Abstract
The cell wall (CW) has been recognized as the major target of aluminum (Al) toxicity. However, the components responsible for Al accumulation and the mechanisms of Al-induced CW function disruption are still elusive. The contribution of different CW components (pectin, hemicellulose 1 [HC1], and HC2) to adsorb Al and the effect of Al on xyloglucan endotransglucosylase/hydrolyase activity were investigated in Arabidopsis (Arabidopsis thaliana) in this study. A fractionation procedure was optimized to effectively extract different CW components, especially to prevent the HC fraction from pectin contamination. When CW materials extracted from Al-treated roots (50 μm Al for 24 h) were fractionated, about 75% of CW Al accumulated in the HC1 fraction. A time-dependent kinetic study showed that only when the HC1 fraction was removed was the amount of Al adsorbed decreased sharply. In vivo localization of xyloglucan endotransglucosylase (XET) activity showed that Al greatly inhibited this enzyme activity within 30 min of exposure, which was concomitant with Al-induced callose deposition in roots. Results from real-time reverse transcription-polymerase chain reaction indicated that three genes may constitute the major contributors to XET activity and that the inhibition of XET activity by Al is caused by transcriptional regulation. These results, to our knowledge for the first time, demonstrate that HC is the major pool for Al accumulation. Furthermore, Al-induced reduction in XET activity could play an important role in Al-induced root growth inhibition.
Aluminum (Al) is the most abundant metal and the third most abundant chemical element in the earth’s crust. When soil pH drops below 5, toxic forms of Al release into soil solution and become a limiting factor of crop growth and yield. It has been estimated that acid soils constitute approximately 50% of the world’s potentially arable land (von Uexküll and Mutert, 1995). Therefore, Al toxicity is the most serious abiotic stress to crop production after drought (Kochian, 1995). The earliest and most dramatic visual symptom of Al toxicity is the inhibition of root elongation. Although it has been shown that Al can alter a series of physiological and biochemical processes, cytoskeleton dynamics disruption, and Ca2+-dependent signal transduction pathway distortion, the primary cause of Al-induced root growth inhibition is still elusive (Kochian, 1995; Matsumoto, 2000; Rengel and Zhang, 2003; Zheng and Yang, 2005).
The cell wall (CW) plays principal roles not only in the regulation of growth and development of plants but also in the perception and expression of Al toxicity. When plant roots are exposed to Al, CW is the first site in contact with Al. Many reports have demonstrated consistently that CW is the major pool of Al accumulation. For example, Clarkson (1967) reported that 85% to 90% of the total Al accumulated by barley (Hordeum vulgare) roots was tightly bound to CWs. Up to 99.9% of the total cellular Al accumulated in the CW of giant algal cells of Chara corallina (Taylor et al., 2000). Furthermore, CW pectin has been implicated as the major Al-binding site (Horst, 1995; Chang et al., 1999). On the other hand, several studies have reported that CW hemicellulose (HC) metabolism is more susceptible to Al stress. For example, Tabuchi and Matsumoto (2001) found that exposure of Al-sensitive wheat (Triticum aestivum ‘Scout 66’) to 10 μm Al for 6 h resulted in the accumulation of HC but not pectin and cellulose. In rice (Oryza sativa), the most significant change of the CW component is also in the HC fraction (Yang et al., 2008). These results suggest that Al very likely targets multiple sites simultaneously, and the major binding component is still not clear. Therefore, comprehensive study to elucidate which part contributes more significantly to Al accumulation than others is urgently needed.
In addition to the accumulation of Al, the properties of the CW have been reported to be affected by Al. The most prominent effect is the decrease of CW extensibility. For example, exposure of wheat roots to Al could decrease the extensibility of CW within 3 h (Ma et al., 2004). However, the biochemistry and molecular basis of Al-induced CW extensibility reduction is unknown. Theoretically, CW extension requires modification of the xyloglucan-cellulose network. Although this process is regulated by a battery of modifying enzymes that collectively provide a mechanism for regulating cell expansion, xyloglucan-metabolizing enzymes, mainly xyloglucan endotransglucosylase/hydrolases (XTHs), are believed to be the important agents for controlling CW strength and extensibility (Rose et al., 2002). To date, the effects of Al on the action of XTHs have not been examined.
In this study, we examined the effect of Al on root growth, CW polysaccharide, Al distribution within the CW, and the ability of different CW components to absorb Al. In addition, we analyzed the effect of Al on XTH activity and XTH gene expression.
