Abstract
The completion of germination in Lepidium sativum and other endospermic seeds (e.g. Arabidopsis [Arabidopsis thaliana]) is regulated by two opposing forces, the growth potential of the radicle (RAD) and the resistance to this growth from the micropylar endosperm cap (CAP) surrounding it. We show by puncture force measurement that the CAP progressively weakens during germination, and we have conducted a time-course transcript analysis of RAD and CAP tissues throughout this process. We have also used specific inhibitors to investigate the importance of transcription, translation, and posttranslation levels of regulation of endosperm weakening in isolated CAPs. Although the impact of inhibiting translation is greater, both transcription and translation are required for the completion of endosperm weakening in the whole seed population. The majority of genes expressed during this process occur in both tissues, but where they are uniquely expressed, or significantly differentially expressed between tissues, this relates to the functions of the RAD as growing tissue and the CAP as a regulator of germination through weakening. More detailed analysis showed that putative orthologs of cell wall-remodeling genes are expressed in a complex manner during CAP weakening, suggesting distinct roles in the RAD and CAP. Expression patterns are also consistent with the CAP being a receptor for environmental signals influencing germination. Inhibitors of the aspartic, serine, and cysteine proteases reduced the number of isolated CAPs in which weakening developed, and inhibition of the 26S proteasome resulted in its complete cessation. This indicates that targeted protein degradation is a major control point for endosperm weakening.
The seed germination process begins with imbibition of the dry seed and is completed when the radicle has emerged through all the layers enveloping the embryo (Finch-Savage and Leubner-Metzger, 2006). In both Arabidopsis (Arabidopsis thaliana) and Lepidium (Lepidium sativum), there are two such layers, an outer dead testa (seed coat) and, beneath that, a layer of living endosperm cells (aleurone layer). Germination in these species has two separate visible stages: first, the testa ruptures, and then the lower hypocotyl/radicle (RAD) extends to complete germination by rupturing the micropylar endosperm layer (CAP) that covers it. A recent publication by Sliwinska et al. (2009) describes how embryo elongation during Arabidopsis seed germination is due to cell expansion growth in a specific zone in the lower hypocotyl/radicle transition region. During the latter process, the CAP weakens through autolysis to reduce the mechanical resistance to radicle protrusion. Biomechanical measurements have been used to record such weakening in species from a variety of different families (Bewley, 1997; Toorop et al., 2000; da Silva et al., 2004; Finch-Savage and Leubner-Metzger, 2006). However, Arabidopsis seeds are too small for such measurements with the techniques used to date, and this has limited progress in linking biomechanical and molecular studies. To overcome this obstacle, we have demonstrated that the larger seeds of Lepidium can be used as a model system for studying both the molecular and biomechanical mechanisms of endosperm cap weakening (Müller et al., 2006, 2009; Linkies et al., 2009). In this work, direct biomechanical measurement has shown that endosperm cap weakening is promoted by GAs and inhibited by abscisic acid (ABA). This endosperm weakening is induced by an early signal from the embryo, after which weakening and lysis proceed as an organ-autonomous process. Further experimentation has shown that in isolated endosperm caps, GA can replace the embryo signal, that de novo GA biosynthesis occurs in the endosperm, and that the weakening is regulated, at least in part, by the GA-ABA ratio.
The genera Lepidium and Arabidopsis both belong to the lineage I clade of the Brassicaceae family and therefore are closely related (Franzke et al., 2009). As may be expected from this close relationship, the above findings in Lepidium are consistent with the known spatial, temporal, and GA-mediated regulation of genes during Arabidopsis seed germination (Yamaguchi et al., 2001; Ogawa et al., 2003; Yamauchi et al., 2004). Separate global expression profiles of the whole embryo and endosperm shortly after radicle emergence in Arabidopsis are also consistent with this pattern of regulation (Penfield et al., 2006). Comparison of the transcriptomes of endosperm and embryo tissues at a single time point of 24 h also showed large differences in expression between the tissues (Okamoto et al., 2010). However, to date, there has been no similar analysis of the changes in these tissues leading to the completion of germination.
To take advantage of their close relationship, we carried out a global transcript analysis of the interaction between individual seed tissues in a time course during germination of Lepidium by cross-species hybridization to a full-genome Arabidopsis array (Linkies et al., 2009). The larger size of Lepidium enabled us to use RNA samples collected specifically from the CAP and RAD to avoid confounding the results with other tissues in the embryo and other regions of the endosperm. This work demonstrated that the CATMA 25K microarrays (Hilson et al., 2004; Allemeersch et al., 2005), which are spotted with PCR-amplified Arabidopsis gene-specific tags (GSTs; 150–500 bp), were effective for comparative genomics by cross-species microarray hybridization with Lepidium. Such cross-species hybridizations for closely related species, using several array platforms, have become an accepted approach where no species-specific arrays are available (for review, see van de Mortel and Aarts, 2006; Bar-Or et al., 2007; Broadley et al., 2008). CATMA microarrays have also been shown to be effective for cross-species microarray hybridization in work by Slotte et al. (2007), in which Capsella bursa-pastoris accessions differing in flowering time were compared at the transcriptome level. This species, like Lepidium and Arabidopsis, is from the lineage I clade of the Brassicaceae (Franzke et al., 2009).
In Linkies et al. (2009), a preliminary analysis of these cross-species Lepidium arrays indicated that ethylene-related transcripts were overrepresented in the lists of regulated genes. The array data, therefore, were used to complement an investigation of the interaction of ethylene with ABA, which resulted in a model for the hormonal regulation of endosperm cap weakening and rupture. In this work, we investigate the importance of the transcription, translation, and posttranslation stages in the regulation of germination through endosperm weakening. We also carry out a full global transcript analysis of the RAD and CAP tissues during the germination process.
RESULTS AND DISCUSSION
The Progression of Germination Is Clearly Linked to Endosperm Weakening That Requires Both Transcription and Translation for Completion in the Whole Seed Population
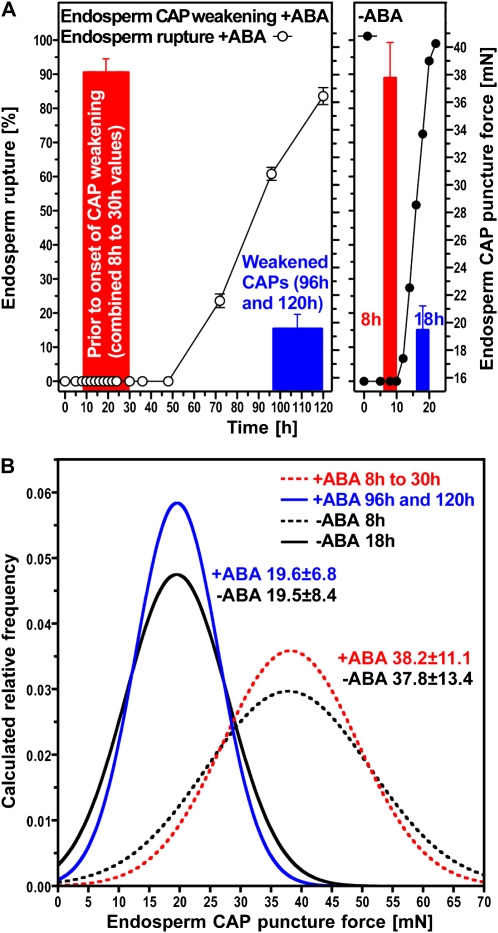
The completion of germination (radicle emergence) in Lepidium is regulated by two opposing forces, the growth potential of the RAD and the resistance to this growth from the seed covering layers (testa and CAP). After testa rupture, the latter is determined by the strength of the endosperm, which can be determined by puncture force measurement, and this progressively decreases toward germination completion (Fig. 1). Onset of endosperm weakening occurs after 8 h of imbibition on medium without hormonal addition (−ABA in Fig. 1), and its progression results in the occurrence of endosperm rupture and germination completion in an increasing proportion of the seed population up to 18 h (Fig. 1A). Both the onset and the completion of endosperm weakening are delayed by the addition of ABA, shifting its onset to more than 30 h of imbibition and its completion to 96/120 h. At the onset of endosperm weakening, there is a high variance in the force required to puncture the endosperm (Fig. 1B). This variance declines as the endosperm weakens in an identical fashion, with and without ABA (Fig. 1B), indicating that ABA has an effect only on the timing of this process. Therefore, ABA provides a means of spreading out the process of endosperm weakening, enabling samples to be taken at several stages, both before and during the process. Overall, these results show that endosperm weakening is not just an imbibition effect but clearly related to the progression of the germination process. Single-tissue analyses of the RAD and CAP, therefore, should provide a means to identify mechanisms underlying the germination process.
Figure 1.
Progression of endosperm CAP weakening both with and without the addition of ABA. A, Endosperm rupture progresses more quickly without ABA, but the mean force required to puncture the CAP is the same in both treatments before and after weakening. B, The distributions of force required to puncture the CAP are the same in both treatments. [See online article for color version of this figure.]
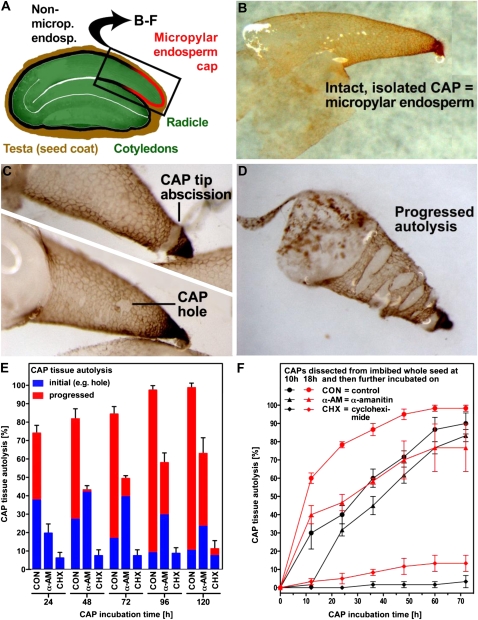
Dry seeds store mRNAs, which are assumed to contain transcripts for genes that are important for both late embryogenesis and early seed germination (Comai et al., 1989; Hughes and Galau, 1989, 1991). Upon imbibition, transcriptional changes take place, and after the first 3 h, huge changes in transcript abundance are already evident in seeds of Arabidopsis (Nakabayashi et al., 2005; Preston et al., 2009; Kimura and Nambara, 2010). Rajjou et al. (2004) have shown that inhibiting this transcription with α-amanitin delays the germination process of whole seeds and inhibits seedling development in Arabidopsis. Transcription inhibitors have also delayed germination in wheat (Triticum aestivum) embryos (Jendrisak, 1980) and endosperm rupture in tobacco (Nicotiana tabacum) seeds (Arcila and Mohapatra, 1992). In contrast, inhibition of translation by cycloheximide entirely blocks the completion of germination in whole seeds (Rajjou et al., 2004). We have utilized the bigger seeds of Lepidium to investigate, in a similar way, the necessity of transcription and translation during endosperm weakening in individual seed tissues. When Lepidium CAPs were dissected from −ABA-imbibed seeds (Fig. 2, A and B) and incubated individually, the initial autolysis caused either hole formation close to where the radicle in an intact seed would penetrate through the endosperm and/or abscission of the CAP tip (Fig. 2C). Subsequent progressive autolysis later disrupts the whole CAP (Fig. 2D). We exploited this situation to observe the influence of the inhibitors α-amanitin and cycloheximide on the progression of endosperm weakening. Unlike when whole seeds are used, there are no problems with the uptake of inhibitors into the tissues using this system.
Figure 2.
The effect of transcription (α-amanitin) and translation (cycloheximide) inhibitors on the progress of autolysis in isolated CAPs. A, Schematic cross-section of a Lepidium seed. B, Isolated CAP (micropylar endosperm). C, Examples of initial autolysis: CAP tip abscission (top) and hole formation (bottom). D, Progressed autolysis. E, The effect of inhibitors on the progress of autolysis in a population of CAPs isolated at 18 h of imbibition. Isolated CAPs were incubated for the times indicated without or with inhibitors. F, The effect of inhibitors on the progress of autolysis (initial + progressed lysis) in a population of CAPs isolated after 10 and 18 h of seed imbibition; subsequent incubation was as indicated. α-AM, α-Amanitin; CHX, cycloheximide.
