Abstract
Folivory is the best studied plant-herbivore interaction, but it is unclear whether the signaling and resistance traits important for the defense of leaves are also important for other plant parts. Larvae of the tobacco stem weevil, Trichobaris mucorea, burrow into stems of Nicotiana attenuata and feed on the pith. Transgenic N. attenuata lines silenced in signaling and foliar defense traits were evaluated in a 2-year field study for resistance against attack by naturally occurring T. mucorea larva. Plants silenced in early jasmonic acid (JA) biosynthesis (antisense [as]-lipoxygenase3 [lox3]; inverted repeat [ir]-allene oxide cyclase), JA perception (as-coronatine insensitive1), proteinase inhibitors (ir-pi), and nicotine (ir-putrescine methyl-transferase) direct defenses and lignin (ir-cad) biosynthesis were infested more frequently than wild-type plants. Plants unable to emit C6 aldehydes (as-hpl) had lower infestation rates, while plants silenced in late steps in JA biosynthesis (ir-acyl-coenzyme A oxidase, ir-opr) and silenced in diterpene glycoside production (ir-geranylgeranyl pyrophosphate synthase) did not differ from wild type. Pith choice assays revealed that ir-putrescine methyl-transferase, ir-coronatine insensitive1, and ir-lox3 pith, which all had diminished nicotine levels, were preferred by larvae compared to wild-type pith. The lack of preference for ir-lox2 and ir-cad piths, suggest that oviposition attraction and vascular defense, rather than pith palatability accounts for the higher attack rates observed for these plants. We conclude that traits that influence a plant’s apparency, stem hardness, and pith direct defenses all contribute to resistance against this herbivore whose attack can be devastating to N. attenuata’s fitness.
The singer-songwriter Paul Simon sang about the “50 ways to leave your lover”; plants have at least as many ways of coping with their insect herbivores, and insect herbivores have at least as many ways of attacking plants, given the great diversity of feeding modes among insect taxa (Strong et al., 1984). However, the vast majority of studies that examine the plant-herbivore interaction have focused on herbivores feeding on leaves: folivores (Haukioja and Koricheva, 2000). Only a few have examined stem feeders, and of these most describe stem feeders on monocotyledonous hosts (Ordás et al., 2002; Soengas et al., 2004; Zhou et al., 2009). Stem feeders on monocotyledenous plants basically feed on modified leaves since most monocots are herbaceous and do not have the ability to increase stem width via secondary growth, and lack stem structures such as pith, and secondary xylem. Many of the stem feeders on dicotyledenous hosts are coleopterans, such as the longhorned beetle Dectes texanus that feeds on soybean (Glycine max) or sunflower (Helianthus annuus) stalks (Charlet et al., 2009), the specialist weevil, Rhyssomatus lineaticollis, found in the stems of the common milkweed, Asclepias syriaca (Fordyce and Malcolm, 2000), or the stem weevil Listronotus setosipennis that consumes stems of Parthenium hysterophorus (Wild et al., 1992). Weevils are the most common stem-boring beetles.
Weevils are classified in the superfamily, Curculionoidea, which contains about 62,000 species and approximately 6,000 described genera (Thompson, 1992; Kuschel, 1995), and as such, they represent one of the most specious groups of herbivorous beetles with the broadest geographical range. They occur in the humid tropics, subaquatic, desert, tundra, and other environments at nearly all latitudes and altitudes with all vegetation types (Arnett et al., 2002). Collectively, weevils feed on nearly every plant taxon (Anderson, 1998; Arnett et al., 2002), all conditions (living, dead, dying, and decaying), and all plant parts such as roots, stems, leaves, flowers, fruits, or seeds (Freude and Harde, 1999; Arnett et al., 2002). The genus Trichobaris is North American in distribution with 12 described species (O'Brien and Wibmer, 1982). Trichobaris compacta for example, feeds within stalks of Datura wrightii as does T. mucorea, albeit within another solanaceaous plant, Nicotiana attenuata (Barber, 1935).
N. attenuata, like other plants, uses both direct and indirect defenses to protect itself against herbivore attack. Direct defenses include toxins, antidigestive proteins, and mechanical barriers that directly affect the susceptibility or performance of herbivores. Proteinase inhibitors (PIs; antidigestive proteins) are inducible by wounding and herbivory and influence herbivore performance by inhibiting insect digestive enzymes (Koiwa et al., 1997; Tamayo et al., 2000). Toxic compounds (e.g. alkaloids such as nicotine, terpenoids, phenolics) poison generalist herbivores and force specialists to invest resources in detoxification mechanisms that, in turn, incur growth and development costs (Walling, 2000; Howe and Jander, 2008).
The vast majority of research on direct defenses has focused on their effects on leaf feeders, but defenses against leaf feeders are likely to be different from those against leaf miners or stem borers, either because the tissues or organs attacked have different fitness values to the plant (McKey, 1979; Zangerl and Bazzaz, 1992) or because internal plant morphology, physiology, and metabolism interferes with the uniform deployment of chemical defenses (Jones et al., 1993). The relative allocation of chemical defenses should depend on the relative fitness value of tissues or structures to the plant and their probability of being attacked (Feeny, 1976; Rhoades and Cates, 1976; Mc Key, 1979; Rhoades, 1979; Nitao and Zangerl, 1987; Zangerl and Bazzaz, 1992; Zangerl and Nitao, 1998). Berenbaum et al. (1986) showed that higher defense investment in reproductive tissues was associated with higher fitness for the plant. Furthermore, tissues that are low in nutrients for herbivores are assumed to have lower concentrations of defense compounds, due to their likely lower probabilities of being attacked. For example, stem tissues, which are thought to be nutritionally deficient as a result of their high cellulose and lignin contents, may be low in defenses (Zangerl and Bazzaz, 1992) but this does not take into account the large negative fitness effects commonly associated with the attack of stems and other structural parts of a plant (Strong et al., 1984; Karban and Baldwin, 1997). Indeed stems may be protected by unique chemistries for exactly this reason. Fordyce and Malcolm (2000) showed that the piths of milkweeds contained nonpolar cardenolids that may be particularly toxic against the larvae of the milkweed weevil R. lineaticollis. Other studies have found stem-associated terpenoids to function as feeding deterrents or repellants (Nordlander, 1990; Lindgren et al., 1996) or toxins (Cook and Hain, 1988; Raffa and Smalley, 1995; Werner, 1995) to bark beetles and pine weevils.
Direct defenses are complemented by the indirect defenses of a plant, like the herbivory-induced emissions of volatile organic compounds that attract carnivorous organisms to feeding herbivores (Dicke, 1999). The induction of predator-attracting plant volatiles was first demonstrated for foliage-feeding mites (Dicke and Sabelis, 1988; Dicke et al., 1990) and caterpillars (Turlings et al., 1990), but found later also for root feeders (van Tol et al., 2001; Rasmann et al., 2005), seed feeders (Steidle et al., 2005), and stem borers (Potting et al., 1995). Stem borer larvae have a broad range of natural enemies, which are able to locate and attack the larvae that feed inside plant tissues (Baker et al., 1949; Smith et al., 1993). Some parasitoids use volatile terpenoid cues to locate stem-boring larvae deep within stems (Roth et al., 1982; Ding et al., 1989; Ma et al., 1992).