RESULTS
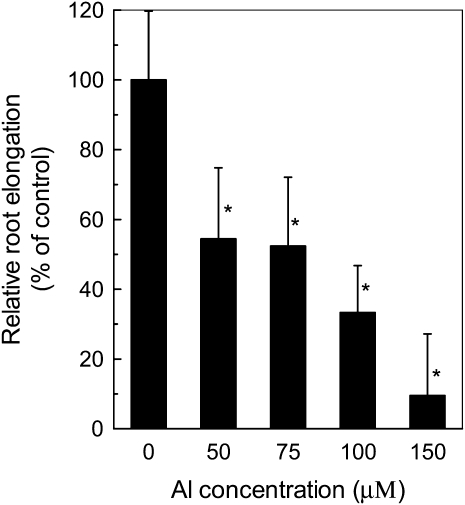
Arabidopsis (Arabidopsis thaliana) seedlings cultivated for 24 h on agar plates with different concentrations of Al exhibited a concentration-dependent inhibition of root growth (Fig. 1). For example, Al inhibited root elongation by about 46% at 50 μm Al. The inhibition was more evident at 100 and 150 μm Al, at which the root elongation was reduced to 33% and 9% of the control, respectively. Therefore, we chose Al concentration at 50 μm for the following experiment.
Figure 1.
The effect of Al on root growth of Arabidopsis. Plants were grown on plates containing 0 or 50 μm Al for 24 h. Root elongation was measured before and after treatment. Data are means ± sd (n = 10). * Significant difference at P < 0.05.
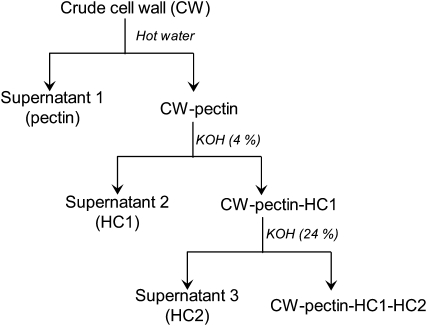
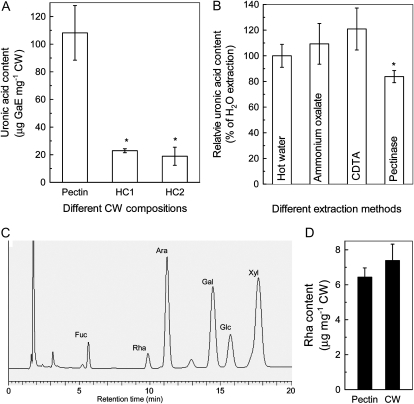
Accumulating evidence indicates that root apoplast plays a critical role in the expression of Al toxicity and/or resistance (Horst et al., 2010). In order to clarify the possible involvement of different CW components in the contribution to Al-induced inhibition of root elongation, a fractionation procedure was adopted to separate the major CW components into pectin, HC1, and HC2 (Fig. 2). To ensure that each component was purely fractionated, we first measured uronic acid content in each fraction. The percentage of uronic acids in pectin, HC1, and HC2 fractions are 72%, 15%, and 13%, respectively (Fig. 3A). Second, we compared the pectin contents in different extraction solutions, including hot water, ammonium oxalate buffer, cyclohexane diamine tetraacetic acid (CDTA), or pectinase, which were frequently reported to isolate pectins from CW materials. The extraction efficiency was similar among hot water, ammonium oxalate buffer, and CDTA, but a slightly lower efficiency was found in pectinase (Fig. 3B). Third, it seems that the monosaccharide Rha is specifically present in pectin domains rhamnogalacturonan (RG)-I and RG-II. Therefore, the ratio of Rha content in the pectin fraction to that of total CW could be another suitable criterion for the extraction efficiency. The high-performance anion-exchange pulsed-amperometric detection method was used to analyze monosaccharide content (Fig. 3C). The amount of Rha in the pectin fraction could account for almost all of the Rha content in the CW (Fig. 3D). Taken together, these results confirmed that the fractionation procedure used in this study is adequate and that the pectin component remaining after hot water extraction should be at least negligible.
Figure 2.
Flow chart for CW component fractionation from isolated CWs. “CW-pectin” represents CW without pectin, and other terms are analogous.
Figure 3.
Purity verification of different CW components. A, Uronic acid content in different CW components. Plants were grown on plates containing 0 or 50 μm Al for 24 h, and CW materials were extracted from roots and fractionated into different components (for details, see Fig. 2 and “Materials and Methods”). B, Uronic acid content in the pectin fraction extracted by different methods. C, High-performance anion-exchange pulsed-amperometric detection profile of monosaccharides in the pectin fraction. D, Rha content in the pectin fraction and CW materials. Data are means ± sd (n = 3). * Significant difference at P < 0.05.
