). At least one line for each transgene showed detectable transcript, although, the chimeric construct containing the EIN4 GAF (cGMP-specific phosphodiesterases, adenylyl cyclases, and FhlA) domain (1411) had low levels of expression (Fig. 7). We examined rescue of growth for all transgenic lines and found that for transgene constructs that were capable of rescuing this phenotype, there was a correlation between transcript level and the amount of growth rescue (data not shown). Therefore, for each construct we chose the line with the highest expression level to examine the rescue of the various seedling phenotypes used in this study.
Figure 7.
Structure of ETR1-EIN4 chimeric constructs used for analyses. A, Domains of full-length ETR1 and EIN4. B, Diagrams of ETR1-EIN4 chimeric constructs used. ETR1 domains are shown dark gray and denoted with a 1 in naming the construct while EIN4 domains are light gray and denoted with a 4. All constructs were cDNA constructs under ETR1 promoter control that were transformed into the etr1-6 etr2-3 ein4-4 triple mutant background as described in the “Materials and Methods.” RNA expression levels for each transgene was analyzed using RT-PCR. The location of primers for each construct is marked above each diagram. Primers used are listed in Supplemental Table S1 Primers for the full-length (cETR1) and truncated ETR1 lacking the receiver domain (cetr1-ΔR) amplified products for both wild-type ETR1 and etr1-6. The cETR1 and cetr1-ΔR transgene transcripts ran as a smaller product (arrows) than the etr1-6 product. Primers for the chimeric receptors were designed with one primer in the EIN4 region and one in the ETR1 region of the construct so that they only amplified the transgene. Transcript levels for β-tubulin in each plant line are shown as a control.
Of the six chimeric transgenes that rescued growth in air, the 4411 and 4414 rescued growth better than the 1411, 1114, 1444, and cetr1-ΔR constructs and the cetr1-ΔR construct was the least effective at rescuing growth in air (Fig. 8A; Table I). Three constructs tested (4111, 1141, 1144) failed to rescue growth. Two of these (1144, 4111) were able to rescue other phenotypes, indicating they produced functional protein. Only 1141 produced a nonfunctional product (see below). In contrast to the rescue of growth, only two constructs (1411, 4111) rescued nutations in the etr1-6 etr2-3 ein4-4 mutant background (Fig. 8B; Table I). The level of rescue by 1411 and 4111 was poor (Fig. 8B) and was approximately half of that observed with the cETR1 transgene (Fig. 3B). Several of the chimeric receptors also rescued the rapid growth recovery rate in the triple mutant. These included 1114, 4411, 1144, and 4414 that rescued the rate of growth recovery to comparable rates seen with the cETR1 and cEIN4 transgenes (Figs. 3D and 8C). Previously we showed that the phosphotransfer-deficient getr1-[D] transgene only partially rescued the rate of growth recovery of the triple mutant (Binder et al., 2004b). Similarly, we found in this study that the truncated cetr1-ΔR construct lacking the receiver domain partially rescued rapid growth recovery (Fig. 8D; Table I). Interestingly, the 1411 construct also partially rescued this phenotype (Fig. 8D; Table I). The low level of rescue could be due to the low expression levels of the 1411 transgene (Fig. 7). We examined two other independent transgenic lines containing the cetr1-ΔR or 1411 transgenes and found that they too had partial rescue of rapid growth recovery (data not shown). Three transgenes (4111, 1141, 1444) failed to rescue the growth recovery phenotype (Fig. 8E; Table I).
Figure 8.