N. attenuata uses a combination of toxins and digestibility reducing defenses, both of which are elicited by herbivore attack. Trypsin protease inhibitors (TPIs) are an effective component of this inducible defensive system that reduces the performance of folivores by targeting their main proteolytic digestive enzymes (van Dam et al., 2000; Glawe et al., 2003; Zavala and Baldwin, 2004; Horn et al., 2005; Zavala et al., 2008; Bezzi et al., 2010). Nicotine, a neurotoxin, generally reduces consumption by a variety of herbivores (Steppuhn et al., 2004). For example, the damage caused by the flea beetle Epitrix hirtipennis was significantly higher for plants silenced in their nicotine production (inverted repeat [ir]-putrescine methyl-transferase [pmt]) compared to wild-type plants (Steppuhn et al., 2008). TPIs and nicotine function synergistically against generalist herbivores that increase their consumption in response to the ingestion of TPIs (Steppuhn and Baldwin, 2007). In addition, N. attenuata uses indirect defenses such as volatile organic compounds, which attract predators of Manduca sexta eggs and larvae (Halitschke et al., 2000; Kessler and Baldwin, 2001).
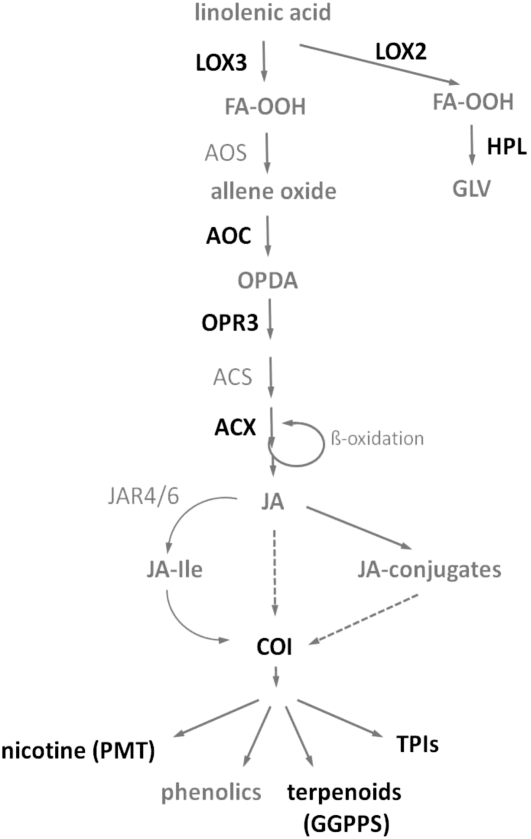
Both direct and indirect defenses are elicited by the jasmonate (JA) signaling cascade. The phytohormone, JA, as well as its precursors and derivatives are synthesized via the octadecanoid pathway (Creelman and Mullet, 1997; Schaller et al., 2004; Wasternack, 2007). Linolenic acid is released from chloroplast membranes in response to different biotic and abiotic stimuli, and is oxygenated by a 13-lipoxygenase (13-LOX). The resulting 13(S)-hydroperoxylinolenic acid is dehydrated by allene oxide synthase and cyclized by allene oxide cyclase (AOC), yielding 12-oxophytodienoic acid (OPDA). The reduction of this cyclopentenone is catalyzed by OPDA reductase (OPR3) in the peroxisome, and followed by three cycles of β-oxidation resulting in JA, which itself serves as a precursor for JA derivatives such as JA-amino acid conjugates or methyl JA (Fig. 1).
Figure 1.
Biosynthetic steps of JA and JA-mediated secondary metabolites of which proteins silenced by RNAi in this study are highlighted. In bold font are the proteins that were silenced by RNAi in isogenic lines of N. attenuata plants that were planted into a field plantation in the plant’s native habitat in the Great Basin Desert, where T. mucorea could freely oviposit on the plants. OPDA, 12-oxo-phytodienoic acid; ACS, acyl-coenzyme A synthetase; HPL, hydroperoxide lyase; JAR, JA resistant; JA-Ile, JA-Ile conjugate.
Plants that have been genetically engineered to accumulate less JA (Halitschke and Baldwin, 2003; Kessler et al., 2004) or are unable to respond to JA (Paschold et al., 2007) have been used to demonstrate that JA acts as a major transducer of signals that are essential for plant defense. To examine more closely the interactions between stem-feeding herbivores and plants, we used N. attenuata plants silenced in various functions known to influence the performance of folivorous insects such as the production and induction of direct and indirect defenses. We compared infestation rates of T. mucorea larvae silenced in production of enzymes involved in early (antisense [as]-lox3, ir-aoc) and late (ir-opr3, ir-acyl-coenzyme A oxidase [acx]) stages of JA biosynthesis or perception (as-coronatine insensitive1 [coi1]), silenced in direct defenses such as PIs (ir-pi) or nicotine (ir-pmt), or silenced in the production of C6 aldehydes (as-hpl, ir-lox2) or diterpene glycosides (DTGs; ir-geranylgeranyl pyrophosphate synthase [ggpps]). In addition, we compared infestation rates of T. mucorea larvae in plants silenced in genes that influence stem characteristics such as the lignin content (ir-cad) of stems, the tissue through which larvae must burrow to enter the pith. Higher infestation rates in plants with diminished direct defenses were correlated with higher pith palatability; so were plants impaired in the early JA biosynthesis steps and JA perception. Ir-cad and ir-lox2 piths on the other hand were not preferred over wild type in pith choice assays by T. mucorea larvae. In comparison however ir-cad plants showed higher infestation rates than as-hpl (as a replacement for ir-lox2) plants in the field, suggesting that the susceptibility of these plants cannot be attributed solely to characteristics of the pith.
RESULTS
Life Cycle of T. mucorea Champion, G.C.
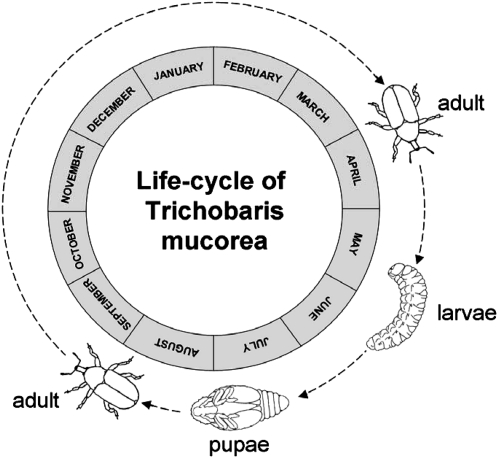
T. mucorea adults are first observed in March emerging from their overwintering sites. Female T. mucorea adults lay their oval, light-yellow eggs at the base of the just-elongating N. attenuata stems from which the almost transparent larvae hatch. The young larva likely feed within a cavity that the female adult beetle creates during oviposition and then tunnels through the vascular bundle and into the pith of its host plant, N. attenuata (Woodside, 1949). Usually only one larva is found per plant, but occasionally (in 10.8% of the plants for the 2009 field season) two or three larvae were found infesting one plant (data not shown). At the end of its feeding period (end of June/beginning of July), larvae burrow into the base of the stem via the hollowed-out pith and excavates a partial exit hole through the vascular bundles and epidermal layers to the outside of the stem, presumably to facilitate the exit of the adult, and pupates. In August the adults emerge from the stems and likely overwinter in dried and senescent N. attenuata stems or in other protected cavities (Figs. 2 and 3).
Figure 2.
