Abstract
In higher plants, the chloroplast NADH dehydrogenase-like complex (NDH) interacts with photosystem I (PSI) to form the NDH-PSI supercomplex via two minor light-harvesting complex I (LHCI) proteins, Lhca5 and Lhca6. Previously, we showed that in lhca5 and lhca6, NDH still associates with PSI to form smaller versions of the NDH-PSI supercomplex, although their molecular masses are far smaller than that of the full-size NDH-PSI supercomplex. In this study, we show that the NDH complex is present in the monomeric form in Arabidopsis (Arabidopsis thaliana) lhca5 lhca6, implying that NDH interacts with multiple copies of PSI. NDH subunit levels were slightly reduced in immature leaves and more drastically (approximately 50%) in mature leaves of the lhca5 lhca6 double mutant compared with the wild type. Chlorophyll fluorescence analyses detected NDH activity of lhca5 lhca6, suggesting that the supercomplex formation is not essential for NDH activity. However, the severe phenotypes of the lhca5 lhca6 proton gradient regulation5 triple mutant in both plant growth rate and photosynthesis suggest that the function of NDH was impaired in this mutant in vivo. Accumulation of NDH subunits was drastically reduced in lhca5 lhca6 when the light intensity was shifted from 50 to 500 μmol photons m−2 s−1. Furthermore, the half-life of NDH subunits, especially that of NDH18, was shorter in monomeric NDH than in the NDH-PSI supercomplex under the high-light conditions. We propose that NDH-PSI supercomplex formation stabilizes NDH and that the process is especially required under stress conditions.
In addition to linear electron transport through complexes of PSII, cytochrome (Cyt) b6f, and PSI, cyclic electron transport around PSI contributes to the light reactions of photosynthesis (Shikanai, 2007a). While linear electron transport produces both ATP and NADPH, PSI cyclic electron transport preferentially produces ATP without accumulation of NADPH; this mechanism is thought to balance the ATP-NADPH ratio for various metabolic reactions (Livingston et al., 2010). Analysis of Arabidopsis (Arabidopsis thaliana) mutants clarified at least two partially redundant pathways of PSI cyclic electron transport in chloroplasts. The main pathway depends on PROTON GRADIENT REGULATION5 (PGR5) and PGR5-Like1 (PGRL1; Munekage et al., 2002; DalCorso et al., 2008), although it is still unclear how these proteins are required for the electron transport (Nandha et al., 2007). Recently, the supercomplex that drives PSI cyclic electron transport was discovered in Chlamydomonas reinhardtii, and this supercomplex was designated PSI-LHCI-LHCII-FNR-Cytb6f-PGRL1 (Iwai et al., 2010). The minor pathway is mediated by the chloroplast NADH dehydrogenase-like complex (NDH), which prevents overreduction of the stroma, especially under stress conditions (Munekage et al., 2004; Shikanai, 2007b).
Chloroplast NDH is thought to have originated from its ancestor, cyanobacterial NDH-1 (Friedrich and Weiss, 1997). Cyanobacteria have two functionally distinct NDH-1 complexes, NDH-1L and NDH-1MS, and chloroplast NDH is related only to cyanobacterial NDH-1L, which is involved in respiration and PSI cyclic electron transport (Battchikova and Aro, 2007). Eleven subunits of the chloroplast NDH complex are homologs to subunits in the mitochondrial complex I and eubacterial NADH dehydrogenase (Matsubayashi et al., 1987; Peng et al., 2011). However, the homologs of the three bacterial subunits (NuoE–NuoG) that function in NADH oxidation are not encoded in the cyanobacterial and higher plant genomes, suggesting that chloroplast and cyanobacterial NDH complexes are equipped with different “catalytic” domains and probably utilize different substrates as electron donors rather than NAD(P)H (Peng et al., 2011).
During the last decade, several novel NDH subunits have been identified in higher plants (Suorsa et al., 2009; Ifuku et al., 2010; Peng et al., 2011). On the basis of the analogy with Escherichia coli and cyanobacterial NDH-1, as well as extensive genetic and biochemical characterization, the chloroplast NDH complex was divided into four categories: membrane, lumen, A, and B subcomplexes (Peng et al., 2011). Although the subunits of the membrane subcomplex and subcomplex A are conserved in cyanobacterial NDH-1L and chloroplast NDH, chloroplast NDH had acquired dozens of novel subunits and these chloroplast-specific subunits are mainly found in the lumen and B subcomplexes (Peng et al., 2011). While the lumen subcomplex includes PsbP-Like protein2, FK-506 Binding Protein16-2 (FKBP16-2), CYCLOPHILIN20-2 (CYP20-2), and At1g14150, subcomplex B contains NDH-DEPENDENT CYCLIC ELECTRON FLOW1 (NDF1)/NDH48, NDF2/NDH45, NDF4, NDF6, NDH18, and At3g01440. Except for CYP20-2, all of the chloroplast-specific subunits are required for the accumulation of the intact NDH complex. The existence of these higher plant-specific subunits suggests that the structure of the NDH complex has changed drastically during the evolution of land plants.
Besides having acquired these novel subunits over the course of evolution, the NDH complex also came to interact with PSI to form the NDH-PSI supercomplex (Peng et al., 2008). The molecular mass of this supercomplex is far greater than 1,000 kD, and it can be separated from other thylakoid membrane complexes by blue native (BN)-PAGE (Peng et al., 2008). Mass analysis of this supercomplex identified the majority of the PSI and NDH subunits as well as two minor light-harvesting complex I (LHCI) proteins, Lhca5 and Lhca6, which show a high degree of similarity to the four major LHCI proteins, Lhca1 to Lhca4 (Peng et al., 2009). Analysis of mutants defective in the expression of Lhca5 and Lhca6 showed that these two minor LHCI proteins are specifically required for the formation of the full-size NDH-PSI supercomplex. In lhca6 RNA interference lines, the full-size NDH-PSI supercomplex was completely missing and NDH subunits accumulated in a smaller supercomplex of approximately 1,000 kD. Although the molecular mass of this supercomplex is far less than that of the full-size NDH-PSI supercomplex, it still associates with PSI (Peng et al., 2009). The smaller version of the NDH-PSI supercomplex was also found in the lhca5 mutant (Peng et al., 2009). These facts suggest that NDH probably associates with multiple copies of PSI.
In this study, we constructed an lhca5 lhca6 double mutant and found that NDH is mainly present as a monomer with a molecular mass of approximately 700 kD in the double mutant, suggesting that NDH interacts with at least two copies of PSI via Lhca5 and Lhca6. Further analyses showed that NDH-PSI supercomplex formation is required for the stability of NDH, especially under high-light conditions.