Rescue of growth, nutations, and growth recovery by chimeric receptor transgenes. The triple etr1-6 etr2-3 ein4-4 were transformed with chimeric cDNA constructs with domains swapped between ETR1 and EIN4 as labeled. All transgenes were under the promoter control of ETR1. For comparison, data from Col (wt), etr2-3 ein4-4 double mutants, and etr1-6 etr2-3 ein4-4 triple mutants are included. A, The growth rates in air of triple mutants transformed with the indicated chimeric receptor are plotted. Growth rate in air was determined from the first hour of growth kinetic measurements prior to the introduction of ethylene. Data represents the average growth rate ± sem. B, Nutations in response to 1 μL L−1 ethylene were measured in etr1-6 etr2-3 ein4-4 triple mutants transformed with the indicated ETR1-EIN4 chimeric receptor. Nutation amplitudes were measured as described in the “Materials and Methods.” The average peak nutation amplitude ± sem is plotted. C to E, Growth kinetic profiles for triple mutants transformed with chimeric receptors are shown. Seedlings were allowed to grow for 1 h in air, followed by the addition of 1 μL L−1 ethylene (down arrow). Two hours later ethylene was removed (up arrow) and seedlings grown in air for an additional 5 h. Growth rate was normalized to growth rate in the air pretreatment and represents the average ± sd for at least four seedlings in three separate experiments. Lines were fitted by hand. For comparison, each section shows the growth kinetic profile for wt (dashed black line), etr2-3 ein4-4 double mutants (dashed gray line), and etr1-6 etr2-3 ein4-4 triple mutants (dotted black line) taken from Figure 3C. In A and B data were analyzed with t tests and increases over the etr1-6 etr2-3 ein4-4 triple mutants were considered statistically significant for P < 0.001 (*) or P < 0.02 (#).
Table I. Overview of phenotypes rescued by receptor transgenes.
| etr1-6 etr2-3 ein4-4 Transformed with: | Phenotype Rescued | ||
| Growth in Aira | Growth Recovery after Removal of Ethyleneb | Ethylene-Stimulated Nutationsa | |
| cETR1 | +++ | +++ | ++ |
| 1411 | ++ | ++ | + |
| cEIN4 | +++ | +++ | − |
| 4411 | +++ | +++ | − |
| 4414 | +++ | +++ | − |
| cETR2 | +++ | +++ | − |
| cERS2 | +++ | + | − |
| 1114 | ++ | +++ | − |
| cetr1-ΔR | + | ++ | − |
| cERS1 | + | + | − |
| 1444 | ++ | − | − |
| 1144 | − | +++ | − |
| 4111 | − | − | + |
| 1141 | − | − | − |
Our results with the receptor isoform transgenes and chimeric ETR1-EIN4 transgenes are summarized in Table I and show that there are four general classes of rescue. One class rescued all three phenotypes. This class only included cETR1 and 1411. A second class rescued growth in air and the growth recovery rate after ethylene removal but did not rescue nutations. This was the largest class of transgenes. The third class of transgenes only rescued one of the three phenotypes. The 1444 construct only rescued growth in air while others only rescued normal growth recovery (1144) or nutations (4111). The fourth class consisted of only the 1141 construct that failed to rescue any phenotype. These results show that growth in air, ethylene-stimulated nutations, and the rate of growth recovery after the removal of ethylene have different requirements for receptor isoforms and ETR1 domains.
DISCUSSION
Previous studies indicate that the five receptor isoforms have both overlapping and distinct roles in the regulation of growth by ethylene (Hua et al., 1995, 1998; Hua and Meyerowitz, 1998; Hall and Bleecker, 2003; Binder et al., 2004b; O’Malley et al., 2005; Xie et al., 2006; Qu et al., 2007; Liu et al., 2010). The basis for these roles is not clear. In this study we examined the receptor requirements that lead to regulation of growth in air, ethylene-stimulated nutations, and recovery of growth after the application of ethylene. We find that these traits have different receptor requirements and different ETR1 domain requirements. Of the three phenotypes examined in this study, the nutation phenotype has the strictest receptor requirements. In contrast, the receptor requirements for growth in air and rapid recovery of growth after removal of ethylene are not as strict. Results from this study also show that the receptors have redundant function during the initial growth inhibition responses to ethylene since none of the receptor mutants or transformants had any obvious alterations in the kinetics of the growth inhibition response upon application of ethylene.
One interesting observation is that ETR1 is not only necessary, but sufficient to regulate ethylene-stimulated nutations. Two obvious differences between ETR1 and the other receptor isoforms are that ETR1 is specifically regulated by RTE1 and it contains both a functional His kinase domain and a receiver domain. While RTE1 is involved in the regulation of nutations, other mechanisms are also involved since the rte1 null mutants still nutate in the presence of ethylene. RTE1 belongs to a small gene family that includes RTE1-HOMOLOG1 (RTH1) so it is possible that RTH1 contributes to the regulation of ethylene-stimulated nutations (Resnick et al., 2006). In contrast, His autophosphorylation followed by phosphotransfer to a receiver domain is not involved in nutations even though the ethylene receptors are structurally related to bacterial two-component receptors that function by this mechanism (West and Stock, 2001). Thus, ETR1 His kinase activity is involved in rapid growth recovery after ethylene removal (Binder et al., 2004b) but not involved in either inhibition of growth (Wang et al., 2003; Binder et al., 2004b) or stimulation of nutations by ethylene (this study).
