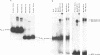Abstract
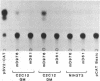
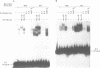
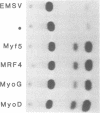
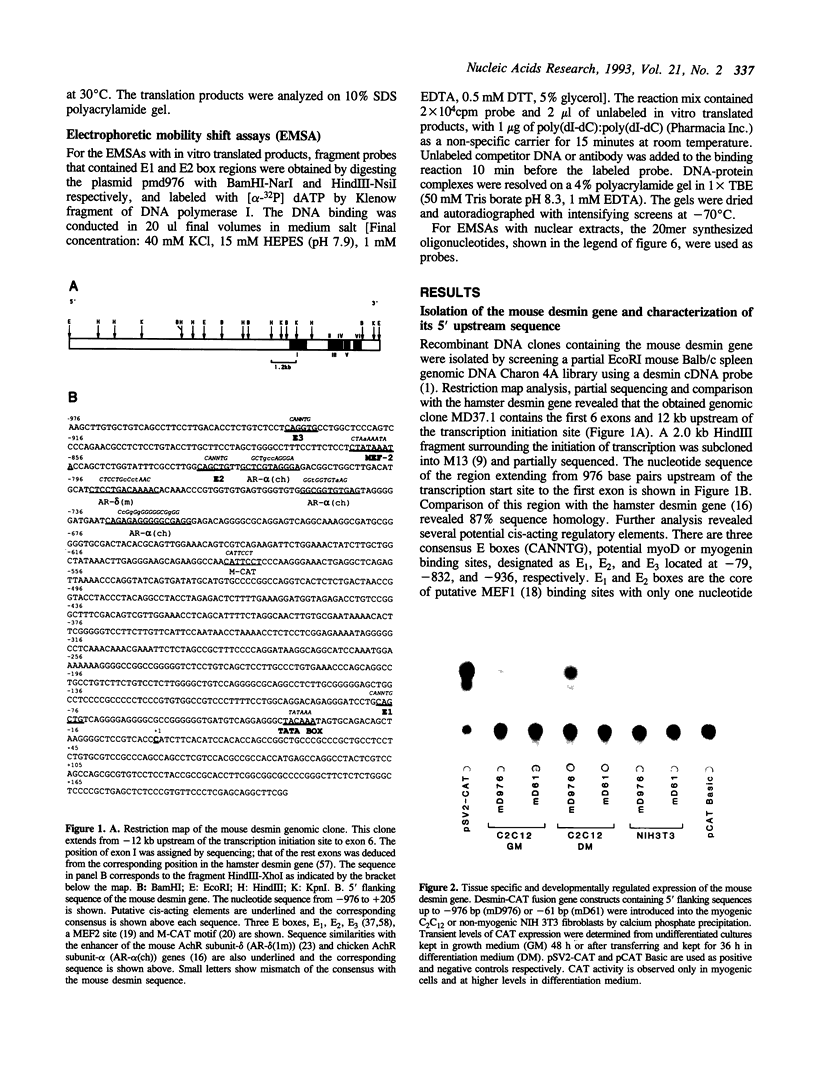
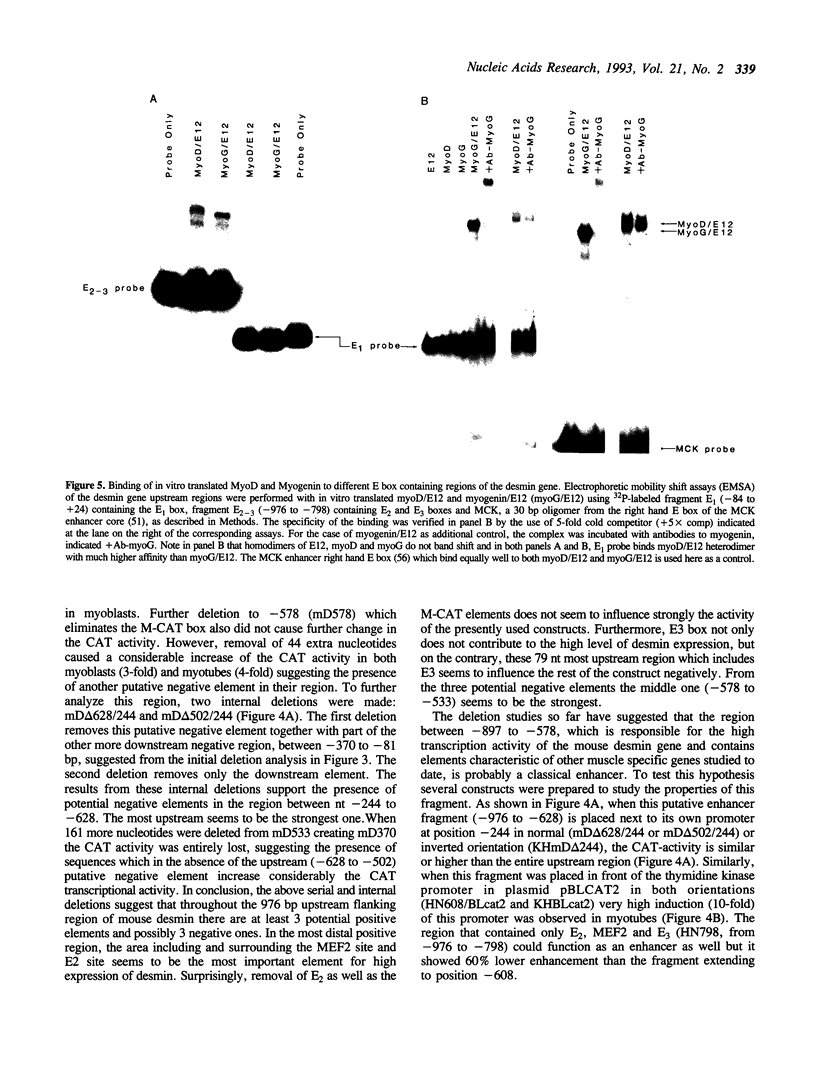
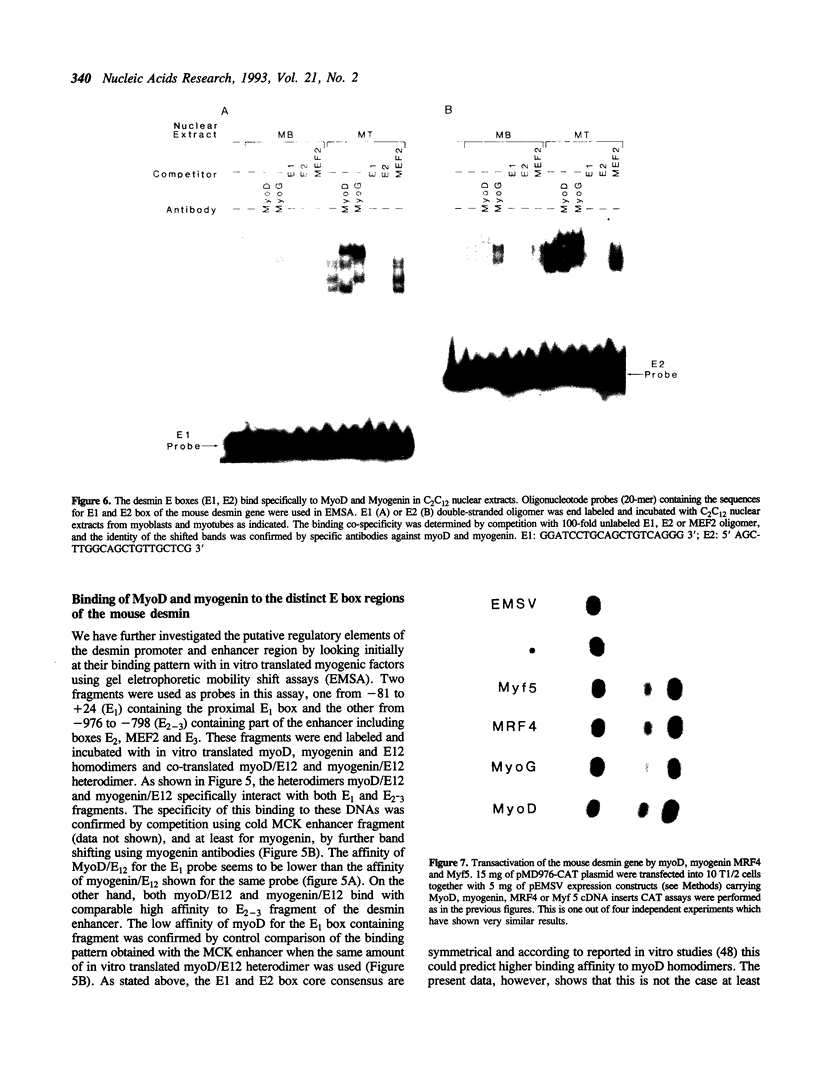
Desmin, the muscle specific intermediate filament (IF) protein, is expressed at low levels in myoblasts and at the onset of differentiation its expression increases several fold. In an effort to explore the mechanism involved in the tissue-specific and developmentally regulated expression of desmin, we have isolated the mouse desmin gene. Sequence analysis of 976 bp 5' flanking region revealed several potential cis-acting elements: 1) Three E boxes (MyoD binding sites), namely, E1, E2 and E3, located at -79, -832 and -936, respectively; 2) one MEF2 binding site at -864; 3) a region with homology to M-CAT motif at -587; 4) several GC boxes. Transient transfections with various 5' flank deletion mutants into C2C12 muscle cells have revealed both positive and negative elements that seem to be involved in the expression of desmin. The first 81 bp upstream of the transcription initiation site, including E1 box, were sufficient to confer muscle specific expression of the desmin gene. The maximal level of expression was achieved by the construct containing up to -897 base pairs. The region between -578 to -976 behaves as a classical enhancer in the absence of which the region between -578 and -81 suppresses CAT activity. Gel electrophoretic mobility shift assays using both C2C12 muscle cell nuclear extracts as well as in vitro translated myoD/E12 and myogenin/E12 heterodimers, showed that both myoD and myogenin bind to the proximal E1 and the distal E2 boxes of the desmin promoter and enhancer respectively. Co-transfection of myoD, myogenin, MRF4 and Myf5, with the desmin-CAT construct into 10T-1/2 cells demonstrated that all these factors could transactivate desmin gene expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Burden S. J. Muscle-specific gene expression controlled by a regulatory element lacking a MyoD1-binding site. Nature. 