Starch is the major storage carbohydrate in higher plants, with many important functions. In photosynthesizing leaves, starch accumulates during the day and is remobilized at night to support continued respiration, Suc export, and growth in the dark (Geiger and Servaites, 1994). In this context, starch has been identified as a major integrator in the regulation of plant growth to cope with continual changes in carbon availability (Sulpice et al., 2009). Its importance is demonstrated by the phenotype of starch-deficient mutants, which grow poorly or even die in short-day conditions (Caspar et al., 1986). In heterotrophic storage organs such as potato (Solanum tuberosum) tubers or developing seeds, starch serves as a longer term carbon store, which is remobilized later in development to support phases of reproductive growth. Since Suc supply to these tissues is fluctuating, regulatory mechanisms are required to stimulate starch synthesis when carbon availability increases (Geigenberger et al., 2004).
In addition to its central role in plant physiology, starch is also of great economical importance (Zeeman et al., 2010). It is the second most abundant biopolymer on earth, after cellulose, and the most important carbohydrate used for food and feed purposes. Therefore, it represents the major resource of our diet and feedstock for many industrial applications, including bioethanol production (Smith, 2008). Understanding starch biosynthesis in plants could pave the way to new strategies to improve crop yield via the use of reverse genetics or marker-assisted breeding (Geigenberger and Fernie, 2006).
Starch metabolism has been the subject of intense research in the past, leading to a sound knowledge of the pathways and the enzymes involved. However, there are many open questions concerning the signals and mechanisms regulating starch metabolism. This review summarizes the current understanding of the regulation of the pathway of starch synthesis in higher plants. The first part will focus on the structure of the pathway, which is an important prerequisite for understanding its regulation. The second part is primarily concerned with signals and mechanisms regulating starch synthesis in response to fluctuations in the carbon and energy status of the plant. The pathway of starch remobilization has recently been reviewed extensively in the literature (Kötting et al., 2010; Zeeman et al., 2010) and will not be covered in any detail here.
THE PATHWAY OF STARCH BIOSYNTHESIS AND ITS SUBCELLULAR COMPARTMENTATION
Starch is an insoluble polymer of Glc residues synthesized inside plastids of higher plants. The pathway of starch synthesis has been clarified in the past and established for many plant species (for review, see Preiss, 1988; Ball and Morell, 2003; James et al., 2003; Geigenberger et al., 2004; Stitt et al., 2010; Vriet et al., 2010; Zeeman et al., 2010; and refs. therein). As outlined in Figure 1, the first committed step involves the conversion of Glc-1-P and ATP to ADP-Glc and inorganic pyrophosphate (PPi), catalyzed by ADP-Glc pyrophosphorylase (AGPase). ADP-Glc acts as the glucosyl donor for different classes of starch synthases (SS), which elongate the α-1,4-linked glucan chains of the two insoluble starch polymers amylose and amylopectin of which the granule is composed. Five distinct SS classes are known in plants: granule-bound SS, which is responsible for the synthesis of amylose; and soluble SS I to IV, responsible for amylopectin synthesis. Amylose is an essentially linear glucan that contains very few α-1,6-branches and makes up to 20% to 30% of normal starch, while amylopectin is more highly branched. Branch points are introduced by two classes of starch-branching enzymes (SBE I and II), which differ in terms of length of the glucan chains transferred and substrate specificities. Interestingly, starch synthesis also involves two types of debranching enzymes, which cleave branch points and are probably involved in tailoring the branched glucans into a form capable of crystallization within the granule. Genetic studies provide evidence that the different isoforms of SS, SBE, and debranching enzyme probably play specific roles in determining the complex structure of starch. They have to act in close coordination to synthesize the crystalline matrix of the starch granule. Intriguingly, SS III and SS IV have recently been implicated to be responsible for starch granule initiation (Szydlowski et al., 2009).
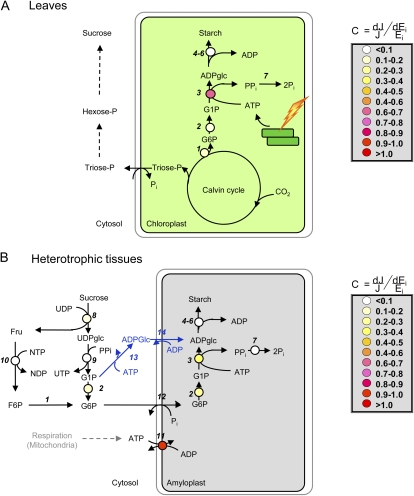
Figure 1.