Current models of ethylene signaling suggest that the receptors stimulate CTR1 which, in turn, acts as a negative regulator of the response pathway. In these models, ethylene inhibits receptor output releasing the inhibition by CTR1. Hence, loss of ethylene receptors mimics the action of ethylene and causes constitutive ethylene responses in air. The observation that etr1 loss-of-function mutants fail to nutate is opposite to what is predicted by these models. Paradoxically, combinatorial loss-of-function mutations in the other receptor isoforms leads to constitutive nutations in air (Binder et al., 2006). We previously proposed two hypotheses to explain this unique role of ETR1 (Binder et al., 2006). One hypothesis is that ethylene induces differential expression of ETR1 in the zone of bending. Prior research has shown that eliminating the ETR1 isoform results in lowered growth (Hua and Meyerowitz, 1998; Cancel and Larsen, 2002) so it is possible that lower expression of ETR1 on one side of the hypocotyl relative to the other side would lead to differential growth rates resulting in bending. Over time, the localized region of altered ETR1 expression moves causing nutational oscillations. A second hypothesis proposes that ETR1 has two functions with one function regulating growth and the other supporting nutations. These hypotheses are not mutually exclusive.
To address these hypotheses, we examined the rescue of nutations in the etr1 etr2 ein4 triple mutant by cDNA transgenes for each of the receptor isoforms. These were all placed under control of the ETR1 promoter to investigate the possibility that differences in expression level or pattern could account for the different roles in ethylene signaling. Only the ETR1 transgene rescued nutations, supporting our prior conclusions that ETR1 is required for ethylene-stimulated nutations. The other transgenes were expressed and produced functional receptors since all of them were able to rescue the reduced growth of the triple mutant and two of them also rescued the rapid growth recovery phenotype. The pattern of rescue for the nutation phenotype by the various receptor transgenes makes it unlikely that differential synthesis of ETR1 in the zone of bending plays a major role in regulating nutational bending. This does not rule out that ETR1 undergoes differential proteolysis leading to differential levels of receptor protein in the zone of bending. It is also possible that the spatial and temporal pattern of expression for another molecule that regulates ETR1 changes in the zone of bending upon stimulation with ethylene. One candidate for this is RTE1 (and perhaps RTH1) since it specifically regulates ETR1.
Four general domains have been identified in ethylene receptors (ethylene binding, GAF, kinase, receiver), although, the specific functions for all of these domains have not been well elucidated. Truncated ETR1 receptors have been used in some studies to uncover roles for these domains. These have shown that the ETR1 kinase domain is likely to be required for signaling leading to growth regulation while the receiver domain is not (Gamble et al., 2002; Qu and Schaller, 2004). Even though the receiver domain of ETR1 is not required for growth inhibition caused by ethylene, it may control sensitivity to ethylene at low doses, suggesting subtle roles for this domain (Qu and Schaller, 2004). However, the use of truncated etr1-1 receptors indicates that the requirement for the kinase domain might not be absolute and other domains are also involved in signaling (Gamble et al., 2002; Xie et al., 2006). We used chimeric ETR1-EIN4 receptor transgene constructs where various domains of EIN4 were swapped into ETR1 to explore the receptor domain requirements for the regulation of the traits examined in this study. Transforming the etr1 etr2 ein4 triple mutants with these various constructs revealed differences in their ability to rescue the three phenotypes examined (summarized in Table I). The expression level for each construct was different, which can make interpretation of the results more complicated. However, there was no obvious correlation between the transcript expression level and the patterns of rescue.