1989 Oct 26;341(6244):716–720. doi: 10.1038/341716a0. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blessing M., Jorcano J. L., Franke W. W. Enhancer elements directing cell-type-specific expression of cytokeratin genes and changes of the epithelial cytoskeleton by transfections of hybrid cytokeratin genes. EMBO J. 1989 Jan;8(1):117–126. doi: 10.1002/j.1460-2075.1989.tb03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T. J., Chakraborty T., Olson E. N. Mutagenesis of the myogenin basic region identifies an ancient protein motif critical for activation of myogenesis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5675–5679. doi: 10.1073/pnas.88.13.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Lazarides E. Characterization and regulation in the expression of a gene coding for the intermediate filament protein desmin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6909–6913. doi: 10.1073/pnas.81.22.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Costa M. L., Mermelstein C. S., Chagas C., Holtzer S., Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Ernst H., Wentworth B., Nadal-Ginard B., Rosenthal N. A muscle-specific enhancer is located at the 3' end of the myosin light-chain 1/3 gene locus. Genes Dev. 1988 Dec;2(12B):1779–1790. doi: 10.1101/gad.2.12b.1779. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Farrell F. X., Sax C. M., Zehner Z. E. A negative element involved in vimentin gene expression. Mol Cell Biol. 1990 May;10(5):2349–2358. doi: 10.1128/mcb.10.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell. 1980 Jan;19(1):263–275. doi: 10.1016/0092-8674(80)90408-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik J. M., Bergsma D. J., Schwartz R. J. Tissue restricted and stage specific transcription is maintained within 411 nucleotides flanking the 5' end of the chicken alpha-skeletal actin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1683–1701. doi: 10.1093/nar/14.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Kaufman S. J., Foster R. F. Replicating myoblasts express a muscle-specific phenotype. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Chow K. L., Fang P., Schwartz R. J. Activation of skeletal alpha-actin gene transcription: the cooperative formation of serum response factor-binding complexes over positive cis-acting promoter serum response elements displaces a negative-acting nuclear factor enriched in replicating myoblasts and nonmyogenic cells. Mol Cell Biol. 1991 Oct;11(10):5090–5100. doi: 10.1128/mcb.11.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersch R., Stellmach V., Stocks C., Giudice G., Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989 Sep;9(9):3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. L., Paulin D. High level desmin expression depends on a muscle-specific enhancer. J Biol Chem. 1991 Apr 5;266(10):6562–6570. [PubMed] [Google Scholar]
- Lilienbaum A., Duc Dodon M., Alexandre C., Gazzolo L., Paulin D. Effect of human T-cell leukemia virus type I tax protein on activation of the human vimentin gene. J Virol. 1990 Jan;64(1):256–263. doi: 10.1128/jvi.64.1.256-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yutzey K. E., Konieczny S. F. Muscle-specific expression of the troponin I gene requires interactions between helix-loop-helix muscle regulatory factors and ubiquitous transcription factors. Mol Cell Biol. 1991 Jan;11(1):267–280. doi: 10.1128/mcb.11.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. A conserved CATTCCT motif is required for skeletal muscle-specific activity of the cardiac troponin T gene promoter. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6404–6408. doi: 10.1073/pnas.85.17.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Pieper F. R., Slobbe R. L., Ramaekers F. C., Cuypers H. T., Bloemendal H. Upstream regions of the hamster desmin and vimentin genes regulate expression during in vitro myogenesis. EMBO J. 1987 Dec 1;6(12):3611–3618. doi: 10.1002/j.1460-2075.1987.tb02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Klarsfeld A., Changeux J. P. Interaction of nuclear factors with the upstream region of the alpha-subunit gene of chicken muscle acetylcholine receptor: variations with muscle differentiation and denervation. EMBO J. 1989 Mar;8(3):687–694. doi: 10.1002/j.1460-2075.1989.tb03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Merlie J. P. A developmental and tissue-specific enhancer in the mouse skeletal muscle acetylcholine receptor alpha-subunit gene regulated by myogenic factors. J Biol Chem. 1991 Nov 25;266(33):22588–22596. [PubMed] [Google Scholar]