Incubation on α-amanitin following dissection slows the progress of autolysis and prevents the completion of the process in a proportion of the CAPs (Fig. 2E). In contrast, cycloheximide completely blocks autolysis of more than 90% of CAPs (Fig. 2E) even during the very late stages of germination (i.e. following dissection at 18 h, when some seeds in the population have already begun autolysis in situ). Comparison of the progression of autolysis in control CAPs dissected at 10 and 18 h suggests that the process occurs more quickly in the presence of the RAD in whole seeds than it does after dissection (i.e. CAPs dissected at 18 h are further progressed than CAPs dissected at 10 h plus 8 h further incubation following dissection; Fig. 2F). If the same treatments are applied to the RAD dissected after 18 h, no inhibition of growth was observed on α-amanitin, but cycloheximide significantly inhibited radicle growth (Supplemental Table S1). These findings show that, in addition to translation, transcription is very important in the CAP, and comparative global transcriptome analysis of both tissues will be very informative.
Arabidopsis CATMA Microarrays Can Be Used Effectively to Investigate Patterns in Lepidium Transcript Expression
To investigate how Lepidium gene transcripts in specific seed tissues are regulated temporally and spatially, we hybridized Lepidium RNA samples to Arabidopsis CATMA 25K microarrays (Complete Arabidopsis Transcriptome Microarray, www.catma.org; Hilson et al., 2004; Allemeersch et al., 2005). The RNA was extracted from specific Lepidium seed tissues (RAD, CAP, and nonmicropylar endosperm [NME]) at defined time points during germination. These tissues were collected after testa rupture, before and during endosperm weakening, but prior to endosperm rupture (i.e. only seeds with intact endosperm were used).
The principal experiment (+ABA arrays) produced samples from seeds imbibed on medium with ABA (10 μm), as this slows the germination process, allowing the dissection at earlier developmental stages (i.e. dissection is not possible before 8 h of imbibition), but without ABA (−ABA arrays), changes that lead to endosperm weakening have already occurred (Linkies et al., 2009). Therefore, we compared RAD and CAP from seeds incubated in medium containing 10 μm ABA at 8, 18, and 30 h leading up to the onset of endosperm weakening, and later at 96 h, just prior to endosperm rupture (Fig. 1). In this experiment, 10 μm ABA slows the germination process but, importantly, does not prevent the completion of germination (radicle emergence). Indeed, the relationship between decreasing endosperm cap puncture force and the increasing percentage of seeds showing endosperm rupture was almost identical with and without ABA, despite the very different rates of this process on these solutions (Linkies et al., 2009; Fig. 1). In a further smaller experiment (−ABA arrays), seeds were imbibed on medium without ABA and samples were prepared at 8 and 18 h from RAD, CAP, and NME. These data were used to confirm results collected in the first experiment and to help aid the identification of CAP-specific gene expression.
Normalized expression values for Lepidium were obtained in the +ABA arrays for 19,794 CATMA probes to which there was significant transcript hybridization (Supplemental Table S2) and in the −ABA arrays for 22,025 probes (Supplemental Table S3). Lepidium gene transcripts that hybridized to these probes were assigned as putative Arabidopsis orthologs, defined by having an Arabidopsis Genome Initiative (AGI) identifier such as At1g62380 and a Gene Ontology (GO) annotation associated with this AGI identifier (www.arabidopsis.org). Henceforth, to avoid repetitive use of the term, putative orthologs in Lepidium will be referred to using AGI annotation. All microarray data, including the normalized intensity values for each microarray, were deposited in ArrayExpress (www.ebi.ac.uk/microarray/; +ABA arrays accession no. E-TABM-743, −ABA arrays accession no. E-TABM-745). To support the use of these cross-species hybridizations, Linkies et al. (2009) verified the transcript expression pattern of the arrays by comparing them with corresponding quantitative reverse transcription-PCR results obtained with independent biological RNA samples from a separate experiment. They concluded that cross-species microarray hybridization with the CATMA platform is a useful and effective tool for heterologous transcriptomics with Lepidium.
There Are Differences in the Pattern of Transcription between the Radicle Tip and the Endosperm Cap, But Much of the Temporal Change Is Common to Both Tissues
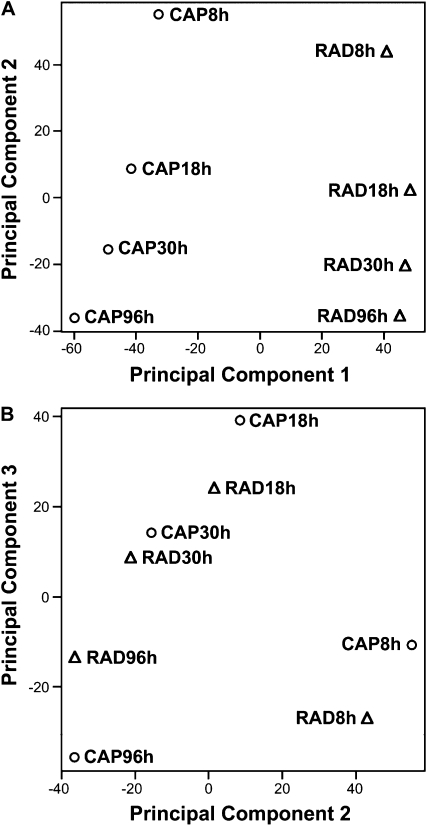
Principal component analysis (PCA) was used to look for global patterns in the Lepidium expression data across all the gene transcripts (Fig. 3). The two components PC1 and PC2 accounted for more than 60% of the variance in gene expression. PC1 clearly separated RAD and CAP (Fig. 3A). PC2 then separated the times in a continuous temporal order. These clear patterns indicate that the data behave in an expected fashion, with greatest differences occurring between the tissues. The comparison indicates that the majority of change in transcript numbers occurs before endosperm weakening (i.e. 8–18 h). The very similar ordering of the time course suggests that much of this change in the earlier stages of germination is common to the two tissues. PC3 confirms the step change between 8 and 18 h with a subsequent smaller progressive change at 18 to 96 h (Fig. 3B). Distances between RAD and CAP are similar at 8 and 18 h, least at 30 h, and then greatest at 96 h, coinciding with the period of endosperm weakening from 30 to 96 h (Fig. 1) and preparation for radicle expansion and emergence.
Figure 3.
The results of PCA applied to the expression of all the Lepidium FR1 gene homologs represented in the +ABA microarrays. A, Principal components 1 and 2 accounted for 42% and 20% of the variance, respectively. B, Principal components 2 and 3 accounted for 20% and 12% of the variance, respectively.
The Majority of the Genes Expressed (“Present”) during Germination Occur in Both Tissues, But Unique Expression Relates to The Specific Functions of the Tissues
To determine whether individual genes were expressed or not, the normalized values for each probe were compared with those for the 912 empty spots on the arrays with a one-sided t test. Probes for which the normalized values were significantly greater than the empty spots (P < 0.05) were considered to be “expressed.” The data shown in Table I are the number of probes on the array that indicate expression based on this criterion. In agreement with the PCA, the majority of genes expressed at any time point are expressed in both the CAP and RAD (common; Table I). Similarly, the majority of genes expressed in successive time points in the same tissue are common. The number of commonly expressed genes range from 8,045 to 10,493. This is a very similar number to that found by Penfield et al. (2006) shortly after radicle emergence in Arabidopsis seeds. They found 9,650 genes in common with approximately 4,000 that were expressed differentially in the embryo or the endosperm. They concluded that patterns of gene expression are broadly similar between the two organs, suggesting similar postgerminative metabolism occurring in these tissues. We show here that this similarity extends to the germination process.
Table I. Numbers of probes on the array that were considered to be significantly expressed.
Values shown are numbers of expressed probes that are unique to the RAD and CAP at each time point and the number of probes that are expressed in both tissues (common). The numbers of probes that are uniquely expressed in any time/tissue combination are shown in parentheses.
| Sample | RAD | Common | CAP |
| 8 h | 4,087 (513) | 9,416 | 917 (154) |
| Common | 10,327 | 8,045 | 9,153 |
| 18 h | 2,004 (229) | 9,515 | 2,542 (298) |
| Common | 10,073 | 8,482 | 10,304 |
| 30 h | 1,903 (145) | 9,479 | 2,328 (285) |
| Common | 10,493 | 8,552 | 10,271 |
| 96 h | 2,601 (322) | 9,809 | 2,228 (321) |
As Penfield et al. (2006) found post germination in Arabidopsis, there are a number of genes that are expressed in one tissue but not the other at all time points leading to germination completion. At 8 h, the number of genes uniquely expressed in the RAD (4,087) is much greater than in the CAP (917). This suggests much greater early transcriptional activity in the RAD than in the CAP. In barley (Hordeum vulgare), Barrero et al. (2009) suggest that the coleorhiza is functionally related to the endosperm cap in Arabidopsis and Lepidium, since it appears to regulate germination by restricting radicle elongation. In this species, they show that 23% of genes are differentially expressed during first 8 h in the coleorhiza but only 16% in the radicle, suggesting a more active role for the former. This is opposite to the results shown here, however, as the coleorhiza initially elongates (grows) with the root before the root penetrates it. This may explain the different pattern of gene expression in the two species, since the CAP of Lepidium shows no such growth.
By 18 and 30 h, this ratio has changed so that the numbers of genes uniquely expressed is 27% and 22% greater, respectively, in the CAP than in the RAD; by 96 h, the number of genes expressed in the two tissues is more similar. To investigate whether these differences were linked to functional specialization of the CAP and RAD tissues, we applied the GO-based seed-specific TAGGIT workflow (Carrera et al., 2007) to identify proportional representations of genes into functional categories (Supplemental Table S4). There are also a number of genes whose expression is unique to each tissue/time combination (Table I, in parentheses), ranging from 145 (30-h RAD) to 513 (8-h RAD). Again, the GO-based seed-specific TAGGIT workflow (Carrera et al., 2007) was used to categorize these genes (Supplemental Table S5).
At 8 h, the numbers of genes represented in TAGGIT categories is greater in the RAD than in the CAP (Supplemental Table S4), which in general reflects the pattern in the total numbers expressed (Table I). There are also tissue-specific differences in the numbers of genes uniquely expressed at each time point. In general, for the RAD, the numbers of genes are greater than in the CAP in the following categories: dormancy related; brassinosteroid; ethylene, cell cycle related; cytoskeleton and translation associated. This reflects the radicle as a growing tissue. Whereas for the CAP after 8 h, the numbers of genes are greater than for the RAD in the following categories: GAs, jasmonic acid, glycolysis, and gluconeogenesis; Krebs cycle; β-oxidation; and stress. This pattern may reflect the function of the endosperm to regulate germination through its autolysis and subsequent death. These differences in the two tissues are broadly similar to those found by Penfield et al. (2006) in postgerminative Arabidopsis tissues. In contrast, there is little difference in the numbers of common genes that are expressed by both tissues in any TAGGIT category between time points, and little pattern is shown in the data (Supplemental Table S4). Similarly, there is little difference and pattern in other genes expressed in common between time points and tissues (data not shown).
There Are Differences in the Numbers of Gene Transcripts That Are Differentially Regulated in the RAD and CAP
The transcript abundance of individual genes in the +ABA array data was compared between the RAD and the CAP at each time point using t tests to identify which genes showed differential expression relative to each other. P values were adjusted for false discovery rate (Benjamini and Hochberg, 1995), and the resulting gene lists (P ≤ 0.10) are given in Supplemental Table S6. These genes were considered to be up- or down-regulated between tissues at the time points specified. The total number of genes that are differentially regulated between the RAD and the CAP increases as the seeds progress toward germination and presumably the functional specialization of the tissues develops (Table II). The numbers of genes that are up-regulated in the RAD and CAP are similar at 8, 18, and 96 h, but at 30 h the number is higher in RAD (1,000) compared with CAP (783). If higher stringency is applied to the analysis, the overall pattern shown in Table II remains the same, but with fewer genes (e.g. for 96 h at P < 0.10, 2,464; at P < 0.05, 1,600 [data not shown]).
