Abstract
Stromules are stroma-filled tubules extending from plastids whose rapid extension toward or retraction from other plastids has suggested a role in interplastidic communication and exchange of metabolites. Several studies point to sporadic dilations, kinks, and branches occurring along stromule length but have not elucidated the underlying basis for these occurrences. Similarly, although specific details on interacting partners have been missing, a consensus viewpoint suggests that stromules increase the interactive surface of a plastid with its cytoplasmic surroundings. Here, using live imaging, we show that the behavior of dynamic, pleomorphic stromules strongly coincides with that of cortical endoplasmic reticulum (ER) tubules. Covisualization of fluorescent protein-highlighted stromules and the ER in diverse cell types clearly suggests correlative dynamics of the two membrane-bound compartments. The extension and retraction, as well as directional changes in stromule branches occur in tandem with the behavior of neighboring ER tubules. Three-dimensional and four-dimensional volume rendering reveals that stromules that extend into cortical regions occupy channels between ER tubules possibly through multiple membrane contact sites. Our observations clearly depict coincidental stromule-ER behavior and suggest that either the neighboring ER tubules shape stromules directly or the behavior of both ER and stromules is simultaneously dictated by a shared cytoskeleton-based mechanism. These new observations strongly implicate the ER membrane in interactions with stromules and suggest that their interacting surfaces might serve as major conduits for bidirectional exchange of ions, lipids, and metabolites between the two organelles.
The presence of stromules (stroma-filled tubules) as plastidic extensions in plant cells is well established (Hanson and Sattarzadeh, 2008). Stromule formation has been suggested as a response to increased subcellular redox stress (Itoh et al., 2010), symbiotic interactions (Fester et al., 2001; Hans et al., 2004; Lohse et al., 2005), elevated temperatures (Holzinger et al., 2007a), virus infection (Caplan et al., 2008), and changed plastid size and plastid density (Pyke and Howells, 2002; Waters et al., 2004). Single or multiple stromules are extended by both chlorophyll-containing and achlorophyllous plastids (Köhler et al., 1997; Tirlapur et al., 1999; Köhler and Hanson, 2000). Stromules have been observed contacting plastids with each other (Menzel, 1994; Arimura et al., 2001; Pyke and Howells, 2002) and an exchange of proteins through stromules has been shown (Köhler et al., 1997, 2000; Tirlapur et al., 1999; Kwok and Hanson, 2003, 2004a). These observations have suggested a role for stromules as transient communication channels between plastids for exchanging metabolites (Köhler et al., 1997, 2000) as well as evidence for the occurrence of a well-connected plastid compartment in plant cells (Menzel, 1994). However, as pointed out by Natesan et al. (2005) the movement of macromolecules between plastids might not be the sole function of stromules since most stromules do not appear to interconnect plastids. In a more generalized viewpoint stromules are believed to increase the plastid surface area exposed to the cell environment. Although pertinent details to prove this viewpoint on stromule function are missing so far, this role seems to be an obvious one since considerable trafficking of ions and metabolites is known to occur between plastids and their surrounding cytoplasm. Moreover, numerous transmission electron micrographs have suggested close associations between stromules and organelles such as the endoplasmic reticulum (ER), mitochondria, and nucleus (Ehara et al., 1985; Bourett et al., 1999; Holzinger et al., 2007a, 2007b; Lütz and Engel, 2007). Indeed aggregation of mitochondria along stromules has been presented as evidence for interactions between the two organelles (Kwok and Hanson, 2004b; Gunning, 2005; Holzinger et al., 2007a, 2007b). Stromule formation and behavior has been associated with cytoskeletal (Menzel, 1994; Gray et al., 2001; Kwok and Hanson, 2003, 2004b; Gunning, 2005) and motor protein dynamics (Gray et al., 2001; Natesan et al., 2009; Sattarzadeh et al., 2009). Despite these singular studies live-imaging-based observations that can visually substantiate our view on the behavior of stromules with respect to other organelles have been lacking. More specifically, while a close association between the ER and the chloroplast outer envelope is known (Andersson et al., 2007; Sandelius et al., 2007), the relationship between stromules and the ER has not been investigated so far.
Stromules are usually presented as filamentous tubules that can be up to 50 to 60 μm in length and be readily distinguished from other more irregular shaped plastid protrusions by a shape index (Holzinger et al., 2007a). They often exhibit transient dilations (also named fluorescent protein package; Hanson and Sattarzadeh, 2008) that appear to move along the stromule length. Stromules routinely extend and retract along linear tracks that are also followed by other organelles during cytoplasmic streaming. In addition, stromules that extend against the cytoplasmic flow have also been observed (Gunning, 2005). However, not all stromules occur as straight tubules. A few studies describe branched stromules (Menzel, 1994; Köhler and Hanson, 2000; Shiina et al., 2000; Arimura et al., 2001; Pyke and Howells, 2002; Gunning, 2005; Holzinger et al., 2007a) but do not elaborate any further on the basis for branching.
Our fluorescent protein-aided time-lapse observations in different cell types in Arabidopsis (Arabidopsis thaliana) and Nicotiana benthamiana focuses on branched stromules. Our observations suggest that stromule branching coincides with the dynamic rearrangement of cortical ER tubules. Subsequent investigations involving simultaneous visualization of GFP-highlighted stromules and red fluorescent protein (RFP)-highlighted ER have revealed that stromules extend and retract within ER-lined channels. A combination of time-lapse confocal laser-scanning microscopy and volume rendering of image stacks clearly conveys the impression that stromule branching occurs in tandem with the dynamic remodelling of contiguous ER tubules possibly through contact points created between the two membranes within the channels. Our observations reinforce views on the role of stromules in increasing the interactive surface of a plastid and suggest the ER as a major interacting partner in the important process of signal and metabolite exchange. We thus lay down a firm foundation for further cell biological investigations on plastid-ER interactions.