RESULTS
NDH Is Present as a Monomer in the lhca5 lhca6 Double Mutant
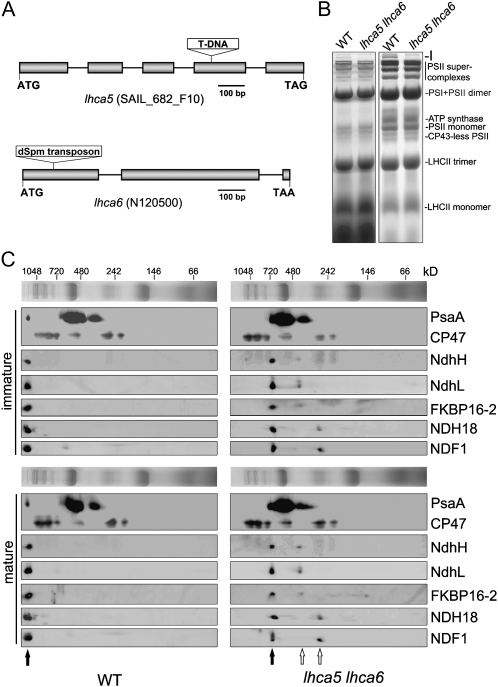
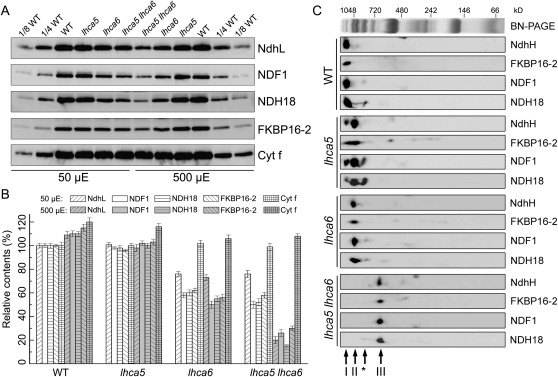
To study the possibility that NDH interacts with multiple PSI complexes via Lhca5 and Lhca6, we constructed an lhca5 lhca6 double mutant. Although RNA interference was previously used to knock down Lhca6 (Peng et al., 2009), a T-DNA insertion line of lhca5 and a dSpm transposon insertion line of lhca6 were obtained from the European Arabidopsis Stock Centre and the Arabidopsis Biological Resource Center, respectively, for convenience of genetic manipulation (Fig. 1A). Reverse transcription-PCR analyses indicate that the expression of Lhca5 and Lhca6 was completely knocked out in the corresponding mutants (data not shown). Consistent with our previous report (Peng et al., 2009), these two mutants did not exhibit any visible phenotype. BN-PAGE was performed to investigate possible structural changes of thylakoid protein complexes between wild-type and lhca5 lhca6 plants. It is clear that the NDH-PSI supercomplex corresponding to band I was absent in the double mutant, whereas other complexes were not affected (Fig. 1B).
Figure 1.
Analysis of thylakoid protein complexes from wild-type (WT) and lhca5 lhca6 double mutant plants. A, Structures of Lhca5 and Lhca6. The positions of the T-DNA insertion in lhca5 and the dSpm transposon insertion in lhca6 were confirmed by sequencing the PCR products. B, Thylakoid membranes were isolated from wild-type and lhca5 lhca6 plants and then separated by BN-PAGE (left panel). After electrophoresis, the gel was stained with Coomassie Brilliant Blue (right panel). Band I is the NDH-PSI supercomplex detected in wild-type plants. C, Thylakoid membrane complexes isolated from immature and mature leaves of wild-type and lhca5 lhca6 plants were separated by BN-PAGE and further subjected to 2D SDS-PAGE. The proteins were probed with specific antibodies against PsaA, CP47, NdhH, NdhL, FKBP16-2, NDH18, and NDF1. The positions of the NDH-PSI supercomplex in the wild type and monomeric NDH in lhca5 lhca6 are indicated by black arrows. The positions of the putative NDH subcomplexes detected in lhca5 lhca6 are indicated by white arrows.
To assign the position of NDH subunits in BN-PAGE, we performed two-dimensional (2D) SDS-PAGE and subsequent immunoblot analyses using specific antibodies (Fig. 1C). Besides the NDH-PSI supercomplex and PSI monomer, a smaller complex containing PsaA was also detected on the BN gel, and it probably corresponds to the PSI core complex, which is detached from the LHCI complex (Romanowska et al., 2008). Consistent with previous reports, NDH subunits comigrate with a trace amount of PSI at the top of the BN gel in wild-type samples (Fig. 1C); this complex corresponds to the full-size NDH-PSI supercomplex (Peng et al., 2008, 2009). Interestingly, all of five NDH subunits tested were detected mainly in a complex of approximately 700 kD in both immature and mature leaves of the lhca5 lhca6 double mutant, and the PSI complex that comigrated with NDH in wild-type thylakoids was absent in lhca5 lhca6 (Fig. 1C). So far, a total of 25 NDH subunits have been identified by various approaches (Rumeau et al., 2005; Ishihara et al., 2007; Ishikawa et al., 2008; Majeran et al., 2008; Shimizu et al., 2008; Peng et al., 2009; Sirpiö et al., 2009a, 2009b; Takabayashi et al., 2009; Suorsa et al., 2010; Yabuta et al., 2010). If we assume that only one copy of each subunit is included in the complex, the calculated molecular mass of NDH is approximately 690 kD, which is almost identical to that of the NDH complex detected in lhca5 lhca6. We propose that the 700-kD complex detected in the lhca5 lhca6 double mutant consists of all the NDH subunits, and we thus refer to the complex as monomeric NDH, which interacts with at least two copies of PSI to form the full-size NDH-PSI supercomplex in wild-type plants (for more details, see “Discussion”).
Besides the NDH monomer, an NDH subcomplex with molecular mass of approximately 450 kD was detected by antibodies against NdhH, NdhL, and FKBP16-2 in the lhca5 lhca6 double mutant (Fig. 1C). In contrast, small amounts of NDH18 and NDF1 were present in another subcomplex with molecular mass of approximately 250 kD. These results imply that the 450-kD complex includes subcomplex A and the lumen subcomplex and that the 250-kD complex contains subcomplex B. Because we have no antibodies against membrane subunits, it is unclear which subcomplex contains the membrane subunits.