Life cycle of the tobacco stem weevil (T. mucorea Champion, G.C.). T. mucorea weevils undergo complete metamorphosis. During March and April, adult T. mucorea females lay eggs on stems of N. attenuata plants from which larvae hatch and burrow into the stem, where they feed on the pith during their entire larval development which lasts for 4 to 5 instars. In the beginning of July, last instar larvae, excavate a partial exit hole through the vascular and epidermal layers of the stem and pupate. The next generations of adult weevils emerge from pupae in August and September and overwinter in the dried and senescent N. attenuata stems or in other protected cavities.
Figure 3.
Developmental stages of the tobacco stem weevil, T. mucorea. A, Dorsal view of the adult weevil. B, Larva feeding on the pith in a stem of a N. attenuata plant. C, Pupal stage. D, Exit hole in a N. attenuata stem from which the adult weevil will emerge.
Infestation Rates of T. mucorea among Different N. attenuata Genotypes
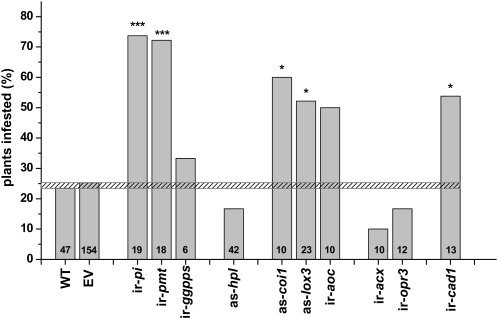
N. attenuata produces many secondary metabolites that function as inducible defenses, most of which are elicited by the JA-signaling pathway. Silencing the genes responsible for the biosynthesis of the JA cascade that mediates the induction of TPIs and nicotine, makes plants highly susceptible to herbivore attack (Halitschke and Baldwin, 2003; Steppuhn et al., 2004; Zavala and Baldwin, 2004) and demonstrates their function as direct defenses (Fig. 1). To evaluate the effect of N. attenuata’s secondary metabolites on T. mucorea, we compared the rates of infestation of T. mucorea larvae among these transformed N. attenuata lines during the 2009 field season (Fig. 4). The infestation percentage in wild-type plants was independent of planting time and averaged 25% ± 1% (se). All plants used for this experiment were planted within 1 week in May. Three subsets of wild-type plants planted on different days within this week showed no differences in T. mucorea infestation (Supplemental Fig. S5). Silencing PI production and nicotine accumulation significantly increased infestations with T. mucorea to 73% (Fisher’s exact probability test; P < 0.001).
Figure 4.
Percentage of isogenic lines of N. attenuata silenced in JA-signaling and JA-mediated defenses infested by T. mucorea larvae. Shown are infestation percentages of N. attenuata plants, with the total number of plants of each genotype in the field plot (e.g. replicates) given at the base of each bar. Plants were considered infested if one or more larvae were found in the stem of a plant at the time of harvest. Dashed line indicates the range of infestation percentage in N. attenuata wild-type and EV plants. CAD1, Cinnamyl alcohol dehydrogenase1; see Figure 1 caption for other abbreviations. Asterisks indicate significant differences among genotypes compared to wild type (Fisher’s exact probability test; * = P < 0.05; ** = P < 0.01; *** = P < 0.001).
To further explore the role of JA signaling in modulating T. mucorea behavior, we used transgenic N. attenuata plants silenced in both early JA biosynthesis steps (including as-lox3 and ir-aoc) as well as late JA biosynthesis steps (ir-opr3 and ir-acx), and JA perception (as-coi1). As-lox3 and ir-aoc plants show up to 2-fold-higher susceptibility to T. mucorea than wild-type plants (as-lox3: P = 0.01; ir-aoc: P = 0.09) whereas infestation rates in ir-acx and ir-opr3 were reduced to 12% and 17%, respectively, and did not differ significantly from wild-type plants (Fig. 4). Plants impaired in JA perception (as-coi1) in turn, had a 2.4-fold-higher infestation rate when compared to wild-type plants (P = 0.027).
The tendency of as-hpl plants to be less frequently infested (P = 0.166) than wild-type plants suggests that the release of green leaf volatiles (GLVs), which are reduced in as-hpl plants to 30% of the amounts released by wild-type plants (Halitschke et al., 2004), might play a role in the T. mucorea-N. attenuata interaction.
Furthermore we investigated the effects of lignin, which confers mechanical strength to cell walls on the performance of T. mucorea by using plants silenced in cinnamyl-alcohol-dehydrogenase (ir-cad) that had significantly reduced lignin content (H. Kaur, I. Galis, and I.T. Baldwin, unpublished data). T. mucorea infested ir-cad plants at a significantly higher rate than wild-type plants, suggesting that decreased stem lignin contents facilitates larval entry (P = 0.0343).
We also examined plants silenced in DTG production (ir-ggpps), as these plants suffer significantly more damage from folivorous herbivores in N. attenuata’s native habitat than wild-type plants (Heiling et al., 2010). T. mucorea weevils however did not discriminate between ir-ggpps and wild-type plants (P = 0.483; Fig. 4).
T. mucorea Pith Choice Tests
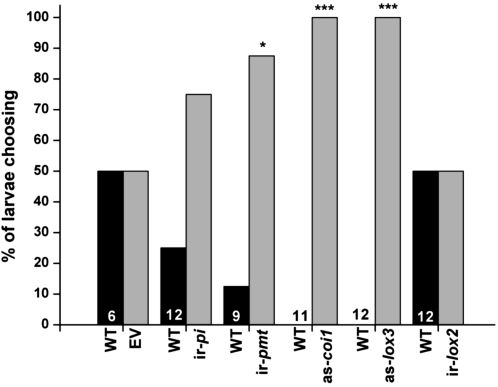
Since T. mucorea larvae consume and tunnel through the pith of N. attenuata plants, and do not feed on leaves, we needed to determine whether differences in food quality of the pith itself or other stem traits that present barriers to the initial infestation steps (epidermal, vascular bundles) were responsible for the differences in observed infestation rates. Therefore pith sandwiches (Supplemental Fig. S1, A and B) were made by pairing split stem halves with their associated pith of one genotype with wild-type stem halves, as shown in Supplemental Figure S1. In 24-h choice tests (Fig. 5), more than twice the number of larvae choose to feed on ir-pi and ir-pmt piths instead of wild-type piths. When given choices between as-coi1/wild type and as-lox3/wild type, the larvae’s choice against wild-type pith was even stronger (P < 0.001). Comparisons between wild-type and empty vector (EV) pith were performed to control for any possible transformation-related differences in pith characteristics; none were found as 50% of the larvae fed on wild-type and 50% on EV pith. Larvae did not distinguish between wild-type and ir-lox2 (plants silenced in LOX2 that supplies hydroperoxide fatty acids to HPL and GLV biosynthesis; Allmann et al., 2010) pith. Ir-lox2 plants were used as a replacement for as-hpl plants since as-hpl plants did not perform well in 2010 field season when the pith assays were conducted. Ir-lox2 plants phenocopy as-hpl plants in their highly reduced GLV emissions compared to wild-type plants. Clear binary choices were made by larvae in 78.9% of the bioassays. In 21.1% of the assays, larvae did not choose between one genotype or the other. These no choice data were excluded from the analysis.
Figure 5.