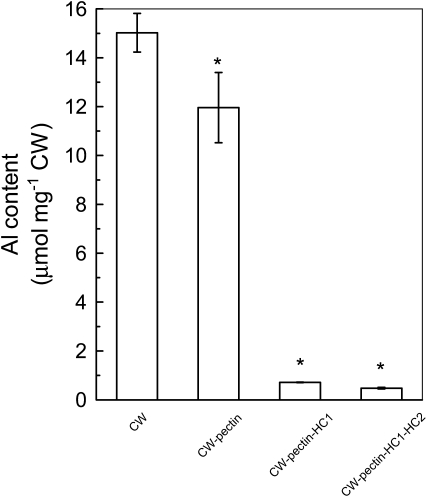
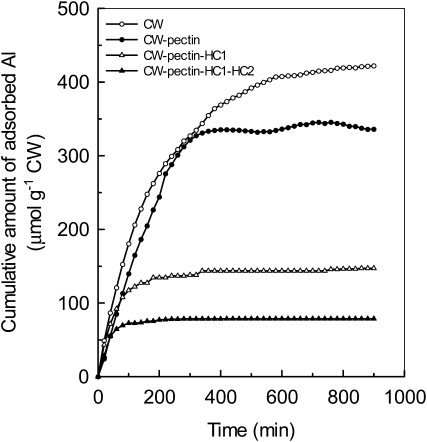
We then measured the ability of different CW components to accumulate Al. First, CW materials were extracted after the roots were treated by Al for 24 h, and Al content in each fraction was determined after fractionation of CW components (Fig. 2). In this experiment, hot water is preferred to ammonium oxalate buffer because of the chelation of Al by oxalate. As shown in Figure 4, about 20% of Al that accumulated in the CW resided in the pectin fraction. However, about 75% of total CW Al was held by the HC1 fraction. Second, a time-dependent kinetic study of Al adsorption was conducted to verify the ability of different CW components to adsorb Al. CW materials were extracted from control roots, and each residue after fractionation was subjected to kinetic studies. As shown in Figure 5, adsorbed Al in pectin, HC1, and HC2 fractions represent 20%, 45%, and 16% of total CW Al, respectively. These results indicated that the HC1 fraction has a greater ability to accumulate Al.
Figure 4.
Al content in different CW components. Plants were grown on plates containing 50 μm Al for 24 h, and CW materials were extracted from roots and fractionated into different residues (for details, see Fig. 2 and “Materials and Methods”). After extraction of Al by 2 m HCl, Al content was determined by inductively coupled plasma-atomic emission spectrometry. Data are means ± sd (n = 3). * Significant difference at P < 0.05.
Figure 5.
Al adsorption kinetics of different CW components. CW materials were extracted from Arabidopsis roots without Al treatment and fractionated into different residues (for details, see Fig. 2 and “Materials and Methods”). Materials were placed into a 2-mL column, and kinetics was determined as described previously (Zheng et al., 2004).
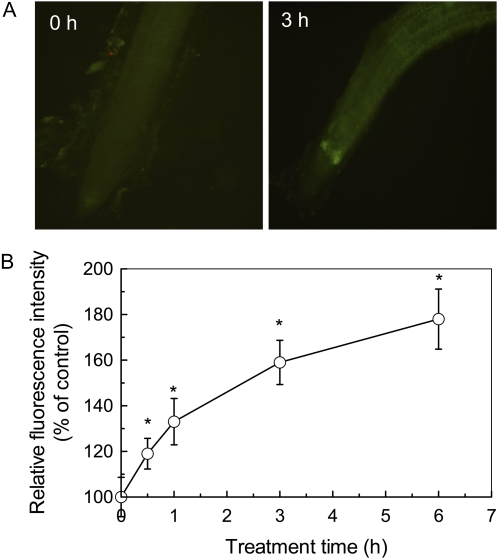
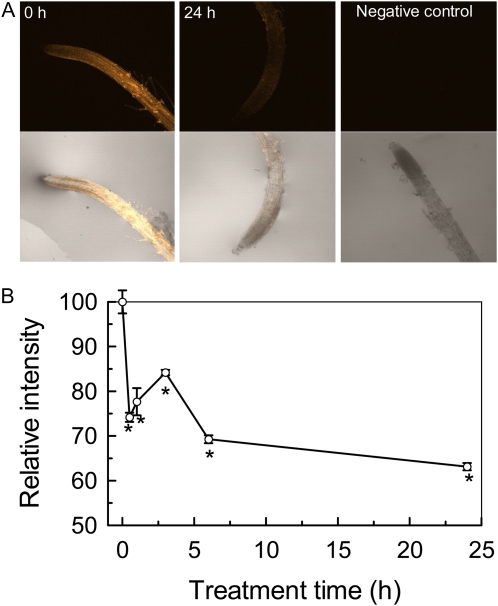
Al rapidly inhibits root elongation by means of interfering with a number of physiological and biochemical processes. It has been proposed that cell expansion requires at least partly xyloglucan endotransglucosylase (XET) or hydrolase (XEH) activities (Rose et al., 2002; Cosgrove, 2005). At present, it is still a challenge to determine whether a member of the XTHs has XET activity or XEH activity. However, the activity of XET can be visualized with the use of fluorescently labeled xyloglucan oligosaccharides (XGO-SRs; Vissenberg et al., 2000, 2003). In this study, we examined the effect of Al on the activity of XET using this method. Because Al exerts its toxic effects rapidly, typically within hours or even minutes of exposure, a time-course experiment was carried out to get detailed information on the relationship between Al toxicity and changes of XET activity. Thus, a more sensitive marker based on the deposition of callose was used to indicate Al-induced apoplastic lesions. As shown in Figure 6A, the deposition of callose was significantly induced by Al treatment. Furthermore, the deposition of callose occurred 30 min after exposure to 50 μm Al (Fig. 6B). As far as the XET activities are concerned, the root segment behind the apex showed bright orange fluorescence in the absence of Al, and the root apex showed very faint fluorescence (Fig. 7A). After 30 min of treatment with 50 μm Al, the fluorescence was greatly decreased, with a further decrease after 24 h of treatment (Fig. 7B). Thus, Al-induced inhibition of XET activity and apoplastic lesions was paralleled. Control roots showed no autofluorescence in response to green light excitation after incubation in MES buffer that lacked XGO-SRs (Fig. 7A).