NDH Subunit Levels in the lhca5 lhca6 Double Mutant
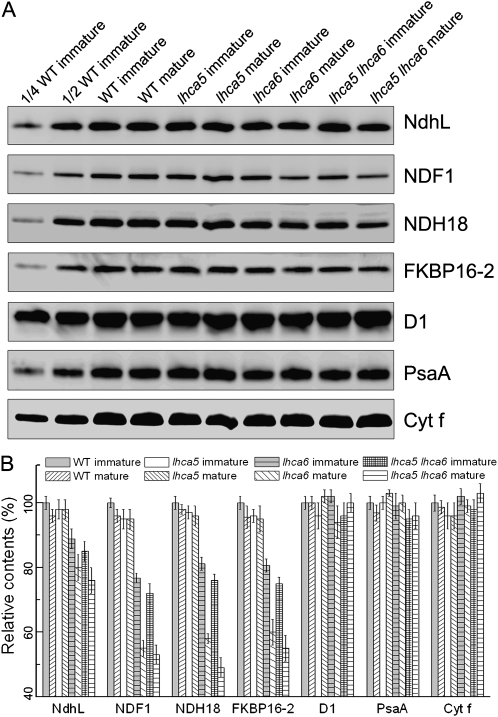
To investigate the steady-state levels of NDH subunits in lhca5 and lhca5 lhca6, the protein blots were probed with antibodies against various NDH subunits (Fig. 2A). As reported previously (Peng et al., 2009), NDH subunit levels were slightly higher in immature than in mature lhca6 leaves (Fig. 2). We also analyzed NDH levels in immature and mature lhca5 and lhca5 lhca6 leaves. In contrast to lhca6, NDH subunit levels were unaffected in both immature and mature leaves in lhca5 (Fig. 2). In lhca5 lhca6, NDH subunit levels were slightly lower than in immature (approximately 80%) and mature (approximately 50%) lhca6 leaves (Fig. 2). These results suggest a minor contribution of Lhca5 in the stabilization or assembly of the NDH complex, at least at 50 μmol photons m−2 s−1.
Figure 2.
Analysis of the NDH complex from wild-type (WT), lhca5, lhca6, and lhca5 lhca6 plants. A, Immunoblot analysis of thylakoid proteins from immature and mature leaves of wild-type, lhca5, lhca6, and lhca5 lhca6 plants. Thylakoid proteins were separated by SDS-PAGE and immunodetected with specific antibodies. Thylakoid proteins were loaded on an equal chlorophyll basis. B, Semiquantitative analysis of thylakoid proteins. Immunoblot results were analyzed with Imagemaster software (Amersham Pharmacia Biotech). The protein levels in the wild-type mature leaves and lhca5, lhca6, and lhca5 lhca6 immature and mature leaves are shown relative to those in the wild-type immature leaves (100%). Values are means ± sd of three independent experiments using different plant materials.
Monomeric NDH Also Localizes to the Stroma Thylakoids
The conservation of Lhca5 and Lhca6 suggests that the NDH-PSI supercomplex is universal among flowering plants (Peng et al., 2009). What is the physiological function of the NDH-PSI supercomplex formation? We know that both NDH and PSI complexes reside in the stroma thylakoids (Lennon et al., 2003). To study the possibility that the NDH complex requires its partner, PSI, for precise localization, we separated the stroma and grana lamellae and then analyzed the localization of NDH by immunoblot analysis (Supplemental Fig. S1). The majority of the NDH-PSI supercomplex (wild type) and monomeric NDH subunits (lhca5 lhca6) were found in the stroma thylakoids, while D1 was mainly localized to the grana lamellae (Supplemental Fig. S1). This result excludes the possibility that NDH-PSI supercomplex formation plays a role in the precise localization of NDH.
Activity of Monomeric NDH in lhca5 lhca6
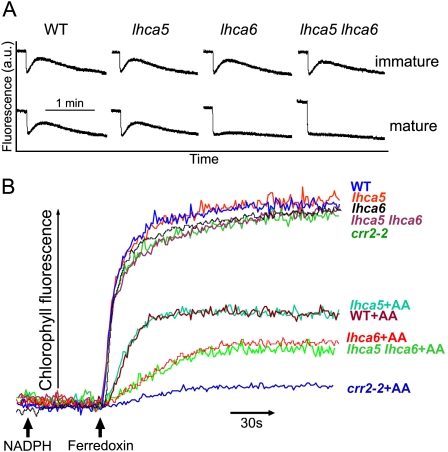
NDH activity is monitored by a transient increase in chlorophyll fluorescence after actinic light (AL) illumination; this phenomenon is due to the reduction of the plastoquinone (PQ) pool by NDH in darkness (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998). Consistent with the previous report (Peng et al., 2009), the transient chlorophyll fluorescence increase could be detected in immature leaves of lhca6, but it was drastically reduced in mature leaves (Fig. 3A). Although the full-size NDH-PSI supercomplex formation was impaired in lhca5 (Peng et al., 2009), the postillumination increase in chlorophyll fluorescence was identical in lhca5 and the wild type (Fig. 3A). Similar to lhca6, the transient increase in chlorophyll fluorescence was detected in immature leaves of lhca5 lhca6, but it was not observed in its mature leaves (Fig. 3A). We conclude that the smaller versions of NDH-PSI supercomplexes present in lhca5 and lhca6 and also the monomeric NDH complex present in lhca5 lhca6 are still functional, at least in immature leaves and in this assay system. Some of the decrease in NDH activity detected in this assay may be explained by the decrease in NDH subunit levels (Fig. 2). However, NDH activity was not detected in mature leaves of lhca5 lhca6, which still accumulate half the NDH subunit levels of the wild type (Figs. 2 and 3A).
Figure 3.
Analyses of the activities of the various NDH complexes. A, Monitoring of NDH activity by chlorophyll fluorescence analysis. Four-week-old plants were illuminated for 5 min with AL (50 μmol photons m−2 s−1). After illumination, NDH activity was monitored as the subsequent transient increase in chlorophyll fluorescence. The fluorescence levels were normalized by the Fm levels. The definition of leaf age is the same as in a previous report (Peng et al., 2009). a.u., Arbitrary units. B, Fd-dependent PQ reduction assay. Increases in chlorophyll fluorescence by the addition of NADPH (0.25 mm) and Fd (5 μm) under weak illumination (1.0 μmol photons m−2 s−1) were monitored in osmotically ruptured chloroplasts (20 μg chlorophyll mL−1) of wild-type (WT), lhca5, lhca6, crr2-2, and lhca5 lhca6 mature leaves. Ruptured chloroplasts were incubated with 10 μm antimycin A before measurement. The maximum fluorescence level in the wild type corresponded to approximately 40% of the Fm level. This is a representative result of three experiments using thylakoid membranes isolated independently.
As an alternative assay of NDH activity, we monitored ferredoxin (Fd)-dependent PQ reduction activity using ruptured chloroplasts isolated from mature leaves (Fig. 3B). In this assay system, PQ is reduced via NDH- and PGR5/PGRL1-dependent pathways, and the latter is specifically inhibited by antimycin A (Munekage et al., 2002). Consistent with the results of in vivo analysis (Fig. 3A), PQ reduction activity was identical in lhca5 and the wild type both in the presence and absence of antimycin A (Fig. 3B). Taken together with the results of in vivo analysis, we conclude that the NDH activity of the smaller version of the NDH-PSI supercomplex detected in lhca5 is comparable to that of the full-size NDH-PSI supercomplex detected in the wild type under the nonstress conditions.
