Abstract
Arabidopsis (Arabidopsis thaliana) NON-RACE-SPECIFIC DISEASE RESISTANCE1 (NDR1), a plasma membrane-localized protein, plays an essential role in resistance mediated by the coiled-coil-nucleotide-binding site-leucine-rich repeat class of resistance (R) proteins, which includes RESISTANCE TO PSEUDOMONAS SYRINGAE2 (RPS2), RESISTANCE TO PSEUDOMONAS SYRINGAE PV MACULICOLA1, and RPS5. Infection with Pseudomonas syringae pv tomato DC3000 expressing the bacterial effector proteins AvrRpt2, AvrB, and AvrPphB activates resistance by the aforementioned R proteins. Whereas the genetic requirement for NDR1 in plant disease resistance signaling has been detailed, our study focuses on determining a global, physiological role for NDR1. Through the use of homology modeling and structure threading, NDR1 was predicted to have a high degree of structural similarity to Arabidopsis LATE EMBRYOGENESIS ABUNDANT14, a protein implicated in abiotic stress responses. Specific protein motifs also point to a degree of homology with mammalian integrins, well-characterized proteins involved in adhesion and signaling. This structural homology led us to examine a physiological role for NDR1 in preventing fluid loss and maintaining cell integrity through plasma membrane-cell wall adhesions. Our results show a substantial alteration in induced (i.e. pathogen-inoculated) electrolyte leakage and a compromised pathogen-associated molecular pattern-triggered immune response in ndr1-1 mutant plants. As an extension of these analyses, using a combination of genetic and cell biology-based approaches, we have identified a role for NDR1 in mediating plasma membrane-cell wall adhesions. Taken together, our data point to a broad role for NDR1 both in mediating primary cellular functions in Arabidopsis through maintaining the integrity of the cell wall-plasma membrane connection and as a key signaling component of these responses during pathogen infection.
Defense signaling in plants following pathogen infection results in the activation of multiple, often parallel, signaling pathways. To accomplish this, the activation of plant resistance signaling requires the utilization of numerous preformed defense responses, systemic and cell-to-cell signaling, and, in many cases, the initiation of gene-for-gene resistance (Knepper and Day, 2010). While examples of the activation and regulation of parallel processes have been described (van Wees et al., 2000), there are numerous instances in which bifurcation exists (Wiermer et al., 2005). In plants, it is now evident that regulation and specificity often have overlapping nodes; the guard hypothesis is one such example (for review, see Chisholm et al., 2006; Jones and Dangl, 2006). In short, host resistance (R) proteins are responsible for the indirect recognition of pathogen effectors by means of “guarding” a virulence target and initiating an effective defense response.
The largest class of R genes found in plants encode for nucleotide-binding site-Leu-rich repeat (NB-LRR) signaling molecules (Moffett, 2009). NB-LRR R proteins have been historically divided into two subgroups, based on the N-terminal presence of either a coiled-coil (CC) domain or a domain with similarity to the Toll IL-1 receptor (TIR) family of proteins (for review, see Takken and Tameling, 2009). While R proteins play a central role in the activation of resistance following pathogen perception, R proteins alone are not sufficient for the initiation of resistance. As such, numerous ancillary proteins and chaperones serving as coactivators of resistance have also been identified (Azevedo et al., 2002; Muskett et al., 2002; Tornero et al., 2002; Hubert et al., 2003; Belkhadir et al., 2004). For example, ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1; Parker et al., 1996) has been shown to mediate defense signaling through the activation of the TIR domain-containing R proteins, while NON-RACE-SPECIFIC DISEASE RESISTANCE1 (NDR1; Century et al., 1995, 1997; Coppinger et al., 2004) has been shown to be required for R proteins that contain the CC domain (Aarts et al., 1998). There are, however, exceptions to this rule; several CC-NB-LRR R proteins that specify resistance to the oomycete pathogen Hyaloperonospora arabidopsidis function independently of NDR1 (McDowell et al., 2000; Bittner-Eddy and Beynon, 2001). Thus, while there appears to be conservation in terms of specificity, function, and activation of signaling through both EDS1 and NDR1 in terms of R protein structure, the full mechanism(s) associated with these pathways is unknown.
Of the two primary signaling components required for the activation of R protein-mediated resistance, the role of EDS1 in defense signaling is best understood. EDS1 serves as a central regulatory protein involved in both biotic and oxidative stress signaling (for review, see Wiermer et al., 2005). In this capacity, EDS1, through its interaction with PHYTOALEXIN-DEFICIENT4 and SENESCENCE-ASSOCIATED GENE101, is required for elicitation of the hypersensitive response (HR) during bacterial infection (Feys et al., 2001, 2005). EDS1 has also been shown to function in halting postinvasive growth of nonpathogenic fungi (Yun et al., 2003) as well as functioning in oxidative stress signaling (Wiermer et al., 2005). Thus, EDS1 can be classified as a broad-spectrum signaling mediator required for the activation of resistance to multiple pathogen types (Hu et al., 2005).
In contrast, the function of NDR1 within the CC-NB-LRR signaling pathway remains unclear. NDR1 is a plasma membrane-localized protein (Century et al., 1997) that undergoes multiple posttranslational modifications, including C-terminal processing (i.e. glycosylphosphatidylinositol anchoring) and N-linked glycosylation (Coppinger et al., 2004). Unlike most glycosylphosphatidylinositol-anchored proteins, NDR1 appears to be unique in that the anchor at the C terminus is resistant to cleavage by phospholipase C and therefore possibly positions NDR1 within the plasma membrane as a “double anchored” protein (Coppinger et al., 2004). This positioning further raises the possibility that NDR1 may play a role in signaling from within the apoplast, transducing the signal from the extracellular space to within the cell, possibly via its association with RPM1-INTERACTING PROTEIN4 (RIN4; Day et al., 2006); however, this hypothesis remains untested. NDR1 has been demonstrated to associate with RIN4 (Day et al., 2006), which has been shown to be required for both the regulation and activation of resistance mediated by at least two members of the CC-NB-LRR class of R proteins (Mackey et al., 2002, 2003; Axtell and Staskawicz, 2003; Belkhadir et al., 2004; Day et al., 2005; Chisholm et al., 2006). The association of RIN4 with the resistance proteins RESISTANCE TO PSEUDOMONAS SYRINGAE PV MACULICOLA1 (RPM1; Grant et al., 1995) and RESISTANCE TO PSEUDOMONAS SYRINGAE2 (RPS2; Kunkel et al., 1993) is believed to maintain the proteins in a signaling-incompetent state (Ellis and Dodds, 2003; Chisholm et al., 2006). The association of NDR1 with a key negative regulator of R protein activation (RIN4) may in fact point to a role for NDR1 as a signal recognition element upstream of the activation of specific R protein signaling (Belkhadir et al., 2004) and, moreover, may signify a role for NDR1 in broader defense and stress-associated responses requiring both NDR1 and RIN4.
The activation of defense signaling through ligand-receptor interactions (e.g. pathogen recognition receptor-pathogen associated molecular pattern [PAMP]) is likely preceded by the mechanical stimulation of responses at the onset of pathogen invasion. For example, in mammalian innate immune and stress signaling, integrins have been characterized as a central signaling component of the mechanosensing apparatus of cells (Kumamoto, 2008). Integrins are ubiquitous plasma membrane receptors that recognize extracellular glycoproteins (e.g. collagen and fibronectins) via the conserved, solvent-exposed Arg-Gly-Asp (RGD) motif (Gee et al., 2008). On the cytoplasmic side, integrins connect the extracellular matrix to the cytoskeleton, and following signal recognition, they can initiate signaling responses associated with cell differentiation (Streuli, 2009) and growth and proliferation (Huveneers et al., 2007). In plants, peptides containing the RGD motif have been shown to block numerous processes, including fungal toxin penetration (Manning et al., 2008), mechanoperception (for review, see Telewski, 2006), cell wall adhesion to the plasma membrane (Canut et al., 1998), and abiotic stress responses (Zhu et al., 1993; for review, see Gao et al., 2007). Similarly, RGD-containing peptides have also been shown to interfere with cell signaling responses initiated during interactions with microorganisms, particularly those involving fungal and oomycete pathogens (Kiba et al., 1998; Mellersh and Heath, 2001; Senchou et al., 2004). While bona fide integrin proteins have not been identified in plants, high-affinity RGD-binding sites have been identified (Canut et al., 1998), and it is hypothesized that these sites mediate plasma membrane-cell wall adhesion.