Schematic representation of the pathway of starch biosynthesis, its subcellular compartmentation, and distribution of flux control in photosynthetic leaves (A) and heterotrophic tissues (B). The reactions of the pathway of starch biosynthesis are catalyzed by the following enzymes: 1, phosphoglucoisomerase; 2, PGM; 3, AGPase; 4, SS; 5, SBE; 6, starch-debranching enzyme; 7, inorganic pyrophosphatase; 8, Suc synthase; 9 UDP-Glc pyrophosphorylase; 10, fructokinase; 11, ATP/ADP translocator; 12, Glc-6-P/Pi translocator; 13, cytosolic AGPase; and 14, ADP-Glc/ADP translocator (steps 13 and 14 are highlighted to be specific for cereal endosperm). The false color symbols represent the relative flux control coefficients (C) of the constituent enzymes, defined as the relation between the fractional change in enzyme activity (Ei) and starch flux (J). Missing symbols represent reactions for which experimental data are missing. Data were taken from Neuhaus and Stitt (1990) and Stitt et al. (2010) for leaves and from Geigenberger et al. (2004) for growing potato tubers.
In most tissues, AGPase is located exclusively in the plastid. In leaves in the light, Glc-1-P is synthesized from Calvin-Benson cycle intermediates via plastidic phosphoglucose isomerase and phosphoglucomutase (PGM), while ATP is provided by photophosphorylation at the thylakoid membrane (Fig. 1A). In nonphotosynthetic tissues such as potato tubers (Fig. 1B), incoming Suc is mobilized by a series of cytosolic reactions to Glc-6-P, which is imported into the amyloplast in counterexchange with inorganic phosphate (Pi) by a Glc-6-P/Pi translocator (Kammerer et al., 1998) and subsequently converted to Glc-1-P via plastidial PGM. The second substrate of AGPase, ATP, is provided by mitochondrial respiration and imported into the plastid via the envelope ATP/ADP exchanger (Tjaden et al., 1998).
By contrast, in cereal seed endosperm, AGPase is mainly located in the cytosol, with a total AGPase activity of about 85% to 95% (James et al., 2003). ADP-Glc synthesized in the cytosol must be imported into the plastid to support starch synthesis. A maize (Zea mays) mutant of the ADP-Glc transporter, brittle1, was identified in 1926. The endosperm of this mutant was characterized by decreased starch content and a collapsed or brittle phenotype (Mangelsdorf and Jones, 1926). Recent transport studies have confirmed that this protein exchanges ADP-Glc in antiport with ADP in maize (Kirchberger et al., 2007) and wheat (Triticum aestivum; Bowsher et al., 2007). Overall, these studies show that cereal endosperm, being the most important source of starch worldwide, employs a unique pathway of starch biosynthesis that requires two additional steps, cytosolic AGPase and ADP-Glc transport, not present in other cereal tissues or noncereal plants (Fig. 1B). It seems likely that control of flux through this pathway will differ from that in other heterotrophic tissues such as potato tubers.
The classical and widely accepted pathway of starch synthesis was recently questioned. It was suggested that in leaves, ADP-Glc is synthesized in the cytosol by Suc synthase, using ADP and Suc as substrates, and then imported into the chloroplast for starch synthesis (Baroja-Fernández et al., 2004; Muñoz et al., 2005). The proposal of this controversial new pathway for starch synthesis led to a considerable debate in the literature (Baroja-Fernández et al., 2005; Neuhaus et al., 2005). Finally, genetic studies in Arabidopsis (Arabidopsis thaliana) showed that the classical pathway is most likely correct (Barratt et al., 2009; Streb et al., 2009). Suc synthase activity in Arabidopsis leaves is too low to account for the rate of starch synthesis, and removal of almost all Suc synthase activity in quadruple sus mutants did not lead to any changes in the levels of ADP-Glc or starch (Barratt et al., 2009). Moreover, subcellular metabolite analysis in growing potato tubers using nonaqueous fractionation showed that more than 95% of the total ADP-Glc is located in the plastid, providing evidence for the classical pathway of starch synthesis to operate also in tubers (A. Tiessen and P. Geigenberger, unpublished data).