Figure 6.
The effect of Al on relative fluorescence intensity of callose accumulation in Arabidopsis. A, Images of callose deposition shown in representative roots after 0 or 3 h of treatment with 50 μm Al. B, Time-course effect of Al stress on callose deposition expressed as relative fluorescence. Data are means ± sd (n = 5–7). * Significant difference at P < 0.05.
Figure 7.
Effect of Al on XET activity. Plants were grown on plates containing 50 μm Al for different times. Roots were subjected to cytochemical assays of XET activity (for details, see “Materials and Methods”). A, Images of XET activity shown as orange fluorescence in representative roots after 0 or 24 h of treatment with 50 μm Al and a negative control assay without xyloglucans. The top and bottom rows represent fluorescence images and merged images, respectively. B, Time course of XET activity changes expressed as relative fluorescence in response to Al stress. Data are means ± sd (n = 7). * Significant difference at P < 0.05.
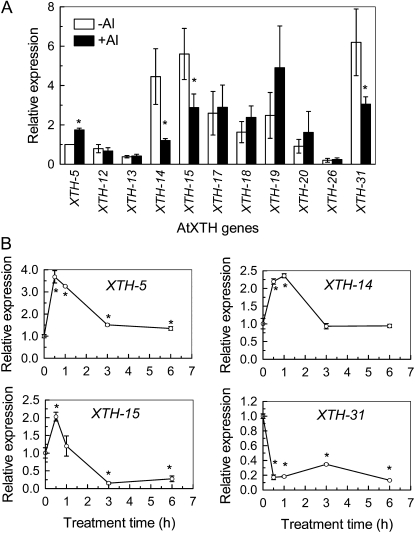
To examine whether transcriptional regulation is involved in the reduction of XET activity during Al stress, 11 out of 33 XTH genes were selected for real-time reverse transcription (RT)-PCR analysis. The selected members of XTH genes were reported to have relatively high expression in roots (Yokoyama and Nishitani, 2001; Becnel et al., 2006). Under normal growth conditions, XTH-14, -15, and -31 showed strongest expression in roots, followed by XTH-17, -18, and -19. However, the other selected genes were relatively weakly expressed in roots. The expression levels of XTH-5, -18, -19, and -20 were slightly increased by 24-h Al treatment, but only XTH-5 expression showed statistically significant up-regulation, whereas the expressions of XTH-14, -15, and -31 were significantly decreased. The expression levels of the other genes were not changed in response to Al stress (Fig. 8A). Thus, XTH-5, -14, -15, and -31 were selected to examine the effect of Al on their expression based on the timing of exposure. Interestingly, only XTH-31 was constitutively repressed by Al treatment; the others showed a transient increase by 1 h of exposure of 50 μm Al and decreased thereafter (Fig. 8B). It appears that within 1 h of treatment, transcriptional regulation of XTH-31 played an exclusive role in the expression of XET activity in response to Al stress, while after longer treatment times (e.g. 24 h of exposure), expressions of XTH-14, -15, and -31 jointly controlled XET activities.
Figure 8.
Effect of Al stress on XTH gene expression. Plants were grown on plates containing 0 or 50 μm Al either for relative long-term treatment (24 h; A) or for a short-term treatment as indicated (B). Total RNAs were extracted from roots and subjected to RT reactions followed by real-time PCR. A, Expression levels with or without Al treatment were compared with the expression level of XTH-5 at the normal condition (−Al), which was assigned an expression level of 1. B, The expression levels of controls (0 h) were used as a calibrator set to 1. Data are means of three independent biological replicates. * Significant difference at P < 0.05.
