Abstract
Leaves of avocado (Persea americana) that develop and persist in deep shade canopies have very low rates of photosynthesis but contain high concentrations of lutein epoxide (Lx) that are partially deepoxidized to lutein (L) after 1 h of exposure to 120 to 350 μmol photons m−2 s−1, increasing the total L pool by 5% to 10% (ΔL). Deepoxidation of Lx to L was near stoichiometric and similar in kinetics to deepoxidation of violaxanthin (V) to antheraxanthin (A) and zeaxanthin (Z). Although the V pool was restored by epoxidation of A and Z overnight, the Lx pool was not. Depending on leaf age and pretreatment, the pool of ΔL persisted for up to 72 h in the dark. Metabolism of ΔL did not involve epoxidation to Lx. These contrasting kinetics enabled us to differentiate three states of the capacity for nonphotochemical chlorophyll fluorescence quenching (NPQ) in attached and detached leaves: ΔpH dependent (NPQΔpH) before deepoxidation; after deepoxidation in the presence of ΔL, A, and Z (NPQΔLAZ); and after epoxidation of A+Z but with residual ΔL (NPQΔL). The capacity of both NPQΔLAZ and NPQΔL was similar and 45% larger than NPQΔpH, but dark relaxation of NPQΔLAZ was slower. The enhanced capacity for NPQ was lost after metabolism of ΔL. The near equivalence of NPQΔLAZ and NPQΔL provides compelling evidence that the small dynamic pool ΔL replaces A+Z in avocado to “lock in” enhanced NPQ. The results are discussed in relation to data obtained with other Lx-rich species and in mutants of Arabidopsis (Arabidopsis thaliana) with increased L pools.
The violaxanthin cycle (V cycle), based on reversible interconversion of zeaxanthin (Z), antheraxanthin (A), and violaxanthin (V), stabilizes nonphotochemical chlorophyll fluorescence quenching (NPQ) and confers photoprotection in most green plants and algae (Demmig et al., 1987; Demmig-Adams and Adams, 1992; Niyogi, 1999; Förster et al., 2001). Since Bungard et al. (1999), an additional xanthophyll cycle (the Lx cycle), involving the α-carotene pathway pigments lutein (L) and lutein epoxide (Lx), has been recognized in many diverse taxa (García-Plazaola et al., 2003; Matsubara et al., 2003, 2008).
The Lx cycle is distinctive in that in some species it was reversible, with kinetics similar to that of the V cycle (Bungard et al., 1999; Matsubara et al., 2001; Esteban et al., 2010), whereas in others it was only very slowly reversible (Matsubara et al., 2005; García-Plazaola et al., 2007; Förster et al., 2009). Leaves that developed and persisted in deep shade canopies of avocado (Persea americana) showed particularly high concentrations of Lx (Esteban et al., 2008). Although deepoxidation of Lx occurred during a few hours of exposure to sunlight and made a small addition to the much larger pool of L (ΔL), little epoxidation of ΔL to Lx was observed overnight, and Lx concentrations were only restored after 3 to 5 weeks in the shade (Förster et al., 2009). In contrast, the V cycle was largely reversible on a diel basis.
Slow reversibility of the Lx cycle in some species led to the hypothesis that augmentation of the L pool by deepoxidation of Lx might “lock in” photoprotection (García-Plazaola et al., 2003; Matsubara et al., 2005). Evidence for persistent, enhanced capacity for NPQ due to ΔL, after A+Z had largely reverted to V, was reported during photosynthetic induction in vivo with leaves of Quercus rubra and Inga marginata (García-Plazaola et al., 2003; Matsubara et al., 2008). These experiments provided the first direct evidence for potentially similar functional roles for ΔL and Z in photoprotection (García-Plazaola et al., 2007; Horton et al., 2008) and presaged the recent demonstration of a Z-like radical cation that appeared when NPQ was engaged in a Z-free, L-enriched Arabidopsis (Arabidopsis thaliana) mutant (Avenson et al., 2008; Li et al., 2009). However, as yet, there have been no quantitative comparisons of the components of NPQ associated with ΔL and A+Z.
Not surprisingly, shade leaves of avocado proved to be extremely responsive to light during treatment and assay. Photosynthetic induction curves at selected, often high, light intensities have been widely used to assess NPQ in relation to xanthophyll pigment composition in Arabidopsis and Chlamydomonas mutants (Niyogi et al., 1998; Pogson et al., 1998; Förster et al., 2001) as well as in Quercus and Inga. These assays proved unsatisfactory for quantitative comparisons of NPQ components in shade leaves of avocado and were confounded by responses of stomata and CO2 supply (Takayama et al., 2008), by photosynthetic induction following treatment, and by xanthophyll deepoxidation during the measurement. In contrast, the light response curve traditionally used to distinguish biochemical and physiological relationships in sun and shade plants (Björkman, 1981) has found less application in these model systems (Russell et al., 1995; Bailey et al., 2001; Pérez-Bueno and Horton, 2008). We tailored rapid light response curves (RLRCs) using chlorophyll fluorescence (Schreiber et al., 1994; White and Critchley, 1999) to the requirements of avocado shade leaves. These minimized deepoxidation during the assay and facilitated quantitative analysis of NPQ while monitoring the opening of stomata and the induction of photosynthetic metabolism following light treatments.
RLRCs were used to differentiate and quantitatively compare three components of the capacity for NPQ in avocado leaves that are expressed following interconversions of xanthophyll pigments. Semantic confusion that has emerged from use of the terms qE (for energy-dependent nonphotochemical quenching) and qN (for nonphotochemical quenching; Horton et al., 1996), particularly in reference to different components of energy-dependent quenching, distinguished by different criteria, in different species and treatments led us to a simple designation for the three types of NPQ in shade leaves of avocado, with subscripts as follows. (1) NPQΔpH in dark-adapted, uninduced leaves with closed stomata that contain high concentrations of Lx and V and extremely low levels of A and Z. We presume that this component is associated with the development of ΔpH in chloroplast thylakoid membranes in the absence of external CO2 (Krause et al., 1982) during assay, prior to deepoxidation of Lx and V. (2) NPQΔLAZ assayed in leaves after modest light exposures that open stomata, induce photosynthetic metabolism, and lead to partial but stoichiometric deepoxidation in the Lx and V cycles. We presume that this component is associated with the stabilization of NPQ by both ΔL and A+Z. (3) NPQΔL in leaves treated as in (2) but assayed after 24 to 72 h in the dark, following almost complete epoxidation of the V cycle (disappearance of A+Z) but not of Lx. We presume that this component is associated with the stabilization of NPQ by ΔL alone.
In this article, we provide the first direct evidence, to our knowledge, that the capacity of NPQΔL is quantitatively similar to NPQΔLAZ, suggesting that a small addition (approximately 5%–10%) to the total L pool (ΔL) substitutes for A+Z and maintains the capacity of elevated NPQ for up to 72 h in the dark. The duration of elevated NPQΔL is determined by the rate of dark metabolism of ΔL by processes other than epoxidation to Lx. Importantly, dark relaxation of NPQΔL is faster than that of NPQΔLAZ and similar to NPQΔpH. These observations are discussed in relation to a common mechanism of L- and Z-based NPQ recently proposed in other studies and in relation to potential functions of L-enhanced photoprotection in shade leaves.