Pith choice assays with T. mucorea larvae given a choice of stems and pith of wild-type and different transformed lines of N. attenuata plants. Individual T. mucorea larvae were placed between two stem halves, one from a wild-type stem and a matching half from a transformed N. attenuata plant (see caption of Fig. 1 for abbreviations and Supplemental Fig. S1 for images of choice assay). The larvae’s choice of pith was determined after 24 h. Numbers in bars reflect replicate numbers. Larval choice was compared with Pearson’s χ2-test; significant differences between the lines are indicated by asterisks (* = P < 0.05; *** = P < 0.001).
Secondary Metabolites in N. attenuata Piths
To investigate the effect of PI production in pith on T. mucorea behavior, the plant piths were analyzed for PI activity after wounding and M. sexta oral secretion (OS) elicitation, a treatment that is known to dramatically increase PI transcripts and activity in leaves (Zavala and Baldwin, 2004; Zavala et al., 2008). Pith of N. attenuata wild-type and ir-pi plants was wounded by making punctures into the stem and adding water only or water and M. sexta OS (as a positive control) to the holes, or left unwounded in controls. No PIs could be detected either in wild-type or in ir-pi piths independent of elicitation treatment (Supplemental Fig. S2).
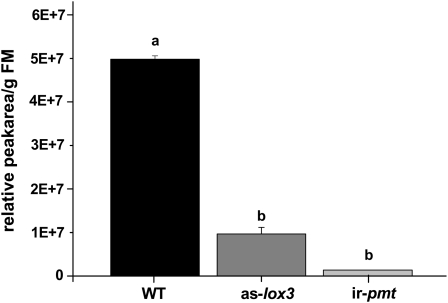
Nicotine on the other hand did show significant differences among wild-type pith and pith from as-lox3 and ir-pmt plants (Fig. 6). While pith of ir-pmt plants had almost no detectable nicotine, as-lox3 pith was also very low with approximately one-fifth the amount of nicotine than that of wild-type pith. These results suggest that nicotine levels play an important role in determining the palatability of piths to T. mucorea larvae.
Figure 6.
Nicotine concentrations of pith from as-lox3 and ir-pmt plants are lower than those from wild-type plants. Values are relative concentrations of nicotine per gram fresh mass (FM) accumulated in the pith of wild-type (black bar), as-lox3 (dark-gray bar), and ir-pmt (light-gray bar) plants. Pith samples were taken from plants growing in a field plot in the Great Basin Desert in Southwestern Utah, which were infested by the natural occurring herbivores. Different letters indicate significant differences among genotypes (ANOVA; P < 0.01).
To determine whether DTG levels differed in the piths of wild-type and ir-ggpps plants, DTG aglycone contents were measured and ir-ggpps pith showed significantly less aglycone than wild-type pith, which had values almost twice those found in ir-ggpps pith (Supplemental Fig. S4). Aglycone contents were measured to rigorously quantify the total DTG amounts since aglycone is the basic fragment ion common to all 16 known DTGs of N. attenuata leaves (Heiling et al., 2010).
The ratio of dry to fresh mass of wild-type N. attenuata leaves and piths was measured (Supplemental Fig. S3). The dry:fresh mass ratio of N. attenuata pith was significantly higher than that of leaves.
DISCUSSION
The life cycles of most organisms are constrained by the seasonality of resources and the timing of N. attenuata’s initiation of stem growth and flowering represents a particularly strong phenological constraint for the tobacco stem weevil. Adults must oviposit just before the initiation of stem elongation so that neonate larvae can enter the newly developing pith to complete development in stems without killing the plant. Since T. mucorea larvae do not survive very long when removed from stems, it is very unlikely that they can move from one plant to another. Hence the adult beetles must be able to identify plants at the rosette stage of growth that will develop sufficiently large stems later in the season for their larvae to complete development. The density of T. mucorea larvae within tobacco stalks differed significantly among different transgenic N. attenuata lines, suggesting that direct and indirect defenses that have been shown to be important against folivores in N. attenuata are also important against this stem-boring herbivore. The analysis revealed many differences between resistance traits important for these two feeding guilds, but the strength of the conclusions that we can draw are constrained by our inability to culture this herbivore and thereby experimentally infest plants.
Infestation can be separated into three distinct stages: (1) identification by adult weevils of plants in the appropriate stage of growth and with the appropriate potential for future growth and oviposition; (2) hatching and larval entrance into the newly developing pith; and (3) growth and completion of development in the pith. Our experiments provide data on all three steps combined (Fig. 4) and the last step (Fig. 5) and from these, we draw inferences about the traits that are relevant for resistance.
The inferences that we can draw from our evaluation of larval preferences on the pith of the different lines are also limited. First, we are limited to one generation of larvae per year, and these must match the availability of the different transgenic lines of plants, and second that many of the larvae were damaged during extraction from stems and couldn’t be used for bioassays. In addition, previous dietary experience (most were harvested from wild-type plants) may influence the preference and performance of larvae. Despite all of these caveats, the consistency of larval choices in the pith assays and the congruence of the field infestation data with the pith choice data were remarkable. We found no evidence of parasitized or predated larvae and hence can say very little about the role of indirect, volatile mediated defenses in the resistance to T. mucorea. Such indirect defenses may be particularly effective against neonate larvae when they are just entering stems and hence are particularly vulnerable to predation. If it was possible to culture T. mucorea, experimental infestations of plants deficient in indirect defenses would be possible and thereby strengthen the inferences that we can currently draw about these resistance traits, which we discuss next.
GLVs can have diverse defense functions such as direct repellents or toxins on insects (De Moraes et al., 2001; Kessler and Baldwin, 2001; Vancanneyt et al., 2001). The reduced infestation rate of N. attenuata plants unable to produce GLVs (as-hpl; Fig. 4), suggests that these plants are invisible to the adult weevils, which may not oviposit on these plants. GLVs are also known to function as feeding stimulants for some lepidopteran larvae, such as M. sexta, which consumes less from as-hpl plants unless the plants are supplemented with synthetic GLVs (Halitschke et al., 2004; Meldau et al., 2009). However for T. mucorea, GLVs do not function as feeding stimulants, as larvae didn’t prefer wild-type piths over ir-lox2 piths (Fig. 5), which like as-hpl plants, are greatly reduced in their GLV’s emissions compared to wild-type plants, because LOX2 specifically supplies hydroperoxide substrates for HPL in N. attenuata (Allmann et al., 2010). We used ir-lox2 plants for the pith choice assays, since as-hpl plants did not perform well during the 2010 field season.
To tunnel into N. attenuata’s stem and feed on the pith, the larvae of T. mucorea must cope with the biomechanical properties of the stem and tissue toughness has long been thought to be an important defense against herbivores (Feeny, 1970; Choong, 1996; Massey et al., 2006; Hanley et al., 2007), perhaps even influencing the composition of insect herbivore communities (Peeters et al., 2007). Lignin dramatically influences the biomechanical properties of plants and is known to influence resistance against insects and pathogens (Kiedrowski et al., 1992; Zabala et al., 2006; Ithal et al., 2007; Lao et al., 2008; Johnson et al., 2009). In N. attenuata stems, a majority of the lignin is produced in basal portions, as is clearly seen from the pattern of accumulation of red pigments in the ir-cad plants (H. Kauer, I. Galis, and I.T. Baldwin, unpublished data). Cinnamyl alcohol dehydrogenase proteins function in lignin biosynthesis and silencing cinnamyl alcohol dehydrogenase in N. attenuata results in higher infestation rates of T. mucorea larvae (Fig. 4). Unfortunately ir-cad plants did not perform well during the 2010 field season and the pith-sandwich experiments shown in Figure 5 could be performed with only three replicates (data not shown). One larva chose ir-cad and one wild-type pith while the third larva did not feed, which suggests that characteristics of the pith in ir-cad plants are not responsible for the greater infestations of these plants and that the ease of penetration through the unlignified vascular bundles of these plants is more likely the explanation.