DISCUSSION
It is still a mater of debate whether the primary target of Al toxicity is apoplastic or symplastic (Horst, 1995; Kochian, 1995). Because Al has a very strong binding affinity for the oxygen donor compounds (Kochian, 1995), it is very likely that Al may target multiple sites simultaneously, which collectively provide a mechanism for root growth inhibition. Considering that the CW is the main site for Al accumulation, it is easy to envision that the CW is the major target site of Al toxicity, especially when the exposure period is short and the concentration of Al is low. In fact, there is ample experimental evidence that Al-induced root growth inhibition is associated with the disruption of CW functions (Horst et al., 2010). For example, Al treatment altered CW polysaccharide metabolism in a number of plant species, such as squash (Cucurbita pepo; Le Van et al., 1994), wheat (Tabuchi and Mastsumoto, 2001), and rice (Yang et al., 2008). Li et al. (2009) reported that disorganization of homogalacturonan is a mechanism for Al-induced root growth inhibition in maize (Zea mays). However, the underlying bases of root growth inhibition in response to Al stress are far from clear. Therefore, although pectin has long been recognized as the main component for binding of Al, a detailed comprehensive comparison has not been performed so far. Here, we provide, to our knowledge, the first evidence that HC contributes the most to Al accumulation in the CW and may play a significant role in Al-induced root growth inhibition in Arabidopsis. Our conclusion is based on the following evidence.
First, HC1 is the major component for Al accumulation. In order to examine which fraction is the major site for Al accumulation, CW was technically fractionated into pectin, HC1, and HC2 fractions and their corresponding residues (Fig. 2). This fractionation method has been frequently used for CW component analysis in a number of plant species (Zhong and Lauchli, 1993; Yang et al., 2008). A time-dependent Al adsorption kinetics was adopted to investigate the contribution of different components to Al adsorption. As shown in Figure 5, the HC1 fraction contributes most significantly to Al accumulation, followed by pectin and the HC2 fraction. A direct measurement of Al content in different CW components also revealed that HC1 is the major pool for Al accumulation (Fig. 4). It should be noted that a moderate toxic condition of Al treatment was used in this study. Exposure of roots to treatment medium containing 50 μm Al caused about 50% inhibition of root elongation (Fig. 1). Under such stress conditions, the roots had actually undergone physiological stress, but it was not lethal, ensuring the reliability of Al distribution. Furthermore, we also compared Al content in different CW components from different plant species, including monocots (wheat and rice) and dicots (buckwheat [Fagopyrum esculentum] and rice bean [Vigna umbellata]), and found that the majority of Al also resided in the HC1 fraction (data not shown). These results, to our knowledge for the first time, clearly demonstrate that HC1 has a greater ability to accumulate Al than the other components of the CW.
According to the review of Fry (2004), pectins are galacturonate (GalA)-rich, acidic polysaccharides (partly methylesterified galacturonan) linked to RG-I and RG-II, which are rich in Ara, Gal, and Rha, while HCs are GalA-free, neutral, or slightly acidic polysaccharides, and major HCs are the xylans, xyloglucans, and mixed-linkage β-(1→3),(1→4)-d-glucans. Thus, one of the criteria to indicate the purity of HC fraction is the low abundance of uronic acids. Some of the uronic acids not extracted by hot water may be GlcA residues of HC rather than GalUA residues of pectin (Fig. 3A; S.C. Fry, personal communication), although the possibility remains that there is a little pectin contamination in the HC fraction. As pectins are a group of polysaccharides that can be extracted by different agents such as hot water, dilute acid, or calcium chelators, different methods were adopted to extract pectin from plant tissues in different studies. In order to verify whether the use of hot water to extract pectins is suitable or effective in this study, we compared the pectin contents in different extraction solution that include hot water, ammonium oxalate buffer, CDTA, and pectinase. The extraction efficiency was similar among hot water, ammonium oxalate buffer, and CDTA, but a slightly lower level was found in pectinase (Fig. 3B). We also tried to isolate pectin by ammonium oxalate buffer at 120°C (in an autoclave) instead of 100°C. The higher the temperature, the more effective the extraction, and the pectin will undergo more backbone cleavage at 120°C than at 100°C (S.C. Fry, personal communication). However, the kinetics study indicated that the amount of accumulated Al by residues after such extraction could account for 80% of CW-accumulated Al, which was not different from that of hot water extraction (data not shown). These results indicate that the method to extract pectin is practicable.