- Quax W., van den Broek L., Egberts W. V., Ramaekers F., Bloemendal H. Characterization of the hamster desmin gene: expression and formation of desmin filaments in nonmuscle cells after gene transfer. Cell. 1985 Nov;43(1):327–338. doi: 10.1016/0092-8674(85)90038-8. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Coutinho L., Amram T., Kolbe M. AP-1/jun binding sites mediate serum inducibility of the human vimentin promoter. Nucleic Acids Res. 1989 Feb 25;17(4):1619–1633. doi: 10.1093/nar/17.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Farrell F. X., Tobian J. A., Zehner Z. E. Multiple elements are required for expression of an intermediate filament gene. Nucleic Acids Res. 1988 Aug 25;16(16):8057–8076. doi: 10.1093/nar/16.16.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry W. M., Vizard D., Weil T., Olson E. N. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988 Jul;8(7):2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Tsay H. J., Schmidt J. Expression of the acetylcholine receptor delta-subunit gene in differentiating chick muscle cells is activated by an element that contains two 16 bp copies of a segment of the alpha-subunit enhancer. EMBO J. 1990 Mar;9(3):783–790. doi: 10.1002/j.1460-2075.1990.tb08174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu H. P., Wang X. M., Ballivet M., Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988 Aug;1(6):527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Kline R. L., Konieczny S. F. An internal regulatory element controls troponin I gene expression. Mol Cell Biol. 1989 Apr;9(4):1397–1405. doi: 10.1128/mcb.9.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutzey K. E., Rhodes S. J., Konieczny S. F. Differential trans activation associated with the muscle regulatory factors MyoD1, myogenin, and MRF4. Mol Cell Biol. 1990 Aug;10(8):3934–3944. doi: 10.1128/mcb.10.8.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]