RESULTS
Stromules Exhibit Sporadic Branching in All Cell Types
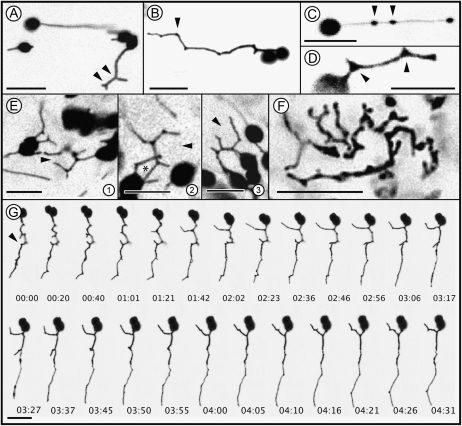
Initially four different fluorescent protein fusions, namely the ferredoxin NADP(H) oxidoreductase (FNR) transit peptide fused to enhanced GFP (FNR-EGFP), the transit peptide from the 16-kD protein of the oxygen-evolving complex fused to enhanced GFP (16-EGFP) (Marques et al., 2004), pt-yk-, and pt-gk (Nelson et al., 2007), each targeted to plastids were used for assessing stromule formation in stable transgenic lines of Arabidopsis and in transient agroinfiltration assays on N. benthamiana leaves. Each fusion protein highlighted stromules efficiently, but based on fluorescence intensity, a clean targeting to the plastid stroma, and similar levels of expression in all cells in the observed tissues, the FNR-EGFP probe and the respective plant line were selected for use in this study. A range of stromule shapes was observed in different cell types of N. benthamiana and Arabidopsis (Fig. 1). Stromules were straight (Fig. 1A), with branches (Fig. 1A, arrowheads), kinked (Fig. 1B, arrowhead), with sporadic, randomly localized elliptical dilations that traversed the tubule length (Fig. 1C, arrowheads; Supplemental Movie S1), or with triangular areas of expansion along tubule length (arrowheads in Fig. 1D, also visible in Fig. 1B at several kinks). Notably, the elliptical dilations (Fig. 1C) within a stromule were observed most frequently in subcortically positioned plastids that moved within the cytoplasmic stream (Supplemental Movie S1). By contrast triangular stroma-filled regions (Fig. 1D) were usually associated with stromules extending from chloroplasts located at or near the cell cortex. Figure 1E (Supplemental Movie S3) depicts several cortically located chloroplasts with kinked stromules exhibiting triangular islands. Time-lapse imaging revealed that the triangular areas are always found at kinks and acted as the initiation sites for stromule branching (left arrowhead in Fig. 1, D and G; n = 40 branching initiation events; Supplemental Movie S2). In some cases of intensive stromule branching and kinking the extending branches overlapped transiently to complete a polygon (Fig. 1E, 2, asterisk). However, this coincidental overlapping of branches did not result in stromule fusion and incomplete polygons were recreated within a few seconds. No such correlations could be drawn for elliptical dilations (Fig. 1C) formed within straight tubules (n = 35 stromules showing elliptical dilations). Interestingly in all of our observations at kinked areas always triangles were formed. We never observed other forms and kinked areas were never found to develop more than three branches (n = 40 stromules showing kinks and branching). This suggested that a typical patterning event formed the basis for their formation as compared to the random shape and size of elliptical dilations that were created transiently within the stromules. Whereas most cells of the upper epidermis, the root cortex, and floral tissue (corolla cells) in transgenic FNR-EGFP Arabidopsis and N. benthamiana plants exhibited similar kinked, branched stromules along with straight ones, cells within the meristematic region of the root were packed with tubules that provided no clear definition of plastid bodies (Fig. 1F). Notably these convoluted tubules also exhibited branch points comparable to the other cell types.
Figure 1.
Confocal microscopic images depicting typical stromule morphology in FNR-EGFP expressing transgenic N. benthamiana or Arabidopsis leaf epidermal, hypocotyl, and root tip cells. A, Straight and branched (arrowheads) stromules in an epidermal hypocotyl cell of N. benthamiana. B, Stromules often exhibit single or multiple kinks as visible at this depiction of a stromule of an epidermal hypocotyl cell of N. benthamiana. These kinks (e.g. arrowhead) are clearly distinguishable from smooth bends. The pictured stromule is slightly bended between several neighboring kinks. C, Oval dilations formed along a straight-extending stromule in an epidermal hypocotyl cell of N. benthamiana (Supplemental Movie S1 shows a group of stromules forming such dilations). D, Triangular stroma-filled dilations (arrowheads) formed at kinks in a cortically located plastid stromule of an epidermal hypocotyl cell. Stromal triangles form initiation points for all newly forming branches (one branch originating from such a triangle is marked by the left arrowhead). E, 1 to 3, Pleomorphic stromules in an epidermal cell of N. benthamiana with several kinking and branching events that suggest the formation of incomplete (open ended) polygonal shapes (black arrowheads). Several such stromule branches come in close contact and suggest a closed polygon (asterisk in 2). However, such events are transient and stromules do not fuse to form complete ER-like polygons. Section 3 shows a polygon shape opening out again (arrowhead). Supplemental Movie S3 depicts such behavior over time. F, Kinks, branches, stromal triangles, and elliptical dilations are observed in other cells such as those in the root tip. The main plastid body, visible in this image of an Arabidopsis cortical root tip cell, is not well defined and the stroma-filled tubules exhibit pleomorphy. G, A time-lapse series of images extending over approximately 4 minutes and 30 s shows the rapid extension, retraction of branches from kinks in a single extended stromule in an epidermal hypocotyl cell of N. benthamiana. Digits at the base of each section denote time in minutes. Scale bar in each image corresponds to 10 μm.
Time-lapse observations (Fig. 1G; Supplemental Movie S4) showed that stromule morphology could change rapidly with multiple branches being extended and retracted within a few seconds. As shown in Figure 1G (arrowhead) many closely placed branches could be created from a single tubule.
Our observations of stroma-filled triangular areas (Fig. 1, D–F) and branches extending from them were strongly reminiscent of three-way junctions created in the cortical ER. These junctions give rise to rapidly extending and retracting tubules for creating polygons that make up the ER mesh (Griffing, 2010). Although stromules do not appear to form completely fused polygons the branching point and the manner of branching is very similar to that exhibited by the ER. This strong resemblance between stromules and the ER tubules was investigated next through simultaneous visualization of both compartments.