The debate on the pathway of starch synthesis raised an interesting problem. It has been found that Arabidopsis pgm1 null mutants with a complete knockout of plastidial PGM still harbor low but significant levels of ADP-Glc and starch (Muñoz et al., 2006; Streb et al., 2009). A possible explanation for the residual starch and ADP-Glc levels in the pgm1 mutant could be import of Glc-1-P into the plastid. Transport studies revealed significant uptake of Glc-1-P into isolated chloroplasts, which explains the low-starch phenotype in the pgm1 mutant, while it seems to be of minor relevance under normal conditions in the wild type (Fettke et al., 2011). Moreover, Glc-6-P/Pi translocator2, a hexose phosphate transporter at the inner chloroplast envelope membrane, has been found to be increased in the pgm1 mutant in the light, most likely due to increased sugar levels under these conditions, compared with the wild type (Kunz et al., 2010). The direct interconnection between cytosolic and plastidial hexose phosphate pools in photosynthesizing leaves suggests so far unnoticed intracellular carbon fluxes toward plastidial starch that may increase the flexibility of plant metabolism when starch synthesis is impaired and sugar supply is increasing (Fettke et al., 2011). Further studies, including nonaqueous fractionation techniques as established for leaves (Gerhardt et al., 1987) and potato tubers (Farré et al., 2001; Tiessen et al., 2002), will be necessary to finally resolve the subcellular distribution of hexose phosphates and ADP-Glc in different tissues, genotypes, and conditions.
DISTRIBUTION OF FLUX CONTROL IN THE PATHWAY
Metabolic control analysis was developed in the early 1970s (Kacser and Burns, 1973) and is probably the most widely used mathematical tool for the study of control in plant systems (ap Rees and Hill, 1994). It quantifies the response of system variables (e.g. fluxes) to small changes in system parameters (e.g. the amount or activity of the individual enzymes). The relative contributions of enzymes to the control of flux in a pathway can be experimentally assessed by systematically creating, for every enzyme in the pathway, a set of plants with a stepwise reduction in the activity of the enzyme. The availability of mutants and transgenic lines with altered expression of the enzymes of the pathway of starch synthesis allowed systematic investigations into the contributions of each step in the pathway to control flux into starch. The work started in the early 1990 in Arabidopsis, driven by the availability of genetic resources, and was recently applied to growing potato tubers (Geigenberger et al., 2004). In Arabidopsis leaves, the majority of control has been found to reside in the reaction catalyzed by AGPase (Neuhaus and Stitt, 1990; Fig. 1A). This is in contrast with potato tubers, where control is shared between AGPase, plastidial PGM, and the plastidial adenylate transporter, with the vast predominance residing in the exchange of adenylates across the amyloplast membrane (Geigenberger et al., 2004; Fig. 1B). The different distribution of flux control in photosynthetic and nonphotosynthetic starch synthesis can be explained, since during photosynthesis the chloroplast can produce sufficient ATP to support starch synthesis, whereas in the amyloplast energy must be imported from the cytosol. In confirmation of these studies, overexpression of a heterologous AGPase (Stark et al., 1992) or plastidial adenylate transporter (Tjaden et al., 1998) led to increased starch accumulation in transgenic potato tubers.
Despite the great economical importance of cereal starch, systematic flux-control studies are lacking for cereal seed endosperm. Although mutants in individual steps of the pathway such as cytosolic AGPase and the brittle1 ADP-Glc transporter have been found to be deficient in starch accumulation (for review, see Jeon et al., 2010), the contributions of these enzymes to the control of flux into starch have not been quantified. Interestingly, when mutated forms of a heterologous AGPase were overexpressed in wheat (Smidansky et al., 2002), rice (Oryza sativa; Sakulsingharoj et al., 2004; Smidansky et al., 2003), and maize (Wang et al., 2007), seed weight and yield were increased. This provides evidence that AGPase is colimiting for overall starch accumulation also in cereal crops (for review, see Hannah and James, 2008).
REGULATORY MECHANISMS
Even when an enzyme has a large control coefficient, it will only be able to contribute to regulation if mechanisms exist to alter the amount or activity of the enzyme. As reviewed in the following section, marked regulatory properties have been found for enzymes involved in starch biosynthesis, especially for AGPase, which is subject to multilevel regulation (Fig. 2).
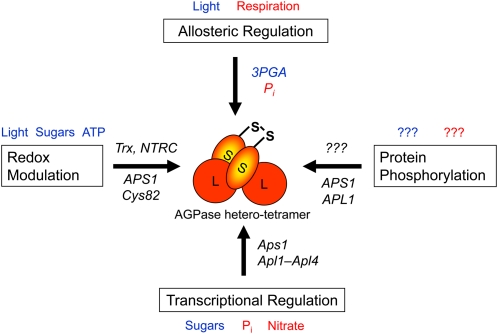
Figure 2.