Table II. The numbers of genes classified in functional categories of the GO-based seed-specific TAGGIT workflow (Carrera et al., 2007) that were up-regulated in the tissue shown at each time point for the +ABA arrays.
| Tags | RAD | CAP | ||||||
| 8 | 18 | 30 | 96 | 8 | 18 | 30 | 96 | |
| Dormancy related | 1 | 7 | 6 | 13 | 15 | 20 | 26 | 25 |
| Germination related | 2 | 3 | 3 | 2 | 0 | 0 | 2 | 3 |
| ABA | 10 | 16 | 22 | 26 | 8 | 6 | 11 | 12 |
| Auxin | 14 | 14 | 14 | 27 | 10 | 13 | 16 | 12 |
| Brassinosteroid | 0 | 0 | 1 | 1 | 2 | 3 | 3 | 2 |
| Cytokinin | 6 | 3 | 2 | 6 | 1 | 1 | 2 | 2 |
| Ethylene | 6 | 7 | 7 | 11 | 2 | 5 | 10 | 8 |
| GA | 6 | 5 | 5 | 5 | 2 | 5 | 3 | 3 |
| Jasmonic acid | 2 | 6 | 3 | 5 | 0 | 1 | 0 | 1 |
| Seed storage proteins/late embryogenesis abundant | 1 | 4 | 4 | 7 | 6 | 10 | 12 | 10 |
| Inhibition of protein degradation | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| Protein degradation | 13 | 19 | 24 | 37 | 18 | 22 | 28 | 35 |
| Heat shock | 2 | 5 | 6 | 6 | 7 | 10 | 14 | 17 |
| Cell wall modification | 19 | 18 | 15 | 24 | 12 | 18 | 19 | 22 |
| Cell cycle related | 7 | 1 | 9 | 7 | 6 | 14 | 14 | 22 |
| Cytoskeleton | 6 | 5 | 8 | 6 | 8 | 8 | 14 | 13 |
| Translation associated | 4 | 1 | 3 | 7 | 63 | 86 | 111 | 137 |
| DNA repair | 1 | 2 | 3 | 6 | 2 | 4 | 7 | 5 |
| Respiration | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| Electron transport | 1 | 2 | 2 | 3 | 0 | 0 | 1 | 2 |
| Pentose phosphate pathway | 2 | 2 | 4 | 3 | 0 | 0 | 0 | 0 |
| Glycolysis and gluconeogenesis | 6 | 9 | 10 | 8 | 0 | 0 | 1 | 4 |
| Krebs cycle | 2 | 3 | 4 | 4 | 1 | 0 | 1 | 1 |
| β-Oxidation | 2 | 2 | 4 | 4 | 0 | 0 | 0 | 0 |
| Stress | 36 | 55 | 53 | 53 | 17 | 24 | 38 | 45 |
| Photosynthesis/chloroplast related | 48 | 60 | 73 | 134 | 34 | 44 | 69 | 121 |
| Unannotated | 1 | 2 | 1 | 4 | 2 | 2 | 4 | 3 |
| Unclassified | 346 | 484 | 496 | 828 | 339 | 438 | 593 | 718 |
| Total genes in lists | 545 | 736 | 783 | 1,238 | 555 | 736 | 1,000 | 1,226 |
| Total genes classified above | 199 | 252 | 287 | 410 | 216 | 298 | 407 | 508 |
| Percentage classified | 36.5 | 34.2 | 36.7 | 33.1 | 38.9 | 40.5 | 40.7 | 41.4 |
To further investigate the functional specialization of the tissue, we applied the seed-specific TAGGIT workflow (Carrera et al., 2007) to the genes up-regulated in either the RAD or the CAP relative to the other (Table II). Although the number of up-regulated genes in both tissues is similar, there are differences in the categories of genes that are overrepresented at all time points. In general, the categories with gene numbers overrepresented in the CAP are related to hormones, aspects of metabolism and reserve mobilization, and stress, whereas the categories with gene numbers overrepresented in the RAD are related to dormancy, late embryogenesis-abundant proteins, aspects of growth, and DNA repair. The most overrepresented category in RAD is translation-associated proteins, with a 26-fold higher number of genes across the four time points than the CAP (397 and 15, respectively). However, when viewing these data, it should be remembered that the actual number of different genes is less than this, since genes can be represented at more than one time point. Protein synthesis and ribosomal protein genes were also highly expressed in the embryo relative to the endosperm in Arabidopsis shortly after germination, and this was linked to a 5-fold higher number of ribosomes in the embryo than the endosperm (Penfield et al., 2006).
There are similar numbers of genes up-regulated in both tissues in the following categories: cell wall modification and protein degradation. This superficial similarity obscures important differences in the details that are explored below. TAGGIT effectively summarizes the proportional representation of seed-specific genes into functional categories; however, the gene lists used are no longer entirely current. In the following sections, we investigate categories identified with TAGGIT but use comprehensive gene lists that extend beyond those known to be seed specific.
Genes Involved in GA and ABA Signaling Networks Have Different Temporal and Spatial Patterns, Consistent with Regulation through Subtle Changes in Hormone Sensitivity
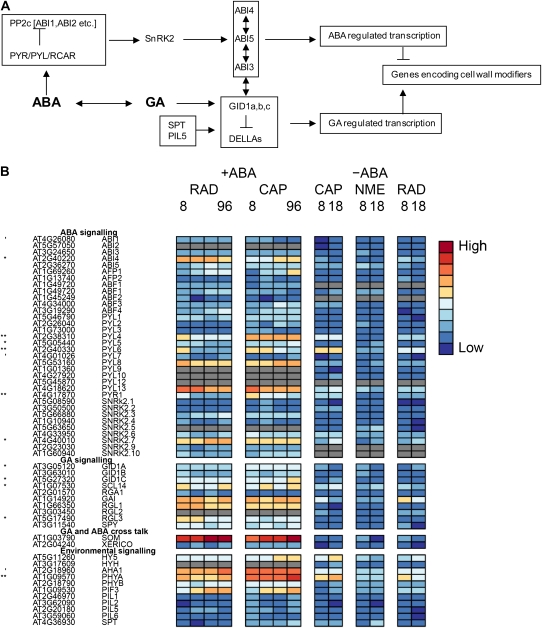
In Linkies et al. (2009), a model for the hormonal regulation of endosperm cap weakening and rupture was constructed for Lepidium. In this model, GA is an embryo signal that releases coat dormancy (if present) and induces the CAP weakening process. Thereafter, weakening is a CAP-autonomous process, with the rate regulated by GA-ABA and ethylene-ABA antagonisms that result in the completion of germination. There are many proteins involved in the regulatory networks controlling this process, and current understanding can be found in several recent comprehensive reviews (Finkelstein et al., 2008; Holdsworth et al., 2008a; Penfield and King, 2009). Hormone signaling, especially that resulting from the dynamic balance of GA and ABA, is a key component of these networks, which are thought to have significant interactions (Kucera et al., 2005; Holdsworth et al., 2008a). Understanding of ABA signal transduction is developing rapidly, and a model has recently emerged in which PYRABACTIN RESISTANCE (PRY)/PYRABACTIN RESISTANCE 1-LIKE (PYL)/REGULATORY COMPONENT OF ABA RECEPTOR receptors bind to ABA to remove the repression by PROTEIN PHOSPHATASE2C of downstream signaling via SNF1-RELATED PROTEIN KINASE to ABRE-regulated gene expression by the transcription factors ABSCISIC ACID INSENSITIVE3 (ABI3), ABI4, and ABI5 (Cutler et al., 2010; Fig. 4A). On the other side of this balance, DELLA proteins repress GA responses and therefore germination (Sun and Gubler, 2004). DELLAs are degraded to remove this repression when they form a complex with GA and GIBBERELLIN INSENSITIVE DWARF1 (GID1) receptors (Hartweck, 2008; Fig. 4A). These signaling networks are influenced by a diverse range of environmental signals during germination, principally temperature and light. Key components of the interaction between these two environmental signals and GA are the two phytochrome-interacting basic helix-loop-helix transcription factors, PHYTOCHROME INTERACTING FACTOR 3-LIKE5 (PIL5) and SPATULA (SPT) these both repress germination in the dark (Penfield et al., 2005).
Figure 4.
Hormone signaling in Lepidium during CAP weakening. A, Schematic to illustrate ABA and GA signaling pathways. B, Heat maps showing the relative abundance of transcripts from genes involved in ABA, GA, and environmental signaling. ′, *, and ** indicate that transcript numbers are significantly different between the tissues on +ABA arrays at P < 0.1, P < 0.05, and P < 0.01, respectively. Genes not present in the data sets are colored gray.
In the Lepidium after-ripened seed used in this work, the kinetics of endosperm rupture is strongly dependent on temperature but not light, and there is no obvious evidence of residual dormancy. This situation contrasts with Arabidopsis seeds, which are light sensitive, and so expression patterns may differ from those anticipated from work on Arabidopsis. Transcript levels of genes encoding the components of the hormone signaling networks in the CAP and RAD during Lepidium germination are shown in Figure 4B. Although germination of our Lepidium seed batch was not responsive to light, phytochrome genes are surprisingly shown to be some of the most highly expressed in both tissues. These genes are up-regulated in the CAP relative to the RAD, in particular PHYTOCHROME A (PHYA). −ABA array results indicate that expression of PHYA is higher in the CAP than in the NME and therefore is CAP specific. In Arabidopsis, SOMNUS (SOM) is thought to encode a component of the phytochrome signal transduction pathway that regulates genes in hormone metabolism and acts as a negative regulator in PHYA-mediated promotion of germination (Kim et al., 2008). However, in Lepidium, SOM is expressed very highly in both tissues, which from its function in Arabidopsis appears counterintuitive in these actively germinating seeds. The reason for these very clear expression patterns with PHYA and SOM in these light-insensitive Lepidium seeds is not clear. PIL5 expression is low, as expected for a negative regulator of germination in this situation. SPT tends to be more highly expressed in the CAP, but transcript levels are low. These results are consistent with the CAP being the principal receptor for environmental signals influencing germination.
In general, genes relating to GA signaling are more highly expressed than those relating to ABA signaling (Fig. 4B), and this is consistent with expectations for nondormant seeds progressing toward the completion of germination. Nevertheless, it is interesting that ABI4 expression is significantly up-regulated in the RAD, whereas ABI5 expression tends to be higher in the CAP, and ABI3 is similarly expressed in both tissues at a low level. This is entirely consistent with the results of Penfield et al. (2006), who showed, using GUS fusions, that in Arabidopsis, ABI3 is expressed in embryo and endosperm, ABI4 expression was specific to the embryo, and although ABI5 was expressed in the embryo and endosperm, expression in the latter was CAP specific. ABI4 is thought to repress lipid breakdown in the seed (Penfield et al., 2006). Another note of interest with ABA signaling is that genes encoding for ABA receptors each exhibit distinct patterns, but where there is a significant differential expression between tissues, for example PYL4, PYL5, PYL6, and PYR1, they are up-regulated in the CAP.
Seeds with an absence of GA receptors fail to germinate (Griffiths et al., 2006; Willige et al., 2007), and by binding to GA and DELLAs, the latter are degraded to derepress germination (Fig. 4A). Genes encoding these receptors are up-regulated in the CAP, in particular GID1A and GID1C, the latter late in the germination process. Interestingly, the reverse is true with DELLA repressor genes, which are expressed more highly in the radicle, in particular RGA-LIKE PROTEIN3 (RGL3) early in the germination process. In Arabidopsis, RGL3 represses testa rupture in response to changes in GA and ABA levels (Piskurewicz and Lopez-Molina, 2009). As with the ABA receptors, each of these genes displays a different temporal pattern, suggesting that regulation occurs through a complex mix of subtle controls with the potential to be highly responsive to the prevailing conditions. Regulation clearly does not result solely from a simple hormone balance but additionally through differing spatial and temporal sensitivity to these hormones generated in the hormone signaling networks.