In the presence of antimycin A, PQ reduction activity of lhca5 lhca6 was lower than that of the wild type but higher than that of chlororespiratory reduction2-2 (crr2-2), in which NDH complex accumulation was impaired due to defective expression of ndhB (Hashimoto et al., 2003). Because NDH subunits were preferentially detected in the 700-kD complex, this residual PQ reduction activity depends on monomeric NDH (Fig. 3B), although NDH activity was not detected in the in vivo analysis of mature leaves (Fig. 3A). Consistent with our previous report (Peng et al., 2009), PQ reduction activity of lhca6 was also lower than that of the wild type but higher than that of crr2-2 (Fig. 3B). Our in vitro assay does not provide exact quantitative information, and we cannot show whether the level of PQ reduction can be explained by the 30% to 50% reduction of NDH subunits in lhca6 and lhca5 lhca6. However, it is clear that the smaller version of the NDH-PSI supercomplex present in lhca6 and the NDH monomer present in lhca5 lhca6 still retain the activity detected in the in vitro system (Fig. 3B).
Accumulation of Monomeric NDH in the pgr5 Mutant Background
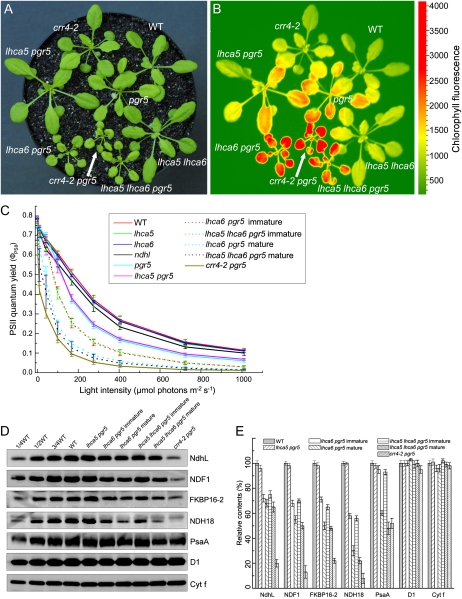
In our previous report (Peng et al., 2009), we showed that plant growth and photosynthesis were drastically impaired in lhca6 pgr5, which accumulated approximately 75% of wild-type levels of NdhH and NdhL. These results suggested that NDH-PSI supercomplex formation may be required for the activity of the NDH complex. However, our in vivo and in vitro analyses of NDH activity in this work challenged this hypothesis (Fig. 3). To further investigate the link between the supercomplex formation and NDH activity, we constructed the lhca5 pgr5 double mutant and the lhca5 lhca6 pgr5 triple mutant. Because NDH is essential for efficient photosynthesis in the pgr5 mutant background, the sizes and steady-state chlorophyll fluorescence levels of the plants reflect NDH activity in vivo (Munekage et al., 2004; Peng et al., 2009). As shown in Figure 4, at a light intensity of 50 μmol photons m−2 s−1, lhca5 lhca6 pgr5 showed retarded growth and displayed high chlorophyll fluorescence, as in lhca6 pgr5 and crr4-2 pgr5 (Fig. 4, A and B), indicating impaired photosynthetic electron transport activity. These results also suggest that NDH activity was drastically impaired in lhca5 lhca6 pgr5 and lhca6 pgr5 as well as in crr4-2 pgr5. In contrast, lhca5 pgr5 grew as well as pgr5 and the wild type and its chlorophyll fluorescence level was slightly higher than that of the wild type, similar to the pgr5 single mutant (Fig. 4, A and B). This result indicates that Lhca5 is not required for NDH activity in vivo even under the pgr5 mutant background.
Figure 4.
Analysis of the lhca5 lhca6 pgr5 triple mutant. A, Visible phenotype of the triple mutant. Plants were grown in a growth chamber (50 μmol photons m−2 s−1, 16-h photoperiod, 23°C) for 4 weeks after germination. B, High-chlorophyll-fluorescence phenotype of the lhca5 lhca6 pgr5 triple mutant. Dark-adapted seedlings of the indicated plants were illuminated at 100 μmol photons m−2 s−1 for 1 min, and a chlorophyll fluorescence image was captured by FluorCAM 700MF. C, Measurement of ΦPSII. ΦPSII was calculated as (Fm′ – Fs)/Fm′. Values are means ± sd (n = 5). D, Analysis of the thylakoid proteins. Thylakoid membrane proteins from the indicated plants were separated by SDS-PAGE and immunodetected with specific antibodies against NdhL, NDF1, FKBP16-2, NDH18, PsaA, D1, and Cyt f. Thylakoid proteins were loaded on an equal chlorophyll basis. E, Semiquantitative analysis of thylakoid proteins as in Figure 2B. The protein levels in the wild-type (WT) plants were defined as 100%. Values are means ± sd (n = 3).
To further characterize the photosynthetic activity of the lhca5 lhca6 pgr5 triple mutant, we analyzed the light intensity dependence of PSII quantum yield (ΦPSII), which provides an estimate of the quantum yield of linear electron flow through PSII (Fig. 4C; Baker, 2008). The ΦPSII levels were similar in wild-type, lhca5, and lhca6 plants, suggesting that Lhca5 and Lhca6 are not required for photosynthetic electron transport. The ΦPSII was not affected in pgr5 below 100 μmol photons m−2 s−1, but it was reduced significantly at higher light intensities compared with the wild type (Fig. 4C), probably due to the restriction of photosynthesis by the ATP level and photodamage of photosystems. In the crr4-2 pgr5 double mutant defective in both pathways of PSI cyclic electron transport, the ΦPSII value was drastically reduced even at 50 μmol photons m−2 s−1. The ΦPSII levels were identical between lhca5 pgr5 and pgr5 plants, and this is consistent with the growth phenotype of lhca5 pgr5 (Fig. 4, A and C). In contrast, the ΦPSII levels were significantly lower in lhca6 pgr5 and lhca5 lhca6 pgr5 than in pgr5, but they were slightly higher than in crr4-2 pgr5. The reduction was more severe in their mature leaves than in immature leaves.
We also determined two other chlorophyll fluorescence parameters, nonphotochemical quenching (NPQ) and 1-qL, which reflects the redox state of the PQ pool (Kramer et al., 2004; Miyake et al., 2009). Compared with the wild type, a slight increase in NPQ was observed in lhca5, lhca6, and ndhl mutants (Supplemental Fig. S2A), as often suggested in the mutants defective in NDH activity (Rumeau et al., 2005). NPQ induction was affected severely in pgr5, as reported previously (Munekage et al., 2002). The similar defect in NPQ induction was also observed in lhca5 pgr5, lhca6 pgr5, and lhca5 lhca6 pgr5 (Supplemental Fig. S2A).