Regulation of plastidial AGPase by multiple mechanisms allows starch synthesis to respond across a range of time scales to a variety of physiological and environmental stimuli. Plastidial AGPase is a heterotetramer that contains two large (APL; 51 kD) and two slightly smaller (APS; 50 kD) subunits, which both have regulatory functions. Top, Allosteric regulation by 3PGA and Pi operates in a time frame of seconds to adjust the rate of starch synthesis to the balance between photosynthesis and Suc synthesis in leaves in the light and Suc breakdown and respiration in tubers. Left, Posttranslational redox modulation involves reversible disulfide bond formation between Cys-82 of the two small APS1 subunits, leading to changes in AGPase activity in response to light and sugar signals in a time frame of minutes to hours. The signaling components leading to redox modulation of AGPase involve Trx and NTRC, which are linked to photoreduced Fdx and interact with different sugar signals. Right, In Arabidopsis leaves, APS1 and APL1 have been identified as potential targets for reversible protein phosphorylation. More studies are needed to investigate the in vivo relevance of this mechanism and the underlying plastidial kinase network. Bottom, Transcriptional regulation in response to changes in carbon and nutrient supply allows more gradual changes in AGPase activity, which may require up to days to develop. Red font indicates inhibition, blue font indicates activation, and question marks indicate unknown (see main text for references).
TRANSCRIPTIONAL REGULATION
Several genes involved in starch synthesis have been found to be subject to transcriptional regulation in diverse tissues such as Arabidopsis leaves (Smith et al., 2004), potato tubers (Geigenberger, 2003a), and cereal endosperm (Mangelsen et al., 2010). Much research in this area has focused on the transcriptional regulation of AGPase, because it is the first committed step in the pathway and reveals a high flux control coefficient (see above). AGPase is a heterotetramer that contains two large (APL; 51 kD) and two slightly smaller (APS; 50 kD) subunits (Okita et al., 1990), which are both required for optimal enzyme activity but have nonequivalent roles in enzyme function (Iglesias et al., 1993). The large subunit plays more of a regulatory role, while the small subunit has both catalytic and regulatory properties (Cross et al., 2004). Multiple isoenzymes of AGPase are present at varying levels in different plant tissues. In Arabidopsis, there are four genes for the large subunit (Apl1–Apl4) and a single gene (Aps1) for the small subunit of AGPase (Crevillén et al., 2003; Hendriks et al., 2003). There is ample evidence that the expression of these genes is temporally and spatially controlled (for review, see Tetlow et al., 2004a). The multiple genes encoding for the large subunits show strong specificity in their expression, being restricted to certain tissues or induced under specific conditions. The differential expression of these subunits in different tissues may produce AGPase with varying degrees of sensitivities to allosteric effectors (see the section on allosteric regulation below), suited to the particular metabolic demands of a given plant tissue (Tetlow et al., 2004a). The expression of AGPase is increased by sugars (Müller-Röber et al., 1990; Sokolov et al., 1998; Tiessen et al., 2003) and decreased by nitrate (Scheible et al., 1997) and phosphate (Nielsen et al., 1998). This may allow starch accumulation to respond to changes in the carbon and nutritional status and to environmental constraints.
To achieve a substantial increase in the rate of starch synthesis, it will be important to increase the expression of a set of enzymes and transporters in the pathway. Comprehensive expression profiling revealed the pathway-wide regulation of the expression of genes involved in Suc-starch interactions in Arabidopsis (Bläsing et al., 2005), potato (Kloostermann et al., 2008), and barley (Hordeum vulgare; Mangelsen et al., 2010). The coordinated regulation of gene expression in source and sink tissues is to a large extent orchestrated by the sugar status. The sensing and signaling mechanisms mediating these processes are largely unknown. In the developing barley endosperm, they involve a WRKY transcription factor, SUSIBA2, which participates in source-sink communication and Suc-mediated regulation of starch synthesis (Sun et al., 2003). In addition to this, ethylene signaling has recently been implicated in the transcriptional regulation of starch synthesis in rice, including the ethylene receptor ETRC (Wuriyanghan et al., 2009) and an AP2/EREBP family transcription factor (Fu and Xue, 2010). Moreover, coexpression analysis implied hypoxia-responsive AP2/EREBP family transcription factors to participate in the regulation of Suc-to-starch interconversion in developing potato tubers, indicating a link between transcriptional regulation of starch synthesis and growth-related hypoxia (F. Licausi, F.M. Giorgi, E. Schmälzlin, B. Usadel, P. Perata, J.T. van Dongen, and P. Geigenberger, unpublished data).