There Is a Complex Pattern of Gene Expression Linked to Proteins Associated with Cell Wall Modification That Underlies CAP Weakening
Many of the differences in gene expression between the CAP and RAD are likely to result from the functional specialization of the whole endosperm as an embryo nutritional tissue during seed development and the RAD as a growing tissue in the germinating seed. However, the CAP in Lepidium is specifically associated with the regulation of germination through endosperm weakening (Müller et al., 2006; Linkies et al., 2009), which requires cell wall modification by cell wall-remodeling proteins (CWRPs). Table II indicates that a similar number of genes in the TAGGIT cell wall modification category are up-regulated in the two tissues at different time points. The total number of genes at all time points is 76 and 71 for CAP and RAD, respectively. However, on closer inspection, there are very different patterns to the expression of CWRP genes in the two tissues (Fig. 5; Supplemental Fig. S1).
Figure 5.
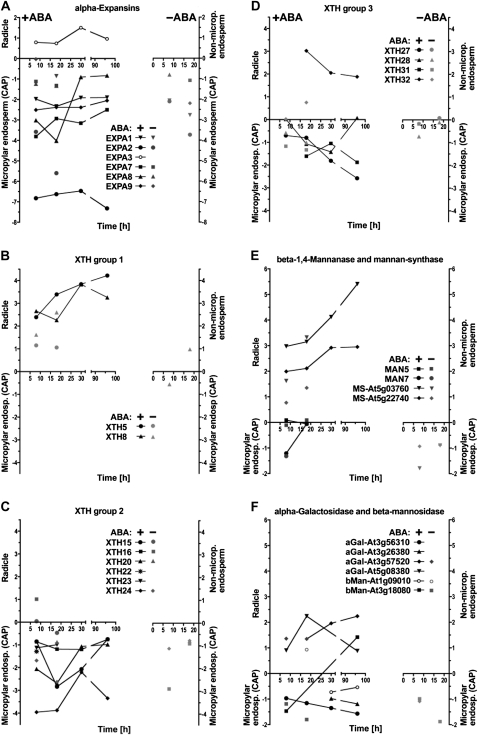
Relative abundance of transcripts in the CAP, RAD, or NME for genes related to cell wall modification. A logarithmic scale is used to quantitatively indicate if a transcript is more abundant in the CAP (below the x axes, negative value; P ≤ 0.1), RAD, or NME (above the x axes, positive value; P ≤ 0.1). A, α-Expansins. B, XTH group 1. C, XTH group 2. D, XTH group 3. E, β-1,4-Mannanase and mannan synthase. F, α-Galactosidase and β-mannosidase. The key identifies genes and which arrays (+ABA or −ABA array, left [+/−ABA] or right [−ABA] part of graphs, respectively) the data are from.
Plant cell expansion growth is driven by water uptake and restricted by the cell wall. The mechanical strength of the plant cell wall determines the shape and the rate and direction of growth of individual cells as well as the mechanical resistance of whole tissues (Fry, 2004; Cosgrove, 2005; Schopfer, 2006; Knox, 2008). The primary cell wall has a fiberglass-like structure with crystalline cellulose microfibrils that are embedded in a matrix of complex polysaccharides, which are divided into two classes: hemicelluloses and pectins. Hemicelluloses are cellulose-binding polysaccharides that, together with cellulose, form a network that is strong yet resilient. Pectins form hydrated gels that push microfibrils apart, easing their sideways slippage during cell growth while also locking them in place when growth ceases. They are important determinants of wall porosity and wall thickness, and they glue cells together in an adhesive layer called the middle lamella. Known wall-remodeling mechanisms include reactive oxygen species-mediated polysaccharide scission, and CWRP actions include enzymatic hydrolysis, transglycosylation, and expansin action. Cell wall loosening is an important developmental process in all stages of plant development, requiring elongation growth or tissue weakening. Examples include seed germination (Bewley, 1997; Nonogaki et al., 2007; Müller et al., 2009), seedling elongation growth (Schopfer, 2006; Schopfer and Liszkay, 2006), and fruit ripening (Fry et al., 2001; Saladié et al., 2007).
Ikuma and Thimann (1963) in their “hatching hypothesis” of seed biology suggested that “the final step in the germination control process is the production of an enzyme whose action enables the tip of the radicle to penetrate through the coat.” In searching for this “hatching enzyme,” evidence has been uncovered for the contribution of various CWRPs, including endo-β-1,4-mannanases (Bewley, 1997; Nonogaki et al., 2000; Iglesias-Fernández et al., 2011) and endo-β-1,3-glucanases (Leubner-Metzger, 2002, 2003; Petruzzelli et al., 2003), as well as for reactive oxygen species (Müller et al., 2009), but most of this work was in solanaceous seeds. However, endosperm weakening is also evident in Brassicaceae seeds, where it is promoted by GA and ethylene and inhibited by ABA (Debeaujon and Koornneef, 2000; Debeaujon et al., 2000; Müller et al., 2006; Bethke et al., 2007; Linkies et al., 2009; Iglesias-Fernández et al., 2011). Based on the timing of GA-inducible transcripts in whole seeds of Arabidopsis, many CWRP genes that remodel hemicellulose are expressed during the early germination phase (Ogawa et al., 2003; Nonogaki et al., 2007). Our tissue-specific transcriptome analysis with Lepidium (Linkies et al., 2009; this work) shows that many of the bigger CWRP families exhibit complex temporal and spatial expression patterns that are presented in the Supplemental Data. Therefore, we restrict our subsequent discussion to a selection of early-expressed hemicellulose-related genes that are abundant during CAP weakening.
Expansins
Expansins are plant cell wall-loosening proteins that disrupt noncovalent bonds that tether matrix polysaccharides to the surface of cellulose microfibrils or to each other (Sampedro and Cosgrove, 2005; Choi et al., 2006). Whatever their biochemical mechanism of action, expansins act in catalytic amounts to stimulate wall polymer creep without causing major covalent alterations of the cell wall. The α-expansins (EXPA) act with a pH optimum around 4. They have possible roles in developmental processes like organ size and elongation growth, fruit tissue softening, and seed germination (Sampedro and Cosgrove, 2005; Choi et al., 2006; Gaete-Eastman et al., 2009; Lizana et al., 2010). Transcripts of the tomato (Solanum lycopersicum) α-expansin SlEXPA4 and its putative ortholog in Datura ferox were specifically expressed in the micropylar endosperm in association with endosperm weakening (Chen and Bradford, 2000; Mella et al., 2004). This transcript expression was promoted by GA but not inhibited by ABA. Where there is a significant difference in the level of expression between the tissues, the majority of Lepidium expansin genes (Fig. 5A; Supplemental Fig. S1A) are expressed more highly in the CAP than in the RAD. This is particularly true for the α-expansins EXPA1, -2, -7, -8, and -9, for which in the +ABA arrays the transcript levels are higher in the CAP compared with the RAD at all time points (Fig. 5A); only EXPA3 was RAD specific. The –ABA arrays show that EXPA1, -2, and -9 are specifically expressed in the CAP but not in the NME. ABA does not down-regulate any of these CAP-specific α-expansin genes. During the early phase of Arabidopsis seed germination, transcripts of AtEXPA1, -2, -8, and -9 accumulate 100- to 500-fold (from 0 to 12 h in whole unstratified seeds), and this induction is promoted by GA, not inhibited by ABA, and mainly localized in the endosperm (Nakabayashi et al., 2005; Penfield et al., 2006; Carrera et al., 2008; Holdsworth et al., 2008a; Preston et al., 2009). In summary, transcript expression analysis during germination of both Lepidium and Arabidopsis shows that α-expansin genes, in particular EXPA2, are induced early in the endosperm cap prior to the onset of weakening and are involved in ABA-insensitive processes that lead to testa rupture and cap weakening (Linkies et al., 2009; this work). Based on their temporal, spatial, and hormonal expression patterns, α-expansins are likely to contribute to processes in the CAP prior to and during endosperm weakening and rupture, but they do not confer the ABA regulation of these processes.
Xyloglucan Endotransglycosylase/Hydrolase
Xyloglucan endotransglycosylase/hydrolase (XTHs) modify xyloglucans, which are part of the hemicellulose network believed to cross-link cellulose microfibrils (Fry, 2004; Cosgrove, 2005). Xyloglucan is the primary XTH substrate, and most XTHs exhibit XET (transferase) activity that breaks and remakes glycosidic bonds in the backbone of xyloglucan. Some XTHs also exhibit XEH (hydrolase) activity and mediate xyloglucan strand breaks, and a few exhibit only XEH activity. Direct unambiguous proof of XTHs inducing wall stress relaxation and extension is still lacking. However, XTHs are implicated in cell wall hemicellulose remodeling leading to loosening (Van Sandt et al., 2007) or stiffening (Maris et al., 2009). XTHs are proposed to have roles in many developmental processes, including cell growth, fruit ripening, and reserve mobilization following germination of xyloglucan-storing seeds (Tiné et al., 2000; Fry, 2004; Nonogaki et al., 2007; Van Sandt et al., 2007). During tomato seed germination, transcript accumulation of SlXET4 was induced in the micropylar endosperm, promoted by GA, but not inhibited by ABA (Chen et al., 2002). The phylogenetic relationship between the 33 members of the Arabidopsis XTH gene family reveals three groups (http://labs.plantbio.cornell.edu/XTH/arabidopsis.html; Becnel et al., 2006). Several XTH genes of Lepidium showed tissue-specific expression during seed germination (Fig. 5, B–D; Supplemental Fig. S1B). The group 2 XTHs 15, 16, 20, 22, 23, and 24 and group 3 XTHs 27, 28, and 31 exhibit stronger expression in the CAP compared with the RAD. In contrast, the group 1 XTHs 5 and 8 and the group 3 XTH32 exhibit stronger expression in the RAD compared with the CAP. During the early phase of Arabidopsis seed germination, of the above-mentioned genes, only transcripts of AtXTH5, -16, and -27 accumulated significantly at 6 h, and AtXTH15, -22, -28, and -31 accumulated significantly at 12 h, while AtXTH20, -23, and -24 were not induced. Only XTH15, -16, -5, and -31 were induced by GA, and only XTH24 was down-regulated by ABA (Nakabayashi et al., 2005; Preston et al., 2009). In summary, transcript expression analysis during seed germination of both Lepidium and Arabidopsis shows that XTH genes are expressed in a complex manner suggesting distinct roles in the RAD and CAP.
Mannans
Mannans are rigidity- and mechanical strength-conferring hemicellulosic polysaccharides present in the endosperm of many seeds (Bewley, 1997; Reid et al., 2003; Nonogaki et al., 2007). The endosperm cell walls of solanaceous seeds contain approximately 60% Man and approximately 10% Gal as galactomannans. Coffee (Coffea arabica) galactomannan contains only approximately 2% Gal, which results in hard and brittle endosperm properties. Mannan polysaccharides could be masked, and this may have prevented the detection of mannan epitopes in Arabidopsis seeds, but genetic evidence has strongly indicated a functional role for mannan in seed development and germination of this species (Marcus et al., 2010; Iglesias-Fernández et al., 2011). Galactomannan biosynthesis in seed endosperms involves β-1-4-mannan synthase and galactomannan galactosyltransferase (Reid et al., 2003; Edwards et al., 2004). The β-1,4-mannan synthases are encoded by the cellulose synthase-like A (CSLA) gene family (Dhugga et al., 2004; Liepman et al., 2005). AtCSLA2 transcripts accumulated in germinating Arabidopsis seeds in a GA-promoted and ABA-unaffected manner (Nakabayashi et al., 2005; Preston et al., 2009). In Lepidium, CSL2 and CSL9 showed a radicle-specific expression during seed germination (Fig. 5E; Supplemental Fig. S1C).