The 1-qL levels were higher in pgr5 than in the wild type, lhca5, lhca6, and ndhl at light intensities of more than 200 μmol photons m−2 s−1 (Supplemental Fig. S2B). 1-qL was higher in crr4-2 pgr5 than in pgr5 at light intensities of more than 50 μmol photons m−2 s−1, indicating that the primary electron-accepting plastoquinone of PSII is highly reduced in crr4-2 pgr5 even at low light intensities. The 1-qL levels were similar between lhca5 pgr5 and pgr5, suggesting that the lhca5 defect scarcely affects NDH activity in vivo. This idea is consistent with the similar 1-qL levels between lhca6 pgr5 and lhca5 lhca6 pgr5 both in immature and mature leaves (Supplemental Fig. S2B). All of the chlorophyll fluorescence analyses suggest that NDH activity is severely impaired in the mature leaves of lhca6 pgr5 and lhca5 lhca6 pgr5. In contrast, the lhca5 defect is unlikely to affect NDH activity even when the phenotype is analyzed in the pgr5 mutant background.
Immunoblot assays were performed to investigate the residual NDH level in these genotypes. The levels of NDH subunits NdhL, NDF1, and FKBP16-2 were reduced to 50% to 75% of the wild-type level in the mature leaves of lhca5 lhca6 pgr5, as in the mature leaves of lhca6 pgr5 (Fig. 4, D and E; Peng et al., 2009), and their immature leaves accumulated higher levels of subunits (Fig. 4, D and E). Interestingly, the NDH18 level was drastically reduced to approximately 25% of the wild-type level in the mature leaves of lhca5 lhca6 pgr5 (Fig. 4, D and E). In mature leaves of lhca6 pgr5, NDH18 accumulated to a slightly higher level than in mature leaves of lhca5 lhca6 pgr5. In contrast, the level of NDH18 was similar to that of other subunits in lhca6 and lhca5 lhca6 (Fig. 2). The low accumulation level of NDH18 in lhca6 pgr5 and lhca5 lhca6 pgr5 may explain the severe phenotype of these two mutants. In contrast to D1 and Cyt f, which were not affected in these plants, the PSI subunit PsaA was reduced to various extents in the mature leaves of lhca6 pgr5 and lhca5 lhca6 pgr5 plants as well as in crr4-2 pgr5 plants. The decrease in PSI levels in these plants would result from PSI photodamage (Munekage et al., 2004; Okegawa et al., 2010).
Accumulation of Monomeric NDH under High-Light Conditions
Accumulation of NDH18 is severely affected in lhca6 pgr5 and lhca5 lhca6 pgr5 mutants (Fig. 4, D and E). Since PGR5 is unlikely to be a component of the NDH complex and to influence the accumulation of NDH18 directly, the stromal overreduction caused by the pgr5 defect may affect the accumulation of NDH18. To further investigate the possibility that the NDH monomer is sensitive to the oxidative stress even in the presence of PGR5, we studied the accumulation of NDH subunits under high-light conditions. For this purpose, wild-type, lhca5, lhca6, and lhca5 lhca6 seedlings cultured at 50 μmol photons m−2 s−1 were exposed to the higher light intensity of 500 μmol photons m−2 s−1 for 2 d, and then the mature leaves were used for immunoblot analyses. By the stress, the maximum photochemical efficiency of PSII (Fv/Fm) level declined from 0.788 ± 0.05 to 0.658 ± 0.21, but this is not the case in pgr5 at low light intensity. The levels of NDH subunits and the Cyt b6f subunit were slightly up-regulated at higher light intensity in the wild type (Fig. 5, A and B), which is in line with a previous report (Tikkanen et al., 2006). However, the levels of NDH subunits were significantly reduced in lhca5 lhca6 seedlings exposed to high-light conditions compared with those cultured in low-light conditions (Fig. 5, A and B), supporting the idea that NDH-PSI supercomplex formation is required for the maintenance of the NDH complex, especially under stress conditions. In addition to NDH18, the levels of other NDH subunits in lhca5 lhca6 were reduced by the increase in light intensity. Although the activity and steady-state level of NDH were not affected in lhca5 under normal light conditions, NDH level did not increase in response to the increase in light intensity (Fig. 5, A and B).
Figure 5.
Analysis of thylakoid proteins from wild-type (WT), lhca5, lhca6, and lhca5 lhca6 plants under high-light conditions. A, Four-week-old plants cultured at 50 μmol photons m−2 s−1 were exposed to high light (500 μmol photons m−2 s−1) for 2 d (16-h photoperiod) in a temperature-controlled chamber at 23°C and then harvested after a 10-h illumination on the last day. Freshly isolated thylakoid proteins were separated by SDS-PAGE and then immunodetected with the specific antibodies indicated. Thylakoid proteins were loaded on an equal chlorophyll basis. B, Semiquantitative analysis of thylakoid proteins as in Figure 2B. The protein levels in the wild type grown at 50 μmol photons m−2 s−1 were defined as 100%. Values are means ± sd of three independent experiments using different plant materials. C, Thylakoid protein complexes isolated from high-light-treated plants were separated by BN-PAGE and then subjected to 2D SDS-PAGE. The proteins were immunodetected with the specific antibodies indicated. Arrows indicate the intact NDH-PSI supercomplex (I), the smaller NDH-PSI supercomplexes detected in lhca5 and lhca6 (II), monomeric NDH detected in lhca5 lhca6 (III), and the putative NDH-PSI subcomplex containing NDH18 and NDF1 detected in lhca5 (*).
To investigate whether the structures of the NDH-PSI supercomplex and monomeric NDH were altered under the high-light conditions, we performed 2D BN/SDS-PAGE and subsequent immunoblot analyses using thylakoids isolated from wild-type and lhca5 lhca6 plants exposed to the high light intensity. The lhca5 and lhca6 single mutants were also included in this assay (Fig. 5C). In the wild type, all of the NDH subunits tested were present in the position of the NDH-PSI supercomplex, suggesting that the structure of the NDH-PSI supercomplex was not affected under the high-light conditions. In lhca5 grown at 50 μmol photons m−2 s−1, NDH subunits were mainly detected in the smaller NDH-PSI supercomplex, with a trace amount of subunits in the intact NDH-PSI supercomplex (Peng et al., 2009). However, under the high-light conditions, an additional subcomplex containing NDF1 and NDH18 but lacking FKBP16-2 and NdhH was detected in lhca5. This finding suggests that Lhca5 also contributes to the stabilization and/or assembly of the NDH complex under the high-light conditions, as does in the absence of Lhca6 (Fig. 1). In lhca6 and lhca5 lhca6, NDH subunits were present in the smaller NDH-PSI subsupercomplex and the monomeric NDH complex, respectively (Fig. 5C). The subcomplexes of 450 and 250 kD detected in lhca6 and lhca5 lhca6 grown at 50 μmol photons m−2 s−1 (Fig. 1C; Peng et al., 2009) were missing when the plants were cultured at 500 μmol photons m−2 s−1 (Fig. 5C).