RESULTS
Optimization of Light Treatments to Manipulate Xanthophyll Pigment Composition in Shade Leaves of Avocado and to Avoid Subsequent Deepoxidation during Assays
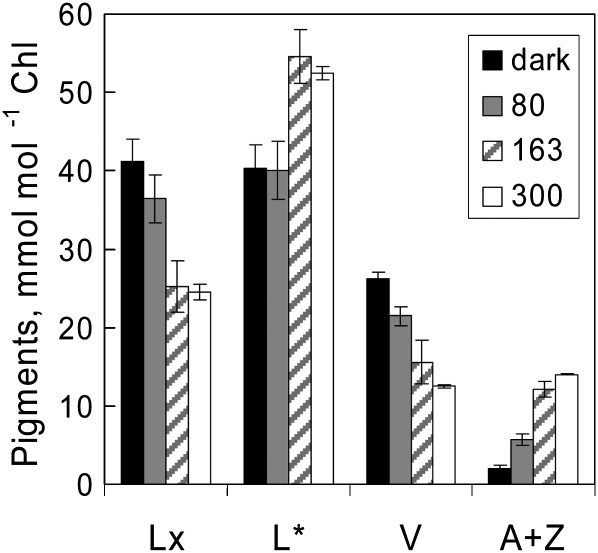
Our previous studies of diel changes in Lx and V cycle pigment composition in avocado leaves suggested that little deepoxidation occurred at light intensities of less than 200 μmol photons m−2 s−1 (Förster et al., 2009). The optimal light intensities needed to bring about quantitatively similar deepoxidation of Lx and V in mature leaves of shade-grown avocado plants without photoinhibition were explored with a single large shade-grown avocado leaf detached in mid afternoon and arranged with the petiole in water in the open greenhouse. The top third of the leaf was covered by aluminum foil as a dark control, and neutral density filters were placed on the remainder of the leaf to give 50- × 50-mm areas exposed to 80 and 163 μmol photons m−2 s−1, leaving other parts of the leaf exposed to the weak afternoon sunlight (300 μmol photons m−2 s−1) for 90 min. As shown in Figure 1, leaf discs from dark control areas contained very little A (1.6 ± 0.2 mmol mol−1 chlorophyll) and only traces of Z (0.5 ± 0.5 mmol mol−1 chlorophyll). Note that the 3- to 5-fold larger pools of L are shown reduced by 100 mmol mol−1 chlorophyll (designated L*) in all figures, unless stated otherwise, to facilitate comparison of stoichiometry with other xanthophylls. Whereas deepoxidation of Lx to L was first evident after exposure to 163 and 300 μmol photons m−2 s−1 (ΔL of approximately 15 mmol mol−1 chlorophyll), quantitatively similar deepoxidation of V to A+Z occurred already at 80 μmol photons m−2 s−1. Therefore, all subsequent actinic light treatments to manipulate the pool sizes of both xanthophyll cycles were done at 120 and 350 μmol photons m−2 s−1 for 60 min.
Figure 1.
Light intensity dependence of Lx and V deepoxidation in a detached avocado leaf on water. Note that values for L* have been reduced by 100 mmol mol−1 chlorophyll to facilitate stoichiometric presentation of the data (i.e. the scale for L* corresponds to 100–160 mmol mol−1 chlorophyll). Parts of the leaf were shaded with aluminum foil as a control (dark), neutral density filters were used to vary light intensity (80 and 163 μmol photons m−2 s−1), and the remainder of the leaf was unshaded during 90 min of exposure to weak afternoon sunlight (300 μmol photons m−2 s−1) in a glasshouse at 28°C. Values shown are means ± se (n = 3).
Measurements of pigments in discs punched at random from control and treated areas of attached mature leaves in nine experiments showed quantitatively similar, almost stoichiometric deepoxidation of Lx and V (Table I). Initial levels of A were close to the limits of detection (0.8 mmol mol−1 chlorophyll), whereas Z was not detectable in most cases. Although levels of residual A+Z after 21 to 72 h of recovery in the dark laboratory (0–5 μmol photons m−2 s−1) were higher, residual A+Z and deepoxidation (DS = A+Z/V+A+Z) was about the same as that produced from deepoxidation of V during the optimized RLRC assays described below. Importantly, measurements of maximum quantum yield of photochemical energy conversion (Fv/Fm) showed that these light exposures caused little photoinhibition (Table I).
Table I. Changes in Lx and V cycle pigments in attached mature leaves of shade-grown avocado following light treatments (1 h at 223 ± 27 μmol photons m−2 s−1) in nine experiments.
Values shown are means ± se (n = 9–13, with two to three replicate leaves in each experiment).
| Parameter | Pigment | |||
| ΔLx | ΔL | ΔV | ΔA+Z | |
| mmol mol−1 chlorophyll | ||||
| Deepoxidation (1 h) | −11.8 ± 2.1 | +10.2 ± 0.6 | −12.5 ± 1.1 | +11.7 ± 1.1 |
| A+Z | A+Z/V+A+Z | Fv/Fm | ||
| Epoxidation (>24 h) | ||||
| Initial dark control | 0.8 ± 0.3 | 0.03 ± 0.01 | 0.808 ± 0.002 | |
| After treatment | 12.5 ± 0.6 | 0.37 ± 0.03 | 0.787 ± 0.002 | |
| After recovery | 3.4 ± 0.6 | 0.11 ± 0.03 | 0.789 ± 0.003 | |
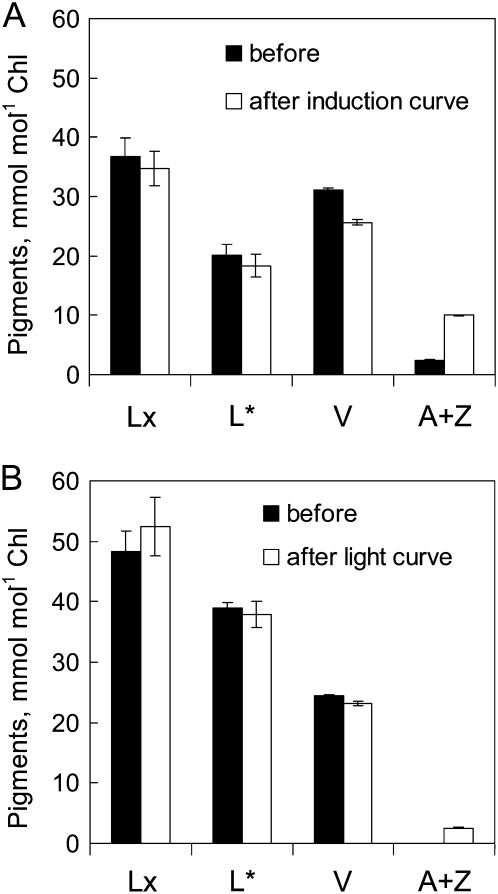
Some deepoxidation of V, but not of Lx, always occurred during chlorophyll fluorescence assays. For example, contrary to the expectations of Takayama et al. (2008), imaging photosynthetic induction for 20 min at 100 μmol photons m−2 s−1 caused substantial deepoxidation of V and increased DS from 0.04 to 0.24 (data not shown). Assuming that each of the 16 1-s saturating flashes used in this protocol delivered 5,000 μmol photons m−2, this assay delivered a total of 200 mmol photons m−2, which is equivalent to the photons delivered in a 6-min photosynthetic induction assay at 300 μmol photons m−2 s−1 with the MINI-PAM (Fig. 2A). This problem had been mitigated previously by infiltration of leaf discs with dithiothreitol, an inhibitor of violaxanthin deepoxidase (Adams et al., 1990), but clearly this was impracticable in our measurements with large attached leaves. Moreover, infiltration with dithiothreitol had been reported to substantially depress Fv/Fm (Matsubara et al., 2008). Seeking a compromise between the actinic light intensity needed to produce a good resolution of kinetic responses and that needed to minimize deepoxidation during assays (Fig. 2B), we chose a RLRC protocol delivering about 60 μmol photons m−2 using eight 30-s steps with actinic light from 0 to approximately 400 μmol photons m−2 s−1.