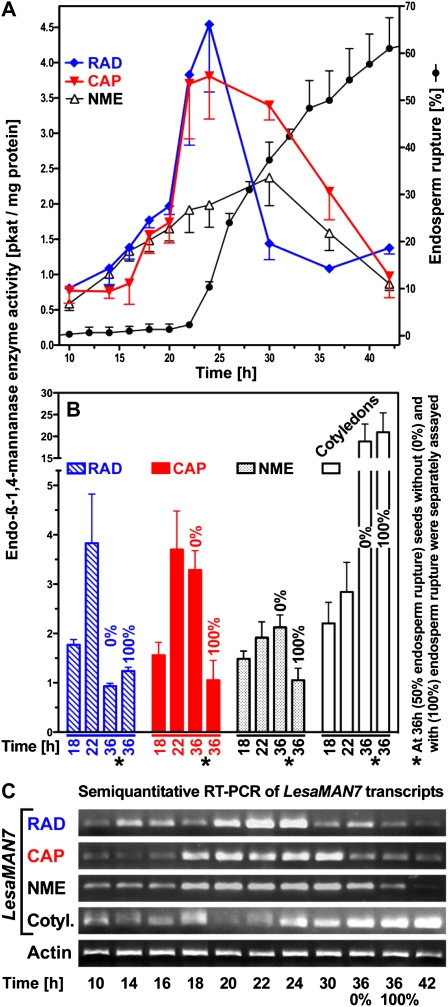
Degradation of mannan and galactomannan polymers involves endo-β-1,4-mannanase, α-galactosidase, and β-mannosidase, all of which have been identified in germinating seeds; several endo-β-1,4-mannanases have hydrolase and endotransglycosylase activity (Schröder et al., 2009). Among the many endo-β-1,4-mannanase isoforms of tomato, the SlMAN2 gene is expressed specifically in the micropylar endosperm prior to radicle emergence in association with enzyme activity accumulation (Nonogaki et al., 2000; Toorop et al., 2000; Gong and Bewley, 2007). This induction is promoted by GA but not inhibited by ABA. Endo-β-1,4-mannanase also accumulates in the micropylar endosperm of Solanum lycocarpum, D. ferox, and coffee and is thought to contribute to endosperm weakening (Bewley, 1997; Nonogaki et al., 2000; Toorop et al., 2000; da Silva et al., 2004; Arana et al., 2006; Pinto et al., 2007). Endo-β-1,4-mannanase enzyme activities of individual tomato micropylar endosperm caps vary at least 100-fold (Still and Bradford, 1997). Although the presence of endo-β-1,4-mannanase enzyme activity in the tomato endosperm cap is consistently associated with radicle emergence, it is not the sole or limiting factor under all conditions. Seed germination of tomato lines overexpressing an endo-β-1,4-mannanase was not promoted (Belotserkovsky et al., 2007). Seed-specific regulation of several endo-β-1,4-mannanases is also known from rice (Oryza sativa; Yuan et al., 2007; Ren et al., 2008). Seven endo-β-1,4-mannanase genes are known in Arabidopsis (Yuan et al., 2007), but of these, only AtMAN7 (At5g66460) transcripts accumulated in whole unstratified seeds, and this induction is promoted by GA but not inhibited by ABA (Nakabayashi et al., 2005; Preston et al., 2009). In agreement with this, transcripts of the Lepidium MAN7 accumulated in the CAP and to a lesser extent in the RAD during seed germination (Fig. 5E; Supplemental Fig. S1C). This induction was not inhibited by ABA, and at 8 h it was stronger in CAP than in the RAD and the NME (Fig. 5E). Figure 6, A and B, show that endo-β-1,4-mannanase enzyme activity accumulated in the CAP and the RAD but less in the NME prior to endosperm rupture. This increasing activity is, at least in part, due to LesaMAN7, as the transcript expression pattern is regulated in a similar manner (Fig. 6C). Late during germination and after endosperm rupture, endo-β-1,4-mannanase enzyme activity and LesaMAN7 transcripts also accumulate in the cotyledons (Fig. 6). In agreement with a role for LesaMAN7 in germination, a recent study shows that Arabidopsis knockout mutants for AtMAN7, -6, and -5 had slower germination than the wild type (Iglesias-Fernández et al., 2011). In seeds of Sisymbrium officinale, which is also a Brassicaceae species, endo-β-1,4-mannanase enzyme activity accumulated in an ethylene- and GA-promoted manner (Iglesias-Fernández and Matilla, 2009).
Figure 6.
Endo-β-1,4-mannanase enzyme activity during CAP weakening. A, Time course of endo-β-1,4-mannanase enzyme activity in separate tissues. B, Endo-β-1,4-mannanase enzyme activity in the RAD, CAP, NME, and cotyledons at three time points. C, The pattern of LesaMAN7 transcript expression in different tissues during CAP weakening. Note that in B and C, values at 36 h are measured separately in tissues from seeds with and without CAP rupture. GenBank accession numbers for LesaMAN7 and LesaACT7 are HQ436349 and HQ436350, respectively. RT, Reverse transcription. [See online article for color version of this figure.]
α-Galactosidases and β-mannosidases contribute to seed galactomannan degradation (Bewley, 1997; Feurtado et al., 2001; Nonogaki et al., 2007), and β-mannosidase enzyme activity has been detected in the micropylar endosperm of tomato seeds, Datura, and coffee (de Miguel et al., 2000; Mo and Bewley, 2002; da Silva et al., 2005). In Arabidopsis seeds, At3g18080 β-mannosidase transcripts accumulate mainly in embryo and At3g57520 α-galactosidase transcripts were abundant between 3 and 24 h in whole seeds (Nakabayashi et al., 2005; Penfield et al., 2006; Preston et al., 2009). Transcripts of Lepidium α-galactosidases and β-mannosidases had complex tissue-specific patterns (Fig. 5F). Taken together, these results support a role for endo-β-1,4-mannanase during the germination of endospermic Brassicaceae seeds.
Cellulase (Endo-β-1,4-Glucanase)
Cellulase (endo-β-1,4-glucanase) activity was detected in tomato, Datura, and coffee seeds (Sanchez et al., 1986; da Silva et al., 2004; Nonogaki et al., 2007). In Datura and coffee, but not in tomato, this was in association with endosperm weakening and germination. Several putative orthologs of Arabidopsis showed a CAP-specific expression pattern during seed germination of Lepidium, while orthologs of At1g70710 and At1g64390 showed a RAD-specific expression (Supplemental Fig. S1D). During the early phase of Arabidopsis seed germination, transcripts of At1g70710 and At1g64390 accumulate approximately 100-fold (from 0 to 24 h of whole unstratified seeds), and this induction is promoted by GA but not appreciably inhibited by ABA (Nakabayashi et al., 2005; Preston et al., 2009). Tomato endosperm cell walls contain up to 10% Ara but little Xyl. Transcripts of a β-d-xylosidase accumulated in the embryo of germinating tomato seeds (Itai et al., 2003; Nonogaki et al., 2007). In Lepidium, β-d-xylosidases (At1g02640, At5g64570, At1g78060, and At5g10560) and α-d-xylosidases showed RAD-specific expression during seed germination, while the β-d-xylosidase At5g49360 was higher in the CAP (Supplemental Fig. S1D). In Arabidopsis, transcripts of β-d-xylosidases accumulated more than 100-fold (from 0 to 24 h of whole unstratified seeds), while the β-d-xylosidase At5g49360 was more than 20-fold induced in the endosperm, and these inductions were promoted by GA but not inhibited by ABA (Nakabayashi et al., 2005; Preston et al., 2009). The transcript expression pattern of pectin-related enzymes in Lepidium has already been discussed by Linkies et al. (2009).
Genes Relating to Protein Degradation and Posttranslational Modification Are Important in the Regulation of Cell Wall Modification
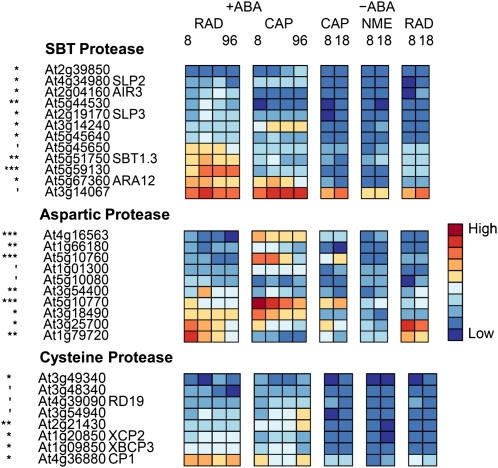
From a review of recent postgenomic data, Holdsworth et al. (2008b) concluded that RNA translation and posttranslational modification provide major levels of control for germination completion. However, there are similar numbers of genes up-regulated in both tissues in the TAGGIT category protein degradation (Table II), but as discussed above, this similarity obscures important differences in details. There are 620 genes tagged in this category, of which 76 are significantly differentially expressed between the two tissues, with 34 and 42 expressed more highly in the CAP and RAD, respectively (Supplemental Fig. S2, A and B). Closer inspection of this cohort of genes reveals a prominent role for the Asp and subtilase families of plant proteases. Therefore, we looked at genes from all members of these two families of plant proteases and included the Cys protease family of enzymes. Members of these classes of proteases are reported by Beers et al. (2004). Figure 7 summarizes those members that are significantly (P < 0.10) differentially expressed between the two tissues. The SBT protease members are predominantly overrepresented in the RAD, while the significant Asp proteases are mainly overrepresented in the CAP. We have also investigated the expression of genes encoding key enzymes in protein modification involving the ubiquitin/26S proteasome E3 ligases, specifically the F-box and REALLY INTERESTING NEW GENE (RING) finger proteins (Supplemental Fig. S2, C and D).
Figure 7.
Heat maps showing relative abundance of transcripts from genes encoding proteases that are differentially expressed between seed tissues. ′, *, **, and *** indicate that transcript numbers are significantly different between the tissues on +ABA arrays at P < 0.1, P < 0.05, P < 0.01, and P < 0.001, respectively.
SBT Proteases
Subtilases are a diverse family of Ser proteases, which number 56 in Arabidopsis and have a high degree of gene duplication and associated redundancy (Rautengarten et al., 2005). This functional redundancy has made it difficult to associate biological function to individual genes, with only two knockout mutants, stomatal density and development1 (sdd1) and abnormal leaf shape (ale1), having recognizable phenotypes. It has been postulated that these encode proteins (SDD1 and ALE1) that act as proprotein convertases yielding bioactive peptides (Berger and Altmann, 2000; Tanaka et al., 2001). In Lepidium, Figure 7 shows predominantly greater expression of the subtilase gene family members in the RAD than in the CAP; one such transcript in Arabidopsis, AUXIN-INDUCED IN ROOT CULTURES3 (At2g04160), has been linked to lateral root emergence (Neuteboom et al., 1999). In Arabidopsis, more than 20 of the family members have been shown to be transcriptionally regulated by light, with the expression of At2g39850 and At5g59130 demonstrating sole dependence on PHYA under far-red light for induction (Zhou, 2009). In Lepidium, both these subtilases are expressed significantly higher in the RAD, whereas PHYA is expressed significantly more highly in the CAP (Fig. 5B), suggesting the possibility of signaling between the two tissues regulated by light.
Asp Proteases
There are 59 Asp proteases identified among the annotated Arabidopsis genes, and little is known about their biological roles (Beers et al., 2004). Subcellular localization may help to elucidate their physiological functions, and a number have been located in the intracellular fluid of the apoplast, with a role in disease resistance signaling (Xia et al., 2004). There is also evidence for a role in seeds. Mutlu et al. (1999) characterized an Asp protease from dry seeds of Arabidopsis and colocated it with the seed storage protein 2S albumin and the vacuolar marker enzyme α-mannosidase. Molecular studies of osmoprimed seeds of cauliflower (Brassica oleracea; Fujikura and Karssen, 1995) identified two Asp proteases with enhanced expression upon priming. A proteomic analysis of Lepidium CAP tissue (Müller et al., 2010) identified Asp proteases as a main class of proteins involved in storage protein degradation. The abundance of one Asp protease was shown to increase from 8 to 18 h during the period of CAP weakening. The authors concluded that this early mobilization of protein bodies in the cap is likely to serve a nonnutritional function in the control of germination (Müller et al., 2010). These observations are consistent with the CAP-specific expression of a number of putative Asp protease transcripts within our data set (Fig. 7).