Stability of Monomeric NDH and the NDH-PSI Supercomplex under the High-Light Conditions
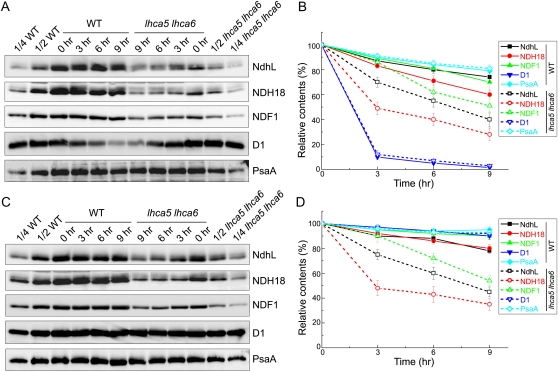
The steady-state accumulation level of the protein complex reflects the balance between its assembly and degradation. To assess which process was affected in lhca5 lhca6, we investigated the degradation of NDH subunits under the high-light conditions in the presence or absence of the protein synthesis inhibitors lincomycin and cycloheximide (Fig. 6). The degradation of the D1 subunit of PSII was accelerated by adding lincomycin and cycloheximide (Fig. 6, A and B), confirming that the inhibitors are functioning in our system. In the absence of the inhibitors, the D1 level was not reduced by the high-light treatment, suggesting efficient repair (Fig. 6, C and D). After the semiquantification of immunoblot signals, it is clear that the degradation of NDH subunits was accelerated in lhca5 lhca6 compared with that in the wild type (Fig. 6, A and B). In contrast, the levels of PsaA were only slightly reduced in the presence of the inhibitors, suggesting that PsaA is stable even under the high-light conditions. These results suggest that NDH subunits in monomeric NDH are unstable under the high-light conditions.
Figure 6.
Analysis of the stability of the NDH monomer and NDH-PSI supercomplex under high-light conditions. A, Leaves from 4-week-old wild-type (WT) and lhca5 lhca6 plants were vacuum infiltrated with lincomycin and cycloheximide for 30 min and then exposed to the high-intensity light (500 μmol photons m−2 s−1) for various times indicated at the top. After the illumination, thylakoids were isolated and subjected to SDS-PAGE and immunodetected with specific antibodies. B, Semiquantitative analysis of thylakoid proteins as in Figure 2B. The protein levels in the wild-type and lhca5 lhca6 leaves are shown relative to those in their leaves before high-light treatment (0 h; 100%). Values are means ± sd (n = 2). C and D, Conditions as in A and B, except that the leaves were vacuum infiltrated with water.
Even in the absence of the inhibitors, the NDH subunit levels in lhca5 lhca6 were drastically decreased under the high-light conditions, and its rate was comparable to that in the presence of the inhibitors (Fig. 6, C and D). This is in contrast to the fact that the reduction of D1 levels is drastically accelerated in the presence of the inhibitors (Fig. 6, A and B). These results suggest that the NDH complex is not repaired rapidly, once it is damaged. We conclude that monomeric NDH is unstable especially under high-light conditions and that the supercomplex formation is required for stabilizing NDH.
Consistent with the low steady-state level of NDH18 in lhca5 lhca6 pgr5 (Fig. 4, D and E), NDH18 in lhca5 lhca6 was more sensitive to the high light intensity than NDF1 and NdhL both in the presence and absence of the inhibitors (Fig. 6). The NDH18 level was also reduced more drastically than the levels of other subunits in the wild type, suggesting that NDH18 is localized to the most sensitive part of the NDH complex by the high-light stress, and the problem is more serious in monomeric NDH.
DISCUSSION
In this study, we investigated the structure and possible physiological functions of the NDH-PSI supercomplex. It has been shown that the molecular masses of the largest PSII supercomplex and PSI monomer are approximately 1,300 and 530 kD, respectively (Ben-Shem et al., 2003; Heinemeyer et al., 2004). Our BN-PAGE showed that the smaller versions of NDH-PSI supercomplexes detected in lhca5 and lhca6 are slightly smaller than the largest PSII supercomplex (Peng et al., 2009). Furthermore, the molecular mass of the NDH complex detected in the lhca5 lhca6 double mutant is approximately 700 kD (Fig. 1C), which likely represents monomeric NDH. These facts imply that only one copy of PSI interacts with monomeric NDH to form the distinct versions of smaller NDH-PSI with molecular masses of approximately 1,200 kD in lhca5 and lhca6, respectively. Detection of 700-kD monomeric NDH in lhca5 lhca6 suggests that NDH interacts with two copies of PSI to form the full-size NDH-PSI supercomplex via Lhca5 and Lhca6, although direct evidence, such as that obtained by electron microscopic analysis, is still lacking.
What is the physiological function of NDH-PSI supercomplex formation? NDH activity was detected in immature leaves of lhca5 lhca6 in vivo (Fig. 3A). NDH-mediated Fd-dependent PQ reduction was also detected in mature leaves of lhca5 lhca6 in vitro (Fig. 3B). Because the in vivo analysis based on chlorophyll fluorescence did not detect any NDH activity in mature leaves of lhca5 lhca6 (Fig. 3A), the in vitro analysis is more sensitive to detect low levels of activity. These results support the conclusion that NDH-PSI supercomplex formation is not essential for NDH activity. This conclusion is inconsistent with the mutant phenotype in the pgr5 background, which suggests that supercomplex formation via Lhca6 is required for the efficient function of NDH in vivo (Fig. 4; Peng et al., 2009). Because Fd is required for NDH-dependent PQ reduction in our ruptured-chloroplast assay (Munekage et al., 2004), it is possible that direct electron channeling occurs between PSI and NDH in the supercomplex via Fd, as in the supercomplex that drives cyclic electron flow in C. reinhardtii (Iwai et al., 2010). Our inconsistent result is partly due to the lack of a reliable method to monitor the rate of NDH-dependent PSI cyclic electron transport quantitatively (Okegawa et al., 2008), and we cannot eliminate the possibility that supercomplex formation is required for the efficient operation of NDH-dependent PSI cyclic electron transport in vivo.