Figure 2.
Deepoxidation of V in detached avocado leaves during chlorophyll fluorescence assay of photosynthetic parameters. Pigment composition of mature shade leaves is shown before and after photosynthetic induction assay at 300 μmol photons m−2 s−1 for 6 min (A) and RLRC assay from 0 to 410 μmol photons m−2 s−1 (B). Note that values for L* have been reduced by 100 mmol mol−1 chlorophyll to facilitate stoichiometric presentation of the data, as in Figure 1 (i.e. the scale for L* corresponds to 100–160 mmol mol−1 chlorophyll). Values shown are means ± se (n = 4).
A Persistent 5% to 10% Addition (ΔL) to the L Pool from Deepoxidation of Lx Sustains Enhanced Capacity for NPQ after Conversion of A+Z to V Overnight
The less intrusive RLRC assays enabled us to follow the enhancement of NPQ in the presence of ΔL under standardized conditions for up to 72 h in the dark. In addition, these curves routinely presented light response profiles of 1−qP (a measure of the redox state of the acceptor side of PSII) and photosynthetic electron transport (ETR). These data helped us ensure that other factors, such as stomatal opening and induction of photosynthesis during light treatments, did not confound the interpretation of NPQ data.
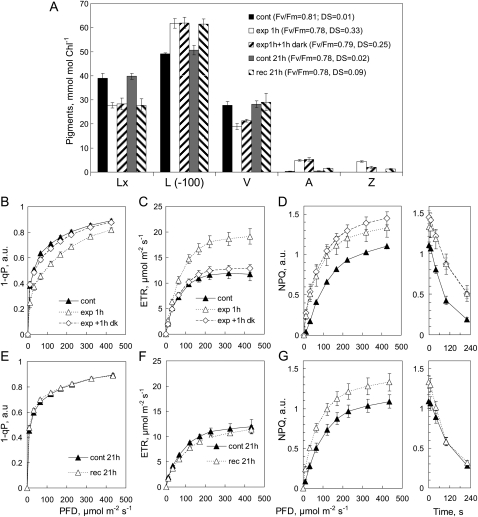
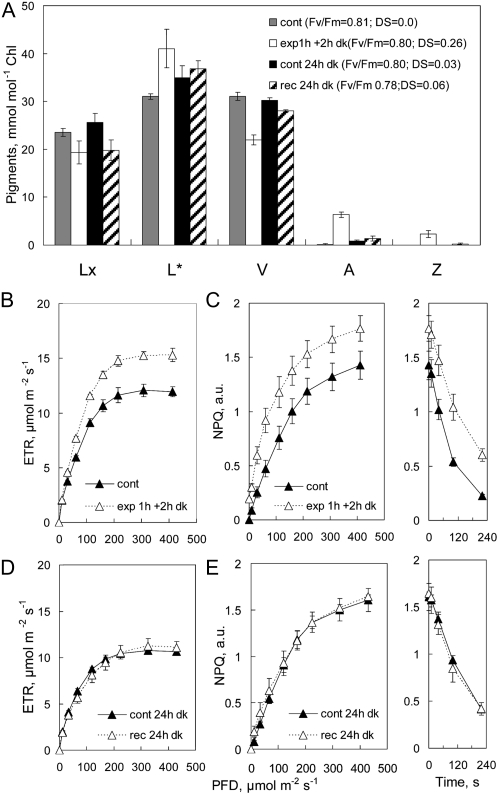
Light treatments caused an initial deepoxidation of approximately 30% of both Lx and V pools with practically no change in PSII efficiency, indicated by steady Fv/Fm (Fig. 3A). Pigment composition changed little during 1 h of dark following treatment. V cycle DS declined by one-third, mainly due to epoxidation of about half the Z pool, without change in the pool of A or L. Although 1−qP was depressed by 20% at low photon flux density (PFD) immediately after exposure, the profiles of control and exposed areas were the same after 1 h in the dark (Fig. 3B), consistent with the initial increase in ETR, which also returned to control levels after 1 h in the dark (Fig. 3C). These responses of 1−qP to light treatment and their relaxation to control values within 1 h of dark were similar in all experiments and are not presented in subsequent graphs. Light treatment led to a relatively larger increase in capacity for NPQ at lower light intensities during assay as well as a higher level of NPQ at light saturation (Fig. 3D). Notably, in the presence of A+Z, NPQ relaxed more slowly in the dark during assay (Fig. 3D). The small decline of the NPQ profile in exposed areas during 1 h of dark may reflect the partial epoxidation of the Z pool (Fig. 3A).
Figure 3.
A, Pigment composition in foil-covered (cont) and light-exposed areas of attached avocado leaves measured immediately following exposure to 150 μmol photons m−2 s−1 for 1 h (exp 1h), after recovery for 1 h in the dark (exp 1h + 1h dark), and in foil-covered (cont 21h) and exposed areas (rec 21h) again after 21 h in the dark. Note that the scale for L* corresponds to 100 to 170 mmol mol−1 chlorophyll. B to G, Changes in photosynthetic parameters measured in RLRCs at each of these pigment sampling times are shown in 1−qP (B and E), ETR (C and F), and NPQ (D and G), with the time course of NPQ relaxation in the dark shown in panels at right in D and G. Values shown are means ± se (n = 3); error bars appear whenever se exceeds symbol size. Values for 1−qP and NPQ were calculated in arbitrary units (a.u.).
In general, L was not metabolized during 24 h in the dark, whereas epoxidation of A+Z was practically complete (Fig. 3A). Photosynthetic parameters in the aluminum foil controls after 21 h in the dark were almost identical to the dark controls at the beginning of the experiment. Profiles of 1−qP (Fig. 3, compare B and E) and ETR (Fig. 3, compare C and F) in control and recovered areas were the same after 1 and 21 h of dark. Enhanced capacity for NPQ persisted in exposed areas after 21 h of dark (Fig. 3G), in spite of the epoxidation of A+Z. Importantly, the kinetics of dark NPQ relaxation now were much the same in control and exposed areas (Fig. 3G). The slower decline of NPQ in the dark following deepoxidation of Lx and V, and its fast relaxation after epoxidation of A+Z, were observed in all experiments with attached leaves. This showed that although ΔL substituted for A+Z and sustained higher capacity for NPQ in the dark, it did not slow the dark relaxation of NPQ observed in the presence of A+Z.