In this work, we describe a role for Arabidopsis (Arabidopsis thaliana) NDR1 in both defense and stress response signaling based on a predicted structure of NDR1 with homology to biotic and abiotic stress-responsive proteins. Using this predicted structural homology and the known positioning of NDR1 in defense signaling, we demonstrate using molecular-genetic, physiological, and cell biology approaches that NDR1 plays a role in electrolyte release in response to bacterial pathogen infection as well as in maintenance of the plasma membrane-cell wall junction. Mutational analysis of NDR1 supports a role for the RGD-like motif, NGD (Asn-Gly-Asp), as a key motif in NDR1 involved in defense signaling following Pseudomonas syringae pv tomato DC3000 (Pst DC3000) infection. Taken together, our data suggest a role for NDR1 in coordinating broader cellular processes in response to stress and bacterial pathogen infection.
RESULTS
NDR1 Shares Predicted Structural Homology with LEA14, an Integrin-Like Protein
Two independent methods were used to generate a predicted structure for NDR1 (Fig. 1A). First, the protein fold recognition server PHYRE (Protein Homology/analogY Recognition Engine; Kelley and Sternberg, 2009) was used to generate a predicted structural model. In the second approach, we utilized the automated homology-modeling server I-TASSER to generate five predicted structures for NDR1 (Supplemental Fig. S1). Both PHYRE and I-TASSER generated similar homology model predictions for NDR1. The outputs of these predictions were then used as a template to guide the structure-function analyses described in this study. Homology modeling and structure threading were used to predict a tertiary fold of NDR1. Once a secondary structure for NDR1 was predicted using PHYRE, homology modeling was performed using MODELER (Martí-Renom et al., 2000) and Chimera (Pettersen et al., 2004). The output of this analysis was then threaded onto the solved structure of the Arabidopsis LATE EMBRYOGENESIS ABUNDANT14 (LEA14) protein (Fig. 1B, pdb_1yyc; Singh et al., 2005), a member of the late embryogenesis family of proteins.
Figure 1.
Homology modeling of NDR1 with the integrin-like protein LEA14. A, Predicted structure of NDR1. B, The solved structure of LEA14 (pdb_1yyc; Singh et al., 2005). C, NDR1 predicted structure threaded onto the solved structure of LEA14, highlighting the predicted structural homology. N denotes N terminal. PHYRE analysis returned an estimated structural homology precision of 95%, with an E-value of 0.008. Additional predicted models are shown in Supplemental Figure S1.
Further analysis of the predicted structure was performed in a domain-by-domain manner, from which we identified several striking similarities with mammalian proteins involved in signal perception and innate immune responses (Supplemental Fig. S2). For example, the large β-sheet torus (Fig. 1A, blue arrows) resembles the core structure of type III fibronectins (Potts and Campbell, 1994). Further modeling (Supplemental Fig. S2) suggests that the primary core structure of NDR1 shares strong structural similarity with the membrane-bound subunit (i.e. fibronectin FNIII domain) of integrin, with the putative transmembrane domains of both NDR1 and integrin connected to large single β-sheets (Hynes, 2009). Adjacent to the three-amino acid α-helix (Fig. 1B, small yellow helix; compare the homologous position in Fig. 1C), we identified the presence of a solvent-exposed RGD-like motif (i.e. NGD) at amino acid positions 178 to 180. In host-fungus interactions, the role of RGD motifs in defense signaling has been characterized as a potential ligand-binding site involved in cell wall-plasma membrane adhesion (Manning et al., 2008; for review, see Dodds et al., 2009). Moreover, RGD sites have also been postulated as both a target and a mode of action for pathogen effector proteins and secretion systems, presumably to facilitate pathogen entry through disrupting cell wall-plasma membrane focal adhesions (Jiménez-Soto et al., 2009; Wang et al., 2009).
NDR1 Plays a Role in Limiting Electrolyte Leakage in Response to Pst DC3000 Infection
Based on the proposed function of LEA proteins in Arabidopsis, we hypothesized that NDR1 may play a broad role in regulating fluid loss. While the precise function of the LEA family of proteins remains elusive, numerous studies have linked LEAs to a variety of biotic and abiotic stress responses (De Meutter et al., 2005; Goyal et al., 2005; Battaglia et al., 2008). In support of this, Singh et al. (2005) proposed LEAs to have a role in stopping or slowing the process of fluid loss in response to wounding or dehydration. Based on the predicted structural homology of NDR1 to LEA14, we hypothesized that defense signaling may be regulated, in part through NDR1, as a function of controlling nutrient availability during pathogen infection (Katagiri et al., 2002). When inoculated with the phytopathogenic bacterium Pst DC3000, ndr1-1 plants show altered electrolyte leakage compared with ecotype Columbia (Col-0) plants. These data are in agreement with similar plant-pathogen studies that have utilized electrolyte leakage as a physiological marker for the HR as well as the activation of defense signaling in plants (Baker et al., 1991; Mackey et al., 2002). While previous methods used to measure electrolyte leakage relied on simple conductance measurements over a relatively short time scale (i.e. 24 h; Supplemental Fig. S3; Supplemental Appendix S1), we chose to examine the changes in leakage in response to disease progression, and not simply in correlation to the HR, by utilizing a method previously applied to abiotic stress (Gilmour et al., 1988). When ndr1-1 plants were dip inoculated with Pst DC3000, the leakage measurements observed paralleled those of Col-0 (Fig. 2A). These data are consistent with previously published bacterial growth data and the enhanced disease susceptibility phenotype(s) (Century et al., 1995) while also indicating a possible alteration in membrane permeability in the ndr1-1 mutant. When plants were inoculated with Pst DC3000 expressing the Cys protease effector protein AvrRpt2 (Axtell et al., 2003), the difference in measured electrolytes between ndr1-1 and Col-0 were in greater contrast, with the most striking difference observed at 2 d postinoculation (Fig. 2B). This result provides further evidence for an alteration in membrane integrity in the ndr1-1 mutant, as a simple increase in programmed cell death cannot explain the dramatic difference in leakage observed; ndr1-1 does not undergo HR in response to AvrRpt2 (Century et al., 1995). The large observed difference in leakage also correlates with the onset of disease symptoms in ndr1-1 (Fig. 2E). To this end, while ndr1-1 does not exhibit a significant increase in leakage, our findings would suggest that the ndr1-1 mutant is unable to restrict leakage induced following Pst DC3000-AvrRpt2 inoculation. This apparent loss in restricting leakage may be a primary mechanism through which AvrRpt2 is able to enhance susceptibility in ndr1-1. Indeed, this hypothesis is further supported by previous results from Freeman and Beattie (2009), which show that Arabidopsis responds to the effector AvrRpm1 by restricting vascular flow to the infected area. Related to the work presented herein, it is noteworthy that both AvrRpm1 and AvrRpt2 are known to target RIN4 (Mackey et al., 2002, 2003), the only previously identified interacting partner of NDR1 (Day et al., 2006). Pst DC3000 expressing the effector proteins AvrPphB (Shao et al., 2003) and AvrB (Mackey et al., 2002) did not elicit a statistically significant difference in the electrolyte release response in ndr1-1 plants compared with Col-0 (Fig. 2, C and D).
Figure 2.
A to D, Enhanced electrolyte leakage in the ndr1-1 mutant following inoculation with Pst DC3000. Levels of electrolyte leakage from Col-0 and ndr1-1 plants in response to DC3000 inoculation are displayed as percentage maximal leakage. Treatments include Pst DC3000 expressing vector control (A), AvrRpt2 (B), AvrB (C), and AvrPphB (D). The dramatic increase in leakage observed in ndr1-1 as compared with Col-0 when inoculated with Pst DC3000 correlates with the onset of disease symptoms. dpi, Days postinoculation. Error bars display sd from four technical replicates from two to three biological replicates. Significance was determined using two-way ANOVA, where asterisks represent statistically significant differences between Col-0 and ndr1-1: * P < 0.05, *** P < 0.001. E, Col-0 and ndr1-1 leaves at 0, 2, 3, and 4 d postinoculation with Pst DC3000 expressing AvrRpt2.