The ethylene-binding and GAF domains generally appear to be interchangeable between ETR1 and EIN4 to support normal growth in air and rapid growth recovery after ethylene is removed. These constructs do not rescue ethylene-stimulated nutations. It is interesting to note that swapping the EIN4 ethylene-binding domain into ETR1 resulted in a minimally functional receptor even though the ethylene-binding domains of EIN4 and ETR1 share a great deal of homology and both are capable of binding ethylene (Schaller and Bleecker, 1995; O’Malley et al., 2005; Wang et al., 2006). This could represent problems with proper protein folding, normal protein-protein interactions, or accelerated proteolysis. When the EIN4 ethylene-binding domain is swapped into ETR1 in combination with other EIN4 domains, a more functional receptor is formed that rescued growth in air and growth recovery, suggesting the EIN4 ethylene-binding domain is functional in these chimeric receptors. In contrast to the chimeric receptors that contained the EIN4 ethylene-binding and GAF domains, chimeric receptors containing the EIN4 kinase domain were minimally or nonfunctional. It is unclear why the constructs containing the EIN4 kinase domain were poor at rescuing phenotypes but this might reflect a structural requirement to have this domain completely in the EIN4 context for proper protein folding or protein-protein interactions. Alternatively, this could reflect a difference in enzymatic output since EIN4 has Ser/Thr kinase activity while ETR1 has His kinase activity (Gamble et al., 1998; Moussatche and Klee, 2004).
Most transgenes rescued both growth in air and rapid growth recovery after the removal of ethylene. However, the cetr1-ΔR transgene did not rescue the growth recovery rate very well. This coupled with the observation that the cERS1 and cERS2 transgenes also rescue this phenotype poorly and our prior results that ers1 and ers2 loss-of-function mutants have normal growth recovery kinetics (Binder et al., 2004b) points to a central role for receptors containing a receiver domain in regulating this phenotype. This is not surprising since our previous work showed that phosphotransfer through the ETR1 receiver domain is involved with normal growth recovery when ethylene is removed (Binder et al., 2004b). Downstream targets for phosphotransfer from ETR1 have yet to be determined however Arabidopsis contains response regulator proteins (Schaller et al., 2008) that are likely targets. The cetr1-ΔR transgene also does not rescue nutations, pointing to a central role for the receiver domain in regulating ethylene-stimulated nutations too. Additionally, most constructs containing the ETR1 receiver domain rescued nutations in the triple mutant while none of the constructs containing the EIN4 receiver domain rescued this phenotype. In contrast, most constructs containing a receiver domain from either receptor rescued rapid growth recovery after ethylene removal. Thus, it appears that the receiver domain of EIN4 can substitute for the ETR1 receiver domain for signaling that leads to growth recovery but not for signaling that leads to ethylene-stimulated nutations.
In the case of the rate of growth recovery, this requirement for the receiver domain involves His kinase activity and phosphotransfer through the conserved Asp on the receiver domain while in the case of nutations, phosphotransfer is not involved. This suggests other functions for the ETR1 receiver domain. The receiver domain of ETR1 has been structurally characterized and has differences from other characterized receiver domains (Müller-Dieckmann et al., 1999). One such difference was noted in the γ-loop that is thought to be important in molecular interactions. This loop could function in coupling between ETR1 and downstream signaling molecules. Thus, this structure might lead to interactions unique to ETR1 that lead to nutations. There is precedence for believing that ETR1 has unique interactions with other molecules (Clark et al., 1998; Urao et al., 2000; Cancel and Larsen, 2002; Gao et al., 2003). Sequence comparison of the receiver domains from ETR1, EIN4, and ETR2 reveals a number of nonconserved amino acid residues found only in the ETR1 receiver domain that could form the basis for this unique ETR1 function.
While the ETR1 receiver domain is important for both the rate of growth recovery and nutations, we hypothesize that the entire ETR1 protein is required for normal nutations since most ETR1-EIN4 chimeric transgene constructs fail to rescue this phenotype even though they rescue other phenotypes. The basis for ETR1’s role in nutations is unknown. Recent studies showed that the ethylene receptors form complexes between isoforms and with other unidentified proteins (Gao et al., 2008; Chen et al., 2010). Of particular note is that ETR1 formed complexes in a domain-dependent fashion with all the domains required (Chen et al., 2010). This suggests a model where the whole ETR1 receptor is required to form protein-protein interactions that, in part, leads to signaling that stimulates nutations. The output of the receptors remains to be determined. The results presented above show that ETR1 has multiple roles in regulating seedling growth and development. The basis for these multiple roles is unknown but clearly each role has unique receptor and receptor domain requirements. Whether the mechanisms underlying these different roles for ETR1 involves multiple outputs from the receptor, modulation of receptor levels or localization, unique protein-protein interactions, or some combination of these factors remains to be determined.