Cys Proteases
A number of Cys proteases that are involved in seed germination have been described in the literature (Cervantes et al., 1994; Helm et al., 2008). Ethylene was shown to induce the expression of a Cys protease responsible for the catabolism of major reserve proteins (Cervantes et al., 1994). Helm et al. (2008) have reported a number of KDEL-Cys proteases involved in programmed cell death and the dismantling of extension scaffolds. This led the authors to the hypothesis that the KDEL-tailed Cys proteases they identified participate in the final cell collapse during programmed cell death by attacking the structural Hyp-rich glycoproteins of the cell wall. In Lepidium, transcript numbers of one of these KDEL-tailed Cys proteases, At3g48340, was significantly up-regulated in the CAP (Fig. 7).
RING Finger E3 Ligases and F-Box Proteins
E3 ligases are the components of the 26S proteasome that confer substrate specificity to the system. The ubiquitin/26S proteasome pathway is important to most aspects of plant biology (Vierstra, 2009), including hormonal signaling (Frugis and Chua, 2002). There are 697 F-box proteins (Gagne et al., 2002) and 469 RING finger proteins (Stone et al., 2005) in the Arabidopsis genome, of which we have transcriptional data for 333 and 327 putative orthologs, respectively, in our Lepidium data set. Transcript abundances from this set that are significantly differentially expressed between the two tissues are shown in Supplemental Figure S2, C and D. There was a greater proportion of both F-box- and E3 ligase-encoding genes up-regulated in the CAP compared with the RAD (2.3- and 2.7-fold, respectively). This suggests that posttranslational modification, in the form of selected proteolysis, performs a more significant role in CAP weakening than in the developing RAD. The role of the 26S proteasome pathway in light and hormonal signaling is well characterized in plants (Vierstra, 2009), and this apparent enrichment of E3 ligase mRNAs may strengthen the argument for these environmental cues playing a substantial role in endosperm weakening and signaling to the developing seedling.
The Effect of Protease Inhibitors
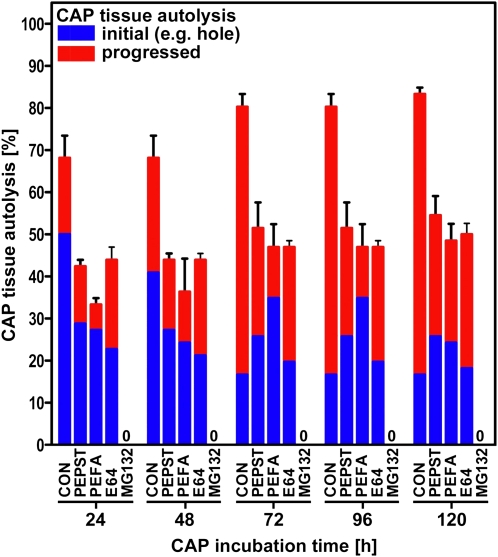
To investigate the role of these proteins in protein degradation, we have monitored the progression of CAP hole formation, as described above, when incubated upon specific protease inhibitors. The CAPs isolated after 12 h of imbibition were then incubated upon 1 μm pepsatin, 4 mm Pefabloc, 28 μm E-64, and 60 μm MG132, which inhibit Asp, Ser, and Cys proteases and the 26S proteasome, respectively (Fig. 8). It is clear from these data that all four classes of proteases investigated have a pronounced affect on endosperm weakening, with the most dramatic effect being the complete cessation of autolysis by the proteasomal inhibitor MG132. The other three inhibitors reduced the number of CAPs exhibiting autolysis to approximately 50% of that shown in the control (84%) over the same time period. This suggests that each class of protease has a specific protein target, and numerous protein targets may be required for complete lysis.
Figure 8.
The effect of inhibitors of specific proteases (pepstatin, Pefabloc, and E64) and a proteasome inhibitor (MG132) on the progression of CAP autolysis. Initial autolysis represents hole formation or tip abscission (Fig. 2C); progressed autolysis represents autolysis of the whole CAP (Fig. 2D). [See online article for color version of this figure.]
The complete inhibition of hole formation and tissue autolysis in those CAPs treated with MG132 suggests that targeted protein degradation is a major control point for endosperm weakening. It is now well established that the ubiquitination pathway plays a role in various hormonal signaling pathways and is involved in the regulation of germination through the degradation of DELLA proteins (Dill et al., 2001); therefore, it could be hypothesized that inhibition of the degradation of an important transcription repressor prevents the cascade of transcriptional activity that we show in our array data. It was recently demonstrated that ABA inhibits CAP hole formation (Linkies et al., 2009), and we show here that the proteasome inhibitor MG132 completely blocks cap hole formation and autolysis. This suggests the involvement of a key transcriptional repressor implicated in several signaling pathways as well as in ABA signaling. Several ubiquitin ligases have been linked to ABA signaling and have the transcription factors ABI3 and ABI5, both important regulators of seed germination, as targets for proteolysis (Lopez-Molina et al., 2003; Holdsworth et al., 2008a; Santner and Estelle, 2010). ABI5 and ABSCISIC ACID INSENSITIVE 5 BINDING PROTEIN (AFP) mRNA and protein levels increase when seeds are treated with ABA, and mutants for ABI5 and ABSCISIC ACID RESPONSIVE ELEMENTS-BINDING FACTOR exhibit seed phenotypes. NOVEL INTERACTOR OF JAZ (NINJA) and AFP are related proteins, and it has been proposed in recent work that the Groucho/Tup1-type family corepressors, including TOPLESS (TLP), are part of a general repressor machinery implicated in several signaling pathways (Liu and Karmarkar, 2008; Pauwels et al., 2010). For jasmonic acid and ABA signaling, NINJA and AFP are proposed to mediate the interaction between transcription factors, ABI5 for ABA, and TLP. The TLP-type corepressors have general functions in plant hormone signaling that are related to transcription factor proteolysis. Supplemental Figure S2C emphasizes the influence of the proteasomal degradation pathway on hormonal signaling. There was significant differential expression between CAP and RAD of genes related to auxin (AUXIN SIGNALING F-BOX3, TRANSPORT INHIBITOR RESPONSE1 [TIR1], and MORE AXILLARY BRANCHES2), jasmonic acid (CORONATINE INSENSITIVE1), and ethylene (EIN3-BINDING F BOX PROTEIN1). All these except TIR1 showed elevated levels of expression in the CAP.
CONCLUSION
We have shown that following rupture of the testa, germination in Lepidium is regulated by the opposing forces of RAD extension and the resistance to this by the surrounding CAP, which progressively declines through autolysis. By 18 h, some seeds have completed autolysis (germination has occurred), but even at this late stage, progress in some seeds can be stopped by inhibiting transcription, and the remainder can be stopped by blocking translation and posttranslational changes. Taken together, these results suggest that this is a control point for germination completion that is very late in the germination process. This late control point, therefore, acts as a gateway to seedling development, but the rate of germination must be determined earlier in the process, as seeds reach this control point at different times, and thus weakening does not determine vigor. Late control is a necessary feature that prevents inappropriate germination when environmental conditions change. The expression of genes involved in hormone signaling networks was shown to have different temporal and spatial patterns consistent with establishing a complex responsive regulation through subtle changes in hormone sensitivity rather than through a crude hormone balance. Genes encoding CWRPs were also expressed in a complex, tissue-specific manner during endosperm weakening, which would allow subtle regulation of weakening and therefore germination completion in response to hormone signals driven by the current ambient environment.
MATERIALS AND METHODS
Plant Material, Germination, and Puncture-Force Measurements
After-ripened Lepidium sativum FR1 (‘Gartenkresse, einfache’) and FR14 (‘Keimsprossen’) seeds (Juliwa) were incubated in petri dishes on two layers of filter paper with 6 mL of one-tenth-strength Murashige and Skoog salts as medium in continuous white light (approximately 100 μmol m−2 s−1) as described by Müller et al. (2006) at the temperatures indicated. Testa rupture and endosperm rupture were scored using a binocular microscope. Puncture-force measurements were performed as described by Müller et al. (2006).
Inhibitor Studies on Endosperm Hole Formation and Autolysis
After-ripened seeds of Lepidium FR1 were incubated in petri dishes on two layers of filter paper with 6 mL of one-tenth-strength Murashige and Skoog salts as medium in continuous white light (approximately 100 μmol m−2 s−1) at 18°C. After 10, 12, and 18 h, the micropylar endosperm was dissected from the seeds for further incubation on 500 μm cycloheximide (Sigma) or 1 μg mL−1 α-amanitin (Sigma). Cycloheximide was dissolved in 50% acetone. Following dilution, 0.1% acetone remained, so this same amount was added to all treatments and the control. Preliminary work determined that this concentration had no influence on germination, hole formation, or radicle growth. In a second experiment, dissection at 12 h was followed by incubation on 1 μm pepstatin (Roche), 4 mm Pefabloc (Roche), 28 μm E64 (Roche), and 60 μm MG132 (Merck). The concentrations used were those recommended by the manufacturer. In preliminary work, 10-fold lower concentrations were also used to test for a lower dose response. The inhibitors were dissolved in methanol, water, water-ethanol, and dimethyl sulfoxide, respectively. Controls for each inhibitor differed and contained the appropriate chemical at less than 0.05%. For every inhibitor and control, at least three replicates of 20 micropylar endosperm caps each were incubated in small petri dishes on two layers of filter paper with 2.5 mL of one-tenth-strength Murashige and Skoog salts as medium with the indicated inhibitor in continuous white light (approximately 100 μmol m−2 s−1) at 18°C. Experiments were repeated to confirm results. Analysis of endosperm autolysis was determined at the times indicated by two categories: beginning of autolysis (initial autolysis) was recorded as soon as one hole was visible, and in nearly all cases that happened just below the tip; progression of autolysis was recorded when more than one hole was visible, which later led to autolysis, resulting in digestion of whole parts of the endosperm.
Endo-β-1,4-Mannanase Enzyme Activity Assay
Seed tissues (RAD, CAP, NME, and cotyledons) were ground in 0.1 m HEPES-0.5 m NaCl buffer (pH 7.5) using an ice-cold mortar. The volume of the HEPES buffer was added at the ratio of fresh weight of tissues (mg):buffer volume (mL) = 1:3. The extract was centrifuged at 4°C for 10 min at 10,000 rpm, and the supernatant was used to assay the activity of endo-β-mannanase as described by Bourgault and Bewley (2002).
Semiquantitative Reverse Transcription-PCR
One microgram of RNA was reverse transcribed using oligo(dT) primer according to the PrimerScript Reverse Transcriptase Kit instructions (TaKaRa). Aliquots of these first-strand cDNAs as templates were used in subsequent PCRs. For the semiquantitative PCR analysis, template volumes were determined that result in equal amplification for the actin reference gene for each sample. For actin, optimal conditions were 27 amplification cycles with 52°C as annealing temperature, forward primer 5′-CTAAAGCCAACAGGGAGA-3′, and reverse primer 5′-TTGGTGCGAGTGCGGTGA-3′. The template volumes determined for actin were used for the semiquantitative PCR analysis of the endo-β-1,4-mannanase (52°C annealing temperature, 35 amplification cycles, forward primer 5′-ACCGATTTCATTGCCAATAACCG-3′, and reverse primer 5′-TGTCGACTTTGTGGCATCAGAGA-3′).