The ndr1-1 Mutant Displays Altered mRNA Expression of Several Biotic and Abiotic Stress-Responsive Genes
Given the differences between the disease phenotypes in the ndr1-1 mutant versus Col-0 plants as well as the observation of enhanced electrolyte leakage, we hypothesized that a relationship between these phenotypes and gene expression may exist. We reasoned that altered expression of genes that are known to be associated with nutrient and water stress, as well as other general abiotic stresses associated with drought tolerance, may be indicators of an altered stasis in ndr1-1. Based on the general role of LEA proteins in both biotic and abiotic stress responses as well as the predicted similarity of NDR1 to LEA14 (Fig. 1), we hypothesized that NDR1 may also play a role in abiotic stress tolerance or may be functionally affected by these stresses. We compiled a candidate list of genes from publicly available microarray and expression data (Sato et al., 2007) whose expression profiles may correlate with our observed electrolyte phenotypes. Two members of the LEA family met our search criteria and were investigated for altered mRNA expression in ndr1-1 plants. LEA group 1 domain-containing protein (At1g32560) showed a nearly 3-fold increase in expression in ndr1-1 mutant plants as compared with Col-0 when inoculated with Pst DC3000 (Fig. 3A). LEA group 1 domain-containing protein also showed altered levels of expression in ndr1-1 plants when inoculated with Pst DC3000 expressing AvrRpt2, but when inoculated with Pst DC3000 expressing AvrPphB, a rapid induction was observed in ndr1-1, leading to a nearly 4-fold increase in mRNA expression at 48 h postinoculation (hpi; Fig. 3A). The expression patterns of LEA14 were nearly identical between Col-0 and ndr1-1 under all conditions, with the exception of Pst DC3000 expressing AvrRpt2, which showed a nearly 3-fold increase in mRNA at 48 hpi in ndr1-1 plants (Fig. 3A). This differential expression in LEA family genes observed between Col-0 and ndr1-1 suggests an increase in cellular stresses occurring within an ndr1-1 cell following pathogen inoculation and may also indicate a similar role for NDR1 in cellular stress responses as that of LEA14.
Figure 3.
Relative mRNA expression of biotic and abiotic responsive genes in ndr1-1 following Pst DC3000 inoculation. A, Altered levels of expression in wild-type Col-0 and ndr1-1 mutant plants of LEA family genes in response to Pst DC3000 or Pst DC3000 expressing AvrRpt2, AvrB, or AvrPphB. B, Expression levels of aquaporin homologs known to be drought responsive in Arabidopsis when inoculated with Pst DC3000 or Pst DC3000 expressing AvrRpt2. Error bars display sd from one to two technical replicates from two biological replicates. Samples were taken at 0, 24, and 48 hpi. Expression is displayed as 0-h average fold for Col-0. Significance was determined using two-way ANOVA, where asterisks represent statistically significant differences between Col-0 and ndr1-1 and pound signs represent statistically significant changes over time: *,# P < 0.05; **,## P < 0.01; ***,### P < 0.001.
Genes associated with water regulation and ion release (e.g. aquaporin homologs; AtPIP1;3, AtPIP1;4, AtPIP1;5, AtPIP2;5, and AtPIP2;6) were also examined. No significant differences in expression were observed between Col-0 and ndr1-1 at 0 hpi (Fig. 3B). However, two of the aquaporin homologs (AtPIP1;4 and AtPIP2;5) showed a rapid induction in ndr1-1 compared with Col-0 when infected with Pst DC3000 alone or expressing AvrRpt2 (Fig. 3B). This finding provides further evidence that NDR1 may play a role in mediating fluid loss, as AtPIP1;4 and AtPIP2;5 have been previously demonstrated to be up-regulated in response to drought stress (Alexandersson et al., 2005). Consistent with our observed electrolyte leakage data, we did not detect any significant differences in the aquaporin homologs tested between Col-0 and ndr1-1 following inoculation with Pst DC3000 expressing either AvrB or AvrPphB (Supplemental Fig. S4). These data further suggest increased water stress within the cells of ndr1-1 plants following inoculation with Pst DC3000. Interestingly, we observed an increase in PDF1.2 mRNA expression at 24 hpi with Pst DC3000 expressing AvrB in the ndr1-1 mutant (Supplemental Fig. S5), which is consistent with previously published observations in RIN4-associated mutants (Cui et al., 2010).
NDR1 Self-Associates in Planta via the Formation of Oligomers
One of the primary characteristics of integrins, and integrin-like proteins, is their association in vivo as dimers (Zhao and Newman, 2001). Based on the predicted structural similarity of NDR1 to integrin-like proteins, we hypothesized that NDR1 exists as multimers in planta. To test this, differentially epitope-tagged (e.g. T7 and hemagglutinin [HA] epitope) NDR1 constructs were expressed in Nicotiana benthamiana using Agrobacterium tumefaciens-mediated transient expression. As shown in Figure 4A, using epitope-tagged NDR1 constructs, we detected a self-association in reciprocal coimmunoprecipitation pull downs, providing another example of integrin-like structure. The band intensity observed for the reciprocal self-associations correlated with differences in total tagged NDR1 protein detection rates (Supplemental Fig. S6).
Figure 4.
NDR1 associates with itself and RIN4 in planta, and this association is unaffected by mutations in the NGD site. Agrobacterium expressing HA:NDR1 or HA:RIN4, along with Agrobacterium expressing T7:NDR1 with substitutions in the NGD motif (wild type, NGD, RGD, or AAA), was infiltrated into N. benthamiana. Tagged proteins were immunoprecipitated from samples taken 48 h after infiltration using anti-HA (NDR1 or RIN4; right lane) or anti-T7 (NDR1; left lane) antibody. Proteins were detected by blotting with anti-T7 (top blot) or anti-HA (bottom blot) HRP-conjugated antibody. A, T7:NDR1 NGD-HA:NDR1. B, T7:NDR1 AAA-HA:NDR1. C, T7:NDR1 RGD-HA:NDR1. D, T7:NDR1 NGD-HARIN4. E, T7:NDR1 AAA-HA:RIN4. F, T7:NDR1 RGD-HA:RIN4. Western blots in Supplemental Figure S6 show relative detection limits.
The NGD Motif Is Not Required for the Interaction of NDR1 with RIN4 or for Self-Association as a Multimeric Protein Complex
RIN4 has been shown to interact with NDR1 (Day et al., 2006). Based on the similarity of the NGD motif to known ligand-binding sites (i.e. the RGD site found in integrins), the possibility exists that this site serves to stabilize the NDR1-RIN4 interaction or as an additional point of interaction between the two associated proteins. Coimmunoprecipitation experiments were performed to isolate the NDR1-RIN4 protein complex (Fig. 4D). T7-tagged NDR1, as well as variants with mutations within the NGD motif (i.e. 178AAA180 and 178RGD180), were tested for association with HA-tagged RIN4. In all cases, an interaction between NDR1 and RIN4 was detected, despite alterations to the NGD motif (Fig. 4, E and F). These data suggest that the NGD motif is not required for the NDR1 association with RIN4 in planta, in agreement with the previous finding that the association occurs within the N-terminal region of NDR1 (Day et al., 2006). Based on the proposed topology of NDR1 (Coppinger et al., 2004), our results are not surprising; moreover, they are consistent with published data describing integrin dimerization and protein association (van der Flier and Sonnenberg, 2001; Zhao and Newman, 2001).
We also tested the impact of mutations in the NGD motif on the NDR1-NDR1 self-association identified in this study. Unlike the NDR1-RIN4 association, the specific residues or motifs required for the formation of multimeric complexes of NDR1 are unknown. HA epitope-tagged NDR1 along with various T7-tagged NDR1 proteins (i.e. 178NGD180, 178AAA180, and 178RGD180) were transiently expressed in N. benthamiana using Agrobacterium-mediated transient expression. Complexes consisting of NDR1 along with NDR1 variants containing an altered NGD site could successfully be isolated using coimmunoprecipitation (Fig. 4, B and C). These data also demonstrate that the NGD site is not required for the formation of self-associated multimeric NDR1 protein complexes.
The ndr1-1 Mutation Has a Reduced Response to the PAMP flg22
While it has long been known that ndr1-1 mutant plants are compromised in resistance to Pst DC3000 as well as to strains expressing several specific bacterial effector proteins (Century et al., 1995), the underlying mechanisms of this process have never been explored. In this study, we explored this through the analysis of the expression of FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1), an early marker of mitogen-activated protein kinase (MAPK) pathway activation (Asai et al., 2002), in both Col-0 and ndr1-1 plants treated with the PAMP flg22. As shown in Figure 5B, the ndr1-1 mutant showed a significantly weakened response to flg22 treatment, as measured by MAPK activation. Furthermore, total protein levels of MAPK3 and MAPK6, both of which are required for priming of the biotic stress response (Beckers et al., 2009), were reduced in ndr1-1 plants treated with flg22 as compared with Col-0 (Fig. 5C).