MATERIALS AND METHODS
l-α-(2-amino ethoxyvinyl)-Gly was supplied by Rohm Haas, Inc. The rte1 mutants were a kind gift of Caren Chang (Resnick et al., 2006). The etr1-6, etr1-7, etr2-3, ers2-3, and ein4-4 mutants were originally obtained from Elliot Meyerowitz (Hua and Meyerowitz, 1998) while ers1-2 was from the Bleecker lab (Hall and Bleecker, 2003; Wang et al., 2003). All mutants are in the Col background except for ers1-2, ers1-3, and ers2-3 that are in the WS background. Combinatorial mutants used in this study have previously been described (Hall and Bleecker, 2003; Wang et al., 2003; Liu et al., 2010). Most are solely in the Col background that was used as the wild-type control. For those mutant combinations containing ers1 or ers2, WS was also used as a wild-type control.
Plasmid Construction
The gETR1, getr1-[HGG], getr1-[D] transgenes as well as chimeric receptors under the promoter control of ETR1 (cETR1, cERS1, cETR2, cEIN4, cERS2) have been described previously (Wang et al., 2003; Binder et al., 2004b, 2006). ETR1-EIN4 chimeric constructs and the truncated ETR1 construct lacking the receiver domain were made as outlined in Supplemental Figure S1. Positions of restriction sites were identified in the ETR1 and EIN4 sequences so that domains could be exchanged with little or no disruption in amino acid sequence. All were cDNA constructs containing the ETR1 promoter.
Generation of Transgenic Lines
The etr1-7 ers1-2 mutants transformed with gETR1, getr1-[HGG], cETR1, cERS1, cETR2, cEIN4, and cERS2 as well as etr1-6 ers2-3 ein4-4 mutants transformed with gETR1 and getr1-[D] have been described previously (Wang et al., 2003; Binder et al., 2004b). All chimeric ETR1-EIN4 constructs or truncated receptors were cloned into the pPZP221 vector and transformed into Arabidopsis (Arabidopsis thaliana) plants using the same methods. Briefly, each cDNA construct was transformed into Agrobacterium tumefaciens strain ABI and transferred into Arabidopsis plants by using the floral-dipping method (Clough and Bent, 1998).
RT-PCR
RT-PCR was carried out to analyze the transcript levels of cETR1, cEIN4, cetr1-ΔR, and each chimeric receptor constructs using primers specific to each construct (Supplemental Table S1). For the cETR1 and cetr1-ΔR transgenes, primers were designed so that the wild-type ETR1 RNA ran as a smaller product than the etr1-6 gene product while the cEIN4 primers were designed to give less amplification of the ein4-4 gene product. This allowed for the detection of transgene expression in these genetic backgrounds since the transgene contains wild-type sequence. For the chimeric receptors, primers were designed with one primer within the sequence of ETR1 and the other within EIN4 sequence so that no product was detected in plants not expressing the specific transgene.
RNA was extracted from 10 or more seedlings using the RNA plant extraction kit from Qiagen and DNA cleaned by the turbo DNase kit from Ambion. PCR amplification was carried out on 100 ng of RNAout using the one-step RT-PCR kit from Qiagen. Products amplified with these primer pairs were run on an 1.5% agarose gel and detected with UV illumination. Transcript levels of β-tubulin were analyzed as a control using primers described previously (Gao et al., 2008).
The methods and primers used to quantitate expression of the cETR1, cERS1, cETR2, cEIN4, and cERS2 transgenes using quantitative RT-PCR have previously been described (Wang et al., 2003; O’Malley et al., 2005).
Seed Preparation
Arabidopsis seeds were surface sterilized by treatment with 70% alcohol for 30 s, placed on sterile filter paper to dry, and then placed on agar plates containing 0.8% (w/v) agar, half-strength Murashige and Skoog basal salt mixture (Murashige and Skoog, 1962), pH 5.7, fortified with vitamins and with no added sugar. Unless otherwise specified, 5 μm l-α-(2-amino ethoxyvinyl)-Gly was added to inhibit ethylene production by the seedlings. Seeds were treated for 2 to 8 d at 4°C, treated with light for 4 to 8 h under continuous fluorescent lights, and then used for time-lapse analysis.
High-Resolution, Time-Lapse Imaging and Analysis of Growth Rate and Nutation Angles
Time-lapse imaging of Arabidopsis hypocotyls was conducted as previously described (Binder et al., 2004a, 2004b, 2006). Images of hypocotyls of etiolated seedlings were captured in the dark with Marlin CCD cameras (Allied Vision Technology) using infrared illumination.