Covisualization of Stromules and the ER Reveals Correlative Behavior
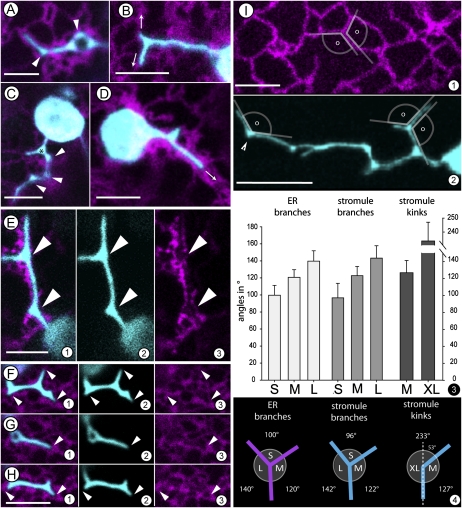
To visualize the ER Agrobacterium tumefaciens (strain GV3101 pMP90) was transformed with ER-targeted RFP-HDEL (Sinclair et al., 2009) and used for agroinfiltration of leaves of N. benthamiana stably transgenic for FNR-EGFP or wild-type plants. Observations were taken between 48 and 60 h postinfiltration on epidermal cells that exhibited cytoplasmic streaming and a dynamic cortical ER. While a certain number of chloroplasts were always localized close to the nucleus a specific directionality in their extension of stromules was not discernable. Chloroplasts that were located in broad cytoplasmic strands were embedded completely within the ER and usually extended one or two straight stromules. By contrast chloroplasts occupying cortical locations sat in a loose cage comprised of connected ER polygons and could extend multiple stromules simultaneously. The tubular areas of most stromules possessed a diameter (0.6 ± 0.1 μm; n = 40) similar to cortical ER tubules (0.5 ± 0.1 μm; n = 40). As noted earlier stromules extended by cortically located chloroplasts were often kinked and displayed triangular junctions (Fig. 2, A–D). Time-lapse imaging of kinked stromules and RFP-highlighted ER clearly showed that branches from stromal triangles always extended concomitantly with neighboring ER tubules, with the tip always remaining in close association with the ER (n = 22 extension events; representative Fig. 2, E and F; Supplemental Movies S7 and S8). It is noteworthy, that in some cases the stromule tip was completely surrounded by an ER cap (e.g. Supplemental Movie S7). The coalignment of stromules and ER tubules was further illustrated by our observation that stromules were never found to be localized within or to protrude into an ER free area like the middle of a large ER polygon. Comparing the angles created by extending stromule branches and kinks with the angles displayed by ER tubules (n = 50 branch points or kinks in each case) further reinforced the observation (Fig. 2I, 1 and 2) of stromule and ER coalignment and similar branching behavior. The range of angles displayed in this correlative behavior is depicted in Figure 2J. In general, kinking occurred when a stromule bent by approximately 53° from the perpendicular (resulting in internal angles: 127°; 233°) whereas a new branch usually formed at nearly right angles to the kinked point (resulting in internal angles: 96°; 122°; 142°; Fig. 2J, 1). The three-way junctions (stromal triangles) thus formed at the base of stromule branches clearly exhibited similar angles as displayed by tubules extending and fusing to form ER polygons (resulting in internal angles: 100°; 120°; 140°; Figure 2J, 1).
Figure 2.
Simultaneous visualization of FNR-EGFP-highlighted stromules (cyan) and RFP-highlighted ER (magenta) in epidermal leaf cells of N. benthamiana transiently expressing the constructs following agroinfiltration suggests strong correlations between stromules and neighboring ER. A to F, Extended stromules aligned with cortical ER tubules. A, A typical stromule that coaligns with parts of an ER polygon in its tip region and forms stromal triangles at kinks along its length. B, A single stromule that branches at the tip along ER-defined directions (arrows). C, A single plastid extending stromules where the one in focus starts branching close to the main body while the more elongated portion forms kinks (arrowheads) and stromal triangles (*). D, A plastid enmeshed in a thick ER strand displaying a stromal triangle that ends within the contiguous ER without extending branches. The stromule is extended along the fast-flowing cytoplasmic stream in this case (arrow). E, A single stromule showing stromal triangles. Single channels (sections E2 and E3) reveal the close association with neighboring ER. Arrowheads mark the stromal triangles filling in triangular gaps of the ER visible in the E3. E1 represents a merge of E2 and E3 sections. F and H, Snapshots taken from a time-lapse confocal image series (Supplemental Movie S8) showing extension (F), retraction (G), and reextension of a stromule. Arrowheads in each section point to the relation between the tips and the contiguous ER. The stromule morphology changes as its surrounding ER undergoes remodeling. Even if the ER stromule alignment develops gaps along its length over time the branch tips always maintain close proximity to the ER. I (1 and 2), Internal and external angles (□) created by the merging of ER tubules into a Y shape that defines three walls of a polygon (section 1). Section 2 depicts a stromule that creates very similar angles through the extension of its branches or the creation of a kink (arrowhead). 3, Measurement of angles created by extended branches of ER, stromules, and stromule kinks as shown in sections I1 and I2 revealed that the angles enclosed by tubular branches can be grouped in three classes ranging from the smallest angle (80°–120° marked as S) to the next larger angle class (110°–140° marked as M) and the largest angle class (130°–160° marked as L). Bar chart presents the respective mean value ± sd. The angle class for stromule kinks is different and consists of wider angles ranging from 210 to 250 (designated as XL). Data on class distribution for ER and stromule branches subjected to Student’s t test provides P values < 0.001 in each case. 4, A schematic depiction of information obtained from I3 on angular measurements suggesting a correlation between stromule branching and ER tubules. The diagrams illustrate the similarity of the ER and stromule branches as well as stromule kinks. In ER and stromule branches S represents the smallest, M the medium, and L the largest angle classes (see section 3). In case of stromule kinks angle XL corresponds to sum of angle S and M depicted for the branches. Dotted line represents the perpendicular. Scale bar = 5 μm in all sections except D2 (where it is 10 μm).
Subjecting the data to a Student’s t test showed that the three angles at a single branch point of a stromule or ER are significantly different and therefore can be grouped according to their size in small (approximately 100°), medium (approximately 120°), and large (approximately 140°) angle classes (P value for comparisons of angles small/medium and medium/large as well as small/large of Fig. 2J, 3, is below 0.001). The angular similarity at branch points of stromules and ER tubules (Student’s t test P values comparing small = 0.130, medium = 0.546, and large = 0.318 angles show no statistically significant differences). The comparable angles between stromules and neighboring ER provided a strong temporal correlation between the two membrane-bound compartments and led us to explore whether they exhibited a precise spatial association too.