ALLOSTERIC REGULATION BY METABOLITES
While there is transcriptional regulation of genes involved in starch metabolism, changes in transcript levels are often not reflected by changes in the respective protein levels or enzyme activities (Geigenberger and Stitt, 2000; Gibon et al., 2004a; Smith et al., 2004). This suggests additional regulatory mechanisms at the posttranscriptional level. The activities of a number of starch metabolic enzymes have been shown to be modulated by effector molecules, which are often metabolic intermediates (Tetlow et al., 2004a). Allosteric regulation of AGPase was discovered in the 1960s (Ghosh and Preiss, 1966) and represents the most highly characterized example of this method of control. The activity of AGPase is generally activated by glycerate-3-phosphate (3PGA) and inhibited by Pi. In photosynthesizing leaves and heterotrophic potato tubers, increasing levels of phosphorylated intermediates typically lead to a marked increase of the 3PGA-Pi ratio. Therefore, activation of AGPase by an increasing 3PGA-Pi ratio allows the rate of starch synthesis to be adjusted in response to changes in the balance between photosynthesis and Suc synthesis in leaves (Stitt et al., 1987) and to changes in the balance between Suc breakdown and respiration in tubers (Geigenberger et al., 1998). The relative sensitivity of AGPase to these allosteric effectors seems to depend on the tissue and subcellular localization. Intriguingly, AGPases from wheat (Tetlow et al., 2004a) and barley endosperm (Doan et al., 1999) have been found to be largely insensitive to allosteric effectors.
POSTTRANSLATIONAL PROTEIN MODIFICATION
Research in the last 10 years has shown that starch biosynthesis is also regulated by posttranslational protein modification. AGPase has been discovered to be subject to posttranslational redox regulation, involving the reversible formation of an intermolecular Cys bridge between Cys-82 of the two small subunits of the heterotetrameric enzyme (Fu et al., 1998; Tiessen et al., 2002). The reduced and active form of AGPase contains the two small subunits as monomers, while the oxidized form contains these subunits as a dimer and displays low activity. Reduction of AGPase leads to dramatic alterations in the kinetic properties of the enzyme, resulting in an increase of the substrate affinities and the sensitivity to the allosteric activator 3PGA, while the sensitivity to inhibition by Pi is decreased (Tiessen et al., 2002). AGPase from potato tubers and pea (Pisum sativum) leaf chloroplasts has been shown to be reduced by thioredoxin (Trx) f and m in vitro (Ballicora et al., 2000; Geigenberger et al., 2005), representing Trx isoforms that also activate enzymes of the Calvin-Benson cycle and other photosynthetic proteins in response to light signals (Schürmann and Buchanan, 2008). Studies in Arabidopsis in the last years revealed that Trxs constitute a small gene family with 10 different isoforms (f1 and -2, m1 to -4, x, y1 and -2, and z) located in the plastid (Gelhaye et al., 2005; Arsova et al., 2010). Studies into Arabidopsis knockout mutants mainly investigated the role of the different Trx isoforms in plant development, showing a function of Trx m3 in meristem (Benitez-Alfonso et al., 2009) and Trx z in chloroplast development (Arsova et al., 2010). More work will be needed to investigate their importance and specificity to regulate AGPase and starch synthesis in photosynthetic leaves as well as in different nonphotosynthetic tissues. More recently, evidence was provided for the involvement of a unique type of NADP-dependent thioredoxin reductase C (NTRC) in the posttranslational redox regulation of AGPase (Michalska et al., 2009). NTRC is an unusual plastid-localized enzyme containing both an NADP-thioredoxin reductase and a Trx domain in a single polypeptide, which has initially been found to supply reductant for detoxifying hydrogen peroxide via peroxiredoxins (Pérez-Ruiz et al., 2006). The study of Michalska et al. (2009) showed that NTRC mediates the reductive activation of AGPase by NADPH in vitro, while NTRC deletion mutants were used to provide evidence that NTRC performs this function also in vivo.