Figure 5.
ndr1-1 exhibits altered PAMP responses. A and B, The expression levels of FRK1 mRNA was analyzed by qRT-PCR in Col-0 and ndr1-1 mutant plants in response to flg22 or mock treatment. Error bars display sd from two technical replicates from one biological replicate. Expression is displayed as untreated average fold for Col-0. Statistical significance was determined using one-way ANOVA followed by Tukey’s test, where asterisks represent statistically significant differences as compared with untreated Col-0 and pound signs represents statistically significant differences between Col-0 and ndr1-1: * P < 0.05; **,## P < 0.01; *** P < 0.001. C, Western blot analysis of MAPK3/6 in Col-0 and ndr1-1 mutant plants in response to flg22 or mock treatment.
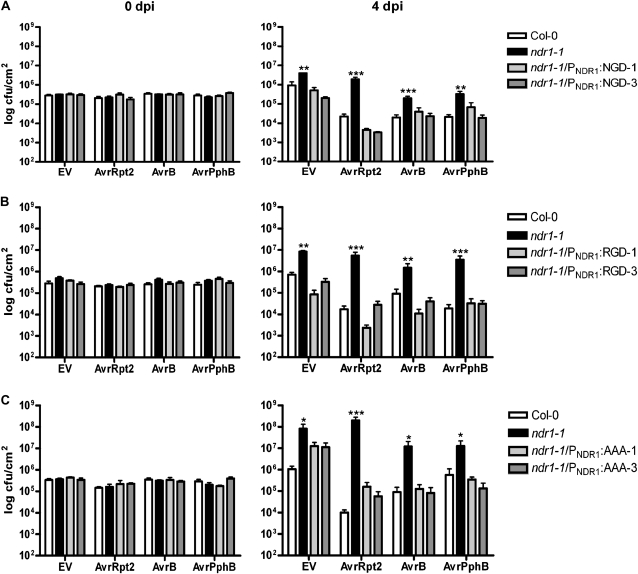
Mutation of the NGD Site in NDR1 May Compromise Resistance to Pst DC3000
Our experimental approach assessing the role of the NGD site in mediating protein-protein interactions revealed no detectable impact on the NDR1-RIN4 or NDR1-NDR1 association (Fig. 4), so the question remains whether mutations in this site alter the overall resistance response to Pst DC3000. To test this, we generated stable T7-tagged transgenic lines in the ndr1-1 mutant background expressing the two mutant variants of NDR1 (i.e. RGD and AAA) at positions 178 to 180 as well as an NDR1 complemented line (i.e. NGD). As shown in Figure 6A, the NGD complemented line displayed resistance to Pst DC3000 and Pst DC3000 expressing each of the three effector proteins described above at a level comparable to wild-type Col-0. Similarly, conversion of the NGD motif to RGD also resulted in a near wild-type level of resistance to all Pst DC3000 strains tested (Fig. 6B). When the NGD motif was changed to AAA, a complementation of resistance to Pst DC3000 overexpressing any of the three effector genes was observed; however, only an intermediate level of resistance to Pst DC3000 was detected (Fig. 6C). The 1 log increase in bacterial growth observed in the AAA lines as compared with Col-0, while not statistically significant under our assigned P value (P < 0.05; Supplemental Table S3), is nonetheless an interesting finding and strengthens the case for the NGD site of NDR1 as playing a critical role in specific defense responses. Pst DC3000 demonstrates an increased growth on ndr1-1 plants as compared with Col-0 (Century et al., 1995). This, together with our observations of the AAA complemented line, suggests that NDR1 may be involved in mediating PAMP-triggered, or basal, immunity. Indeed, this hypothesis is further supported by the alteration observed in the MAPK pathway response to the PAMP flg22 in the ndr1-1 mutant (Fig. 5). The inability of the AAA complemented lines to fully complement resistance would suggest that this site may in fact play a role in resistance signaling to Pst DC3000, possibly through mediating association with an as yet unidentified ligand, similar to the function of integrin RGD-binding sites (Plow et al., 2000).
Figure 6.
Growth of Pst DC3000 in Arabidopsis is altered by mutations to the NGD site of NDR1. Bacterial growth assay of Pst DC3000 (EV) and Pst DC3000 expressing AvrRpt2, AvrB, or AvrPphB dip inoculated on ndr1-1 lines complemented with native NDR1 promoter with NDR1 NGD (ndr1-1/PNDR1:NGD; A), NDR1 RGD (ndr1-1/PNDR1:RGD; B), or NDR1 AAA (ndr1-1/PNDR1:AAA; C). Growth was assayed at 0 and 4 d postinoculation. Growth is expressed as log cfu cm−2. Error bars display sd from three technical replicates from two biological replicates. Statistical significance was determined using unbalanced two-way ANOVA (model included in Supplemental Fig. S7), where asterisks represent statistically significant differences between Col-0 and ndr1-1: * P < 0.1, ** P < 0.01, *** P < 0.0001 (for P values, see Supplemental Table S3).
ndr1-1 Mutant Plants Have Altered Cell Wall Adhesions
Several pieces of evidence point to a role for NDR1 in the adhesion of the plasma membrane to the cell wall: predicted structural similarity to integrins as well as the putative orientation of NDR1 within the plasma membrane (Coppinger et al., 2004). To test this hypothesis, 8-d-old ndr1-1 and Col-0 hypocotyls were visualized before and after CaCl2-induced plasmolysis to assess the integrity of plasma membrane-cell wall adhesion. As shown in Figure 7A, a distinctive concave shape was observed in the membranes of Col-0 hypocotyl cells undergoing plasmolysis, with obvious attachments to the cell wall still visible (Fig. 7A, arrows). These data are in agreement with previously observed results using hypocotyls or suspension-cultured cells (Canut et al., 1998; Gouget et al., 2006). Surprisingly, CaCl2-induced plasmolysis of the ndr1-1 mutant was significantly altered (Fig. 7, A and C), resulting in the complete detachment of the plasma membrane from the cell wall, yielding spherical protoplasts with no remaining attachments (convex plasmolysis). Complementation of the ndr1-1 mutant with a constitutively expressed (i.e. 35S) GFP:NDR1 fusion protein restored the cell wall attachment phenotype to the wild type (Fig. 7B).
Figure 7.
ndr1-1 mutant plants exhibit altered plasmolysis and plasma membrane-cell wall focal adhesions. A, Time course (left to right: 0 [before], 2, and 5 min after treatment) of 8-d-old Col-0 or ndr1-1 CaCl2-plasmolyzed hypocotyls. In contrast to plasmolyzed wild-type Col-0 hypocotyl cells, which reveal plasma membrane-cell wall attachments (arrows), the plasma membrane of ndr1-1 cells quickly loses adhesion following the induction of plasmolysis, yielding spherical protoplasts within the cell wall. Bars = 25 μm. B, Complemented ndr1-1 mutant line constitutively expressing a GFP:NDR1 protein exhibits wild-type plasma membrane-cell wall adhesions (arrows). Differential interference contrast (top panel) and confocal-differential interference contrast overlay (bottom panel) images of CaCl2-induced plasmolysis are shown. Arrows indicate Hechtian strand formation, illustrating significant physical linkages with the plasma membrane and cell wall. Bars = 30 μm. C, Hechtian strands are absent, or significantly reduced, in the ndr1-1 mutant plant. Bar = 10 μm.
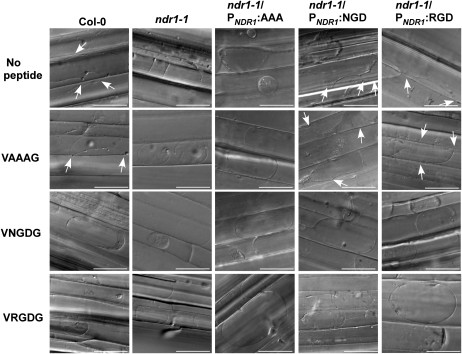
Disruption of plasma membrane-cell wall adhesion has been previously correlated with the function of the RGD motif (Canut et al., 1998). Moreover, it is well established that the conversion from concave to convex plasmolysis can be induced in cells with the addition of exogenous RGD-containing or RGD-binding peptides (Canut et al., 1998; Mellersh and Heath, 2001; Senchou et al., 2004; Gouget et al., 2006). As shown in Figure 8, addition of exogenous peptides did not alter the plasmolysis phenotype in ndr1-1 hypocotyls. Interestingly, the addition of the VNGDG peptide prior to the addition of CaCl2 was able to alter the plasmolysis pattern of Col-0 hypocotyls from concave to convex in the same manner as the addition of the VRGDG peptide, suggesting that the NGD motif might function similarly to the RGD motif. In addition, complemented ndr1-1 lines expressing either PNDR1:NGD or PNDR1:RGD behaved similarly to Col-0 without peptide treatment as well as when treated with exogenous peptides. Conversely, PNDR1:AAA complemented lines responded in the same manner as ndr1-1 under both treatment conditions (Fig. 8), strengthening the case for the NGD motif functioning in adhesion in NDR1.