For growth response kinetics measurements, seedlings were treated for 1 h with ethylene-free air followed by 2 h with ethylene to examine the growth inhibition kinetics. This was followed by 5 h ethylene-free air to allow for growth recovery. Images were captured every 5 min for 8 h. To determine growth rate of the hypocotyl, the height in pixels of each seedling in each frame was analyzed using custom software written by Edgar Spalding (Parks and Spalding, 1999; Folta and Spalding, 2001) as previously described (Binder et al., 2004a, 2004b). Basal growth rate in air was determined from the first hour of measurements prior to the introduction of ethylene. Experiments under all conditions were repeated in at least three separate experiments and at least four seedlings were measured.
For nutational bending experiments, seedlings were treated with ethylene for 22 to 24 h and electronic images were captured every 15 min. The angles of hypocotyls were measured manually as previously described (Binder et al., 2006). Since nutations represent a sinusoidal oscillation of bending over time, the nutation amplitude was determined by measuring the change in angle from the peak of each oscillation to the midline of the sine wave. For each seedling, the peak nutational amplitude was determined. Experiments under all conditions were repeated in at least three separate experiments and at least six seedlings examined. Measurements were statistically analyzed using t tests.
Ethylene Concentration Measurements
Ethylene concentrations were determined using a Hewlett-Packard 6890 gas chromatograph with an HP Plot/Q column with ethylene as a calibration standard (Hall et al., 1999).
Arabidopsis Genome Initiative accession numbers for genes described in this article are: ETR1, At1g66340; ERS1, At2g40940; EIN4, At3g04580; ETR2, At3g23150; ERS2, At1g04310; CTR1, At5g03730; RTE1, At2g26070; and RTH1, At3g51040.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Domain structure of the truncated and chimeric receptors.
Supplemental Table S1. Primer sequences used for RT-RCR.
Acknowledgments
We thank E.G.B. Bickford, R. Lacey, J. Luthar, C. O’Leary, B. Ramirez, and G. Woo for technical assistance.
References
- Abeles F, Morgan P, Saltveit MJ. (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB. (2004a) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’Malley RC, Wang W, Zutz TC, Bleecker AB. (2006) Ethylene stimulates nutations that are dependent on the ETR1 receptor. Plant Physiol 142: 1690–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’malley RC, Wang WY, Moore JM, Parks BM, Spalding EP, Bleecker AB. (2004b) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. (2007) The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancel JD, Larsen PB. (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chen Y-F, Randlett MD, Findell JL, Schaller GE. (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277: 19861–19866 [DOI] [PubMed] [Google Scholar]
- Chen Y-F, Shakeel SN, Bowers J, Zhao X-C, Etheridge N, Schaller GE. (2007) Ligand-induced degradation of the ethylene receptor ETR2 through a proteasome-dependent pathway in Arabidopsis. J Biol Chem 282: 24752–24758 [DOI] [PubMed] [Google Scholar]
- Chen YF, Gao Z, Kerris RJ, III, Wang W, Binder BM, Schaller GE. (2010) Ethylene receptors function as components of high-molecular-mass protein complexes in Arabidopsis. PLoS ONE 5: e8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y-H, Yoo S-D. (2007) ETHYLENE RESPONSE 1 histidine kinase activity of Arabidopsis promotes plant growth. Plant Physiol 143: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang XX, Chang C. (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95: 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dong C-H, Jang M, Scharein B, Malach A, Rivarola M, Liesch J, Groth G, Hwang I, Chang C. (2010) Molecular association of the Arabidopsis ETR1 ethylene receptor and a regulator of ethylene signaling, RTE1. J Biol Chem 285: 40706–40713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-H, Rivarola M, Resnick JS, Maggin BD, Chang C. (2008) Subcellular co-localization of Arabidopsis RTE1 and ETR1 supports a regulatory role for RTE1 in ETR1 ethylene signaling. Plant J 53: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE. (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE. (2002) Mutational analysis of the ethylene receptor ETR1: role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wen C-K, Binder BM, Chen Y-F, Chang J, Chiang Y-H, Kerris RJ, III, Chang C, Schaller GE. (2008) Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J Biol Chem 283: 23801–23810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZY, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Grefen C, Städele K, Růzicka K, Obrdlik P, Harter K, Horák J. (2008) Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant 1: 308–320 [DOI] [PubMed] [Google Scholar]