Mitochondrial complex I is homologous to chloroplast NDH and forms a supercomplex with dimeric complex III (Dudkina et al., 2006). Several possible functions of this supercomplex have been proposed, including the enhancement of electron transfer rates, the stabilization of individual complexes, and assisting the complex insertion into the mitochondrial membranes (Boekema and Braun, 2007). PSI level is reduced in crr4-2 pgr5 (Fig. 4, D and E) and crr2 pgr5 (Okegawa et al., 2010), probably due to the photoinhibition of PSI. A similar phenotype was found in lhca5 lhca6 pgr5 (Fig. 4, D and E), suggesting the occurrence of oxidative stress in these plants even at low light intensity. A drastic decrease of the NDH18 level in mature leaves of lhca6 pgr5 and lhca5 lhca6 pgr5 strongly suggests that NDH-PSI supercomplex formation is required for the maintenance of NDH, especially in stress conditions. This idea was also supported by results showing that accumulation of NDH is lowered to approximately 50% of the wild-type level in lhca5 lhca6 mature leaves (Fig. 2) and more drastically (to approximately 25% of the wild-type level) when plants are exposed to high-intensity light (Fig. 5). Oxidative stresses caused by the pgr5 defect and by the high light in the presence of PGR5 may be different, and we cannot specify the direct reason for the phenotype. Because the NDH-PSI supercomplex level is more severely affected in mature leaves than in immature leaves in lhca5 lhca6 (Fig. 2), it is likely that it is the stability rather than the assembly that is affected. This idea was strongly supported by the experimental evidence indicating that degradation of NDH subunits is more accelerated in NDH monomer than in the NDH-PSI supercomplex (Fig. 6). We detected subcomplexes of 450 and 250 kD in the lhca6 and lhca5 lhca6 plants grown at 50 μmol photons m−2 s−1 (Fig. 1C; Peng et al., 2009), but these subcomplexes were missing when the plants were cultured at 500 μmol photons m−2 s−1 (Fig. 5C). We cannot eliminate the possibility that the supercomplex formation is required for the efficient assembly of NDH and the 450- and 250-kD subcomplexes are assembly intermediates. However, the NDH subunit levels are more drastically affected in mature leaves than in immature leaves of lhca6 and lhca5 lhca6 (Fig. 2). It is most likely that the 450- and 250-kD subcomplexes are produced by the degradation of NDH, and they are unstable especially at high light intensity (Figs. 1C and 5C).
We propose that the supercomplex formation with PSI stabilizes NDH. A similar phenomenon was also discovered in mammalian mitochondria, in which complex I interacts with complex III and the assembly of complex I is independent of complex III, but complex I is unstable in the absence of complex III (Acín-Pérez et al., 2004). It is still unclear how NDH-PSI supercomplex formation protects NDH from stress-induced inactivation and subsequent degradation. A possible target of the stress is NDH18, which contains one transmembrane domain and is included in subcomplex B (Peng et al., 2009). The severe phenotype of crr4-2 pgr5 suggests the critical function of NDH in alleviating stromal overreduction caused by the defect in pgr5 (Fig. 4). Even in wild-type leaves, environmental stress causes similar stromal overreduction, where NDH function is essential. However, monomeric NDH is unstable under stress conditions, possibly via the inactivation of NDH18. During the evolution of flowering plants, plants may have had to make the NDH complex more resistant to oxidative stresses, possibly via supercomplex formation with PSI.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotypes Columbia-gl1 and Columbia-0 were grown in soil in a growth chamber (50 μmol photons m−2 s−1, 16-h photoperiod, 23°C) for 3 to 4 weeks. Insertion sites of the SAIL T-DNA line for Lhca5 (SAIL_682_F10) and the dSpm transposon line for Lhca6 (N120500) were confirmed by PCR and direct sequencing of the PCR products. For the high-light treatment, 4-week-old intact plants were subjected to 500 μmol photons m−2 s−1 light conditions for 2 d in a temperature-controlled chamber at 23°C (16-h photoperiod) and then harvested after 10 h of illumination on the last day. Immature and mature leaves were defined previously (Peng et al., 2009).
Thylakoid Membrane Preparation, BN-PAGE, and Immunoblot Analysis
Chloroplasts and thylakoids were isolated as described previously (Munekage et al., 2002). BN-PAGE and subsequent 2D SDS-PAGE immunoblot analysis were performed as described previously (Peng et al., 2008, 2009). For immunoblot analysis, thylakoid proteins were loaded on an equal chlorophyll basis. Signals were detected using an ECL Plus Western Blotting Detection Kit (GE Healthcare; http://www.gehealthcare.com) and visualized by an LAS3000 chemiluminescence analyzer (Fuji Film; http://www.fujifilm.com). Immunoblots were quantified by Imagemaster software (Amersham Pharmacia Biotech) using three independent plant materials except for Figure 6 (n = 2).
Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence was measured using a MINI-PAM portable chlorophyll fluorometer (Walz). The transient increase in chlorophyll fluorescence after turning off AL was monitored as described previously (Shikanai et al., 1998). To investigate the dependence of ΦPSII, NPQ, and 1-qL on light intensity, measuring light (650 nm; 0.1 μmol photons m−2 s−1) was used to excite the minimum fluorescence at open PSII centers in the dark-adapted state (Fo). A saturating pulse of white light (0.8 s; 8,000 μmol photons m−2 s−1) was applied to determine the maximum fluorescence at closed PSII centers in the dark (Fm) or during illumination (Fm′). The steady-state fluorescence level (Fs) was recorded during AL illumination (15–1,000 μmol photons m−2 s−1). These photosynthetic parameters were recoded 2 min after the change of AL intensity. Fv/Fm was calculated as (Fm – Fo)/Fm. ΦPSII was calculated as (Fm′ – Fs)/Fm′. NPQ was calculated as (Fm – Fm′)/Fm′. 1 − qL, the fraction of PSII centers with reduced primary quinone acceptor, was calculated as 1 − {ΦPSII/(1 – ΦPSII)} × [(1 – Fv/Fm)/(Fv/Fm)] × (NPQ + 1) (Miyake et al., 2009). For imaging of chlorophyll fluorescence, the seedlings were first kept in the dark for 20 min and then AL light was turned on (100 μmol photons m−2 s−1). After illumination for 1 min, an image of chlorophyll fluorescence was captured by a FluorCAM 700MF (Photon System Instruments). Fd-dependent PQ reduction activity was measured in ruptured chloroplasts as described previously (Munekage et al., 2004; Peng et al., 2009). As electron donors, 5 μm maize (Zea mays) Fd (Sigma-Aldrich) and 0.25 mm NADPH (Sigma-Aldrich) were used. Antimycin A (Sigma-Aldrich) at a concentration of 10 μm was added before measurement.
Stability of the NDH Monomer and the NDH-PSI Supercomplex
Detached leaves of 4-week-old Arabidopsis plants were vacuum infiltrated with water or a solution containing 100 μg mL−1 lincomycin (an inhibitor of plastid-encoded protein synthesis) and 20 μg mL−1 cycloheximide (an inhibitor of nucleus-encoded protein synthesis) for 30 min. After incubation, leaves were floated on their respective infiltration solutions and illuminated for 0, 3, 6, and 9 h under light conditions of 500 μmol photons m−2 s−1 and 23°C. After the treatment, thylakoids were isolated and subjected to immunoblot analysis.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Lhca5 (At1g45474) and Lhca6 (At1g19150).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of the location of the NDH complex in wild-type and lhca5 lhca6 plants.
Supplemental Figure S2. Light intensity dependence of NPQ and 1-qL.
Acknowledgments
We thank H. Mi, T. Endo, and A. Makino for providing antibodies. We also thank the European Arabidopsis Stock Centre and the Arabidopsis Biological Resource Center for providing the mutant seeds.