Enhanced Capacity for NPQ Persists for up to 72 h in the Dark without A+Z, While ΔL Is Always Detectable
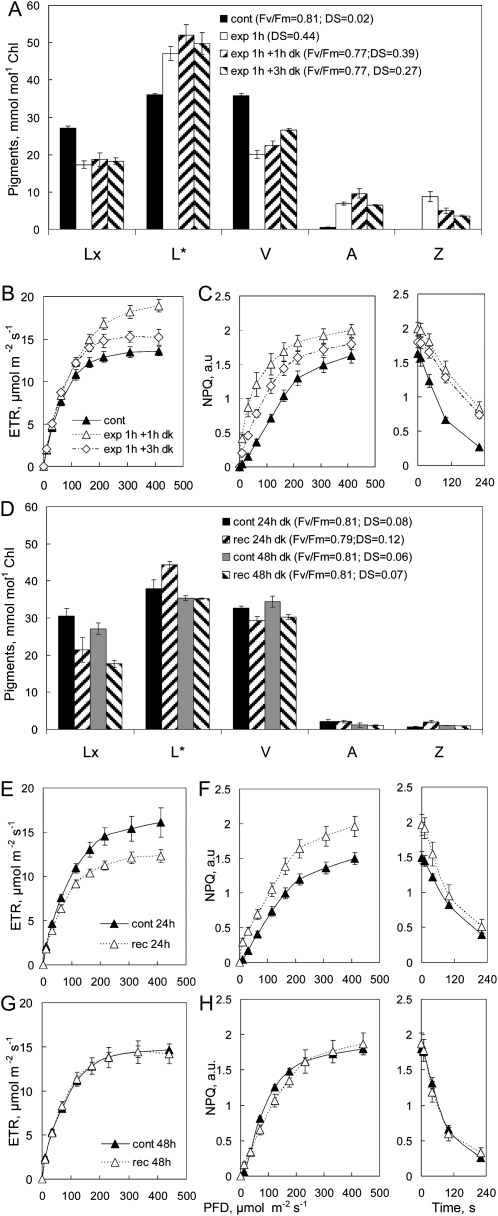
Younger, fully expanded leaves with lower Lx content (Fig. 4A) showed much the same deepoxidation of Lx and V after light treatments and the commencement of epoxidation of A+Z during 1 and 3 h in the dark. Photosynthetic induction in the light treatment increased ETR during assay, which declined to near control levels after 3 h of dark (Fig. 4B). NPQ increased following light treatment and then declined slightly, as the level of Z began to decrease after 3 h in the dark, but the slower dark relaxation of NPQ persisted (Fig. 4C). Epoxidation of A+Z was largely complete after 24 h of dark but ΔL was still detectable (Fig. 4D). Although ETR of treated areas was somewhat lower than in controls after 24 h of dark (Fig. 4E), persistent ΔL sustained higher capacity for NPQ (Fig. 4F), with relaxation kinetics similar to controls. Metabolism of ΔL between 24 and 48 h reestablished control levels of L (Fig. 4C), and this was reflected in identical profiles of ETR and NPQ (Fig. 4, G and H). Clearly, the capacity for enhanced NPQ persisted only as long as ΔL was detectable, and interestingly, the metabolism of ΔL between 24 and 48 h of dark was not due to epoxidation to Lx.
Figure 4.
Enhancement of NPQ is lost when ΔL is metabolized after 48 h of dark. A, Pigment composition measured in aluminum foil control (cont) after exposure to 120 μmol photons m−2 s−1 (exp) and after 1 and 3 h in the dark (dk). Note that the scale for L* corresponds to 100 to 160 mmol mol−1 chlorophyll. B and C, ETR (B) and NPQ (C) assayed in RLRCs in control and treated leaves at times as in A. D to H, Pigment composition (D) and ETR (E and G) and NPQ (F and H) measured again after 24 h (E and F) and 48 h (G and H) of recovery in the dark. The time course of NPQ relaxation in the dark following the RLRC assay is shown in columns at right in C, F, and H. Values shown are means ± se (n = 4); error bars appear whenever se exceeds symbol size.
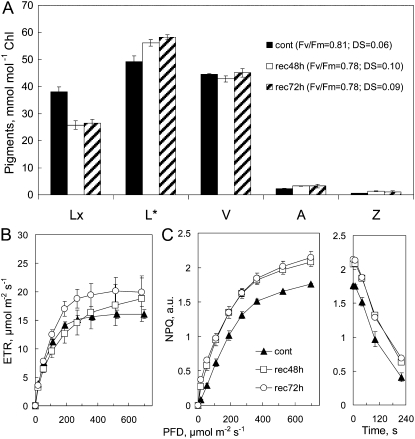
The longest period of enhanced capacity for NPQ associated with sustained ΔL in our experiments was found in isolated leaf discs 72 h after the start of the actinic light treatment (Fig. 5). Light treatment produced a persistent 30% decrease in Lx and a less than stoichiometric, but still substantial, increase in ΔL after 48 and 72 h in the dark. The levels of V, A, and Z were the same as controls (Fig. 5A), and A+Z formed during the light treatment was epoxidized to V within 24 h in the dark (data not shown). The ETR observed after 48 and 72 h in the dark was slightly greater than in controls (Fig. 5B), showing that elevated NPQ capacity (Fig. 5C) was not an artifact of reduced ETR. The relaxation of NPQ was the same as controls throughout.
Figure 5.
Enhanced NPQ and retention of ΔL from Lx persists for up to 72 h in the dark in leaf discs in the absence of A+Z. Discs from four leaves on a plant dark adapted overnight were floated on lower epidermis uppermost and exposed to white light-emitting diodes from below (1 h at 220 μmol photons m−2 s−1) and then allowed to recover on water in the dark. A, Pigment composition of controls (cont) and samples after 48 and 72 h (rec48h and rec72h); samples were collected after RLRC assay. Note that L* has been adjusted by 50 mmol mol−1 chlorophyll, so the scale for L corresponds to 0 to 120 mmol mol−1 chlorophyll. B, Photosynthetic parameters assayed in RLRC on controls and after 48 and 72 h of recovery in the dark. Values shown are means ± se (n = 4); error bars appear whenever se exceeds symbol size.
We were able to eliminate sustained ΔL accumulation and the associated enhanced NPQΔL by pretreatment of a plant in the dark laboratory for 11 d (0–5 μmol photons m−2 s−1) prior to light treatment (1 h at 200 μmol photons m−2 s−1). Prolonged dark pretreatment did not alter deepoxidation of Lx and V during exposure or epoxidation of A+Z overnight (Fig. 6A). The response of ETR and NPQ to the light treatment was as found in all previous experiments (Fig. 6, B and C), as was the slower dark relaxation of NPQ in the presence of ΔL and A+Z. However, prolonged dark pretreatment specifically accelerated the metabolism of the pool of ΔL. Both pools of ΔL and A+Z were metabolized overnight, no enhancement of NPQ was detected during assays (Fig. 6, B and C), and, as expected, relaxation kinetics of NPQ were the same as in controls.
Figure 6.
Prolonged dark pretreatment promotes metabolism of ΔL overnight and prevents sustained NPQ. A, Pigment composition shows that ΔL is metabolized within 24 h. Note that the scale for L* corresponds to 100 to 150 mmol mol−1 chlorophyll. B and C, Measurements of photosynthetic parameters in RLRCs confirm the normal induction of ETR (B) and enhanced NPQ (C) following deepoxidation. D and E, However, following overnight metabolism of ΔL without deepoxidation and epoxidation of A+Z, ETR and NPQ return to control levels. The time course of NPQ relaxation in the dark is shown in panels at right in C and E. Values shown are means ± se (n = 3); error bars appear whenever se exceeds symbol size.