Second, XET activity was inhibited by Al stress. XTHs are a family of enzymes that catalyze molecular grafting and/or the hydrolysis of xyloglucans (Rose et al., 2002). Xyloglucan is the most abundant HC in dicotyledons; it functions as a load-bearing cross-link among microfibrils and plays a central role in modulating the mechanical properties of CWs (Nishitani, 1997). It has been frequently reported that CW extensibility is decreased by Al stress, and this has been implicated in the mechanism of Al-induced root growth inhibition. However, the mechanisms responsible for the Al-dependent decrease of extensibility are unknown. Two possible scenarios have been proposed. One is that Al causes changes of CW polysaccharides metabolism, and the other is that Al interferes with the action of enzymes responsible for CW extensibility. Because of the ample experimental evidence of the accumulation of polysaccharides in response to Al stress, the prevailing opinion supports that interruption of CW deformation by Al causes decreased extensibility. However, from our results here (Fig. 7), another scenario can be envisioned. CW extension requires cleavage of the xyloglucan backbone and the reformation of the newly synthesized xyloglucan chain. XET is the major enzyme responsible for this process. The inhibition of XET activity will result in the failure of cleavage of the xyloglucan backbone and subsequent binding of a new xyloglucan chain, which not only causes the accumulation of HC polysaccharides but also hampers CW extension. Because the inhibition of XET activity by Al occurred rapidly (within 30 min), which was in line with the deposition of callose (Figs. 6 and 7), an indicator of apoplastic lesions of Al toxicity, this reinforces the importance of XET activity changes in the expression of Al toxicity. Recently, experimental evidence supporting that a specific member of the XTH protein family plays an indispensable role in the cell elongation process has been reported (Osato et al., 2006; Liu et al., 2007).
The Arabidopsis genome contains 33 XTHs. Different XTHs have diverse and distinct expression patterns in terms of tissue specificity and developmental, hormonal, or environmental stimuli. Using real-time RT-PCR, Yokoyama and Nishitani (2001) revealed that XTH-5, -12, -13, -14, -17, -18, -19, -20, -26, and -31 exhibited root-specific expression. Based on Genevestigator data, Becnel et al. (2006) found that at least 21 members of the XTHs have robust root expression, especially XTH-14, -15, and -31. According to these reports, 11 out of 33 XTHs were chosen for transcriptional analysis of Al-induced reduction of XET activity in this study. Only three gene expression levels were selectively reduced by a 24-h treatment with 50 μm Al, which resulted in 50% inhibition of root elongation (Fig. 1), implying that XTH-14, -15, and -31 may be the major contributors to XET activity changes in response to Al stress (Fig. 8). This is in line with a previous supposition (Becnel et al., 2006). A time-course experiment indicated that only XTH-31 was constitutively suppressed by Al stress; however, the expression of XTH-14 and -15 showed transient up-regulation but suppression after a longer time. We suppose that XTH-31 may contribute most importantly to XET activity and that the initial increase of other genes may function to complement XTH-31 function. On the other hand, a few XTH gene expression levels were up-regulated by Al stress. For instance, the expression of XTH-5 was increased by about 3.5-fold within 1 h of exposure and remained about 1.5-fold increased after 24 h of exposure (Fig. 8). At present, we still have little information on the functions of individual XTH gene in terms of root growth regulation and development. These need further study.
Understanding the control of cell elongation is a key step in elucidating the mechanism of plant growth and development and may provide important information on how Al-induced root growth inhibition proceeds. It is believed that regulation of CW properties is the most critical step in the regulation of cell elongation. To our knowledge, for the first time we have demonstrated here that Al targets HC1 easily and inhibits XET activity, which will be the key process responsible for Al-induced root growth inhibition in Arabidopsis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana ecotype Columbia) was used in this study. Seeds were surface sterilized and germinated on an agar-solidified nutrient medium in petri dishes. The nutrient medium was based on Murashige and Skoog (1962) salts. The final pH was adjusted to 4.5. The seeds were vernalized at 4°C for 2 d. Petri dishes were placed into a growth chamber, positioned vertically, and kept under controlled environmental conditions at 24°C, 140 μmol m−2 s−1, and a 16/8-h day/night regime.
Effect of Al on Root Growth
Seedlings with a root length of 1 cm were selected and transferred to petri dishes containing agar-solidified CaCl2 (0.5 mm) medium with different Al concentrations (0, 50, 75, 100, and 150 μm total concentration of Al in the form of AlCl30.6H2O). Root length measurements were collected using a digital camera connected to a computer. Data were quantified and analyzed by Photoshop 7.0 (Adobe Systems).
Callose Determination
For determination of callose, seedlings were placed in petri dishes containing agar-solidified CaCl2 (0.5 mm) medium containing 50 μm Al for different times. After treatment, callose accumulation was determined according to Kudlicka and Brown (1997).
Cytochemical Assay
The XET action of XTH was determined according to Vissenberg et al. (2000). In brief, roots were incubated in a 6.5 μm XGO-SR mixture (XLLG-SR > XXLG-SR > XXXG-SR; for nomenclature, see Fry et al., 1993; for the synthesis, see Fry, 1997) dissolved in 25 mm MES buffer at pH 5.5 for 1 h. The assay was followed by a 10-min wash in ethanol:formic acid:water (15:1:4, v/v/v) to remove any remaining unreacted XGO-SRs; a further incubation overnight in 5% formic acid was performed to remove apoplastic, non-wall-bound XGO-SRs. Samples were mounted on glass slides and examined with a laser-scanning confocal microscope (LSM 510; Zeiss) using excitation light of 540 nm.