Figure 8.
Application of exogenous peptides can alter the plasma membrane-cell wall adhesion in wild-type Col-0. Application of exogenous VNGDG or VRGDG peptide results in reduced plasma membrane-cell wall adhesions in Col-0, ndr1-1/PNDR1:NGD, and ndr1-1/PNDR1:RGD hypocotyl cells. Both ndr1-1 and ndr1-1/PNDR1:AAA hypocotyls are unaltered in adhesion phenotypes upon addition of purified VRGDG, VNGDG, and VAAAG peptides. Arrows indicate cell wall adhesion points. Differential interference contrast microscopy images were collected 5 min after the induction of plasmolysis. Bars = 25 μm.
DISCUSSION
NDR1 was identified more than 15 years ago in a screen for enhanced disease susceptibility mutants in Arabidopsis following Pst DC3000 inoculation (Century et al., 1995). Numerous studies have speculated on a role for NDR1 in defense signaling (Belkhadir et al., 2004; Coppinger et al., 2004; Zhang et al., 2004; Day et al., 2006); however, a functional role has remained elusive. Our primary goal in this study was to look beyond the general defense signaling processes with which NDR1 is known to be associated and focus on determining a broader physiological role for NDR1. This knowledge would both give insight into the general role of NDR1 and also enable us to hypothesize on its function in plant disease resistance signaling.
Studies have shown that NDR1 is required for a specific subset of resistance signaling pathways in plants (Innes, 1998). A conceptual model has evolved that suggests that a biochemical role for NDR1 must encompass a multitude of specific signaling pathways associated with the activation of resistance. For example, NDR1 is required for the activation of a majority of CC-NB-LRR R proteins in Arabidopsis (Aarts et al., 1998). However, there are exceptions to this requirement (McDowell et al., 2000; Bittner-Eddy and Beynon, 2001). With this in mind, how do we broadly assign a role for NDR1 in defense signaling while also explaining exceptions to the rule?
The association of NDR1 with RIN4 provides a possible mechanism through which NDR1 participates in defense signaling (Day et al., 2006). As a required component of signaling mediated by three CC-NB-LRR resistance proteins (e.g. RPM1, RSP2, and RPS5), association with RIN4 physically links NDR1 to two of these R proteins: RPM1 and RPS2 (Axtell and Staskawicz, 2003; Mackey et al., 2003). Day et al. (2006) speculated that the NDR1-RIN4 association might serve as a protein scaffold, directly or indirectly linking multiple signaling components at the plasma membrane. The association of NDR1 with RIN4, as well as the association of RIN4 with both RPM1 and RPS2, provides the potential for the assembly of a multiprotein complex at the plasma membrane.
The control of electrolyte release during host-pathogen interactions (Baker et al., 1991; Mackey et al., 2002) as well as the desiccation responses induced in plants as a result of pathogen infection (Wright and Beattie, 2004) led us to hypothesize that NDR1 may play a role in similar, associated responses. This proved successful, as our data suggest a role for NDR1 in electrolyte release following infection with Pst DC3000 (Fig. 2, A–D). However, our data do not fully define the precise role of NDR1 in the general process of nutrient release. The simplest explanation, given the proposed role of NDR1 in membrane adhesion, may be that the absence of NDR1 results in a weakened membrane system, one that is unable to prevent or restrict leakage in response to a virulent pathogen. Alternatively, NDR1 may be associated with additional proteins, such as membrane-associated transporters and/or regulatory processes, in the host plants, which themselves are involved in nutrient release-associated mechanisms. In support of this, recent work by Liu et al. (2009) identified several interacting partners of RIN4, thereby potentially linking NDR1 to a multitude of host cell processes, including guard cell dynamics and membrane potential.
The predicted structural homology of NDR1 with LEA14 (Fig. 1) and, by inference, with integrins (Supplemental Fig. S2) prompted us to investigate a general role for NDR1 in stress responses that are regulated by the LEA family of proteins. In plants, LEA proteins have been described as playing a role in a broad range of biotic and abiotic responses, including salt tolerance, as well as cold, heat, and drought stress (De Meutter et al., 2005; Goyal et al., 2005; Battaglia et al., 2008). Our observation of an increase in LEA14 mRNA expression in ndr1-1 plants in response to Pst DC3000 infection may in fact suggest that ndr1-1 plants undergo increased water stress during pathogen infection. We reason that this increase in LEA14 expression may be an attempt by the plant to compensate for the increased electrolyte leakage observed in ndr1-1 plants. Therefore, we hypothesize that NDR1 plays a role in limiting fluid loss. A similar role was proposed for LEA14 in response to wounding and dehydration (Singh et al., 2005). Changes in expression patterns of LEA group 1 domain-containing transcripts further strengthen the argument for NDR1 in the regulation of abiotic stress tolerance, while increases in the expression of PIP1;4 and PIP2;5 (Fig. 3) demonstrate an increase in drought stress within the cells of ndr1-1 mutant plants inoculated with Pst DC3000 expressing AvrRpt2. Linking the physiological and phenotypic observations with the mRNA expression data provides a more complete picture of the role NDR1 plays in processes associated with electrolyte release, water movement, drought tolerance, and pathogen response.
In mammals, integrins are viewed as the primary signal response elements, having critical roles in adhesion signaling (Huveneers and Danen, 2009). Based on the predicted structural homology with the fibronectin protein fold, we hypothesized that NDR1 may be involved with the plasma membrane-cell wall network. Based on the double anchor model (Coppinger et al., 2004), we hypothesized that NDR1 may function by mediating signaling processes or play a role in plasma membrane-cell wall adhesion. During compatible or incompatible plant-pathogen interactions, adhesion between the plasma membrane and cell wall can be disturbed or strengthened, respectively, and elicitation of defense responses is dependent on adherence (Mellersh and Heath, 2001). Our work shows that ndr1-1 mutant plants lack observable adhesion points between the plasma membrane and cell wall under CaCl2-induced plasmolysis (Fig. 7A). Interestingly, we observed that addition of peptides with the NDR1 motif NGD (i.e. VNGDG) to Col-0 hypocotyls prior to plasmolysis resulted in conversion from the concave to the convex plasmolysis phenotype. This is in agreement with previous work described by Canut et al. (1998), who characterized the role of RGD peptides in the disruption of plasma membrane-cell wall adhesion. Furthermore, complemented ndr1-1 lines expressing NDR1 with the wild type NGD, or mutant RGD, site behave, as one would predict, with both NDR1 variants able to complement the mutation and thus restore normal plasmolysis. Conversely, substituting AAA into the NGD site mirrored the lack of adhesion and convex plasmolysis observed in ndr1-1 mutant plants.
In support of our proposed role for NDR1 in mediating these processes, there are numerous examples describing the role of plasma membrane-cell wall adhesion in basic plant physiology (Gouget et al., 2006), mediating resistance during host-pathogen interactions (Mellersh and Heath, 2001), as well as a potential virulence target during pathogen invasion (Pieterse et al., 1992; Senchou et al., 2004). In each of these instances, exogenous application of RGD peptides altered host cell membrane dynamics. In this study, the addition of exogenous peptides (e.g. VNDGG, VRGDG, or VAAAG), while significantly altering plasma membrane-cell wall adhesion, did not alter the HR observed in either Col-0 or ndr1-1 plants (Supplemental Table S1). This finding indicates that the role of NDR1 in adhesion does not fully account for the ndr1-1 mutant’s loss of HR in response to the effector AvrRpt2. Based on the role(s) NDR1 plays in defense signaling processes initiated by a diverse array of plant pathogens, we hypothesize that the role of NDR1 in these processes are in (1) mediating plasma membrane-cell wall adhesion, (2) regulation of fluid movement in response to pathogen infection, (3) the perception of mechanical stimuli initiated at the plasma membrane-cell wall interface, and (4) the transmission of this signal from the apoplast to/through the plasma membrane either via its association with RIN4 or other associated proteins.
The proposed role of NDR1 in mediating leakage may partially explain the differential requirements for NDR1 in CC-NB-LRR-mediated resistance to bacterial pathogens and to the oomycete pathogen H. arabidopsidis (McDowell et al., 2000; Bittner-Eddy and Beynon, 2001). Wright and Beattie (2004) have previously shown that limiting leaf water availability results in restricted P. syringae growth, thereby supporting our hypothesis that the inability of ndr1-1 mutants to restrict leakage provides a more suitable environment to support bacterial growth. Given the different mechanisms of infection and disease progression between a bacterial pathogen and an obligate oomycete pathogen such as H. arabidopsidis, it is not surprising that NDR1 would have a much stronger effect on defense responses associated with a specific subset of CC-NB-LRR proteins.