RNA Isolation from Lepidium Seed Tissues
For each sample, approximately 1,000 Lepidium CAP, approximately 1,000 NME, or approximately 100 RAD were collected at the times indicated, frozen in liquid nitrogen, and stored at −80°C. Total RNA extraction was carried out by the cetyl-trimethyl-ammonium bromide method followed by quantity and quality control analyses as described (Chang et al., 1993). Four biological replicate RNA samples were used for downstream applications.
Microarray Experimental Design
We carried out two separate microarray experiments. The first compared CAP and RAD at 8, 18, 30, and 96 h of imbibition on 10 μm ABA and were termed +ABA arrays. The second compared CAP, NME, and RAD at 8 and 18 h of imbibition on germination medium without ABA and were termed –ABA arrays. Each experiment used four biological replicates. Hybridizations were carried out according to the description below and Linkies et al. (2009). For the −ABA array experiment, the two time points for each tissue were directly compared on four microarrays, balanced for color. For each tissue in the +ABA array experiment, all time points were directly compared with each other on one microarray each, and for each time point, the two tissues were compared on one microarray. Each treatment was balanced for color. This design can be thought of as four interlinked loops.
Cross-Species CATMA Microarrays and Lepidium RNA Hybridization
RNA was prepared in the following way for microarray hybridization. The Ambion MessageAmp II aRNA Amplification Kit (AM1751; Applied Biosystems) was used according to the manual, with 2 μg of Lepidium FR1 total RNA as template to generate antisense amplified RNA, called aRNA (Van Gelder et al., 1990). The quality and quantity of the aRNA was checked by running an aliquot on a 2100 Bioanalyzer (Agilent). The microarrays used carried GST fragments generated using gene-specific primers identified by the CATMA Consortium (http://www.catma.org; Hilson et al., 2004; Allemeersch et al., 2005). CATMA version 2 arrays with 24,576 GSTs were used for the –ABA array experiment, while CATMA version 3 arrays with 30,343 GSTs were used for the +ABA array experiment. The aRNA was labeled and the CATMA microarrays were hybridized according to the method described by Lim et al. (2007) and Linkies et al. (2009). The microarrays were scanned using an Affymetrix 428 array scanner at 532 nm (Cy3) and 635 nm (Cy5). Scanned data were quantified using Imagene version 4.2 software (BioDiscovery; http://www.biodiscovery.com/). Microarray data were deposited in ArrayExpress (http://www.ebi.ac.uk/microarray/) under accessionpt?> numbers E-TABM-745 (−ABA arrays) and E-TABM-743 (+ABA arrays).
RNA Microarray Data Handling and Analysis
Data from the two experiments (+ABA arrays and −ABA arrays) were analyzed separately using a similar approach but differing in line with the different experimental designs used and the availability of genomic DNA hybridization data for CATMA version 3 arrays used in the ABA experiment (Linkies et al., 2009). In both cases, spot intensity data from Imagene were analyzed using the limma package in Bioconductor (Smyth, 2005). There was an initial screen to the data that removed all probes that could not be assigned to an Arabidopsis (Arabidopsis thaliana) gene defined by having an AGI identifier and associated with this a TAIR 7 GO annotation (http://www.arabidopsis.org). Background correction was performed using the “normexp” method, which is analogous to robust multiarray average. Within-array normalization (Smyth and Speed, 2003) was performed using print tip loess. In the −ABA array experiment, between-array normalization was performed using quantile normalization on the “A” values. For the +ABA array experiment, probes that had shown no significant response in the genomic DNA microarrays (Linkies et al., 2009) were weighted out of the normalization and analysis. The two filtering steps resulted in lists (Supplemental Tables S2 and S3) containing 19,794 and 22,025 genes for the +ABA arrays and −ABA arrays, respectively. These gene lists were then used in all downstream analyses. For both experiments, the data were analyzed as a linear model (Smyth, 2004), and for the −ABA arrays experiment, the analysis was adjusted for the intraspot correlation. The variance estimates were adjusted using empirical Bayes estimates of the per-spot variability for use in differential expression analyses. Estimates of the transcript numbers (intensities) for individual spots (genes) on the +ABA and −ABA arrays (Supplemental Tables S1 and S2) were compared across sample time points and tissues using F tests to identify those whose intensity had significantly changed. Statistical significance of differences was assessed using the approach of Benjamini and Hochberg (1995) to control the false discovery rate at the level of 10%. The genes identified were considered to be up- or down-regulated between tissues at the same time or different time points. PCA was performed in R for each experiment to compare the tissues and time points across the probes.
Functional Categorization of Genes
Gene lists were created for genes that were significantly expressed (present) on the arrays and significantly up- or down-regulated between tissues using procedures described at appropriate points in the text. In order to investigate whether these genes were linked to functional specialization categories, the lists were subjected to the GO-based established seed-specific TAGGIT workflow (Carrera et al., 2007) to identify proportional representations of genes in functional categories.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers HQ436369 (LesaMAN7) and HQ436350 (LesaACT7).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Heat maps showing the relative abundance of transcripts from genes encoding cell wall modification proteins.
Supplemental Figure S2. Heat maps showing the relative abundance of transcripts from genes encoding proteins associated with posttranslational modification.
Supplemental Table S1. Effect of α-amanitin and cycloheximide on radicle growth.
Supplemental Table S2. Normalized mean abundance of transcripts from genes identified as present and used in the analysis of +ABA arrays (19,794 genes).
Supplemental Table S3. Normalized mean abundance of transcripts from genes identified as present and used in the analysis of –ABA arrays (22,025 genes).
Supplemental Table S4. Proportional representation in functional categories of expressed (present) genes on +ABA arrays.
Supplemental Table S5. Proportional representation in functional categories of genes with expression on +ABA arrays that is unique to each tissue/time combination.
Supplemental Table S6. Genes on +ABA arrays that show differential expression between tissues.
Acknowledgments
We thank Cassandra Cadman (Warwick University), Meike Wenk and Anita Rott (University of Freiburg), and Jianqing Zhang (South China Agricultural University) for expert technical help. We also thank Alex Tabrett and Jim Beynon for enabling access to CATMA arrays at Warwick University.
References
- Allemeersch J, Durinck S, Vanderhaeghen R, Alard P, Maes R, Seeuws K, Bogaert T, Coddens K, Deschouwer K, Van Hummelen P, et al. (2005) Benchmarking the CATMA microarray: a novel tool for Arabidopsis transcriptome analysis. Plant Physiol 137: 588–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana MV, de Miguel LC, Sánchez RA. (2006) A phytochrome-dependent embryonic factor modulates gibberellin responses in the embryo and micropylar endosperm of Datura ferox seeds. Planta 223: 847–857 [DOI] [PubMed] [Google Scholar]
- Arcila J, Mohapatra SC. (1992) Effect of protein-synthesis inhibitors on tobacco seed-germination and seedling emergence. J Plant Physiol 139: 460–466 [Google Scholar]
- Bar-Or C, Czosnek H, Koltai H. (2007) Cross-species microarray hybridizations: a developing tool for studying species diversity. Trends Genet 23: 200–207 [DOI] [PubMed] [Google Scholar]
- Barrero JM, Talbot MJ, White RG, Jacobsen JV, Gubler F. (2009) Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiol 150: 1006–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel J, Natarajan M, Kipp A, Braam J. (2006) Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol 61: 451–467 [DOI] [PubMed] [Google Scholar]
- Beers EP, Jones AM, Dickerman AW. (2004) The S8 serine, C1A cysteine and Al aspartic protease families in Arabidopsis. Phytochemistry 65: 43–58 [DOI] [PubMed] [Google Scholar]
- Belotserkovsky H, Berger Y, Shahar R, Wolf S. (2007) Specific role of LeMAN2 in the control of seed germination exposed by overexpression of the LeMAN3 gene in tomato plants. Planta 227: 199–209 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300 [Google Scholar]
- Berger D, Altmann T. (2000) A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev 14: 1119–1131 [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL. (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143: 1173–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. (1997) Breaking down the walls: a role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci 2: 464–469 [Google Scholar]
- Bourgault R, Bewley JD. (2002) Gel diffusion assays for endo-β-mannanase and pectin methylesterase can underestimate enzyme activity due to proteolytic degradation: a remedy. Anal Biochem 300: 87–93 [DOI] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Graham NS, Bowen HC, Emmerson ZF, Fray RG, Iannetta PPM, McNicol JW, May ST. (2008) Evidence of neutral transcriptome evolution in plants. New Phytol 180: 587–593 [DOI] [PubMed] [Google Scholar]
- Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, Holdsworth MJ. (2008) Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J 53: 214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Holman T, Medhurst A, Peer W, Schmuths H, Footitt S, Theodoulou FL, Holdsworth MJ. (2007) Gene expression profiling reveals defined functions of the ATP-binding cassette transporter COMATOSE late in phase II of germination. Plant Physiol 143: 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes E, Rodríguez A, Nicolás G. (1994) Ethylene regulates the expression of a cysteine proteinase gene during germination of chickpea (Cicer arietinum L.). Plant Mol Biol 25: 207–215 [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Chen F, Bradford KJ. (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124: 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ. (2002) A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J Exp Bot 53: 215–223 [DOI] [PubMed] [Google Scholar]
- Choi D, Cho HT, Lee Y. (2006) Expansins: expanding importance in plant growth and development. Physiol Plant 126: 511–518 [Google Scholar]
- Comai L, Dietrich RA, Maslyar DJ, Baden CS, Harada JJ. (1989) Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell 1: 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- da Silva EAA, Toorop PE, Nijsse J, Bewley JD, Hilhorst HWM. (2005) Exogenous gibberellins inhibit coffee (Coffea arabica cv. Rubi) seed germination and cause cell death in the embryo. J Exp Bot 56: 1029–1038 [DOI] [PubMed] [Google Scholar]
- da Silva EAA, Toorop PE, van Aelst AC, Hilhorst HWM. (2004) Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta 220: 251–261 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M. (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122: 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel L, Burgin MJ, Casal JJ, Sánchez RA. (2000) Antagonistic action of low-fluence and high-irradiance modes of response of phytochrome on germination and beta-mannanase activity in Datura ferox seeds. J Exp Bot 51: 1127–1133 [DOI] [PubMed] [Google Scholar]
- Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, et al. (2004) Guar seed beta-mannan synthase is a member of the cellulose synthase super gene family. Science 303: 363–366 [DOI] [PubMed] [Google Scholar]
- Dill A, Jung HS, Sun TP. (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards ME, Choo TS, Dickson CA, Scott C, Gidley MJ, Reid JSG. (2004) The seeds of Lotus japonicus lines transformed with sense, antisense, and sense/antisense galactomannan galactosyltransferase constructs have structurally altered galactomannans in their endosperm cell walls. Plant Physiol 134: 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurtado JA, Banik M, Bewley JD. (2001) The cloning and characterization of alpha-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. J Exp Bot 52: 1239–1249 [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. (2006) Seed dormancy and the control of germination. New Phytol 171: 501–523 [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59: 387–415 [DOI] [PubMed] [Google Scholar]
- Franzke A, German D, Al-Shehbaz IA, Mummenhoff K. (2009) Arabidopsis family ties: molecular phylogeny and age estimates in Brassicaceae. Taxon 58: 425–437 [Google Scholar]
- Frugis G, Chua NH. (2002) Ubiquitin-mediated proteolysis in plant hormone signal transduction. Trends Cell Biol 12: 308–311 [DOI] [PubMed] [Google Scholar]
- Fry SC. (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161: 641–675 [DOI] [PubMed] [Google Scholar]
- Fry SC, Dumville JC, Miller JG. (2001) Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem J 357: 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura Y, Karssen CM. (1995) Molecular studies on osmoprimed seeds of cauliflower: a partial amino-acid-sequence of a vigor-related protein and osmopriming-enhanced expression of putative aspartic protease. Seed Sci Res 5: 177–181 [Google Scholar]
- Gaete-Eastman C, Figueroa CR, Balbontin C, Moya M, Atkinson RG, Herrera R, Moya-Leon MA. (2009) Expression of an ethylene-related expansin gene during softening of mountain papaya fruit (Vasconcellea pubescens). Postharvest Biol Technol 53: 58–65 [Google Scholar]