DISCUSSION
Previous studies suggested that the presence of Lx and V cycles in shade canopy leaves of woody plants from Mediterranean forests (García-Plazaola et al., 2003) and tropical American forests (Matsubara et al., 2008) indicated that photoconversion of Lx to L may lock in photoprotection overnight after restoration A+Z to V. The pronounced difference in the kinetics of epoxidation of L and A+Z in the Lx-rich shade leaves of avocado (Esteban et al., 2008; Förster et al., 2009) offered an ideal system in which to probe the role in photoprotection of the particular fraction of L (ΔL from Lx) that persists after epoxidation of A+Z. This article presents, to our knowledge, the first comprehensive and quantitative analysis using nonintrusive RLRCs to differentiate three states of the capacity for NPQ associated with distinct xanthophyll pigment compositions in these plants and substantially extends our understanding of this enigmatic role of L in vivo.
The three pigment composition states were established by exposing shade leaves of avocado to modest, nonphotoinhibitory light treatments (120–350 μmol photons m−2 s−1). The transitions between NPQΔpH (in the absence of deepoxidation of xanthophylls), to NPQΔLAZ (after stoichiometric deepoxidation of Lx and V), and to NPQΔL (persistence of ΔL following overnight epoxidation of A+Z) were assayed using sensitive RLRCs in which a range of 30-s exposures to increasing PFD was tailored to minimize the deepoxidation of xanthophyll pigments during assay. The nonintrusive assays were supported with simple tests, such as the “Vaseline patch,” that help monitor physiological (closure of stomata) and biochemical (induction of photosynthetic metabolism) processes with the potential to confound interpretations of NPQ capacity in relation to pigment composition in vivo. We did not use routine photosynthetic induction curves to analyze changes in NPQ in shade-grown avocado leaves because they produced higher deepoxidation of V (Fig. 2). As an aside, we strongly recommend that deepoxidation should be routinely assessed during NPQ induction assays at high PFD.
Four functional aspects of photoprotection that have arisen from this investigation of avocado shade leaves will be discussed. First, we will explore comparative quantitative aspects of the substitution of A+Z by ΔL to sustain enhanced capacity for NPQ for up to 72 h in the dark and expand earlier hypotheses that deepoxidation of Lx to L locks in photoprotection (García-Plazaola et al., 2003, 2007; Matsubara et al., 2005, 2007, 2008). Second, and contrary to expectations, we briefly discuss the demonstration that the duration of enhanced NPQ capacity was determined by the metabolism of ΔL by processes other than its slow epoxidation to Lx. Third, we point out that in avocado leaves, deepoxidation of V to A+Z slows the dark relaxation of NPQ during assay, whereas ΔL from deepoxidation of Lx does not affect NPQ relaxation kinetics. Fourth, we suggest some implications of sustained NPQ capacity with the above properties for photoprotection in shade canopies and light use efficiency during sun flecks.
Past research has been focused on the extent to which Z-independent components of chlorophyll fluorescence quenching (NPQΔpH) participate in NPQ even after deepoxidation of xanthophylls. There is a debate as to whether these components are additive or substitutive (Adams et al., 1990; Bilger and Björkman 1994; Gilmore et al., 1998; Li et al., 2004) and whether common or separate mechanisms are involved (Pogson et al., 1998; Finazzi et al., 2004). Recent evidence suggests that both involve common antenna-based mechanisms (Johnson et al., 2009), and moreover, at least two distinct quenching mechanisms involving Z (type I and type II) seem to be involved. In the type I mechanism, Z in the L2 position of minor antenna complexes is a direct quencher of excited chlorophyll, as demonstrated by a distinctive radical cation (Avenson et al., 2008). In the type II mechanism, Z is an allosteric modulator of the ΔpH sensitivity of NPQ involving xanthophyll exchanges in the L1 position of light-harvesting complexes (Lhcs; Johnson et al., 2009). It is beyond the scope of our data to speculate further on these mechanistic details.
We took a simple approach to quantitative estimation of the relative NPQ capacity using the total areas under RLRC of NPQ versus PFD to compare NPQΔpH and NPQΔLAZ in avocado leaves. Comparison of controls with those after 1 h of exposure and after 1 h of dark with similar amounts of ΔL and A+Z shows an increase of 45.1 ± 1.5% (n = 4 experiments) in NPQΔLAZ compared with NPQΔpH. In other words, in these assays, the capacity of Z (and ΔL)-independent NPQΔpH in control leaves was about 69% of NPQΔLAZ after deepoxidation of Lx and V. Compared with leaves with persistent ΔL after 21 to 24 h of dark recovery, NPQΔL was increased by 36.6% ± 2.5% over NPQΔpH. Even after 48 h of dark recovery, NPQΔL was still 84% of NPQΔLAZ. Thus, our analyses provide evidence that ΔL effectively and almost completely substitutes for A+Z in stabilizing the capacity for NPQ in avocado.
The mechanistic aspects of substitution of A+Z by ΔL are little known. Analyses of shade leaves of Inga sapindoides (Matsubara et al., 2007) showed that Lx was the principal xanthophyll in LHCII complexes. In these experiments, deepoxidation of part of the Lx pool to L on exposure to 200 μmol photons m−2 s−1 for 30 min led to the replacement of Lx with L in the peripheral V1 site of LHCII trimers as well as in the internal L2 site of both monomeric and LHCII trimers in both photosystems. It was concluded that the slowly reversible Lx↔L exchange took place at the same binding sites as reversible V↔A+Z exchange. We suggest that the observed sustained high capacity for NPQΔL is based on substitution of A+Z with L in these sites, but further evidence for the association of ΔL, A, and Z with specific LHCII proteins in avocado is needed before this substitution can be integrated into general models of regulated excitation dissipation in antenna complexes (de Bianchi et al., 2010).
Whereas the structural roles of L in the antennae of the photosynthetic apparatus have been the focus of much past research, the photoprotective function of L has remained enigmatic (Gilmore, 2001). Analyses of Arabidopsis mutants for a decade or so had not uncovered direct evidence of a role for L in NPQ. Although lutein-deficient2 (lut2) mutants show less NPQ (Pogson et al., 1998) and although other mutants with lower than wild-type L content show impaired NPQ, mutants with slightly enhanced L and enhanced NPQ (Pogson and Rissler, 2000; Niyogi et al., 2001) have been difficult to evaluate, principally because none were markedly deficient in Z. A recently constructed suppressor of the npq1 mutant, suppressor of zeaxanthinless1, lacks Z, has low levels of V and A, accumulates more L and α-carotene than the wild type, and retains about 50% wild-type NPQ (Li et al., 2009). The authors state: “Analysis of the carotenoid radical cation formation and leaf absorbance changes strongly suggest that the higher amount of lutein substitutes for zeaxanthin in qE, implying a direct role in qE.” In the light of these observations in Arabidopsis, it seems that a compelling next step is to examine shade leaves of avocado in the NPQΔpH, NPQΔLAZ, and NPQΔL states to discover whether distinctive leaf absorbance changes, and changes in carotene radical cation formation (Avenson et al., 2008; Li et al., 2009), persist in vivo following the substitution of A+Z by ΔL.