References
- Acín-Pérez R, Bayona-Bafaluy MP, Fernández-Silva P, Moreno-Loshuertos R, Pérez-Martos A, Bruno C, Moraes CT, Enríquez JA. (2004) Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell 13: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Battchikova N, Aro E-M. (2007) Cyanobacterial NDH-1 complexes: multiplicity in function and subunit composition. Physiol Plant 131: 22–32 [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Frolow F, Nelson N. (2003) Crystal structure of plant photosystem I. Nature 426: 630–635 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Braun H-P. (2007) Supramolecular structure of the mitochondrial oxidative phosphorylation system. J Biol Chem 282: 1–4 [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J 17: 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D. (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132: 273–285 [DOI] [PubMed] [Google Scholar]
- Dudkina NV, Heinemeyer J, Sunderhaus S, Boekema EJ, Braun HP. (2006) Respiratory chain supercomplexes in the plant mitochondrial membrane. Trends Plant Sci 11: 232–240 [DOI] [PubMed] [Google Scholar]
- Friedrich T, Weiss H. (1997) Modular evolution of the respiratory NADH:ubiquinone oxidoreductase and the origin of its modules. J Theor Biol 187: 529–540 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T. (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36: 541–549 [DOI] [PubMed] [Google Scholar]
- Heinemeyer J, Eubel H, Wehmhöner D, Jänsch L, Braun HP. (2004) Proteomic approach to characterize the supramolecular organization of photosystems in higher plants. Phytochemistry 65: 1683–1692 [DOI] [PubMed] [Google Scholar]
- Ifuku K, Ishihara S, Sato F. (2010) Molecular functions of oxygen-evolving complex family proteins in photosynthetic electron flow. J Integr Plant Biol 52: 723–734 [DOI] [PubMed] [Google Scholar]
- Ishihara S, Takabayashi A, Ido K, Endo T, Ifuku K, Sato F. (2007) Distinct functions for the two PsbP-like proteins PPL1 and PPL2 in the chloroplast thylakoid lumen of Arabidopsis. Plant Physiol 145: 668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N, Takabayashi A, Ishida S, Hano Y, Endo T, Sato F. (2008) NDF6: a thylakoid protein specific to terrestrial plants is essential for activity of chloroplastic NAD(P)H dehydrogenase in Arabidopsis. Plant Cell Physiol 49: 1066–1073 [DOI] [PubMed] [Google Scholar]
- Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J. (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Kofer W, Koop HU, Wanner G, Steinmüller K. (1998) Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet 258: 166–173 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE. (2004) New fluorescence parameters for the determination of q(a) redox state and excitation energy fluxes. Photosynth Res 79: 209–218 [DOI] [PubMed] [Google Scholar]
- Lennon AM, Prommeenate P, Nixon PJ. (2003) Location, expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher-plant plastids. Planta 218: 254–260 [DOI] [PubMed] [Google Scholar]
- Livingston AK, Cruz JA, Kohzuma K, Dhingra A, Kramer DM. (2010) An Arabidopsis mutant with high cyclic electron flow around photosystem I (hcef) involving the NADPH dehydrogenase complex. Plant Cell 22: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Zybailov B, Ytterberg AJ, Dunsmore J, Sun Q, van Wijk KJ. (2008) Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Mol Cell Proteomics 7: 1609–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi T, Wakasugi T, Shinozaki K, Yamaguchi-Shinozaki K, Zaita N, Hidaka T, Meng BY, Ohto C, Tanaka M, Kato A.et al (1987) Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Mol Gen Genet 210: 385–393 [DOI] [PubMed] [Google Scholar]
- Miyake C, Amako K, Shiraishi N, Sugimoto T. (2009) Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system: relationship among the fraction of open PSII centers, non-photochemical quenching of Chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol 50: 730–743 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T. (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN. (2007) The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta 1767: 1252–1259 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T. (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49: 825–834 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Kobayashi Y, Shikanai T. (2010) Physiological links among alternative electron transport pathways reducing and oxidizing plastoquinone in Arabidopsis. Plant J 63: 458–468 [DOI] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Takami T, Shikanai T. (2009) Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 21: 3623–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Shimizu H, Shikanai T. (2008) The chloroplast NAD(P)H dehydrogenase complex interacts with photosystem I in Arabidopsis. J Biol Chem 283: 34873–34879 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Peng L, Yamamoto T, Shikanai T. (2011) Structure and biogenesis of the chloroplast NAD(P)H dehydrogenase complex. Biochim Biophys Acta (in press) [DOI] [PubMed] [Google Scholar]
- Romanowska E, Kargul J, Powikrowska M, Finazzi G, Nield J, Drozak A, Pokorska B. (2008) Structural organization of photosynthetic apparatus in agranal chloroplasts of maize. J Biol Chem 283: 26037–26046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G. (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 17: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T. (2007a) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58: 199–217 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2007b) The NAD(P)H dehydrogenase complex in photosynthetic organisms: subunit composition and physiological function. Funct Plant Sci Biotechnol 1: 129–137 [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A. (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95: 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Peng L, Myouga F, Motohashi R, Shinozaki K, Shikanai T. (2008) CRR23/NdhL is a subunit of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol 49: 835–842 [DOI] [PubMed] [Google Scholar]
- Sirpiö S, Allahverdiyeva Y, Holmström M, Khrouchtchova A, Haldrup A, Battchikova N, Aro E-M. (2009a) Novel nuclear-encoded subunits of the chloroplast NAD(P)H dehydrogenase complex. J Biol Chem 284: 905–912 [DOI] [PubMed] [Google Scholar]
- Sirpiö S, Holmström M, Battchikova N, Aro E-M. (2009b) AtCYP20-2 is an auxiliary protein of the chloroplast NAD(P)H dehydrogenase complex. FEBS Lett 583: 2355–2358 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Sirpiö S, Aro E-M. (2009) Towards characterization of the chloroplast NAD(P)H dehydrogenase complex. Mol Plant 2: 1127–1140 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Sirpiö S, Paakkarinen V, Kumari N, Holmström M, Aro E-M. (2010) Two proteins homologous to PsbQ are novel subunits of the chloroplast NAD(P)H dehydrogenase. Plant Cell Physiol 51: 877–883 [DOI] [PubMed] [Google Scholar]
- Takabayashi A, Ishikawa N, Obayashi T, Ishida S, Obokata J, Endo T, Sato F. (2009) Three novel subunits of Arabidopsis chloroplastic NAD(P)H dehydrogenase identified by bioinformatic and reverse genetic approaches. Plant J 57: 207–219 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Piippo M, Suorsa M, Sirpiö S, Mulo P, Vainonen J, Vener AV, Allahverdiyeva Y, Aro E-M. (2006) State transitions revisited: a buffering system for dynamic low light acclimation of Arabidopsis. Plant Mol Biol 62: 779–793 [DOI] [PubMed] [Google Scholar]
- Yabuta S, Ifuku K, Takabayashi A, Ishihara S, Ido K, Ishikawa N, Endo T, Sato F. (2010) Three PsbQ-like proteins are required for the function of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol 51: 866–876 [DOI] [PubMed] [Google Scholar]