Our results clearly point to an important role played by chemical defenses in the pith. Pith, with its marginally higher dry mass content than leaves (Supplemental Fig. S3) was thought to be unprotected starch, located in a tissue with a low metabolic rate (Lavee and Galston, 1968), consistent with the expectations of a role in storage. T. mucorea larvae however show clear preferences for the pith of different transgenic lines. Ir-pi and ir-pmt plants for example show a positive correlation between their infestation rates (Fig. 4) and palatability (pith choice test; Fig. 5; Supplemental Fig. S1), suggesting that chemical traits of the pith is as important as the characteristics of the layers that surround and protect the pith.
In wild type as well as in ir-pi pith, and even in OS-elicited pith, PI levels were not detectable (Supplemental Fig. S2). van Dam et al. (2001) showed that PI levels in stems of N. attenuata did not increase after methyl-JA treatment and were at basal levels. The reason why these authors detected trace levels of PIs in the stems is likely because they included the epidermis of the stem in the analysis. Why T. mucorea larvae prefer ir-pi plants in the pith choice tests remains unclear. One explanation could be an altered total protein content as it has been shown for the nectar of ir-pi plants (Bezzi et al., 2010).
In contrast to PIs, wild-type pith contains substantial amount of nicotine and the concentration of this toxin is substantially reduced in the pith of ir-pmt plants (Fig. 6) that likely accounts for the strong preference for ir-pmt piths by T. mucorea larvae. Both nicotine and PIs are inducibly regulated by the JA-signaling cascade, the first biosynthetic step of which is catalyzed by LOXs. In N. attenuata the wound- and herbivore-elicited increase in JA accumulation is dependent on the expression of NaLOX3 (Fig. 1) and pith nicotine levels are significantly lower in as-lox3 plants (Fig. 6). Since nicotine is a very effective deterrent to T. mucorea larvae, it is not surprising, that as-lox3 plants have significantly higher infestation rates (Fig. 4), and are preferred over wild-type plants (Fig. 5).
Another early JA biosynthetic enzyme is AOC (Fig. 1). Like ir-lox3, ir-aoc plants are more susceptible to T. mucorea larvae although the effects are not as strong (Fig. 4). For Arabidopsis and potato (Solanum tuberosum) leaves it is known that OPDA (the precursor of JA) as well as the levels of the related compound, dinore OPDA also increase after wounding or herbivore attack (Weber et al., 1973; Reymond et al., 2000, 2004), suggesting a direct defensive role of OPDA (Koch et al., 1999; Stintzi et al., 2001). OPDA is not produced in both ir-lox3 and ir-aoc lines, which might account for the higher infestation rates by T. mucorea larvae in comparison to wild-type plants. When the JA biosynthetic pathway is dissected into early and late steps, additional patterns emerge. N. attenuata plants silenced in the late steps of JA biosynthesis (ir-opr and ir-acx; Fig. 1) do not show higher infestation rates by T. mucorea larvae (Fig. 4) and these plants are still able to produce OPDA, consistent with the hypothesis that OPDA functions as a direct defense in resisting T. mucorea attack.
Recent studies provide evidence that the mechanism of OPDA signaling is distinct from that involved in the perception of JA-derived signals such as JA-Ile (Taki et al., 2005; Thines et al., 2007). Much of our understanding of the role of JAs in plant-insect interactions has come from the analysis of mutants that fail to perceive JA/methyl JA (Devoto and Turner, 2005). In particular, mutants that are defective in the COI1 gene are impaired in all JA-signaling processes and are highly susceptible to a variety of herbivores (Reymond et al., 2000; Stintzi et al., 2001; Li et al., 2004; Chen et al., 2005; Mewis et al., 2005; Paschold et al., 2007; Zarate et al., 2007). N. attenuata ir-coi plants are also highly susceptible to T. mucorea larvae and almost 60% of all plants of this genotype had T. mucorea larvae in their stems in the 2009 field season. Moreover, the ir-coi pith was highly preferred to the pith of wild-type plants, which might be explained by the positive feedback loop between COI signaling and JA biosynthesis described in Paschold et al. (2007). Recent work with JA and OPDA has shown that both function as signaling molecules that elicit the expression of overlapping but distinct sets of genes; JA induces a set of COI1-dependent genes, while OPDA elicits a set of largely COI1-independent genes (Stintzi et al., 2001; Taki et al., 2005).
From this study, it is clear that we have underestimated the ability of dicots to defend their piths and that the defenses used differ subtly from those used to protect leaves.
MATERIALS AND METHODS
Plant Material and Growing Conditions
Wild-type Nicotiana attenuata Torr. Ex. Watson. plants originated from seeds collected on the Desert Inn ranch in Utah in 1988 (Baldwin, 1998). Seeds of wild-type and genetically transformed plants were sterilized and incubated in 0.1 m gibberellic acid (www.carl-roth.de) and 1:50 diluted liquid smoke (v/v; House of Herbs) before being germinated on Gamborg’s B5 medium (Duchefa) as described previously (Kruegel et al., 2002). The transformed plants have been fully characterized in previous work and the following lines were used: ir-coi1, which is unable to perceive JA (Paschold et al., 2007); as-lox3 silenced in 13-lox, ir-aoc, ir-opr3, and ir-acx (M. Kallenbach, G. Bonaventure, and I.T. Baldwin, unpublished data), which are silenced in various steps in JA biosynthesis (Halitschke and Baldwin, 2003); ir-pmt silenced in the putrescine N-methyltransferase genes, nicotine biosynthetic enzymes (Steppuhn et al., 2004); as-tpi silenced in TPI, TPIs production (Zavala and Baldwin, 2004); and as-hpl silenced in hydroperoxide lyase that is essential for C6-volatile production (Halitschke et al., 2004); ir-cad silenced in a key step in lignin production (H. Kaur, I. Galis, and I.T. Baldwin, unpublished data); and ir-ggpps, silenced in a key step in DTG production (Heiling et al., 2010).
Fifteen days after germination, seedlings were transferred into previously hydrated 50-mm peat pellets (Jiffy 703, http://www.jiffypot.com) and gradually adapted to the environmental conditions of high sun and low relative humidity of the Great Basin Desert habitat over 14 d by keeping the seedlings in the shade. Adapted size-matched seedlings were transplanted into an irrigated field plot at the Lytle Ranch Preserve. Seedlings were watered every other day until roots were established. The release of transgenic plants was carried out under Animal and Plant Health Inspection Service release 06-242-03r.
For experiments shown in Figures 6 and 7, seeds were germinated on Gamborg’s B5 at a 26°C/16 h 155 μm s−1 m−2 light: 24°C/8 h dark cycle (Percival). Plants were grown in the glasshouse with a day/night cycle of 16 (26°C–28°C)/8 (22°C–24°C) h under supplemental light from Master Sun-T PIA Agro 400 or Master Sun-T PIA Plus 600 W Na lights (Philips).