A still unanswered key question is how the partitioning of the majority of the L pool to structural functions in Lhcs is regulated and how a small additional pool (ΔL) participates in excitation dissipation. This bears some analogy to the situation in the Arabidopsis mutants lut2, where V cycle pigments substitute for the structural roles of L (Pogson et al., 1996), and aba1-3 and aba1-4, which are constitutively enriched in Z and depleted in V+A, but only a small fraction of the Z pool seems to be involved in NPQ photoprotection (Hurry et al., 1997). Also, this may be related to the still ill-defined fraction of the L and Z pools that mitigate photooxidation (Havaux and Niyogi, 1999; Johnson et al., 2007) and the contribution of these reactive oxygen species-scavenging activities to photoprotection (Förster et al., 2005; Dall’Osto et al., 2006). It remains to be seen if the recently described partitioning (Lepetit et al., 2010) of xanthophylls in diatoms between protein-associated fractions (involved in NPQ) and lipid-dissolved fractions (involved in reactive oxygen species scavenging) can be achieved with avocado thylakoids. Both L and Z serve similar roles in visual systems (Kim et al., 2006), and photooxidation products of both xanthophylls have been identified in retinas of the human eye (Khachik et al., 1997). We observed earlier that sudden exposure of shade-grown avocado leaves to sunlight was initially accompanied by a decline in the total L pool in spite of Lx deepoxidation (Förster et al., 2009), which could be explained by photooxidation of L. It would be of interest to investigate the occurrence of L (and Z) photooxidation products in avocado leaves under these conditions.
The transition from higher capacity NPQΔL to lower capacity NPQΔpH after prolonged darkness was associated with the disappearance of ΔL. Against expectation, the decline of ΔL was not coupled to the epoxidation of ΔL to Lx and restoration of the Lx pool. Interestingly, metabolism of ΔL occurred much faster when leaves were pretreated with 11 d in the dark laboratory prior to light exposure, when ΔL was fully metabolized in 24 h of darkness without epoxidation (Fig. 6). In previous experiments (Förster et al., 2009), slow recovery of Lx pools in prolonged shade after deepoxidation in sunlight was not simply related to the epoxidation of L or the accumulation of the biosynthetic precursor α-carotene, as this pool remained constant and the total L pool declined more than twice as rapidly as the Lx pool recovered. Obviously, other pathways to metabolize ΔL became dominant in prolonged dark. Little is known about the degradation of xanthophyll pigments, although carotenoid cleavage dioxygenases and nonenzymatic degradation have been implicated in some tissues (Cazzonelli and Pogson, 2010). At present, we cannot explain the dark induction of L metabolism.
Another intriguing aspect of the NPQ-pigment relationships was that relaxation kinetics of NPQ were delayed specifically by the presence of A+Z, whereas both NPQΔpH and NPQΔL relaxation rates were equally fast in the absence of A+Z. This seems consistent with previous evidence that Z slows the relaxation of qE in isolated chloroplasts (Nocter et al., 1991). More recently, Johnson et al. (2008) reported that in wild-type Arabidopsis and a Z-accumulating β-carotene hydroxylase overexpression line (sChy B), slower relaxation in the dark after assay was not related to the absolute Z pool itself but to higher DS. Other contributing factors to the slow relaxation of NPQΔLAZ cannot be ruled out. For example, the slower decay of NPQΔLAZ might reflect postillumination interactions with leaf respiratory metabolism and could reflect slowly declining energization of chloroplast thylakoids in darkness, when chloroplast and/or mitochondrial ATP pools sustain NPQ in the dark through a ΔpH maintained by ATP hydrolysis rather than photosynthetic electron transport (Gilmore and Björkman, 1995).
Persistent, ΔL-enhanced capacity for NPQ was first recognized in shade leaves of Quercus and Inga examined in the field (García-Plazaola et al., 2003; Matsubara et al., 2008) and has been confirmed in some Inga species in the Eden Project in Cornwall, United Kingdom (C. Nichol and C.B. Osmond, unpublished data), as well as in avocado shade leaves in orchards in tropical Eastern Australia (C.B. Osmond, B. Förster, and J. Leonardi, unpublished data). The deeply shaded canopies of the latter retain up to 30 leaves on shoots, with photosynthetic capacities and pigment compositions that are comparable to those of shade-grown plants examined here. The modest light treatments used to bring about xanthophyll pigment conversions in our greenhouse experiments correspond reasonably to changes in shade canopy light environment following upper canopy disturbance in storms and to changes following routine orchard canopy-pruning practices (Whiley et al., 2002; C.B. Osmond, B. Förster, and J. Leonardi, unpublished data). The 30-s steps in our RLRCs reasonably correspond to sunlight fleck experiences in tropical canopies (Pearcy, 1990) and to sun fleck measurements in avocado orchards in California (M.V. Mickelbart, W. Stilwell, M.L. Arpaia, and R. Heath, unpublished data). The effect of ΔL on locking in a higher capacity of NPQΔL in the shade for several days, without the penalty of slower relaxation associated with the presence of Z, may offer a lower penalty in light energy use efficiency during sequences of sun flecks than the slower relaxing NPQΔLAZ (Zhu et al., 2004). These consequences of the slowly reversible Lx cycle need to be evaluated in relation to the reversible engagement of the V cycle in these plants. For example, we found previously that sunlight exposure of avocado shade leaves results in sustained low Fv/Fm (Förster et al., 2009) and persistently higher L and A+Z. Separation of the contributions of slowly reversible Z- and/or L-related NPQ from xanthophyll-independent, low-efficiency photoinactivated PSII centers to these observations will be discussed in a subsequent article.
In conclusion, this study established improved assay methods to analyze xanthophyll pigment-NPQ relationships in avocado shade leaves that resolved three different NPQ components based on the associated xanthophyll deepoxidation state. Based on these distinctions, we were able to provide novel, direct evidence for a special role of the fraction ΔL of the L pool that had been generated by Lx deepoxidation in conferring higher capacity for NPQ induction while allowing rapid dark relaxation, which implies a direct role of L in photoprotection and may improve the ability of avocado shade leaves to persist during frequent sun flecks. Evaluation of these processes during shade-to-sun acclimation in avocado shade leaves under laboratory and field conditions will be presented elsewhere.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seedlings of avocado (Persea americana ‘Edranol’) were purchased from Vallance’s Nursery and maintained in 20-L containers of potting soil with regular irrigation and additions of slow-release nutrients. The seedlings were kept in a shaded section of a temperature-controlled glasshouse for 24 months (18°C night/29°C day) and pruned to the main stem 6 to 12 months prior to experiments. The light environment of upper and lower canopy leaves in the shade enclosure, measured with a LI-190 sensor (www.licor.com), was 30 to 50 and 15 to 30 μmol photons m−2 s−1, respectively, when horizontal full sun in the outer greenhouse was 1,100 μmol photons m−2 s−1 at noon.
Fully expanded leaves ranged from 20 to 35 cm in length and were thinner and deeper green in the shade than in the sun. Transverse sections confirmed that shade-grown avocado leaves were heterobaric to the level of fourth class veins with a single layer of palisade cells above two to four layers of spongy mesophyll cells and had numerous small stomata restricted to the underside. Imaging experiments showed that this anatomy conferred markedly heterogeneous, small-scale differences in NPQ during photosynthetic induction and light response curves. However, this heterogeneity was effectively integrated over areas of 1 to 2 cm2, and similar results were obtained from imaging and spot measurement techniques (Takayama et al., 2008). Photosynthetic parameters were routinely measured from the upper epidermis, but measurements from the lower epidermis were not significantly different.