While much work remains to fully define the role of NDR1 in plant defense signaling, this work provides a foundation for moving forward with additional biochemical analyses. To date, the genetic role of NDR1 in defense signaling has been narrowly defined without having a biochemical function for NDR1. As such, the role of NDR1 has been defined based almost exclusively on the absence (e.g. ndr1-1 mutant) of the protein. In total, our work proposes a role for NDR1 in mediating electrolyte leakage and, in part, regulating processes associated with desiccation and drought tolerance, processes that are primary tenets of plant disease resistance and abiotic stress response. A role for LEA14 may be the preservation of nutrient and fluid loss during biotic and abiotic stresses (Singh et al., 2005), and NDR1 may have a similar role while also having evolved parallel functionality as an integral signaling component of the plant defense network, possibly linking both effector-triggered immunity- and PAMP-triggered immunity-induced signaling following P. syringae infection.
MATERIALS AND METHODS
Homology Modeling and Structure Threading
The primary amino acid sequence of NDR1 (AT3G20600) was submitted to the PHYRE protein fold recognition server (http://www.sbg.bio.ic.ac.uk/phyre/). The predicted structure was analyzed using the MODELER comparative homology-modeling software (Martí-Renom et al., 2000). The output of this analysis, a predicted model of all nonhydrogen atoms based on spatial restraints, was then viewed using Chimera (Pettersen et al., 2004). Image overlays were performed using the solved NMR structure of LEA14 (AT1G01470; 1yyca; Singh et al., 2005). Further analysis (Supplemental Fig. S1) was performed by submitting the primary amino acid sequence of NDR1 to I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/), an internet-based structure prediction service.
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants were grown at 20°C under a 12-h/12-h light/dark cycle at 60% relative humidity in a Bio Chambers model FLX-37 growth chamber. Nicotiana benthamiana plants were grown under the same conditions.
DNA Cloning and Mutagenesis
Cloning of DNA constructs was performed using standard protocols. Site-directed mutagenesis of NDR1 was performed according to previously published protocols (Day et al., 2005) modified from the QuikChange PCR Mutagenesis Kit (Stratagene). Two site-directed mutations within the NGD site of NDR1, located at amino acids 178 to 180, were constructed. A template plasmid (i.e. pTOPO-NDR1) containing the open reading frame of NDR1 flanked by SalI (5′) and SacI (3′) restriction enzyme sites was used in combination with the DNA oligonucleotide primer sets labeled RGD and AAA in Supplemental Table S1. Following 18 cycles (95°C for 1 min, 55°C for 3 min, 68°C for 5 min) on a Bio-Rad MyCycler thermal cycler (Bio-Rad Laboratories) using Pfu Turbo DNA polymerase (Clontech), the product was then treated with DpnI for 1 h at 37°C. Five microliters of the digested DNA reaction was transformed into Escherichia coli DH5α cells and grown on Luria-Bertani medium containing 100 μg mL−1 kanamycin overnight at 37°C. Mutant NDR1 constructs were cloned into either the native promoter vector pDDNDR (Coppinger et al., 2004) or the 35S binary vector pMD-1 with an N-terminal T7 epitope tag (Day et al., 2005). To make pDDNDR native promoter constructs, primers were designed to add a 5′ T7 epitope tag and SalI site and a 3′ SpeI site (Supplemental Table S2). Amplicons from the site-directed mutant constructs were ligated into pGEM T-EASY vector (Promega) and subsequently digested with SalI and SpeI and ligated into pDDNDR (Coppinger et al., 2004). DNA plasmids were transformed and maintained in E. coli DH5α cells. The fidelity of all DNA constructs was confirmed by DNA sequencing (ABI 3730 Genetic Analyzer; Applied Biosystems). Agrobacterium tumefaciens strain GV3101 (pMP90) (Lazo et al., 1991) and strain C58C1 (Tai et al., 1999; for N. benthamiana expression) were transformed with wild-type and NDR1 mutant constructs by electroporation.
All native, wild-type NDR1 T7 and HA fusion constructs were previously demonstrated to be functional and to fully complement the ndr1-1 mutation (Coppinger et al., 2004; Day et al., 2006).
Cloning of NDR1 Mutant Constructs
Two site-directed mutations within the NGD site of NDR1, located at amino acids 178 to 180, were constructed using a quick-change PCR approach. A template plasmid (i.e. pTOPO-NDR1) containing the open reading frame of NDR1 flanked by SalI (5′) and SacI (3′) restriction enzyme sites was used in combination with the DNA oligonucleotide primer sets labeled RGD and AAA in Supplemental Table S1. Following 18 cycles (95°C for 1 min, 55°C for 3 min, 68°C for 5 min) on a Bio-Rad MyCycler thermal cycler using Pfu Turbo DNA polymerase (Clontech), the product was then treated with DpnI for 1 h at 37°C. Five microliters of the digested DNA reaction was transformed into E. coli DH5α cells and grown on Luria-Bertani medium containing 100 μg mL−1 kanamycin overnight at 37°C. The resultant site-directed mutant constructs were digested with SalI and SacI restriction enzymes and ligated into the respective sites in the 35S binary vector pMD-1-T7, which incorporates a 5′ T7 epitope tag (Day et al., 2005). To make pDDNDR native promoter constructs, primers were designed to add a 5′ T7 epitope tag and SalI site and a 3′ SpeI site (Supplemental Table S1). Amplicons from the site-directed mutant constructs were ligated into pGEM T-EASY vector (Invitrogen) and subsequently digested with SalI and SpeI and ligated into pDDNDR (Coppinger et al., 2004). DNA plasmids were transformed and maintained in E. coli DH5α cells. A. tumefaciens strain GV3101 (pMP90) (Lazo et al., 1991) and strain C58C1 (Tai et al., 1999; for N. benthamiana expression) were transformed with wild-type and NDR1 mutant constructs by electroporation.
All native, wild-type NDR1 T7 and HA fusion constructs were previously demonstrated to be functional and to fully complement the ndr1-1 mutation (Coppinger et al., 2004; Day et al., 2006).
Arabidopsis Transformation
Flowering Arabidopsis plants were transformed and selected for homozygosity, as described by Clough and Bent (1998), on Murashige and Skoog medium containing 1% Bacto agar and 25 μg mL−1 kanamycin.
Pathogen Inoculation and Growth Assays
Pseudomonas syringae pv tomato DC3000 strains containing pVSP61 (empty vector) or pVSP61-containing AvrRpt2, AvrB, or AvrPphB were described previously (Kunkel et al., 1993; Simonich and Innes, 1995). To assay for bacterial growth, 4-week-old plants were dip inoculated in bacterial suspensions of 3 × 107 colony-forming units (cfu) mL−1. Leaves were preselected and marked to ensure that analyses were performed on developmentally similar leaves. At 0 and 4 d postinoculation, three leaf discs of 0.7 cm diameter were collected from a single plant into a microcentrifuge tube containing 1 mm MgCl2 + 0.1% Triton X-100. Bacterial growth assays were performed as described by Tornero and Dangl (2001) with the modification of plating 5 μL instead of 2 μL, as described by Tian et al. (2009). Results were analyzed for significance using SAS (version 9.2; SAS Software) using an ANOVA model modified from Tsuda et al. (2008; Supplemental Fig. S7). Log10-transformed bacterial titer counts were compared using Tukey’s test.
Electrolyte Leakage
Electrolyte leakage was measured in 4- to 5-week-old plants using a protocol modified from Gilmour et al. (1988). Leaves were preselected and marked based on similarity in size before plants were dip inoculated at 3 × 107 cfu mL−1. After inoculation, plants were covered with a clear plastic dome for 30 min before the 0-h time point, when leaves were removed. The remaining pots were left covered for another 2.5 h. A single leaf was removed from a plant and a disc (0.7 cm diameter) was harvested using a number 3 cork borer. Excised leaf discs were floated in a bath of sterile deionized water and quickly swirled before being placed in a tube containing 3 mL of sterile deionized water. Four plants were used for each replicate. Tubes containing leaf discs were shaken on an orbital rocker at 35 rpm for 3 h. After 3 h, leaf discs were removed and the solution was assayed for conductance using a conductance meter (Traceable 23226-505; VWR Scientific). Leaf discs were frozen at −80°C for 1 h. After the freeze cycle, the leaf punch was returned to the original sample tube and rocked for an additional 3 h at room temperature. After 3 h, the leaf punch was removed and the conductance was measured, recorded as total leakage. Electrolyte leakage was recorded and calculated as percentage leakage of total (i.e. first reading/second reading) adjusted to percentage maximal.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from leaves using the RNeasy Plant Mini Kit (Qiagen). First-strand cDNA was synthesized from 1 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed on a Mastercycler ep Realplex real-time PCR system (Eppendorf) using HotStart-IT SYBR Green qPCR Master Mix (2×; USB). Cycle time for all replicates was 95°C for 2 min followed by 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 45 s. Data were analyzed by two-way ANOVA using Prism 4 (GraphPad Software) with outliers removed by Grubb’s test (α = 0.05) utilizing the QuickCalcs online outlier calculator (GraphPad Software; http://www.graphpad.com/quickcalcs/Grubbs1.cfm). Aquaporin homolog primers were used are described (Alexandersson et al., 2010). All primer sets utilized are listed in Supplemental Table S2.