Stromules Extend, Retract, and Accommodate within ER-Lined Channels
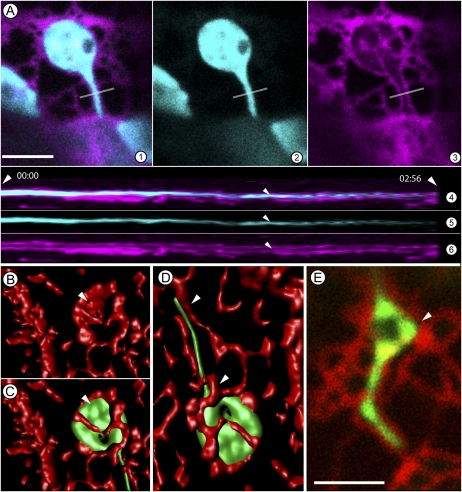
Earlier observations on live cells (Figs. 1 and 2) provided two views of the chloroplast; one, where they are largely embedded within the bulked ER and being carried along the cytoplasmic flow while extending long unbranched tubules, and the second where they occupy a cortical location that pushes them against the cortical ER mesh. Notably the cortical ER exhibits rapid rearrangements constantly during which short tubules extend and retract, form polygons and broad islands with large cisternae. As noted chloroplasts in the cortical region produce kinked and branched stromules, a comparison of confocal images in two channels (Fig. 3A, 1–3; Supplemental Movie S6) showed ER envelopes around plastids and a clear ER-lined channel within which a stromule is extended (Fig. 3A, 3). As depicted through a kymograph (Fig. 3A, 4–6) of the cutting plane (white line) depicted in Figure 3A the extended stromule maintains constant contact with the ER channel over several minutes. Nevertheless, the constant remodeling of ER polygons invariably creates gaps between a tubule and the ER channel (e.g. between arrowheads, Fig. 2E). Three-dimensional (3D; x, y, z axes) volume rendering over time was used for achieving a better spatiotemporal understanding of the suggested relationship between the two tubular membrane extensions (Fig. 3, B–D). Figure 3B depicts a rendering of the ER to show the cup shape within which a chloroplast is nestled (arrowhead in Fig. 3C). The stromule extended by the chloroplast passes through a narrow ER channel (Fig. 3C). The close ER-stromule relationship is depicted in Figure 3D and shows possible points of contact and detachment. A time-lapse series with an extending and retracting stromule suggest that over time, areas of the channel become detached from the stromule to form small gaps. In situations where these gaps get created near converging walls of an ER polygon a triangular space is created. As shown in Figure 3E the stromule can fill in such triangular spaces to create the often-observed stromal triangles. Whether a similar event occurs in concave spaces created through ER-stromule detachment within a rapidly flowing ER containing cytoplasmic stream to create elliptical dilations of stromules is presently unclear. However, it is noteworthy that such filling in of regions enclosed by a polygon is a common feature of cortical ER organization and creates ER islands with wide cisternae (Griffing, 2010). A similar behavior exhibited by stromules suggests that they might maintain a strong attachment to the ER membrane during this process.
Figure 3.
Spatial association of plastids and stromules with the ER suggested through confocal image stacks and their 3D volume rendering. A, A portion of an N. benthamiana epidermal cell shows a single chloroplast embedded in an ER basket (magenta) with its stromule (cyan) being extended along an ER-lined channel. Section 1 is a merge of sections 2 and 3. The gray line in the sections marks the slice on the y axis used for building the kymograph presented in sections 4 to 6. The kymograph clearly shows the channel (small arrowheads) and its continuity at that position during the observation time of 2:56 minutes (large arrowheads). B to D, A 3D surface rendering of a plastid enmeshed within the cortical ER (red) in a leaf epidermal cell of N. benthamiana (B). B shows the ER cup (arrowhead) within which the plastid body C sits. The image in B is completed with the chloroplast and its stromule in location. Note that the 3D volume rendering clearly brings out the spatial correlations and shows ER tubules that localize on top of the plastid as well as the stromule. D, The complete image of a fully extended tubule taken after a few minutes shows a certain degree of realignment where the middle portion of the stromule is in contact with the ER on one side only the other side has become free of the ER restraint. Portions suggesting connectivity at stromule base and tip are indicated by arrowheads. E, A similar stromule as observed in D undergoes space-filling activity in an area that has become available after ER remodeling (arrowhead). The space that has been filled in by the stromule dilation is triangular through the boundary defined by neighboring ER. Size bar = 5 μm.
Extension and retraction is a characteristic feature of all stromules and we focused on this aspect next. Time-lapse observations on 40 stromules showed that their tips were always contiguous to or in contact with an extending ER tubule. Rapid retraction was observed if an extending stromule failed to encounter a concomitantly extending ER tubule. Thus stromule branch initials that emanated within an expanding ER polygon were quickly retracted to the plastid body if the extension could not maintain alignment with an ER tubule. Alternatively, stromules also retracted upon encountering bulked-up ER during the merging and remodeling of ER strands. In many such events the retraction of a stromule was quickly followed by the formation of new protuberances (called beaks; Gunning, 2005) from the plastid (Supplemental Movie S9). Fresh stromules were extended by the plastid if the ER remodeling created tubules that could be used for alignment and extension (n = 25 observations). Interestingly, multiple beaks could be produced in such cases, suggesting that stromule extension does not occur from a fixed location on a plastid but depends upon a contact and pull mechanism that involves neighboring ER tubules (Supplemental Movie S9).
DISCUSSION
In ascribing possible roles to stromules certain morphological clues provided by these dynamic extensions have not been rigorously pursued. One such detail that has been noted but not followed through is the extension and retraction of branches along the length of stromules. We were intrigued by the stromule branching phenomenon and decided to investigate it.
Branch initiation by stromules is rapid; several branches can be extended from a single tubule and like the main stromule tip each branch can extend and retract independently. While observing branched stromules we noticed the strong similarity that they exhibit to the tubules that create the polygonal mesh of the cortical ER. Interestingly both ER tubules and stromules possess very similar diameters (0.5 and 0.6 μm). Moreover our measurements show that branching points of stromules and ER exhibit similar angles, suggesting a correlation between both organelles. This lead was followed through simultaneous visualization of fusion proteins targeted to plastids and the ER lumen, respectively, and as described here provides interesting correlations that suggest an extensive stromule ER coalignment during stromule extension, retraction, and branching.