Experimental Protocols
Small shade-grown avocado trees were moved to the dark laboratory overnight prior to light treatments to alter xanthophyll pigment composition, but one experiment was done with a plant maintained in the dark laboratory for 11 d. Sets of two or three adjacent attached leaves of similar size and age in the canopy were partly covered with aluminum foil and/or black cloth prior to illumination of the rest of the leaf at 120 to 350 μmol photons m−2 s−1 for 60 min using wide-beam, high-efficiency fluorescent spotlights (Philips PAR38 23W; 2700K warm white). A small leaf disc was punched from foil control and exposed areas of each leaf (or from the larger leaf discs) for pigment analysis before light treatment and again at the end of the treatment, then after 1 to 3 h of dark to relax photosynthetic induction following light treatment, and after 21 to 72 h of recovery in darkness. As found previously (Förster et al., 2009), large avocado leaves were remarkably robust with respect to sampling for pigment analysis. Moreover, photosynthetic parameters measured adjacent to, or remote from, disc sampling areas were similar in both short-term (hours) and long-term (days) experiments.
Preliminary gas-exchange measurements of photosynthesis in leaves of shade-grown plants during induction at 100 μmol photons m−2 s−1 at 25°C were very noisy, even at the slowest practicable flow rates. Stomata opened slowly to low conductance that was responsive to CO2 (20, 7, and 3 mmol m−2 s−1 in 100, 400, and 700 μL L−1 CO2, respectively, after 20 min). Photosynthetic CO2 assimilation saturated at 1.5 to 2.0 μmol m−2 s−1 after 5 min in 700 μL L−1 CO2, reached 1.0 μmol m−2 s−1 after 20 min in 400 μL L−1 CO2, and remained negative in 100 μL L−1 CO2. Chlorophyll fluorescence imaging during the above experiments confirmed that NPQ was sensitive to [CO2] and increased most rapidly to highest values in 100 μL L−1 (Takayama et al., 2008). Mature leaves taken from the shade enclosure during the light period showed higher NPQ when a Vaseline patch was applied to the lower epidermis. Indeed, we used this simple technique to confirm that stomata were closed in leaves dark adapted overnight (no response of ETR or NPQ to a Vaseline patch) and that stomata were open and photosynthesis was induced following the above actinic light treatments (decreased ETR and increased NPQ in response to a Vaseline patch).
Chlorophyll fluorescence assays of photosynthetic parameters in mature leaves of shade-grown avocado were made with the Photosynthesis Yield Analyzer MINI-PAM fitted with a 2030-B leaf clip holder (www.walz.com). Photosynthetic induction curves with Arabidopsis (Arabidopsis thaliana) grown under similar light environments have been routinely run at 1,000 to 1,500 μmol photons m−2 s−1 (Pogson et al., 1998). Previous light response curve assays using chlorophyll fluorescence have ranged from dwell times of 10 s at PFD of 0 to 2,500 μmol photons m−2 s−1 (White and Critchley, 1999; peas [Pisum sativum] grown at 200 μmol photons m−2 s−1) to 180 s at PFD of 0 to 2,000 μmol photons m−2 s−1 (Pérez-Bueno and Horton, 2008; Arabidopsis grown at 120 μmol photons m−2 s−1). Leaves of shade-grown avocado were extremely responsive to actinic light intensity and duration during measurement, and these protocols led to photoinhibition, manifest as reductions in ETR during photosynthetic induction curves with greater than 300 μmol photons m−2 s−1 and in RLRCs with dwell times of 60 to 120 s at each PFD. More important was our observation that measurable deepoxidation of V, but not of Lx, occurred during both assays and was always greater during induction assays. A compromise was reached between the actinic light intensity needed to produce acceptable kinetic responses in ETR and NPQ during RLRC assays and that causing the least deepoxidation of V. We settled for eight steps of 30-s duration over the range from 0 to 400 μmol photons m−2 s−1, followed by measurements at four intervals during 240 s in the dark.
Calculation of Photosynthetic Parameters
The automated photosynthetic induction curve and RLRC protocols in the MINI-PAM fluorimeter measured intrinsic chlorophyll fluorescence (F) and maximum fluorescence yield during a saturating flash (Fm) in dark-adapted leaves to calculate the maximum quantum yield of photochemical energy conversion: Fv/Fm = (Fm−F)/Fm. Photosynthetic electron transport was calculated from fluorescence yield in saturating flashes under actinic light (Fm′) from the quantum yield of photochemical energy conversion (ΔF/Fm′ = Fm′−F/Fm′) and the PFD measured at that spot using the quantum sensor of the leaf clip (adjusted for absorptance of 0.85 and assuming equal light absorption in PSII and PSI). After light exposures to alter the xanthophyll pigment composition of leaves, all NPQ data were recalculated using Fm measured on the leaf kept in the dark overnight and/or Fm from aluminum foil-shaded areas of the leaf during and after treatment. Likewise, 1−qP = 1−((Fm′−F)/(Fm′−Fo′)) was recalculated using the minimum value of F obtained within 100 s after actinic light was switched off as Fo′.
Pigment Analyses
Leaf discs (1 cm diameter) were punched from treated and control areas of leaves at the times specified in each experiment. Discs were wrapped in foil and immediately frozen in liquid nitrogen. Pigments were extracted from individual discs in a microfuge tube with 0.6 mL of ethyl acetate:acetone (60:40, v/v) and shaken at 30 Hz for 2 min with a stainless steel ball (2 mm diameter) before addition of 0.5 mL of water prior to a 5-min centrifugation at 13,000 rpm. The pigment containing the upper layer was transferred to a fresh microfuge tube, centrifuged as before, and 0.1 mL of the pigment solution was placed in vials for HPLC analysis on an Agilent 1100 fitted with a Waters Spherosorb ODS2 column, using a linear gradient from 100% to 33% acetonitrile:water (90:10 [v/v] with 0.1% triethanolamine) into ethyl acetate over 31 min. Pigments were identified by retention times and spectra, and carotenoid concentrations were calculated using conversion factors for A440 obtained with pure pigments, determined by Dr. Shizue Matsubara (ICG-III). The L pool in shade leaves of avocado was 3- to 5-fold greater than that of the other xanthophylls, so values for L have been reduced by 100 mmol mol−1 chlorophyll (marked L* in graphical presentations) to facilitate more sensitive comparisons of stoichiometric relationships. As found previously (Förster et al., 2009), there were scarcely detectable changes in neoxanthin, α- or β-carotene pool, or chlorophyll a/b ratio in the course of any of the 24- to 72-h experiments. In most cases, only Lx and V cycle pigment concentrations are reported.
Acknowledgments
We are grateful to Kotaro Takayama for his preliminary investigations of the photosynthetic properties of shade-grown avocado leaves using chlorophyll fluorescence imaging, to Steve Dempsey for maintenance of the plant material, and to Fred Chow for the use of his dark laboratory facilities.