Gene Expression Analysis
Total RNA was isolated from root apices (0–10 mm) using TRIzol (Invitrogen). cDNA was prepared from 1 μg of total RNA using the PrimeScript RT reagent kit (Takara). For real-time RT-PCR analysis, 1 μL of 10-fold-diluted cDNA was used for the quantitative analysis of gene expression performed with SYBR Premix ExTaq (Takara) with pairs of gene-specific primers (Table I). Each cDNA sample was run in triplicate. Expression data were normalized with the expression level of the tubulin gene (forward, 5′-AAGTTCTGGGAAGTGGTT-3′; reverse, 5′-CTCCCAATGAGTGACAAA-3′).
Table I. Gene-specific primer pairs used for real-time RT-PCR.
| Gene Namea | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
| XTH-5 | TTTGTCGTCCCAGAACTCAGA | CGTATCGGCACATCATCCAC |
| XTH-12 | TGTGGACTACACTTAATTCAAATCAGCT | GTTGAGGTTGCATTCAGTGGGT |
| XTH-13 | TGTGGACTACCCTTAACTCAAATCAGTA | GTTGAGATTGCATTCCGTAGGC |
| XTH-14 | ATTTTAAGAGATTCCCTCAAGGCC | CATGTAATATCGTATCTCAGAAACACCA |
| XTH-15 | GAAGTCCAGAGTCTGATGAACACATATTA | CCAGGAATGCTTTATTGATCTTGAC |
| XTH-17 | AGTTCAAGAACCCCGAGGCGAT | TTGCCCAATGCTCGGCTTCC |
| XTH-18 | TGCATGTCTATGCGGGTAGC | TCCATCTCTGTCGCGCACCT |
| XTH-19 | ATGGGAAGCAGAGCATTG | TTGCGGGACAAACTGAC |
| XTH-20 | TAATCACGTGGCATTGTCTTTGTA | AATTTATTTAGCGCAAATACTCAGCTAG |
| XTH-26 | AGCTGACGAAGATGCAAAAGATAA | CATTCCGGAGGCATAACACCT |
| XTH-31 | TGTCACTCTTTGGCTCG | ACCTCATCGTGGTCTCC |
Gene names were adopted according to Yokoyama and Nishitani (2001).
CW Extraction and Fractionation
Extraction of crude CW materials and subsequent fractionation of CW components were carried out according to Zhong and Lauchli (1993) with minor modifications. As depicted in Figure 2, pectin was extracted twice by hot water for 1 h each and pooling the supernatants (supernatant 1; pectin). The pellet (CW-pectin) was subjected to triple extraction with 4% KOH for a total time of 24 h and pooling the supernatants (supernatant 2; HC1). The resulting pellet (CW-pectin-HC1) was subsequently extracted with 24% KOH for a total time of 24 h and pooling the supernatants (supernatant 3; HC2), and the residue was considered the cellulose fraction (CW-pectin-HC1-HC2). For comparison of pectin extraction efficiency by hot water with other extracts, pectin was extracted from CW materials by the following methods: 0.5% ammonium oxalate buffer (pH 4) in a boiling water bath (twice each for 1 h); 50 mm CDTA (pH 7.5) containing 0.02% thimerosal, incubated overnight at 25°C; and 1% pectinase plus 0.1% bovine serum albumin with constant agitation at 30°C overnight.
Uronic Acid Measurement
Uronic acid content in each supernatant was assayed according to Blumenkrantz and Asboe-Hansen (1973) using GalUA (Sigma) as a standard.
Al Content Measurement
Al content in each pellet was extracted by 2 n HCl for 24 h with occasional shaking. Al concentrations in the extracts were determined by inductively coupled plasma-atomic emission spectrometry (IRIS/AP optical emission spectrometer).
Monosaccharide Analysis
Pectin fractions or CW samples were hydrolyzed in 2 m trifluoroacetic acid for 1 h at 120°C. Trifluoroacetic acid was removed by a rotary evaporator at 40°C. The residues were redissolved in 1 mL of MilliQ water. The monosaccharide component was determined by high-performance anion-exchange chromatography with pulsed-amperometric detection using a PA20 column (Dionex) as described previously (Øbro et al., 2004). Monosaccharide standards included l-Fuc, l-Rha, l-Ara, d-Gal, d-Glc, and d-Xyl and were purchased from Sigma. A standard curve was performed before the analysis of each batch of samples.
Adsorption Kinetics
In order to determine the adsorption ability of different CW components to Al, a total of 5 mg of CW materials or its corresponding residues (Fig. 2) was placed into a 2-mL column equipped with a filter at the bottom. The adsorption solution consisted of 20 μm AlCl3 in 0.5 mm CaCl2 at pH 4.5. The solution was sipped by a peristaltic pump set at a speed of 4 mL per 20 min after running through a 2-mL column holding the CW samples. The adsorption solutions were collected at 20-min intervals, and Al in the adsorption solutions was measured spectrophotometrically with pyrocatechol violet according to Kerven et al. (1989) with some modification (Zheng et al., 2004). The kinetics study was carried out twice independently, and one set of adsorption curves is presented in “Results.”