Using large-scale proteomics screens in Arabidopsis and other species, further starch-related proteins have been identified as potential Trx targets. This includes two enzymes of the starch synthesis pathway in wheat endosperm, the brittle1 ADP-Glc transporter and SBE IIa (Balmer et al., 2006), implying redox regulation of starch biosynthesis also to be present in cereal endosperm tissue. This may not involve cytosolic AGPase, since its small subunit is lacking the conserved regulatory Cys-82 (Hendriks et al., 2003). While redox regulation seems to be restricted to plastidial AGPase, more studies are clearly needed to investigate this type of regulation in cereal seeds. In addition to this, various enzymes involved in starch degradation have been found to be redox regulated, which may imply a coordinated regulation of starch synthesis and degradation by redox signals (for review, see Kötting et al., 2010).
More recent studies implicate reversible protein phosphorylation to play a role in the regulation of starch metabolism. In isolated amyloplasts from wheat endosperm, several enzymes involved in starch biosynthesis have been found to be phosphorylated, including different isoforms of SS and SBE (Tetlow et al., 2004b, 2008). Large-scale phosphoproteome profiling provides evidence for an extension of the role of protein phosphorylation to starch metabolic enzymes in maize (Grimaud et al., 2008) and Arabidopsis (Heazlewood et al., 2008; Lohrig et al., 2009; Reiland et al., 2009; Kötting et al., 2010). In Arabidopsis, several proteins involved in the pathway of starch biosynthesis in leaves have been identified as potential targets for reversible protein phosphorylation, such as phosphoglucose isomerase (At4g24620), PGM (At5g51820), AGPase small subunit (At5g48300) and AGPase large subunit (At5g19220), and SS III (At1g11720). More studies are needed to investigate the in vivo relevance of this mechanism. Several protein kinases and phosphatases have recently been identified to be potentially located in the plastid (Schliebner et al., 2008; Baginsky and Gruissem, 2009). Reverse genetic approaches will be necessary to identify whether they are involved in posttranslational modification of starch biosynthetic enzymes. In this respect, the possible interaction between redox regulation and protein phosphorylation is also an interesting avenue to follow (Bräutigam et al., 2009).
PROTEIN COMPLEX FORMATION
In the developing cereal endosperm, some of the enzymes of the starch biosynthetic pathway have been found to form protein complexes. Heterocomplexes comprising specific isoforms of SS and SBE have been identified in wheat (Tetlow et al., 2004b, 2008) and maize (Hennen-Bierwagen et al., 2008), and some complexes also have been found to include AGPase and starch phosphorylase (Tetlow et al., 2008; Hennen-Bierwagen et al., 2009). While the underlying mechanisms for complex formation are largely unresolved, there is evidence that the physical association of these proteins depends on their phosphorylation status (Tetlow et al., 2004b; Liu et al., 2009). Complex formation may serve to orchestrate the activities of the different SS and SBE isoforms acting on a common amylopectin substrate, which may help to improve the efficiency of starch polymer construction. However, direct evidence is lacking for the in vivo relevance and the physiological importance of these complexes for starch synthesis in the developing endosperm. Moreover, it is unclear whether similar starch enzyme complexes exist in other tissues. Intriguingly, enzymes previously unknown to be involved in plastidial starch synthesis also have been found within a complex from maize endosperm, including pyruvate:phosphate dikinase and Suc synthase (Hennen-Bierwagen et al., 2009). Further studies are needed to evaluate the significance of these results. Pyruvate:phosphate dikinase is generating PPi, and it has been suggested that an increase in the PPi concentration may lead to inhibition of AGPase activity in the plastid. However, the plastidial concentration of PPi in cereal endosperm is unknown, and its determination would require the adoption of the nonaqueous fractionation method to cereal endosperm tissues.