Our observations indicate clear correlations between ER tubules and stromules. At this early stage of the work two possible interpretations are suggested. The first explanation relies on the intimate relationship shown to exist between the plastid envelope and the neighboring ER (Andersson et al., 2007; Sandelius et al., 2007). Specific ER regions that are involved in such membrane contact sites (MCSs) have been isolated and both lipid transfer as well as protein-protein interactions have been demonstrated at the MCSs (reviewed by Holthuis and Levine, 2005). Our own observations of single chloroplasts sitting within loose ER cages confirm this (Supplemental Movie S5). Considering that stromules are double-membrane-bound extensions of the plastid (Holzinger et al., 2008) the ER plastid-envelope relationship can be readily extrapolated to suggest that MCSs might persist on extending stromules. Indeed Figure 3 and Supplemental Movies S6 to S8 show stromules threading their way into ER-lined channels. In this scenario the stromule behavior might be considered passive, as it would be dictated by the activity of neighboring ER. It is also possible to imagine that multiple contacts points might be created between the two membranes so that the extent of stromule extension and retraction become guided by contact with contiguous ER tubules. Thus over time the alignment of stromules and ER tubules might remain quite tight (Supplemental Movie S6) or gaps might appear where the two membrane surfaces transiently lose contact with each other. In agreement with Andersson et al. (2007), our observations suggest that surface adherence between ER tubules and stromules exists only at certain points and not along the entire length of the stromule. Further as suggested in Figure 4 while small gaps between the ER channel and an extended stromule might form the basis for a transient dilation of the stromule it seems imperative that for stromule extension its tip must maintain contact with the ER. Loss of tip contact generally leads to stromule retraction. Interestingly stromules might retract in short steps or in a single step that takes them all the way back to the parent plastid surface. The number of MCSs and their positioning along the stromule might again dictate this behavior. Our time-lapse observations are strongly suggestive of these events but the actual contact points between the two membranes have not been resolved in this study. Nevertheless, it is noteworthy that rapid freeze-fracture-based images (Mclean et al., 1988; Whatley et al., 1991) and transmission electron micrographs of extended stromules also indicate these possibilities (Holzinger et al., 2008). A demonstration of MCSs between the ER membrane and the plastid envelope would be very important for proving this conjecture. As suggested by the findings of Gao et al. (2006) and Jouhet and Gray (2009) different proteins localized on the chloroplast outer envelope might provide clues to the occurrence of MCSs. Our investigations are now focused on a thorough spatiotemporal characterization of these proteins and their relationship with the ER during stromule formation.
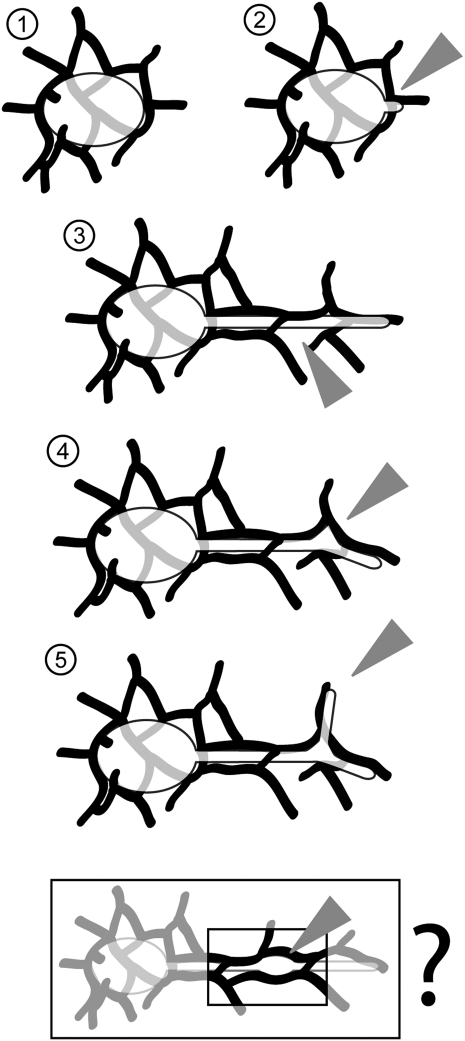
Figure 4.
A model for ER-dependent stromule initiation, elongation, kink formation, and branching suggested by observations presented here. 1, A loose mesh comprising cortical ER tubules surrounds plastids localized within the cortical regions of a cell. 2, A new stromule is initiated at possible MCSs between the plastid outer envelope and the surrounding ER. Stromule extension relies on this initial tip contact and alignment with a neighboring ER tubule. 3, Over time the ER network gets remodeled. ER strands can enmesh the stromule completely within a channel. Alignment of stromules with an ER tubule dictates the angle of its extension. Subsequent ER remodeling can however result in open areas (arrowhead) where contact between stromule and ER membrane can be lost temporarily. Stromules might dilate rapidly to fill in these areas. 4, At points where ER-stromule contacts develop near ER branches (arrowhead) kinks are initiated in the stromule. Filling in of these kinked areas results in the formation of stromal triangles (arrowhead). 5, Stromal triangles are the initiation points for new stromule branches, which in turn align with extending ER tubules (arrowhead). Although further proof is required the box provides a speculative extrapolation of section 4 for the formation of oval dilations (arrowhead) along straight stromules that are extended by plastids that are part of a rapid cytoplasmic stream along with bulked ER. As discussed the model does not exclude an involvement of F-actin as a shared component between ER and stromules.
A second possible explanation for the observed similarity in branching patterns and dynamic behavior is based on the fact that both stromules and the ER rely upon the actin cytoskeleton for their dynamic behavior (Kwok and Hanson, 2003; Sparkes et al., 2009). Like the ER cage shown in Figure 3 and Supplemental Movie S5 an actin cage surrounds individual plastids (Kandasamy and Meagher, 1999). Thus our observations on the coincidental behavior of stromules and the ER might reflect their shared affinity for the actin cytoskeleton. Observations implicating class XI myosins as driving force for stromule and ER dynamics (Natesan et al., 2009; Ueda et al., 2010) lend further support to this conjecture. Notably the myosin XI-K implicated in ER motility (Ueda et al., 2010) is different from the myosin XI-F found associated with stromules (Sattarzadeh et al., 2009). These reports raise an interesting and testable possibility that is discussed further.