References
- Adams WW, III, Demmig-Adams B, Winter K. (1990) Relative contributions of zeaxanthin-related and zeaxanthin-unrelated types of ‘high-energy-state’ quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol 92: 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR. (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283: 3550–3558 [DOI] [PubMed] [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213: 794–801 [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O. (1994) Relationships among violaxanthin deepoxidation, thylakoid membrane conformation, and nonphotochemical chlorophyll fluorescence quenching in leaves of cotton (Gossypium hirsutum L.). Planta 193: 238–246 [Google Scholar]
- Björkman O. (1981) Responses to different quantum flux densities. Lange OL, Nobel PS, Osmond CB, Ziegler H, Encyclopedia of Plant Physiology: Physiological Plant Ecology. I. Responses to the Physical Environment, Vol 12A. Springer, Berlin, pp 57–l [Google Scholar]
- Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P, Scholes JD. (1999) Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA 96: 1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli CI, Pogson BJ. (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci 15: 266–274 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R. (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bianchi S, Ballottari M, Dall’Osto L, Bassi R. (2010) Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 38: 651–660 [DOI] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krüger A, Czygan F-C. (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84: 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- Esteban R, Jiménez MS, Morales D, Jiménez ET, Hormaetxe K, Becerril J-M, Osmond B, García-Plazaola J-I. (2008) Short- and long-term modulation of the lutein epoxide and violaxanthin cycles in two species of the Lauraceae: sweet bay laurel (Laurus nobilis L.) and avocado (Persea americana Mill.). Plant Biol (Stuttg) 10: 288–297 [DOI] [PubMed] [Google Scholar]
- Esteban R, Matsubara S, Soledad-Jiménez M, Morales D, Brito P, Lorenzo R, Fernández-Marin B, Becerril J-M, García-Plazaola JI. (2010) Operation and regulation of the lutein epoxide cycle in seedlings of Ocotea foetans. Funct Plant Biol 37: 859–869 [Google Scholar]
- Finazzi G, Johnson GN, Dall’Osto L, Joliot P, Wollman FA, Bassi R. (2004) A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci USA 101: 12375–12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster B, Osmond CB, Boynton JE. (2001) Very high light resistant mutants of Chlamydomonas reinhardtii: responses of photosystem II, nonphotochemical quenching and xanthophyll pigments to light and CO2. Photosynth Res 67: 5–15 [DOI] [PubMed] [Google Scholar]
- Förster B, Osmond CB, Pogson BJ. (2005) Improved survival of very high light and oxidative stress is conferred by spontaneous gain-of-function mutations in Chlamydomonas. Biochim Biophys Acta 1709: 45–57 [DOI] [PubMed] [Google Scholar]
- Förster B, Osmond CB, Pogson BJ. (2009) De novo synthesis and degradation of Lx and V cycle pigments during shade and sun acclimation in avocado leaves. Plant Physiol 149: 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Plazaola J-I, Matsubara S, Osmond CB. (2007) The lutein epoxide cycle in higher plants: its relationship to other xanthophyll cycles and possible functions. Funct Plant Biol 34: 754–779 [DOI] [PubMed] [Google Scholar]
- García-Plazaola J-I, Olano JM, Hernández A, Becerril JM. (2003) The operation of the lutein epoxide cycle correlates with energy dissipation. Funct Plant Biol 30: 319–324 [DOI] [PubMed] [Google Scholar]
- Gilmore AM. (2001) Xanthophyll cycle-dependent nonphotochemical quenching in photosystem II: mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein. Photosynth Res 67: 89–101 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Björkman O. (1995) Temperature sensitive coupling and uncoupling of ATPase-mediated nonradiative energy dissipation: similarities between chloroplasts and leaves. Planta 197: 646–654 [Google Scholar]
- Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee G. (1998) Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a fluorescence lifetime distributions and intensity in thylakoids. Biochemistry 37: 13582–13593 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV. (2008) Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states?. FEBS J 275: 1069–1079 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Hurry V, Anderson JM, Chow WS, Osmond CB. (1997) Accumulation of zeaxanthin in abscisic acid-deficient mutants of Arabidopsis does not affect chlorophyll fluorescence quenching or sensitivity to photoinhibition in vivo.. Plant Physiol 113: 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Davidson PA, Ruban AV, Horton P. (2008) The xanthophyll pool size controls the kinetics of nonphotochemical chlorophyll quenching in Arabidopsis thaliana. FEBS Lett 582: 262–266 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Havaux M, Triantaphylidès C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P. (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282: 22605–22618 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Pérez-Bueno ML, Zia A, Horton P, Ruban AV. (2009) The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol 149: 1061–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. (1997) Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 38: 1802–1811 [PubMed] [Google Scholar]
- Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. (2006) Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res 82: 828–839 [DOI] [PubMed] [Google Scholar]
- Krause GH, Vernotte C, Briantais JM. (1982) Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae: resolution of two components. Biochim Biophys Acta 679: 116–124 [Google Scholar]
- Lepetit B, Volke D, Gilbert M, Wilhelm C, Goss R. (2010) Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol 154: 1905–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK. (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK. (2009) Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21: 1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Gilmore AM, Osmond CB. (2001) Diurnal and acclimatory responses of violaxanthin and lutein epoxide in the Australian mistletoe Amyema miquelii. Aust J Plant Physiol 28: 793–800 [Google Scholar]
- Matsubara S, Krause GH, Seltmann M, Virgo A, Kursar TA, Jahns P, Winter K. (2008) Lutein epoxide cycle, light harvesting and photoprotection in species of the tropical tree genus Inga. Plant Cell Environ 31: 548–561 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Morosinotto T, Bassi R, Christian AL, Fischer-Schliebs E, Lüttge U, Orthen B, Franco AC, Scarano FR, Förster B, et al. (2003) Occurrence of the lutein-epoxide cycle in mistletoes of the Loranthaceae and Viscaceae. Planta 217: 868–879 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Morosinotto T, Osmond CB, Bassi R. (2007) Short- and long-term operation of the lutein-epoxide cycle in light-harvesting antenna complexes. Plant Physiol 144: 926–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, Bassi R, Osmond B. (2005) Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may ‘lock-in’ lutein-based photoprotection during acclimation to strong light. J Exp Bot 56: 461–468 [DOI] [PubMed] [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Shih C, Soon Chow W, Pogson BJ, Dellapenna D, Björkman O. (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67: 139–145 [DOI] [PubMed] [Google Scholar]
- Nocter G, Rees D, Young AJ, Horton P. (1991) The relationship between zeaxanthin, energy-dependent quenching of chlorophyll fluorescence, and trans-thylakoid pH gradient in isolated chloroplasts. Biochim Biophys Acta 1057: 320–330 [Google Scholar]
- Pearcy RW. (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41: 421–453 [Google Scholar]
- Pérez-Bueno ML, Horton P. (2008) The role of lutein in the acclimation of higher plant chloroplast membranes to suboptimal conditions. Physiol Plant 134: 227–236 [DOI] [PubMed] [Google Scholar]
- Pogson BJ, McDonald KA, Truong M, Britton G, DellaPenna D. (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Niyogi KK, Björkman O, DellaPenna D. (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA 95: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Rissler HM. (2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton GGR, Chow WS, Anderson JM, Osmond CB. (1995) Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol 107: 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. (1994) Chlorophyll fluorescence as a non intrusive indicator for rapid assessment of in-vivo photosynthesis. Schulze E-D, Caldwell MM, , Ecophysiology of Photosynthesis. Springer, Berlin, pp 49–70 [Google Scholar]
- Takayama K, Osmond B, Omasa K. (2008) Imaging heterogeneity of xanthophyll independent non-photochemical quenching during photosynthetic induction in shade-grown leaves of avocado (Persea americana L.). Allen JF, Gant E, Golbeck JH, Osmond B, , Photosynthesis: Energy from the Sun. 14th International Congress on Photosynthesis. Springer, Dordrecht, The Netherlands, pp 681–685 [Google Scholar]
- Whiley AW, Schaffer B, Wolstenholme BN.editors (2002) The Avocado: Botany, Production and Uses. CABI Publishing, Oxford [Google Scholar]
- White AJ, Critchley C. (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59: 63–72 [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP. (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55: 1167–1175 [DOI] [PubMed] [Google Scholar]