REGULATION OF STARCH BIOSYNTHESIS IN RESPONSE TO LIGHT SIGNALS
In the chloroplast of leaves, starch is synthesized during the day and degraded during the night. This requires a tight regulation of the pathways of starch synthesis and degradation in response to light signals. Two different mechanisms are acting on AGPase to turn starch synthesis on in the light and off in the dark. First, illumination of leaves or isolated chloroplasts leads to rapid redox activation of AGPase, which is completely reversed in the dark (Hendriks et al., 2003). Using transgenic Arabidopsis plants expressing a mutated AGPase where the regulatory Cys-82 of APS1 has been replaced by Ser, genetic evidence has been provided that redox regulation contributes to the coordination of starch synthesis and breakdown during the light/dark cycle, allowing complete inactivation of AGPase in the dark (Stitt et al., 2010). Second, allosteric regulation of AGPase by changes in the plastidial concentrations of 3PGA as activator and Pi as inhibitor provides a further mechanism for light/dark modulation of starch biosynthesis. 3PGA is the first fixation product of the Calvin-Benson cycle, and its concentration in the chloroplast stroma will increase when the fixation cycle is turned on in the light and decrease when it is turned off in the dark (Gerhardt et al., 1987). Pi will change inversely to 3PGA. Recently, overexpression of a mutated form of AGPase that is more sensitive to allosteric activation led to an increase in transitory starch synthesis, demonstrating the importance of the regulatory properties of AGPase for the regulation of diurnal starch synthesis in Arabidopsis leaves (Obana et al., 2006). Allosteric regulation and redox regulation will act synergistically on AGPase to achieve the activation of starch synthesis in the light and complete inactivation in the dark. First, redox regulation leads to changes in the sensitivity of the enzyme to its allosteric effectors, which are in line with changes in their concentrations in the chloroplast stroma in response to light/dark alterations. Second, studies with isolated chloroplasts show that light-dependent redox activation of AGPase itself is promoted by the allosteric activator 3PGA (Hendriks et al., 2003). This indicates a very close interaction between redox and allosteric regulation of AGPase to achieve a very efficient on/off regulation of starch synthesis in response to light/dark changes. The underlying mechanisms for the stimulation of redox activation of AGPase by 3PGA are unclear at the moment but may involve modification of the midpoint redox potential of the regulatory Cys-82 by metabolites, as shown for photosynthetic enzymes (Scheibe, 1991).
Light-dependent redox activation of AGPase resembles the light activation of enzymes of the Calvin-Benson cycle and related photosynthetic processes (Fig. 3). Photosynthetic electron transport leads to reduction of ferredoxin (Fdx), and reducing equivalents are transferred by ferredoxin:thioredoxin reductase (FTR) to Trx f and m, which activate target enzymes by reduction of regulatory disulfides (Schürmann and Buchanan, 2008). Both starch synthesis and the Calvin-Benson cycle are activated by reduced Trx f, allowing photosynthesis and end product synthesis to be coordinately regulated in response to light via the same signaling pathway. Studies on Arabidopsis knockout mutants will be required to evaluate the significance of the different plastidial Trx isoforms (see above) and FTR for redox activation of AGPase and starch synthesis during light/dark changes.
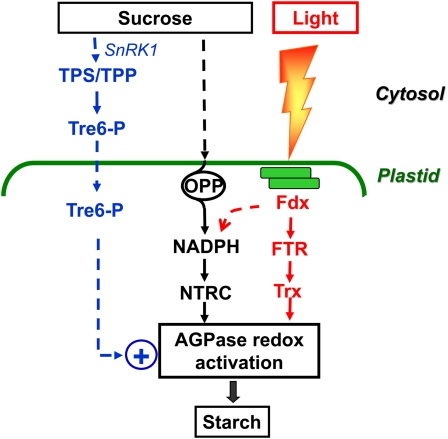
Figure 3.
Posttranslational redox regulation of starch biosynthesis in response to light and sugar signals. Light activation of starch synthesis involves posttranslational redox activation of AGPase in the chloroplast (Hendriks et al., 2003) and resembles the light activation of enzymes of the Calvin-Benson cycle and related photosynthetic processes. Photosynthetic electron transport leads to reduction of Fdx, and reducing equivalents are transferred by FTR to Trx f or m, which activate target enzymes by the reduction of regulatory disulfides. NTRC, containing both an NADP-Trx reductase and a Trx in a single polypeptide, serves as an alternate system for transferring reducing equivalents from NADPH to AGPase, thereby enhancing storage starch synthesis (Michalska et al., 2009). In the light, NTRC is mainly linked to photoreduced Fdx via Fdx-NADP reductase (identified with the dashed arrow) and complements the FTR/Trx system in activating AGPase. In the dark or under conditions in which light reactions are impaired, NTRC is primarily linked to sugar oxidation via the initial reactions of the oxidative pentose phosphate pathway (OPP) and in this way regulates AGPase independently of the Fdx/Trx system. Redox activation of AGPase is also induced by Suc, which operates in leaves in the light and in nonphotosynthetic tissues (Tiessen et al., 2002; Hendriks et al., 2003). Tre-6-P acts an intracellular signal, linking Suc in the cytosol with AGPase in the plastid (Kolbe et al., 2005; Lunn et al., 2006). In the working model, an increase in Suc is sensed in the cytosol, leading to an increase in the level of Tre-6-P by modulating Tre-6-P synthase (TPS) and/or Tre-6-P phosphatase (TPP). Tre-6-P is taken up into the plastid and promotes NTRC- and/or FTR/Trx-dependent redox activation of AGPase by a yet unresolved mechanism. SnRK1 is also implicated in this Suc signaling pathway, although its specific role in signal transduction is not fully resolved yet (Tiessen et al., 2003; Jossier et al., 2009; Zhang et al., 2009). How SnRK1 and Tre-6-P interact in this signaling cascade is unclear and may depend on the tissue.