Statistics
All statistical analyses were conducted with SAS software (SAS Institute). Means were compared by t test at P < 0.05 in all cases.
Acknowledgments
We are grateful to Prof. Stephen C. Fry (Institute of Cell and Molecular Biology, University of Edinburgh) for kindly donating XGO-SRs and for valuable suggestions on CW fractioning. Thanks are also given to two anonymous reviewers for their valuable comments to improve the quality of this work.
References
- Becnel J, Natarajan M, Kipp A, Braam J. (2006) Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol 61: 451–467 [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Chang Y-C, Yamamoto Y, Matsumoto H. (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22: 1009–1017 [Google Scholar]
- Clarkson DT. (1967) Interactions between aluminum and phosphorus on root surfaces and cell wall material. Plant Soil 27: 347–356 [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Fry SC. (1997) Novel 'dot-blot' assays for glycosyltransferases and glycosylhydrolases: optimization for xyloglucan endotransglycosylase (XET) activity. Plant J 11: 1141–1150 [Google Scholar]
- Fry SC. (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161: 641–675 [DOI] [PubMed] [Google Scholar]
- Fry SC, Aldington S, Hetherington PR, Aitken J. (1993) Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol 103: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernahr Bodenkd 158: 419–428 [Google Scholar]
- Horst WJ, Wang Y, Eticha D. (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot (Lond) 106: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Halman PS, Kokot S. (1989) Aluminium determination in soil solution. II. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic ligands. Aust J Soil Res 27: 91–102 [Google Scholar]
- Kochian LV. (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kudlicka K, Brown RM., Jr (1997) Cellulose and callose biosynthesis in higher plants. I. Solubilization and separation of (1,3)- and (1,4)-β-glucan synthase activities from mung bean. Plant Physiol 115: 643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Yang JL, Zhang YJ, Zheng SJ. (2009) Disorganized distribution of homogalacturonan epitopes in cell walls as one possible mechanism for aluminium-induced root growth inhibition in maize. Ann Bot (Lond) 104: 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-B, Lu S-M, Zhang J-F, Liu S, Lu Y-T. (2007) A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 226: 1547–1560 [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen RF, Nagao S, Tanimoto E. (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45: 583–589 [DOI] [PubMed] [Google Scholar]
- Matsumoto H. (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200: 1–46 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–496 [Google Scholar]
- Nishitani K. (1997) The role of endoxyloglucan transferase in the organization of plant cell walls. Int Rev Cytol 173: 157–206 [DOI] [PubMed] [Google Scholar]
- Øbro J, Harholt J, Scheller HV, Orfila C. (2004) Rhamnogalacturonan I in Solanum tuberosum tubers contains complex arabinogalactan structures. Phytochemistry 65: 1429–1438 [DOI] [PubMed] [Google Scholar]
- Osato Y, Yokoyama R, Nishitani K. (2006) A principal role for AtXTH18 in Arabidopsis thaliana root growth: a functional analysis using RNAi plants. J Plant Res 119: 153–162 [DOI] [PubMed] [Google Scholar]
- Rengel Z, Zhang WH. (2003) Role of dynamics of intracellular calcium in aluminium toxicity syndrome. New Phytol 159: 295–314 [DOI] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43: 1421–1435 [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Matsumoto H. (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112: 353–358 [DOI] [PubMed] [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. (2000) Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol 123: 987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van HL, Kuraishi S, Sakurai N. (1994) Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol 106: 971–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Martinez-Vilchez IM, Verbelen J-P, Miller JG, Fry SC. (2000) In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell 12: 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Van Sandt V, Fry SC, Verbelen JP. (2003) Xyloglucan endotransglucosylase action is high in the root elongation zone and in the trichoblasts of all vascular plants from Selaginella to Zea mays. J Exp Bot 54: 335–344 [DOI] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E. (1995) Global extent, development and economic impact of acid soils. Date RA, Grundon NJ, Raymet GE, Probert ME, , Plant-Soil Interactions at Low pH: Principles and Management. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 5–19 [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ. (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R, Nishitani K. (2001) A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol 42: 1025–1033 [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Lin XY, Yang JL, Liu Q, Tang C. (2004) The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistance wheat (Triticum aestivum L.) cultivar. Plant Soil 261: 85–90 [Google Scholar]
- Zheng SJ, Yang JL. (2005) Target sites of aluminum phytotoxicity. Biol Plant 49: 321–331 [Google Scholar]
- Zhong H, Lauchli A. (1993) Changes of cell wall component and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44: 773–778 [Google Scholar]