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. (2002) The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA 99: 11519–11524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XM, Bewley JD. (2007) Sorting out the LeMANs: endo-beta-mannanase genes and their encoded proteins in tomato. Seed Sci Res 17: 143–154 [Google Scholar]
- Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, et al. (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweck LM. (2008) Gibberellin signaling. Planta 229: 1–13 [DOI] [PubMed] [Google Scholar]
- Helm M, Schmid M, Hierl G, Terneus K, Tan L, Lottspeich F, Kieliszewski MJ, Gietl C. (2008) KDEL-tailed cysteine endopeptidases involved in programmed cell death, intercalation of new cells, and dismantling of extensin scaffolds. Am J Bot 95: 1049–1062 [DOI] [PubMed] [Google Scholar]
- Hilson P, Allemeersch J, Altmann T, Aubourg S, Avon A, Beynon J, Bhalerao RP, Bitton F, Caboche M, Cannoot B, et al. (2004) Versatile gene-specific sequence tags for Arabidopsis functional genomics: transcript profiling and reverse genetics applications. Genome Res 14: 2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJJ. (2008a) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179: 33–54 [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Finch-Savage WE, Grappin P, Job D. (2008b) Post-genomics dissection of seed dormancy and germination. Trends Plant Sci 13: 7–13 [DOI] [PubMed] [Google Scholar]
- Hughes DW, Galau GA. (1989) Temporally modular gene expression during cotyledon development. Genes Dev 3: 358–369 [DOI] [PubMed] [Google Scholar]
- Hughes DW, Galau GA. (1991) Developmental and environmental induction of Lea and LeaA mRNAs and the postabscission program during embryo culture. Plant Cell 3: 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Fernández R, Matilla A. (2009) After-ripening alters the gene expression pattern of oxidases involved in the ethylene and gibberellin pathways during early imbibition of Sisymbrium officinale L. seeds. J Exp Bot 60: 1645–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Fernández R, Rodríguez-Gacio MC, Barrero-Sicilia C, Carbonero P, Matilla A. (2011) Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta 233: 25–36 [DOI] [PubMed] [Google Scholar]
- Ikuma H, Thimann KV. (1963) The role of the seed-coats in germination of photosensitive lettuce seeds. Plant Cell Physiol 4: 169–185 [Google Scholar]
- Itai A, Ishihara K, Bewley JD. (2003) Characterization of expression, and cloning, of beta-D-xylosidase and alpha-L-arabinofuranosidase in developing and ripening tomato (Lycopersicon esculentum Mill.) fruit. J Exp Bot 54: 2615–2622 [DOI] [PubMed] [Google Scholar]
- Jendrisak J. (1980) The use of alpha-amanitin to inhibit in vivo RNA synthesis and germination in wheat embryos. J Biol Chem 255: 8529–8533 [PubMed] [Google Scholar]
- Kim DH, Yamaguchi S, Lim S, Oh E, Park J, Hanada A, Kamiya Y, Choi G. (2008) SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20: 1260–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Nambara E. (2010) Stored and neosynthesized mRNA in Arabidopsis seeds: effects of cycloheximide and controlled deterioration treatment on the resumption of transcription during imbibition. Plant Mol Biol 73: 119–129 [DOI] [PubMed] [Google Scholar]
- Knox JP. (2008) Mapping the walls of the kingdom: the view from the horsetails. New Phytol 179: 1–3 [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15: 281–307 [Google Scholar]
- Leubner-Metzger G. (2002) Seed after-ripening and over-expression of class I beta-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta 215: 959–968 [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G. (2003) Functions and regulation of beta-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res 13: 17–34 [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K. (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Kim Y, Breeze E, Koo JC, Woo HR, Ryu JS, Park DH, Beynon J, Tabrett A, Buchanan-Wollaston V, et al. (2007) Overexpression of a chromatin architecture-controlling AT-hook protein extends leaf longevity and increases the post-harvest storage life of plants. Plant J 52: 1140–1153 [DOI] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, et al. (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21: 3803–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Karmarkar V. (2008) Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci 13: 137–144 [DOI] [PubMed] [Google Scholar]
- Lizana XC, Riegel R, Gomez LD, Herrera J, Isla A, McQueen-Mason SJ, Calderini DF. (2010) Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.). J Exp Bot 61: 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Kinoshita N, Chua N-H. (2003) AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev 17: 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Blake AW, Benians TAS, Lee KJD, Poyser C, Donaldson L, Leroux O, Rogowski A, Petersen HL, Boraston A, et al. (2010) Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J 64: 191–203 [DOI] [PubMed] [Google Scholar]
- Maris A, Suslov D, Fry SC, Verbelen JP, Vissenberg K. (2009) Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. J Exp Bot 60: 3959–3972 [DOI] [PubMed] [Google Scholar]
- Mella RA, Burgin MJ, Sanchez RA. (2004) Expansin gene expression in Datura ferox L. seeds is regulated by the low-fluence response, but not by the high-irradiance response, of phytochromes. Seed Sci Res 14: 61–71 [Google Scholar]
- Mo BX, Bewley JD. (2002) Beta-mannosidase (EC 3.2.1.25) activity during and following germination of tomato (Lycopersicon esculentum Mill.) seeds: purification, cloning and characterization. Planta 215: 141–152 [DOI] [PubMed] [Google Scholar]
- Müller K, Job C, Belghazi M, Job D, Leubner-Metzger G. (2010) Proteomics reveal tissue-specific features of the cress (Lepidium sativum L.) endosperm cap proteome and its hormone-induced changes during seed germination. Proteomics 10: 406–416 [DOI] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Tintelnot S, Leubner-Metzger G. (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47: 864–877 [DOI] [PubMed] [Google Scholar]
- Mutlu A, Chen X, Reddy SM, Gal S. (1999) The aspartic proteinase is expressed in Arabidopsis thaliana seeds and localized in the protein bodies. Seed Sci Res 9: 75–84 [Google Scholar]
- Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E. (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41: 697–709 [DOI] [PubMed] [Google Scholar]
- Neuteboom LW, Veth-Tello LM, Clijdesdale OR, Hooykaas PJ, van der Zaal BJ. (1999) A novel subtilisin-like protease gene from Arabidopsis thaliana is expressed at sites of lateral root emergence. DNA Res 6: 13–19 [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Chen F, Bradford K. (2007) Mechanisms and genes involved in germination sensu stricto. Bradford K, Nonogaki H, , Seed Development, Dormancy and Germination. Blackwell, Oxford, pp 264–304 [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ. (2000) A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123: 1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Tatematsu K, Matsui A, Morosawa T, Ishida J, Tanaka M, Endo TA, Mochizuki Y, Toyoda T, Kamiya Y, et al. (2010) Genome-wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of Arabidopsis using tiling arrays. Plant J 62: 39–51 [DOI] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Josse EM, Kannangara R, Gilday AD, Halliday KJ, Graham IA. (2005) Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr Biol 15: 1998–2006 [DOI] [PubMed] [Google Scholar]
- Penfield S, King J. (2009) Towards a systems biology approach to understanding seed dormancy and germination. Proc Biol Sci 276: 3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Li Y, Gilday AD, Graham S, Graham IA. (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Müller K, Hermann K, Leubner-Metzger G. (2003) Distinct expression patterns of beta-1,3-glucanases and chitinases during the germination of solanaceous seeds. Seed Sci Res 13: 139–153 [Google Scholar]
- Pinto LVA, Da Silva EAA, Davide AC, De Jesus VAM, Toorop PE, Hilhorst HWM. (2007) Mechanism and control of Solanum lycocarpum seed germination. Ann Bot (Lond) 100: 1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U, Lopez-Molina L. (2009) The GA-signaling repressor RGL3 represses testa rupture in response to changes in GA and ABA levels. Plant Signal Behav 4: 63–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E. (2009) Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant Cell Physiol 50: 1786–1800 [DOI] [PubMed] [Google Scholar]
- Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D. (2004) The effect of α-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol 134: 1598–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Steinhauser D, Büssis D, Stintzi A, Schaller A, Kopka J, Altmann T. (2005) Inferring hypotheses on functional relationships of genes: analysis of the Arabidopsis thaliana subtilase gene family. PLoS Comput Biol 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JSG, Edwards ME, Dickson CA, Scott C, Gidley MJ. (2003) Tobacco transgenic lines that express fenugreek galactomannan galactosyltransferase constitutively have structurally altered galactomannans in their seed endosperm cell walls. Plant Physiol 131: 1487–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YF, Bewley JD, Wang XF. (2008) Protein and gene expression patterns of endo-beta-mannanase following germination of rice. Seed Sci Res 18: 139–149 [Google Scholar]
- Saladié M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, Xiaolin R, Labavitch JM, Shackel KA, Fernie AR, et al. (2007) A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol 144: 1012–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Cosgrove DJ. (2005) The expansin superfamily. Genome Biol 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RA, Demiguel L, Mercuri O. (1986) Phytochrome control of cellulase activity in Datura ferox L. seeds and its relationship with germination. J Exp Bot 37: 1574–1580 [Google Scholar]
- Santner A, Estelle M. (2010) The ubiquitin-proteasome system regulates plant hormone signaling. Plant J 61: 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P. (2006) Biomechanics of plant growth. Am J Bot 93: 1415–1425 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A. (2006) Plasma membrane-generated reactive oxygen intermediates and their role in cell growth of plants. Biofactors 28: 73–81 [DOI] [PubMed] [Google Scholar]
- Schröder R, Atkinson RG, Redgwell RJ. (2009) Re-interpreting the role of endo-beta-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann Bot (Lond) 104: 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska E, Bassel GW, Bewley JD. (2009) Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J Exp Bot 60: 3587–3594 [DOI] [PubMed] [Google Scholar]
- Slotte T, Holm K, McIntyre LM, Lagercrantz U, Lascoux M. (2007) Differential expression of genes important for adaptation in Capsella bursa-pastoris (Brassicaceae). Plant Physiol 145: 160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article 3 [DOI] [PubMed] [Google Scholar]
- Smyth G. (2005) Limma: linear models for microarray data. Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W , Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, New York, pp 397–420 [Google Scholar]
- Smyth GK, Speed T. (2003) Normalization of cDNA microarray data. Methods 31: 265–273 [DOI] [PubMed] [Google Scholar]
- Still DW, Bradford KJ. (1997) Endo-β-mannanase activity from individual tomato endosperm caps and radicle tips in relation to germination rates. Plant Physiol 113: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Hauksdóttir H, Troy A, Herschleb J, Kraft E, Callis J. (2005) Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 137: 13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Gubler F. (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y. (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128: 4681–4689 [DOI] [PubMed] [Google Scholar]
- Tiné MAS, Cortelazzo AL, Buckeridge MS. (2000) Xyloglucan mobilisation in cotyledons of developing plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinoideae). Plant Sci 154: 117–126 [DOI] [PubMed] [Google Scholar]
- Toorop PE, van Aelst AC, Hilhorst HWM. (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51: 1371–1379 [PubMed] [Google Scholar]
- van de Mortel JE, Aarts MGM. (2006) Comparative transcriptomics: model species lead the way. New Phytol 170: 199–201 [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. (1990) Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA 87: 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sandt VST, Suslov D, Verbelen JP, Vissenberg K. (2007) Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot (Lond) 100: 1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EMN, Maier A, Schwechheimer C. (2007) The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YJ, Suzuki H, Borevitz J, Blount J, Guo ZJ, Patel K, Dixon RA, Lamb C. (2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO J 23: 980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun TP. (2001) Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J 28: 443–453 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16: 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Yang XH, Lai JR, Lin H, Cheng ZM, Nonogaki H, Chen F. (2007) The endo-beta-mannanase gene families in Arabidopsis, rice, and poplar. Funct Integr Genomics 7: 1–16 [DOI] [PubMed] [Google Scholar]
- Zhou Z. (2009) Study of light dependent Arabidopsis phytochrome A signal transduction through FHY1 and its downstream gene expression regulation. PhD thesis. State University of New York, Binghamton [Google Scholar]