Based on our present understanding different myosin motors can possibly use the same F-actin track for movement and thus their respective cargo may appear to move over similar paths. However, the presence of a particular myosin on a particular region of an actin strand as well as the relative rates of movement between different myosin proteins might vary. In a comparative analysis these parameters might create a relative lag for the movement of one of the cargoes. In this study despite consistently observing ER-like patterns created by stromules through numerous time-lapse recordings we found that in most cases an ER organization appeared to be already in place before a stromule extends or retracts. We speculate that ER tubules start becoming organized into a particular pattern using a specific F-actin track and their exclusive myosin motor. A stromule follows the same F-actin track through the activity of a different myosin motor and thus also gets organized in a very similar, ER-like pattern. In this scenario the underlying F-actin cytoskeleton is able to fashion both the ER and the stromule. As proposed through numerous observations on membrane surfaces (Holthuis and Levine, 2005) the MCSs created between the two membrane surfaces might aid in the alignment process and also act as conduits for lipid and protein exchange. Our future endeavor will be to test these conjectures rigorously.
The nature and behavior of stromules in diverse plant species have suggested that they serve to increase the membrane surface area of the respective plastid and thus provide greater capability of interacting with other organelles and the surrounding cytosol. Indeed, mitochondria and peroxisomes are routinely observed near plastids and in certain cases have displayed a more than coincidental colocalization with stromules (Kwok and Hanson, 2004b; Gunning, 2005; Sage and Sage, 2009). Biochemical roles associated with mitochondria and peroxisomes also favor interpretations of stromules being involved in increasing organelle interactions. Nevertheless, contacts between the organelles are generally transient and notably, plastids, especially chloroplasts, are generally 3 to 10 times larger than mitochondria and peroxisomes. As such plastids already provide a large surface area for small organelles to interact. It seems rather implausible that very long extensions should be created by plastids for increasing contact with much smaller subcellular components. An equally valid possibility is that small organelles encounter stromules as they move around the cytoplasm and aggregate around them transiently. During such interactions it is possible that both organelles extend their interactive surface by forming tubular protrusions. Indeed peroxules (Sinclair et al., 2009) and matrixules (Scott et al., 2007) have been described but so far their interactions with stromules have not been described. Independent of the underlying mechanism the ER and stromule coalignment reported here results in their increased contact, which clearly is an absolute requirement for the bidirectional exchange of important signals, lipids, proteins, and metabolites between plastids and the endomembrane system.
The formation of dilations that rapidly and randomly change their location within a long stromule is a frequent observation. Our observations allow a reinterpretation of these dilated packets (Pyke and Howells, 2002; Hanson and Sattarzadeh, 2008) as they now take into account not just the stromules but also their relationship with neighboring ER tubules. Notably the lumen of both stromules and ER tubules is filled with fluids that exert an outward-directed pressure on the membrane and provide turgidity to the tubule. A channel, such as the ER-lined channel (Fig. 3) within which a stromule extends can act as a narrow confining space. A gap in the ER channel (Figs. 3 and 4) can create a localized region of reduced pressure on the tubular stromule. Release of constraining pressure on a tubule can cause it to dilate locally until a juxtaposed equivalent force once again restrains the swelling. Our time-lapse observations (Fig. 3; Supplemental Movie S7) suggest this to be the case and reinforce our view of the ER acting as a bounding channel for stromules while ER remodeling produces small gaps that get rapidly filled in by stromule dilations. The shape of the area filled in is clearly determined by the shape of the gap. Although the rapid movement of subcortical cytoplasm does not allow us to provide evidence for a similar scenario being involved in the creation of elliptical dilations in stromules our observations on stroma-filled triangles within ER-defined polygons (Figs. 2E and 3E) do suggest it as a strong likelihood.
In conclusion our observations suggest a strong underlying basis for stromule branching behavior. While reinforcing the role of the ER as an essential organelle involved in bidirectional exchange of metabolites and signals between plastids and the endomembrane system we suggest that stromules rather than the surface of the main plastid body act as the major conduits in this interaction. Finally, our findings pave the way for exploring more details on the ER-stromule interactions during plant development and response to environmental cues.
MATERIALS AND METHODS
Plasmids and Constructs
For visualization of plastid morphology in transient expression and transgenic plants the previously described FNR-EGFP construct (Marques et al., 2003), consisting of the transit peptide of the ferredoxin NADP(H) oxidoreductase (source Spinacia oleracea) N-terminally fused to EGFP was used. For transient expression and generation of transgenic plant lines the FNR-EGFP protein fusion was placed between the BamHI and XbaI sites of the modified pRT100 expression cassette (Überlacker and Werr, 1996). The respective T-DNA vector (35S-FNRtp-EGFP-term::pGreenII0129) was generated by swapping the complete expression cassette consisting of FNR-EGFP into pGreenII0129 (Hellens et al., 2000) using the PstI sites of both vectors.
The RFP-HDEL construct for labeling the ER has been described previously (Sinclair et al., 2009).
The binary vectors created for both constructs were introduced into the disarmed Agrobacterium tumefaciens strain GV3101-pMP90 (Koncz and Schell, 1986) by electroporation.
Plant Material
Previously reported transgenic Arabidopsis (Arabidopsis thaliana) ecotype Columbia lines (Marques et al., 2004) expressing the FNR-EGFP protein fusion (Marques et al., 2003) were used for experiments utilizing Arabidopsis. Other Arabidopsis lines highlighting plastid stroma, which were tested but not further utilized in this study are 16-EGFP (Marques et al., 2003, 2004), pt-yk, and pt-gk (Arabidopsis Biological Resource Center stock numbers CS16267 and CS16266, respectively; Nelson et al., 2007).
For analysis of stromules and ER in Nicotiana benthamiana transient expression in wild type as well as transgenic FNR-EGFP-expressing plants were used. For generation of transgenic FNR-EGFP-expressing N. benthamiana plants, sterile explants were transformed according to Clementine (2006) using A. tumefaciens strain GV3101::pMP90 harboring the respective T-DNA plasmid.
Plant Growth Conditions
For examination of roots of N. benthamiana as well as Arabidopsis the seeds were germinated and grown on 1% agar-gelled Murashige and Skoog (1962) medium, supplemented with 3% Suc and with a pH adjusted to 5.8. Plants were maintained in growth chambers according to Sinclair et al. (2009). Seedlings were used between 8 and 12 d following germination.