Plant Treatments
For experiments presented in Figure 4, plants of all genotypes were allowed to elongate to provide possibilities for egg deposition by adult Trichobaris mucorea weevils. At the end of the field season when all plants were in the late-flowering stage of growth, the plants were dug up and all branches were slit lengthwise and T. mucorea larvae were counted. All lines tested were randomized across the field plot and fully interspersed among EV/wild-type plants as controls.
Data shown in Figure 6 were obtained by treating plants grown in the glasshouse. To simulate the damage from T. mucorea larvae feeding, stems were mechanically wounded with an insect pin (1 mm in diameter) to produce nine puncture wounds along a 10-cm section of the stem starting from the base of the stem just above the attachment of the rosette leaves. These puncture wounds were immediately filled with 20 μL of Manduca sexta OS (diluted 1:1 with water) or with water only with a syringe. Control plants remained untreated. Pith was excavated from stems with a razor blade.
Pith Choice Assays
To experimentally determine the preferences of T. mucorea larvae among the different piths of the different genotypes, the stems of the different genotypes shown in Figure 5 were slit lengthwise and combined with size-matched mirror image halves of stems of wild-type plants (see Supplemental Fig. S1 for the experimental setup). One-third to one-fourth instar larva was placed into an approximate 1-mm3 crevice excavated into the pith on both halves of the stem sandwich. This paired design with wild-type piths was chosen to allow for the use larvae of different instars. The two halves were wrapped together with parafilm (www.parafilm.com). Stems were kept hydrated and held in a vertical position by placing them on wet tissues inside of 500 mL polystyrene clear food boxes. After 24 h, the parafilm wrapping was removed and both stem halves were inspected for feeding damage. Damage was scored as a binary decision, if the larvae had made a clear pith feeding choice after 24 h or as a no choice if both piths were fed upon. The results of trials with larvae that did not feed within 24 h were not included in the analysis.
Protein Measurement
PI activities were analyzed via the radial diffusion assay described by Jongsma et al. (1993) using bovine trypsin (Sigma) dissolved in agar. For PI activities different solutions of increasing solutions of soybean (Glycine max) trypsin inhibitor (Boehringer Mannheim) were used to obtain a reference curve. Therefore protein from N. attenuata wild-type pith and leaves and ir-pi pith was extracted. In short, approximately 150 mg of the plant material was crushed in liquid N2, and 300 μL of protein extraction buffer (Jongsma et al., 1993) was for the tissue. After vortexing and centrifugation at 4°C for 20 min at 12,000g the clear supernatants were transferred to fresh Eppendorf tubes and kept on ice until analysis. Protein concentration was determined by the Bradford method (Bradford, 1976) with bovine serum albumin (Sigma) as a standard.
DTG and Nicotine Measurements
DTGs were measured as described in Gaquerel et al. (2010), but instead of leaf material, 100 mg of fresh pith material was ground in liquid nitrogen and DTGs were extracted by homogenizing in FastPrep tubes containing 900 mg of lysing matrix (BIO 101; Vista) and 1 mL extracting buffer (50 mm acetate buffer, pH 4.8, containing 40% methanol). Samples were homogenized twice by shaking at 6.5 ms−1 for 45 s (FastPrep FP 120; Thermo Savant). Homogenized samples were centrifuged at 16,000g for 20 min at 4°C. Supernatant was centrifuged a second time for 20 min to remove any remaining particles. DTG measurements were conducted on a HPLC/electrospray ionization-time of flight-mass spectrometer (Agilent) using a Dionex Acclaim 2.2 μm 120A 2.1 × 150 mm column (Dionex) with an injection volume of 4 μL. Mixtures of two solvents were used to elute analytes from the column: A (Millipore water, 0.05% formic acid, and 0.01% acetonitrile) and B (acetonitrile, 0.05% formic acid). HPLC-grade acetonitrile was purchased from Malinckrodt Baker (www.mallbaker.com), formic acid from Fluka (www.sigmaaldrich.com), and ultrapure water was obtained from a Millipore model Milli-Q Advantage A10. The following binary gradient was applied: 0 to 0.5 min isocratic 95% A, 5% B; 0.5 to 6.5 min linear gradient to 80% B; isocratic for 3.5 min. The flow rate was 300 μL min−1. Eluted compounds were detected by a MicroToF mass spectrometer (Bruker Daltonik) equipped with an electrospray ionization source in positive ion mode. Typical instrument settings were as follows: capillary voltage, 4,500 V; capillary exit, 130 V; dry gas temperature, 200°C; dry gas flow, 10 L min−1. Ions were detected from mass-to-charge ratio (m/z) 200 to 1,400 at a repetition rate of 2 Hz. Mass calibration was performed using sodium formate clusters (10 mm solution of NaOH in 50%/50% v/v isopropanol/water containing 0.2% formic acid). Quantification was performed using Bruker QuanAnalysis software (Bruker Daltonik). Extracted ions used for the quantification of all DTGS present in N. attenuata where m/z = 271.24 ± 0.01 at 5.5 ± 2 min, which is as a positive fragment ion common to all of the DTGs (Heiling et al., 2010).
Nicotine was analyzed as described for DTG measurements. Ions were detected from m/z of 163.123 ± 0.01 at 2.1 ± 0.2 min in positive mode.
Pith and Leaf Dry:Fresh Mass Ratios
Mass of fresh and dry plant material was obtained by excising approximately 100 mg of S1 leaves from 10 individual N. attenuata wild-type plants and placing them individually into Eppendorf tubes. Approximately 100 mg of excised pith, obtained from 10-cm basal stem segments were also placed individually into Eppendorf tubes. Leaf and pith samples were dried in a drying oven for 6 d at 80°C. Masses were determined before and after drying to calculate water loss.
Statistical Analysis
Data presented in Figure 6 and Supplemental Figure S4 were analyzed with Statview 5.0 (SAS Institute). Data were transformed if they did not meet the assumption of homoschedacity. All other statistical analyses were performed using the publically available software R (R Development Core45) and the libraries therein (http://www.r-project.org/).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Experimental setup for T. mucorea pith choice assay.
Supplemental Figure S2. PI activity in N. attenuata leaves and pith.
Supplemental Figure S3. Dry mass:fresh mass of leaves and pith of N. attenuata.
Supplemental Figure S4. Diterpene aglycone levels in pith of GGPPS plants are lower than in pith of wild-type N. attenuata plants.
Supplemental Figure S5. Planting times and average infestation percentages of T. mucorea in N. attenuata wild-type plants.
Acknowledgments
We thank Brigham Young University for use of their field station, the Lytle Ranch Preserve, the Animal and Plant Health Inspection Service for constructive regulatory oversight, and Dr. Matthias Schöttner for invaluable technical assistance.