HR Assay
The HR was assayed as described by Century et al. (1995), slightly modified, by hand infiltration of Arabidopsis leaves with Pst DC3000 using a needleless syringe at a concentration of 1 × 107 cfu cm−2, following a 6- or 12-h pretreatment by hand infiltration of 5 mm VAAAG, VNGDG, or VRGDG peptide solution in 1 mm MgCl2 buffer or a mock control of only buffer. The leaves were evaluated for tissue collapse 20 h after infiltration of the bacteria.
Plasmolysis
Plasmolysis experiments were performed based on the methods of Gouget et al. (2006) using 8-d-old etiolated hypocotyls with the cotyledons and roots removed. Hypocotyl sections were immersed in 50 mm Tris (pH 8.0) for 1 h at room temperature, rinsed in sterile distilled water, and then stained with 0.05% neutral red for 5 to 30 min. Sections were rinsed in sterile distilled water and mounted on a coverslip in 15 μL of sterile water. A second coverslip was placed on top of the section, offset to the first coverslip. To plasmolyze the cells, 15 μL of 1.0 m CaCl2 was placed on the sample where the second coverslip overlapped the first, allowing the solution to cover the sample via capillary action. Stained, plasmolyzed hypocotyls were observed with an Olympus IX-71 inverted microscope, and images were acquired with an Olympus DP70 camera. Images were processed and adjusted for contrast using Canvas X (ACD Systems). Approximately 100 hypocotyls of each genotype were observed in total from more than five biological replicates. For peptide addition experiments, the same method as described above was followed, with the exception that the hypocotyls were mounted in 15 μL of 5 mm peptide in distilled water. Peptides (VRGDG, VAAAG, and VNGDG) were synthesized by EZ-Biolabs at a purity of greater than 99%.
Tagged Protein Constructs and Coimmunoprecipitation
Coimmunoprecipitation experiments were performed as described by Day et al. (2006), with slight modifications. In brief, A. tumefaciens strains expressing the epitope-tagged (e.g. T7 or HA) NDR1, RIN4, and NDR1 mutant constructs fused to a 35S promoter were infiltrated into 5-week-old leaves on N. benthamiana at a final, individual concentration of 4 × 108 cells mL−1. Leaves were incubated at room temperature for 40 h, after which time 16 1-cm2 leaf discs were harvested into liquid nitrogen and held at −80°C until processing. Samples were processed according to Day et al. (2005).
T7 epitope monoclonal and T7 horseradish peroxidase (HRP)-conjugated antibodies were purchased from Novagen. HA epitope monoclonal antibody was purchased from Covance. HA HRP-conjugated antibody and protease inhibitors were purchased from Roche.
MAPK Western Blotting
To detect MAPK3/6 activity, 40 μg of total protein was loaded onto a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane, followed by incubation for 1 h with an antibody specific for anti-pTEpY (catalog no. 9101S; Cell Signaling Technology). An anti-rabbit HRP secondary antibody was used for detection on film.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g20600 (NDR1), At1g01470 (LEA14), At1g32560 (LEA group 1 domain-containing protein), At2g39010 (PIP2;6), At3g54820 (PIP2;5), At4g23400 (PIP1;5), At4g00430 (PIP1;4), At1g01620 (PIP1;3), At2g14610 (PR1), At5g44420 (PDF1.2), At3g03600 (RPS2), and At2g19190 (FRK1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Predicted structural models of Arabidopsis NDR1 using I-TASSER and PHYRE homology-modeling servers.
Supplemental Figure S2. Homology modeling of the predicted β-sheet torus of NDR1 with LEA14 and the fibronectin type III domain of human integrin β-4 (10.2210/pdb2yrz/pdb).
Supplemental Figure S3. Electrolyte leakage conductance measurements.
Supplemental Figure S4. Expression of aquaporin homolog family mRNAs following inoculation with Pst DC3000 expressing AvrB or AvrPphB.
Supplemental Figure S5. Expression of NDR1, defense marker genes, and RPS2 in Arabidopsis following inoculation with Pst DC3000.
Supplemental Figure S6. Expression levels of the HA- and T7-tagged NDR1 constructs utilized.
Supplemental Figure S7. Statistical model for the analysis of bacterial growth assays.
Supplemental Table S1. The effect of exogenous peptide application on the HR in Col-0 and ndr1-1 mutant plants.
Supplemental Table S2. Primers used for qRT-PCR as well as primer sets for the development of the RGD and AAA site-directed mutant constructs.
Supplemental Table S3. P values of Pst DC3000 growth assays.
Supplemental Appendix S1. Electrolyte leakage: supplemental conductance method.
Acknowledgments
We thank Katie Porter for technical support on qRT-PCR analyses as well as Wenzhao Yang for assistance with the statistical analysis of bacterial growth assays.
References
- Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA 95: 10306–10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Danielson JAH, Rade J, Moparthi VK, Fontes M, Kjellbom P, Johanson U. (2010) Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J 61: 650–660 [DOI] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P. (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59: 469–484 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ. (2003) Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol Microbiol 49: 1537–1546 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Staskawicz BJ. (2003) Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112: 369–377 [DOI] [PubMed] [Google Scholar]
- Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K, Schulze-Lefert P. (2002) The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295: 2073–2076 [DOI] [PubMed] [Google Scholar]
- Baker CJ, O’Neill NR, Keppler LD, Orlandi EW. (1991) Early responses during plant-bacteria interactions in tobacco cell suspensions. Phytopathology 81: 1504–1507 [Google Scholar]
- Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148: 6–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers GJM, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21: 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Subramaniam R, Dangl JL. (2004) Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol 7: 391–399 [DOI] [PubMed] [Google Scholar]
- Bittner-Eddy PD, Beynon JL. (2001) The Arabidopsis downy mildew resistance gene, RPP13-Nd, functions independently of NDR1 and EDS1 and does not require the accumulation of salicylic acid. Mol Plant Microbe Interact 14: 416–421 [DOI] [PubMed] [Google Scholar]
- Canut H, Carrasco A, Galaud J, Cassan C, Bouyssou H, Vita N, Ferrara P, Pont-Lezica R. (1998) High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana link the cell wall. Plant J 16: 63–71 [DOI] [PubMed] [Google Scholar]
- Century K, Holub EB, Staskawicz BJ. (1995) NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and fungal pathogen. Proc Natl Acad Sci USA 92: 6597–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century K, Shapiro AD, Repetti PP, Dahlbeck D, Holub E, Stascawicz BJ. (1997) NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278: 1963–1965 [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coppinger P, Repetti PP, Day B, Dahlbeck D, Mehlert A, Staskawicz BJ. (2004) Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J 40: 225–237 [DOI] [PubMed] [Google Scholar]
- Cui H, Wang Y, Xue L, Chu J, Yan C, Fu J, Chen M, Innes RW, Zhou JM. (2010) Pseudomonas syringae effector protein AvrB perturbs Arabidopsis hormone signaling by activating MAP kinase 4. Cell Host Microbe 7: 164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B, Dahlbeck D, Huang J, Chisholm ST, Li D, Staskawicz BJ. (2005) Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell 17: 1292–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B, Dahlbeck D, Staskawicz BJ. (2006) NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell 18: 2782–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meutter J, Robertson L, Parcy F, Mena M, Fenoll C, Gheysen G. (2005) Differential activation of ABI3 and LEA genes upon plant parasitic nematode infection. Mol Plant Pathol 6: 321–325 [DOI] [PubMed] [Google Scholar]
- Dodds PN, Rafiqi M, Gan PH, Hardham AR, Jones DA, Ellis JG. (2009) Effectors of biotrophic fungi and oomycetes: pathogenicity factors and triggers of host resistance. New Phytol 183: 993–1000 [DOI] [PubMed] [Google Scholar]
- Ellis J, Dodds P. (2003) Plant pathology: monitoring a pathogen-targeted host protein. Curr Biol 13: R400–R402 [DOI] [PubMed] [Google Scholar]
- Feys BJ, Moisan LJ, Newman MA, Parker JE. (2001) Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20: 5400–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, Cabral A, Parker JE. (2005) Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Beattie GA. (2009) Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana. Mol Plant Microbe Interact 22: 857–867 [DOI] [PubMed] [Google Scholar]