For Agrobacterium-mediated transient expression N. benthamiana wild-type or FNR-EGFP transgenic plants were grown on soil at 23°C in 8-h light (150 μE) 16-h dark cycles. For experiments young expanded leaves of 4- to 6-week-old N. benthamiana wild-type or FNR-EGFP transgenic plants were used.
Transient Expression
For infiltration of N. benthamiana leaf tissue A. tumefaciens (strain GV3101::pMP90) harboring the binary vector was cultured on yeast extract-beef extract plates containing antibiotics for two days. The bacteria were collected from plates and suspended in Agrobacterium infiltration media (10 mm MgCl2, 5 mm MES, pH 5.3, 150 μm acetosyringone) using a pipette tip. The bacteria suspension was incubated for 1 to 2 h at room temperature and its optical density at 600 nm adjusted to 0.8 prior to infiltration. For expression of two or more constructs, bacteria suspensions were mixed in equal amounts prior to infiltration. As target tissue young expanded leaves of 4- to 6-week-old N. benthamiana wild-type or FNR-EGFP transgenic plants were used. The Agrobacteria were introduced into the plant tissue by using a needleless syringe. For microscopic analysis leaf discs of infiltrated tissue were harvested, vacuum treated to remove air bubbles, and mounted in tap water 30 to 48 h post infiltration.
Microscopy
For live imaging, seedlings were placed in a glass depression slide while leaf discs were observed on a plain slide under a glass coverslip mounted in tap water. Confocal microscopy was performed using a Leica TCS-SP5 setup with a 488-nm Ar laser and a 543-nm HeNe laser (Leica) and a Leica DM6000B microscope equipped with a 40×·water immersion lens (numerical aperture 0.80). Images were obtained in a 1,024 × 512 pixel format in x/y/z and x/y/time dimensions, and processed using proprietary LEICA software. Fluorescent emission collection was at 490 to 510 nm for GFP, 500 to 522 nm for yellow fluorescent protein, 558 to 596 nm for RFP, and 600 to 680 nm for chlorophyll.
Post-Acquisition Image Processing
All images and movies were cropped and processed for brightness/contrast as complete image or stack either using Adobe Photoshop CS3 (http://www.adobe.com) or the ImageJ distribution Fiji (http://pacific.mpi-cbg.de/wiki/index.php/Fiji). Adobe Photoshop was used for annotation of movies. Angles of ER and stromule branches as well as kinks were measured using the angle tool of Fiji. Imaris software (v. 6.4.0; Bitplane AG) was used to render 3D ER and plastid reconstructions from confocal optical stacks and time series. Figures were assembled using Adobe Illustrator CS3 (http://www.adobe.com).
All experiments reported here have been repeated at least four times. Wherever applicable the total number of observations (n = 20 or more) has been provided. The Student’s t test was carried out using SigmaPlot (www.SigmaPlot.com).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Movie S1. Straight stromules extended by subcortically located chloroplasts in an epidermal cell of N. benthamiana seedling hypocotyl.
Supplemental Movie S2. A single stromule in a hypocotyl epidermal cell of N. benthamiana seedling forms kinks along its length.
Supplemental Movie S3. The movie shows numerous chloroplasts extending kinked and branched stromules in a lower leaf epidermal cell of N. benthamiana.
Supplemental Movie S4. A single stromule from an epidermal hypocotyl cell of N. benthamiana seedling that forms numerous branch points along its length and exhibits branch extension and rapid retraction.
Supplemental Movie S5. The ER mesh (magenta) surrounding chloroplasts (cyan) localized in the cortical region of a cell from the lower leaf epidermis of an N. benthamiana plant.
Supplemental Movie S6. A single stromule being extended from a chloroplast nestled within an ER mesh in a lower leaf epidermal cell of N. benthamiana.
Supplemental Movie S7. A stromule in a lower leaf epidermal cell of N. benthamiana plant that displays filling in of a triangular region (*) within an ER polygon and the extension and retraction in tandem with the neighboring ER.
Supplemental Movie S8. Stromule in a lower leaf epidermal cell of N. benthamiana displaying stromal triangles, branch extension, and retraction in coordination with neighboring ER.
Supplemental Movie S9. A chloroplast in an epidermal cell of N. benthamiana exhibiting beaks (Gunning, 2005) that appear transiently from different points of the plastid surface.
Acknowledgments
We thank Dr. Annett Strauss (former Institute of Genetics, Martin-Luther University of Halle-Wittenberg, Germany) for helping to generate transgenic N. benthamiana plants.
References
- Andersson MX, Goksör M, Sandelius AS. (2007) Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J Biol Chem 282: 1170–1174 [DOI] [PubMed] [Google Scholar]
- Arimura S, Hirai A, Tsutsumi N. (2001) Numerous and highly developed tubular projections from plastids observed in tobacco epidermal cells. Plant Sci 160: 449–454 [DOI] [PubMed] [Google Scholar]
- Bourett TM, Czymmek KJ, Howard RJ. (1999) Ultrastructure of chloroplast protuberances in rice leaves preserved by high-pressure freezing. Planta 208: 472–479 [Google Scholar]
- Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar SP. (2008) Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132: 449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementine T. (2006) Nicotiana (Nicotiana tabaccum, Nicotiana benthamiana). InWang K, ed, Agrobacterium Protocols, Ed 2, Vol 1. Humana Press, Totowa, NJ, pp 143–154 [Google Scholar]
- Ehara T, Osafune T, Hase E. (1985) Interactions between the nucleus and cytoplasmic organelles during the cell-cycle of Euglena-gracilis in synchronized cultures. III. Association between the nucleus and chloroplasts at an early stage in the cell-cycle under photoorganotrophic conditions. 2. Plant Cell Physiol 26: 1155–1165 [Google Scholar]
- Fester T, Strack D, Hause B. (2001) Reorganization of tobacco root plastids during arbuscule development. Planta 213: 864–868 [DOI] [PubMed] [Google Scholar]
- Gao H, Sage TL, Osteryoung KW. (2006) FZL, an FZO-like protein in plants, is a determinant of thylakoid and chloroplast morphology. Proc Natl Acad Sci USA 103: 6759–6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Sullivan JA, Hibberd JM, Hansen MR. (2001) Stromules: mobile protrusions and interconnections between plastids. Plant Biol 3: 223–233 [Google Scholar]
- Griffing LR. (2010) Networking in the endoplasmic reticulum. Biochem Soc Trans 38: 747–753 [DOI] [PubMed] [Google Scholar]
- Gunning BE. (2005) Plastid stromules: video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma 225: 33–42 [DOI] [PubMed] [Google Scholar]