References
- Allmann S, Halitschke R, Schuurink RC, Baldwin IT. (2010) Oxylipin channelling in Nicotiana attenuata: lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ 33: 2028–2040 [DOI] [PubMed] [Google Scholar]
- Anderson RS. (1998) Assessment of species diversity in the Montane Cordillera Ecozone. Smith IM, Scudder GGE, , Weevils (Curculionoidea). Ecological Monitoring and Assessment Network, Burlington, ON, Canada [Google Scholar]
- Arnett RH, Thomas MC, Skelley PE. (2002) Polyphaga: Scarabaeoidea through Curculionoidea, Vol 2 CRC Press, Boca Raton, FL [Google Scholar]
- Baker WA, Bradley WG, Clark OA. (1949) Biological Control of the European Corn Borer. Tech. Bull. U.S. Dep. Agric., Washington, DC, pp 185 [Google Scholar]
- Baldwin IT. (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95: 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber HS. (1935) The Tobacco and Solanum Weevils of the Genus Trichobaris. Misc. Pub. U.S. Dep. Agric., Washington, DC, pp 21–28 [Google Scholar]
- Berenbaum MR, Zangerl AR, Nitao JK. (1986) Constraints in chemical coevolution: wild parsnip and the parsnip webworm. Evolution 6: 1215–1228 [DOI] [PubMed] [Google Scholar]
- Bezzi S, Kessler D, Diezel C, Muck A, Anssour S, Baldwin IT. (2010) Silencing NaTPI expression increases nectar germin, nectarins, and hydrogen peroxide levels and inhibits nectar removal from plants in nature. Plant Physiol 152: 2232–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Charlet LD, Aiken RM, Miller JF, Seiler GJ. (2009) Resistance among cultivated sunflower germplasm to stem-infesting pests in the central Great Plains. J Econ Entomol 102: 1281–1290 [DOI] [PubMed] [Google Scholar]
- Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA. (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc Natl Acad Sci USA 102: 19237–19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong MF. (1996) What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Funct Ecol 10: 668–674 [Google Scholar]
- Cook SP, Hain FP. (1988) Toxicity of host monoterpenes to Dendroctonus frontalis and Ips calligraphus (Coleoptera, Scolytidae). J Entomol Sci 23: 287–292 [Google Scholar]
- Creelman RA, Mullet JE. (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355–381 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH. (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 577–580 [DOI] [PubMed] [Google Scholar]
- Devoto A, Turner JG. (2005) Jasmonate-regulated Arabidopsis stress signalling network. Physiol Plant 123: 161–172 [Google Scholar]
- Dicke M. (1999) Evolution of induced indirect defense of plants. Tollrian R, Harvell CJ, , The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, NJ, pp 62–88 [Google Scholar]
- Dicke M, Sabelis MW. (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38: 148–165 [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA. (1990) Plant strategies of manipulating predator-prey interactions through allelochemicals—prospects for application in pest-control. J Chem Ecol 16: 3091–3118 [DOI] [PubMed] [Google Scholar]
- Ding D, Swedenborg PD, Jones RL. (1989) Plant odor preferences and learning in Macrocentrus grandii (Hymenoptera, Braconidae), a larval parasitoid of the european corn-borer, Ostrinia nubilalis (Lepidoptera, Pyralidae). J Kans Entomol Soc 62: 164–176 [Google Scholar]
- Feeny P. (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51: 565–581 [Google Scholar]
- Feeny P. (1976) Plant apparency and chemical defense. Recent Adv. Phytochem. 10: 1–40 [Google Scholar]
- Fordyce JA, Malcolm SB. (2000) Specialist weevil, Rhyssomatus lineaticollis, does not spatially avoid cardenolide defenses of common milkweed by ovipositing into pith tissue. J Chem Ecol 26: 2857–2874 [Google Scholar]
- Freude H, Harde KW. (1999) Rhynchophora I (Bruchidae–Curculionidae I), Vol 10 Spektrum Akademischer Verlag, Heidelberg, Germany [Google Scholar]
- Gaquerel E, Heiling S, Schoettner M, Zurek G, Baldwin IT. (2010) Development and validation of a liquid chromatography-electrospray ionization-time-of-flight mass spectrometry method for induced changes in Nicotiana attenuata leaves during simulated herbivory. J Agric Food Chem 58: 9418–9427 [DOI] [PubMed] [Google Scholar]
- Glawe GA, Zavala JA, Kessler A, Van Dam NM, Baldwin IT. (2003) Ecological costs and benefits correlated with trypsin protease inhibitor production in Nicotiana attenuata. Ecology 84: 79–90 [Google Scholar]
- Halitschke R, Baldwin IT. (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36: 794–807 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT. (2000) Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124: 408–417 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Ziegler J, Keinänen M, Baldwin IT. (2004) Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant J 40: 35–46 [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8: 157–178 [Google Scholar]
- Haukioja E, Koricheva J. (2000) Tolerance to herbivory in woody vs. herbaceous plants. Evol Ecol 14: 551–562 [Google Scholar]
- Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT. (2010) Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell 22: 273–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Patankar AG, Zavala JA, Wu JQ, Dolecková-Maresová L, Vujtechová M, Mares M, Baldwin IT. (2005) Differential elicitation of two processing proteases controls the processing pattern of the trypsin proteinase inhibitor precursor in Nicotiana attenuata. Plant Physiol 139: 375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. (2007) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact 20: 510–525 [DOI] [PubMed] [Google Scholar]
- Johnson MTJ, Smith SD, Rausher MD. (2009) Plant sex and the evolution of plant defenses against herbivores. Proc Natl Acad Sci USA 106: 18079–18084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Hopper RF, Coleman JS, Krischik VA. (1993) Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93: 452–456 [DOI] [PubMed] [Google Scholar]
- Jongsma MA, Bakker PL, Stiekema WJ. (1993) Quantitative determination of serine proteinase inhibitor activity using a radial diffusion assay. Anal Biochem 212: 79–84 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago [Google Scholar]
- Kessler A, Baldwin IT. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305: 665–668 [DOI] [PubMed] [Google Scholar]
- Kiedrowski S, Kawalleck P, Hahlbrock K, Somssich IE, Dangl JL. (1992) Rapid activation of a novel plant defense gene is strictly dependent on the Arabidopsis RPM1 disease resistance locus. EMBO J 11: 4677–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W. (1999) Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol 121: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiwa H, Bressan RA, Hasegawa PM. (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2: 379–384 [Google Scholar]
- Kruegel T, Lim M, Gase K, Halitschke R, Baldwin IT. (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12: 177–183 [Google Scholar]
- Kuschel G. (1995) A phylogenetic classification of Curculionoidea to families and subfamilies: memoirs of the entomological society of Washington; biology and phylogeny of Curculionoidea. 14: 5–33 [Google Scholar]
- Lao M, Arencibia AD, Carmona ER, Acevedo R, Rodríguez E, León O, Santana I. (2008) Differential expression analysis by cDNA-AFLP of Saccharum spp. after inoculation with the host pathogen Sporisorium scitamineum. Plant Cell Rep 27: 1103–1111 [DOI] [PubMed] [Google Scholar]
- Lavee S, Galston AW. (1968) Structural physiological, and biochemical gradients in tobacco pith tissue. Plant Physiol 43: 1760–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao YF, McCaig BC, Wingerd BA, Wang JH, Whalon ME, Pichersky E, Howe GA. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren BS, Nordlander G, Birgersson G. (1996) Feeding deterrence of verbenone to the pine weevil, Hylobius abietis (L) (Col, Curculionidae). Journal of Applied Entomology-Zeitschrift Fur Angewandte Entomologie 120: 397–403 [Google Scholar]