- Hall AE, Bleecker AB. (2003) Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 15: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB. (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Shakeel S, Schaller G. (2007) Ethylene receptors: ethylene perception and signal transduction. J Plant Growth Regul 26: 118–130 [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ. (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 51: 458–467 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Li H, Guo H. (2007) Molecular basis of the ethylene signaling and response pathway in Arabidopsis. J Plant Growth Regul 26: 107–117 [Google Scholar]
- Liu Q, Xu C, Wen C-K. (2010) Genetic and transformation studies reveal negative regulation of ERS1 ethylene receptor signaling in Arabidopsis. BMC Plant Biol 10: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Cui M-L, Sun H-J, Takada K, Mori H, Kamada H, Ezura H. (2006) Subcellular localization and membrane topology of the melon ethylene receptor CmERS1. Plant Physiol 141: 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche P, Klee HJ. (2004) Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. J Biol Chem 279: 48734–48741 [DOI] [PubMed] [Google Scholar]
- Müller-Dieckmann H-J, Grantz AA, Kim S-H. (1999) The structure of the signal receiver domain of the Arabidopsis thaliana ethylene receptor ETR1. Structure 7: 1547–1556 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, Bleecker AB. (2005) Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J 41: 651–659 [DOI] [PubMed] [Google Scholar]
- Parks BM, Spalding EP. (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett JM, Cvetkovska M, Makenson P, Xing T, Regan S. (2009a) Arabidopsis ethylene receptors have different roles in Fumonisin B1-induced cell death. Physiol Mol Plant Pathol 74: 18–26 [Google Scholar]
- Plett JM, Mathur J, Regan S. (2009b) Ethylene receptor ETR2 controls trichome branching by regulating microtubule assembly in Arabidopsis thaliana. J Exp Bot 60: 3923–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Hall BP, Gao Z, Schaller GE. (2007) A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BMC Plant Biol 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Schaller GE. (2004) Requirement of the histidine kinase domain for signal transduction by the ethylene receptor ETR1. Plant Physiol 136: 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick JS, Wen C-K, Shockey JA, Chang C. (2006) REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci USA 103: 7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivarola M, McClellan CA, Resnick JS, Chang C. (2009) ETR1-specific mutations distinguish ETR1 from other Arabidopsis ethylene receptors as revealed by genetic interaction with RTE1. Plant Physiol 150: 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang CR, Medrano LJ, Bleecker AB, Meyerowitz EM. (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ, Shiu S-H. (2008) Two-component signaling elements and histidyl-aspartyl phosphorelays. The Arabidopsis Book 6: e0112, doi/10.1199/tab.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Roberts K. (2004) Growth regulators and the control of nucleotide sugar flux. Plant Cell 16: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Miyata S, Yamaguchi-Shinozaki K, Shinozaki K. (2000) Possible His to Asp phosphorelay signaling in an Arabidopsis two-component system. FEBS Lett 478: 227–232 [DOI] [PubMed] [Google Scholar]
- Voet-van-Vormizeele J, Groth G. (2008) Ethylene controls autophosphorylation of the histidine kinase domain in ethylene receptor ETR1. Mol Plant 1: 380–387 [DOI] [PubMed] [Google Scholar]
- Wang W, Esch JJ, Shiu SH, Agula H, Binder BM, Chang C, Patterson SE, Bleecker AB. (2006) Identification of important regions for ethylene binding and signaling in the transmembrane domain of the ETR1 ethylene receptor of Arabidopsis. Plant Cell 18: 3429–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hall AE, O’Malley R, Bleecker AB. (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AH, Stock AM. (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci 26: 369–376 [DOI] [PubMed] [Google Scholar]
- Xie F, Liu Q, Wen C-K. (2006) Receptor signal output mediated by the ETR1 N terminus is primarily subfamily I receptor dependent. Plant Physiol 142: 492–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Lin Z, Grierson D. (2008) Tomato ethylene receptor-CTR interactions: visualization of NEVER-RIPE interactions with multiple CTRs at the endoplasmic reticulum. J Exp Bot 59: 965–972 [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu Q, Xie F, Wen C-K. (2007) RTE1 is a Golgi-associated and ETR1-dependent negative regulator of ethylene responses. Plant Physiol 145: 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]