- Hans J, Hause B, Strack D, Walter MH. (2004) Cloning, characterization, and immunolocalization of a mycorrhiza-inducible 1-deoxy-d-xylulose 5-phosphate reductoisomerase in arbuscule-containing cells of maize. Plant Physiol 134: 614–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A. (2008) Dynamic morphology of plastids and stromules in angiosperm plants. Plant Cell Environ 31: 646–657 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Holthuis JCM, Levine TP. (2005) Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6: 209–220 [DOI] [PubMed] [Google Scholar]
- Holzinger A, Buchner O, Lütz C, Hanson MR. (2007a) Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma 230: 23–30 [DOI] [PubMed] [Google Scholar]
- Holzinger A, Kwok EY, Hanson MR. (2008) Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem Photobiol 84: 1324–1335 [DOI] [PubMed] [Google Scholar]
- Holzinger A, Wasteneys GO, Lütz C. (2007b) Investigating cytoskeletal function in chloroplast protrusion formation in the arctic-alpine plant Oxyria digyna. Plant Biol (Stuttg) 9: 400–410 [DOI] [PubMed] [Google Scholar]
- Itoh RD, Yamasaki H, Septiana A, Yoshida S, Fujiwara MT. (2010) Chemical induction of rapid and reversible plastid filamentation in Arabidopsis thaliana roots. Physiol Plant 139: 144–158 [DOI] [PubMed] [Google Scholar]
- Jouhet J, Gray JC. (2009) Interaction of actin and the chloroplast protein import apparatus. J Biol Chem 284: 19132–19141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, Meagher RB. (1999) Actin-organelle interaction: association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskeleton 44: 110–118 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. (1997) Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Hanson MR. (2000) Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci 113: 81–89 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Schwille P, Webb WW, Hanson MR. (2000) Active protein transport through plastid tubules: velocity quantified by fluorescence correlation spectroscopy. J Cell Sci 113: 3921–3930 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J. (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Kwok EY, Hanson MR. (2003) Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J 35: 16–26 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. (2004a) GFP-labelled Rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J Exp Bot 55: 595–604 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. (2004b) In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biol 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse S, Schliemann W, Ammer C, Kopka J, Strack D, Fester T. (2005) Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol 139: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütz C, Engel L. (2007) Changes in chloroplast ultrastructure in some high-alpine plants: adaptation to metabolic demands and climate? Protoplasma 231: 183–192 [DOI] [PubMed] [Google Scholar]
- Marques JP, Dudeck I, Klösgen RB. (2003) Targeting of EGFP chimeras within chloroplasts. Mol Genet Genomics 269: 381–387 [DOI] [PubMed] [Google Scholar]
- Marques JP, Schattat MH, Hause G, Dudeck I, Klösgen RB. (2004) In vivo transport of folded EGFP by the DeltapH/TAT-dependent pathway in chloroplasts of Arabidopsis thaliana. J Exp Bot 55: 1697–1706 [DOI] [PubMed] [Google Scholar]
- Mclean B, Whatley JM, Juniper BE. (1988) Continuity of chloroplast and endoplasmic-reticulum membranes in chara and equisetum. New Phytol 109: 59–65 [Google Scholar]
- Menzel D. (1994) An interconnected plastidom in Acetabularia—implications for the mechanism of chloroplast motility. Protoplasma 179: 166–171 [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio agsays with tohaoco tissue cultures. Physiol Plant 15: 437–497 [Google Scholar]
- Natesan SK, Sullivan JA, Gray JC. (2005) Stromules: a characteristic cell-specific feature of plastid morphology. J Exp Bot 56: 787–797 [DOI] [PubMed] [Google Scholar]
- Natesan SKA, Sullivan JA, Gray JC. (2009) Myosin XI is required for actin-associated movement of plastid stromules. Mol Plant 2: 1262–1272 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A. (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Pyke KA, Howells CA. (2002) Plastid and stromule morphogenesis in tomato. Ann Bot (Lond) 90: 559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sage RF. (2009) The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol 50: 756–772 [DOI] [PubMed] [Google Scholar]
- Sandelius AS, Andersson MX, Goksor M, Tjellstrom H, Wellander R. (2007) Membrane contact sites: physical attachment between chloroplasts and endoplasmic reticulum revealed by optical manipulation. Chem Phys Lipids 149: S42–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattarzadeh A, Krahmer J, Germain AD, Hanson MR. (2009) A myosin XI tail domain homologous to the yeast myosin vacuole-binding domain interacts with plastids and stromules in Nicotiana benthamiana. Mol Plant 2: 1351–1358 [DOI] [PubMed] [Google Scholar]
- Scott I, Sparkes IA, Logan DC. (2007) The missing link: inter-organellar connections in mitochondria and peroxisomes? Trends Plant Sci 12: 380–381 [DOI] [PubMed] [Google Scholar]
- Shiina T, Hayashi K, Ishii N, Morikawa K, Toyoshima Y. (2000) Chloroplast tubules visualized in transplastomic plants expressing green fluorescent protein. Plant Cell Physiol 41: 367–371 [DOI] [PubMed] [Google Scholar]
- Sinclair AM, Trobacher CP, Mathur N, Greenwood JS, Mathur J. (2009) Peroxule extension over ER-defined paths constitutes a rapid subcellular response to hydroxyl stress. Plant J 59: 231–242 [DOI] [PubMed] [Google Scholar]
- Sparkes I, Runions J, Hawes C, Griffing L. (2009) Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21: 3937–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirlapur UK, Dahse I, Reiss B, Meurer J, Oelmüller R. (1999) Characterization of the activity of a plastid-targeted green fluorescent protein in Arabidopsis. Eur J Cell Biol 78: 233–240 [DOI] [PubMed] [Google Scholar]
- Überlacker B, Werr W. (1996) Vectors with rare-cutter restriction enzyme sites for expression of open reading frames in transgenic plants. Mol Breed 2: 293–295 [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I. (2010) Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Fray RG, Pyke KA. (2004) Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J 39: 655–667 [DOI] [PubMed] [Google Scholar]
- Whatley JM, Mclean B, Juniper BE. (1991) Continuity of chloroplast and endoplasmic-reticulum membranes in Phaseolus-vulgaris. New Phytol 117: 209–217 [Google Scholar]