- Ma RLZ, Swedenborg PD, Jones RL. (1992) Host-seeking behavior of Eriborus terebrans (Hymenoptera, Ichneumondidae) toward the european corn borer and the role of chemical stimuli. Ann Entomol Soc Am 85: 72–79 [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. (2006) Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. J Anim Ecol 75: 595–603 [DOI] [PubMed] [Google Scholar]
- Mc Key D. (1979) The distribution of secondary compounds within plants. Rosenthal GA, Janzen DH, , Herbivores: Their Interaction with Secondary Plant Metabolites, Ed 1 Academic Press, New York, pp 56–133 [Google Scholar]
- Meldau S, Wu JQ, Baldwin IT. (2009) Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol 181: 161–173 [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. (2005) Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitao JK, Zangerl AR. (1987) Floral development and chemical defense allocation in wild parsnip (Pastinaca-Sativa). Ecology 68: 521–529 [Google Scholar]
- Nordlander G. (1990) Limonene inhibits attraction to alpha-pinene in the pine weevils Hylobius abietis and H. pinastri. J Chem Ecol 16: 1307–1320 [DOI] [PubMed] [Google Scholar]
- O'Brien CW, Wibmer GJ. (1982) Annotated checklist of the weevils (Curculionidae sensu lato) of North America, Central America and the West-Indies Coleoptera Curculionoidea. Mem Am Entomol Inst 34: 1–10 [Google Scholar]
- Ordás B, Butrón A, Soengas P, Ordás A, Malvar RA. (2002) Antibiosis of the pith maize to Sesamia nonagrioides (Lepidoptera: Noctuidae). J Econ Entomol 95: 1044–1048 [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51: 79–91 [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Sanson G, Read J. (2007) Leaf biomechanical properties and the densities of herbivorous insect guilds. Funct Ecol 21: 246–255 [Google Scholar]
- Potting RPJ, Vet LEM, Dicke M. (1995) Host microhabitat location by stem-borer parasitoid Cotesia flavipes—the role of herbivore volatiles and locally and systemically induced plant volatiles. J Chem Ecol 21: 525–539 [DOI] [PubMed] [Google Scholar]
- Raffa KF, Smalley EB. (1995) Interaction of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle fungal complexes. Oecologia 102: 285–295 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732–737 [DOI] [PubMed] [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. (2004) A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DF. (1979) Evolution of plant chemical defense against herbivores. Rosenthal GA, Janzen DH, , Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York, pp 3–54 [Google Scholar]
- Rhoades DF, Cates RG. (1976) Toward a general theory of plant antiherbivore chemistry. Recent Adv Phytochem. 10: 168–213 [Google Scholar]
- Roth JP, King EG, Hensley SD. (1982) Plant, host, and parasite interactions in the host selection sequence of the Tachinid Lixophaga diatraeae (Diptera, Tachinidae). Environ Entomol 11: 273–277 [Google Scholar]
- Schaller F, Schaller A, Stintzi A. (2004) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23: 179–199 [Google Scholar]
- Smith JM, Wiedenmann RN, Overholt WA. (1993) Parasites of Lepidopteran Stemborers of Tropical Graminaceous Plants. ICIPE Sciences Press, Nairobi, Kenya, p 89 [Google Scholar]
- Soengas P, Butrón A, Revilla P, Ordás A, Malvar RA. (2004) Performance of crosses among flint maize populations under infestation by Sesamia nonagrioides (Lepidoptera: Noctuidae). J Econ Entomol 97: 1438–1443 [DOI] [PubMed] [Google Scholar]
- Steidle JLM, Fischer A, Gantert C. (2005) Do grains whisper for help? Evidence for herbivore-induced synomones in wheat grains. Entomol Exp Appl 115: 239–245 [Google Scholar]
- Steppuhn A, Baldwin IT. (2007) Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett 10: 499–511 [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. (2004) Nicotine’s defensive function in nature. PLoS Biol 2: E217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Schuman MC, Baldwin IT. (2008) Silencing jasmonate signalling and jasmonate-mediated defences reveals different survival strategies between two Nicotiana attenuata accessions. Mol Ecol 17: 3717–3732 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DRJ, Lawton JH, Southwood TRE. (1984) Insects on Plants: Community Patterns and Mechanisms. Blackwell Scientific Publications, Oxford [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al. (2005) 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139: 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo MC, Rufat M, Bravo JM, San Segundo B. (2000) Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of Spodoptera littoralis larvae. Planta 211: 62–71 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J. (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Thompson RT. (1992) Observations on the morphology and classification of weevils (Coleoptera, Curculionoidea) with a key to major groups. J Nat Hist 26: 835–891 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Hadwich K, Baldwin IT. (2000) Induced responses in Nicotiana attenuata affect behavior and growth of the specialist herbivore Manduca sexta. Oecologia 122: 371–379 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT. (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27: 547–568 [DOI] [PubMed] [Google Scholar]
- van Tol R, van der Sommen ATC, Boff MIC, van Bezooijen J, Sabelis MW, Smits PH. (2001) Plants protect their roots by alerting the enemies of grubs. Ecol Lett 4: 292–294 [Google Scholar]
- Vancanneyt G, Sanz C, Farmaki T, Paneque M, Ortego F, Castañera P, Sánchez-Serrano JJ. (2001) Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc Natl Acad Sci USA 98: 8139–8144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling LL. (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction, and action in plant stress response, growth and development. Ann Bot (Lond) 100: 691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Arens D, Grosch W. (1973) Identifizierung von lipoxigenase-isoenzymen als carotinoxidasen. Z Lebensm Unters Forsch 152: 152–154 [Google Scholar]
- Werner RA. (1995) Toxicity and repellency of 4-allylanisole and monoterpenes from white spruce and tamarack to the spruce beetle and eastern larch beetle (Coleoptera, Scolytidae). Environ Entomol 24: 372–379 [Google Scholar]
- Wild CH, McFadyen RE, Tomley AJ, Willson BW. (1992) The biology and host specificity of the stem boring weevil Listronotus setosipennis (Coleoptera, Curculionidae) a potential biocontrol agent for Parthenium hysterophorus (Asteracea). Entomophaga 37: 591–598 [Google Scholar]
- Woodside AM. (1949) The tobacco stalk borer in Western Mexico. J Econ Entomol 42: 63–67 [Google Scholar]
- Zabala G, Zou JJ, Tuteja J, Gonzalez DO, Clough SJ, Vodkin LO. (2006) Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Bazzaz FA. (1992) Theory and pattern in plant defense allocation. Fritz RS, Simms EL, , Plant Resistance of Herbivores and Pathogens. Ecology, Evolution, and Genetics. The University of Chicago Press, Chicago, pp 363–391 [Google Scholar]
- Zangerl AR, Nitao JK. (1998) Optimal defence, kin conflict and the distribution of furanocoumarins among offspring of wild parsnip. Evol Ecol 12: 443–457 [Google Scholar]
- Zarate SI, Kempema LA, Walling LL. (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. (2004) Fitness benefits of trypsin proteinase inhibitor expression in Nicotiana attenuata are greater than their costs when plants are attacked. BMC Ecol 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Giri AP, Jongsma MA, Baldwin IT. (2008) Digestive duet: midgut digestive proteinases of Manduca sexta ingesting Nicotiana attenuata with manipulated trypsin proteinase inhibitor expression. PLoS ONE 3: e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Qi J, Ren N, Cheng J, Erb M, Mao B, Lou Y. (2009) Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J 60: 638–648 [DOI] [PubMed] [Google Scholar]