- Gao H, Gong Y, Yuan Y. (2007) RGD-dependent mechanotransduction of suspension cultured Taxus cell in response to shear stress. Biotechnol Prog 23: 673–679 [DOI] [PubMed] [Google Scholar]
- Gee EP, Ingber DE, Stultz CM. (2008) Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. PLoS ONE 3: e2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Hajela RK, Thomashow MF. (1988) Cold acclimation in Arabidopsis thaliana. Plant Physiol 87: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, Pont-Lezica R, Canut H. (2006) Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol 140: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Tunnacliffe A. (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL. (1995) Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269: 843–846 [DOI] [PubMed] [Google Scholar]
- Hu G, deHart AK, Li Y, Ustach C, Handley V, Navarre R, Hwang CF, Aegerter BJ, Williamson VM, Baker B. (2005) EDS1 in tomato is required for resistance mediated by TIR-class R genes and the receptor-like R gene Ve. Plant J 42: 376–391 [DOI] [PubMed] [Google Scholar]
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL. (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 22: 5679–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EHJ. (2009) Adhesion signaling-crosstalk between integrins, Src and Rho. J Cell Sci 122: 1059–1069 [DOI] [PubMed] [Google Scholar]
- Huveneers S, Truong H, Danen EHJ. (2007) Integrins: signaling, disease, and therapy. Int J Radiat Biol 83: 743–751 [DOI] [PubMed] [Google Scholar]
- Hynes RO. (2009) The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes RW. (1998) Genetic dissection of R gene signal transduction pathways. Curr Opin Plant Biol 1: 299–304 [DOI] [PubMed] [Google Scholar]
- Jiménez-Soto LF, Kutter S, Sewald X, Ertl C, Weiss E, Kapp U, Rohde M, Pirch T, Jung K, Retta SF, et al. (2009) Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog 5: e1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. (2002) The Arabidopsis thaliana-Pseudomonas syringae interaction. The Arabidopsis Book 1: e0039, doi/10.1199/tab.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4: 363–371 [DOI] [PubMed] [Google Scholar]
- Kiba A, Sugimoto M, Toyoda K, Ichinose Y, Yamada T, Shiraishi T. (1998) Interaction between cell wall and plasma membrane via RGD motif is implicated in plant defense responses. Plant Cell Physiol 39: 1245–1249 [Google Scholar]
- Knepper C, Day B. (2010) From perception to activation: the molecular-genetic and biochemical landscape of disease resistance signaling in plants. The Arabidopsis Book 8: e012, doi/10.1199/tab.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA. (2008) Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol 6: 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Bent AF, Dahlbeck D, Innes RW, Staskawicz BJ. (1993) RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell 5: 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo GR, Stein PA, Ludwig RA. (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/Technology 9: 963–967 [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G. (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. (2003) Arabidopsis RIN4 is the target of the type III virulence effector AvrRpt2 and modulates RPS2 mediated resistance. Cell 112: 379–389 [DOI] [PubMed] [Google Scholar]
- Mackey D, Holt BF, Wiig A, Dangl JL. (2002) RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108: 743–754 [DOI] [PubMed] [Google Scholar]
- Manning VA, Hamilton SM, Karplus PA, Ciuffetti LM. (2008) The Arg-Gly-Asp-containing, solvent-exposed loop of Ptr ToxA is required for internalization. Mol Plant Microbe Interact 21: 315–325 [DOI] [PubMed] [Google Scholar]
- Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Sali A. (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biomol Struct 29: 291–325 [DOI] [PubMed] [Google Scholar]
- McDowell JM, Cuzick A, Can C, Beynon J, Dangl JL, Holub EB. (2000) Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J 22: 523–529 [DOI] [PubMed] [Google Scholar]
- Mellersh DG, Heath MC. (2001) Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell 13: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett P. (2009) Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res 75: 1–33, 228–229 [DOI] [PubMed] [Google Scholar]
- Muskett PR, Kahn K, Austin MJ, Moisan LJ, Sadanandom A, Shirasu K, Jones JDG, Parker JE. (2002) Arabidopsis RAR1 exerts rate-limiting control of R gene mediated defenses against multiple pathogens. Plant Cell 14: 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ. (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8: 2033–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, de Wit PJGM, Govers F. (1992) Molecular aspects of the potato-Phytophthora infestans interaction. Neth J Plant Pathol 98: 85–92 [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. (2000) Ligand binding to integrins. J Biol Chem 275: 21785–21788 [DOI] [PubMed] [Google Scholar]
- Potts JR, Campbell ID. (1994) Fibronectin structure and assembly. Curr Opin Cell Biol 6: 648–655 [DOI] [PubMed] [Google Scholar]
- Sato M, Mitra RM, Coller J, Wang D, Spivey NW, Dewdney J, Denoux C, Glazebrook J, Katagiri F. (2007) A high-performance, small-scale microarray for expression profiling of many samples in Arabidopsis-pathogen studies. Plant J 49: 565–577 [DOI] [PubMed] [Google Scholar]
- Senchou V, Weide R, Carrasco A, Bouyssou H, Pont-Lezica R, Govers F, Canut H. (2004) High affinity recognition of a Phytophthora protein by Arabidopsis via an RGD motif. Cell Mol Life Sci 61: 502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233 [DOI] [PubMed] [Google Scholar]
- Simonich MT, Innes RW. (1995) A disease resistance gene in Arabidopsis with specificity for the avrPph3 gene of Pseudomonas syringae pv. phaseolicola. Mol Plant Microbe Interact 8: 637–640 [DOI] [PubMed] [Google Scholar]
- Singh S, Cornilescu CC, Tyler RC, Cornilescu G, Tonelli M, Lee MS, Markley JL. (2005) Solution structure of a late embryogenesis abundant protein (LEA14) from Arabidopsis thaliana, a cellular stress-related protein. Protein Sci 14: 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH. (2009) Integrins and cell fate determination. J Cell Sci 122: 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai TH, Dahlbeck D, Clark ET, Gajiwala P, Pasion R, Whalen MC, Stall RE, Staskawicz BJ. (1999) Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc Natl Acad Sci USA 96: 14153–14158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken FL, Tameling WI. (2009) To nibble at plant resistance proteins. Science 324: 744–746 [DOI] [PubMed] [Google Scholar]
- Telewski FW. (2006) A unified hypothesis of mechanoperception in plants. Am J Bot 93: 1466–1476 [DOI] [PubMed] [Google Scholar]
- Tian M, Chaudry F, Ruzicka DR, Meagher RB, Staiger CJ, Day B. (2009) Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol 150: 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Dangl JL. (2001) A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. Plant J 28: 475–481 [DOI] [PubMed] [Google Scholar]
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. (2008) Interplay between MAMP-triggered and SA-mediated defense responses. Plant J 53: 763–775 [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. (2001) Function and interactions of integrins. Cell Tissue Res 305: 285–298 [DOI] [PubMed] [Google Scholar]
- van Wees SC, de Swart EA, van Pelt JA, van Loon LC, Pieterse CM. (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97: 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cao Y, Zheng G. (2009) Mutation in the RGD motif decreases the esterase activity of Xcc_est. Biotechnol Lett 31: 1445–1449 [DOI] [PubMed] [Google Scholar]
- Wiermer M, Feys BJ, Parker JE. (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8: 383–389 [DOI] [PubMed] [Google Scholar]
- Wright CA, Beattie GA. (2004) Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc Natl Acad Sci USA 101: 3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BW, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, Loake GJ. (2003) Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J 34: 768–777 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dorey S, Swiderski M, Jones JDG. (2004) Expression of RPS4 in tobacco induces an AvrRps4-independent HR that requires EDS1, SGT1 and HSP90. Plant J 40: 213–224 [DOI] [PubMed] [Google Scholar]
- Zhao T, Newman PJ. (2001) Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J Cell Biol 152: 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK, Shi J, Singh U, Wyatt SE, Bressan RA, Hasegawa PM, Carpita NC. (1993) Enrichment of vitronectin- and fibronectin-like proteins in NaCl-adapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant J 3: 637–646 [PubMed] [Google Scholar]