A further signaling pathway contributing to light-dependent redox activation of AGPase is provided by NTRC, which uses NADPH to reduce AGPase (Michalska et al., 2009). Arabidopsis knockout mutants showed that 40% to 60% of the light activation of AGPase and the associated increase in starch synthesis are attributable to NTRC. In the light, NTRC is linked to photoreduced Fdx via Fdx:NADPH reductase and complements the classical FTR/Trx system in activating AGPase (Fig. 3). Conversely, photoreduced Fdx has two options to activate AGPase in the light: FTR/Trx and Fdx:NADPH reductase/NADPH. This versatility enables AGPase to respond to dynamic changes in the level of reduction of both activators and chloroplasts to adjust to changes in a wider variety of conditions (see also the section on mitochondrial effects on starch biosynthesis below).
REGULATION OF STARCH BIOSYNTHESIS IN RESPONSE TO SUGAR SIGNALS
Changes in the light/dark cycle will also lead to strong alterations in the carbon balance of the plant. Moreover, plants experience massive fluctuations of carbon availability when the rate of photosynthesis is modified due to changes in light intensity/quality, daylength, or abiotic stress conditions or when the rate of carbon use is changed for growth and development. This is buffered by accumulation and remobilization of starch as a carbon reserve, integrating changes in the balance between carbon supply and growth (Gibon et al., 2009; Sulpice et al., 2009; Stitt et al., 2010). In leaves, sugar-dependent regulation allows starch synthesis to be linked to photosynthetic activity and carbon export rates to growing tissue. Starch synthesis also has to be regulated during the day in a manner to provide sufficient carbon for growth and metabolism in the following night. If plants are immediately shifted to short-day conditions allowing less photosynthesis per day, sugars are depleted during the night, leading to a temporary restriction of carbon utilization for growth, which is then followed by an accumulation of sugars and a stimulation of starch biosynthesis in the subsequent photoperiod (Gibon et al., 2004b). In nonphotosynthetic storage organs such as growing potato tubers, starch synthesis has to be regulated in response to fluctuations in the supply of Suc from the leaves due to changes in the light/dark cycle, sink-source alterations, or developmental changes (Geigenberger and Stitt, 2000; Tiessen et al., 2002). If more carbon is available, starch synthesis is specifically activated to channel a greater proportion of the incoming Suc into starch.
Transcriptional regulation will be involved in long-term acclimation of starch metabolism to changes in the carbon status and photoperiodic signals (Bläsing et al., 2005; Gibon et al., 2009; Graf et al., 2010; Harmer, 2010). However, it is unlikely that this mechanism will contribute significantly to the more short-term regulation of starch synthesis in response to diurnal changes in sugar levels, since changes in transcripts within this time frame are in most cases not followed by changes in protein levels in leaves or tubers (Geigenberger and Stitt, 2000; Gibon et al., 2004a; Smith et al., 2004). In both Arabidopsis leaves (Gibon et al., 2004b; Kolbe et al., 2005) and growing potato tubers (Tiessen et al., 2002), the stimulation of starch synthesis in response to a change in sugar levels occurred already within 1 to 2 h and involved posttranslational redox activation of AGPase. In leaves, redox activation of AGPase increased during the day as leaf sugar levels increased, an effect that is more pronounced when carbon utilization for growth is restricted (Hendriks et al., 2003; Gibon et al., 2004b). External feeding of Suc or Glc to leaves in the dark showed that sugar-dependent redox activation of AGPase and stimulation of starch synthesis occur independently of light (Hendriks et al., 2003; Kolbe et al., 2005). Moreover, activation of AGPase in leaves (Hendriks et al., 2003) and growing tubers (Tiessen et al., 2002) was closely correlated with the sugar content across a range of physiological and genetic manipulations. Light led to an additional activation in leaves, showing both sugar- and light-dependent redox activation of AGPase to be additive (Hendriks et al., 2003). As demonstrated for potato tubers, Suc-dependent redox activation of AGPase can override allosteric regulation by changes in the 3PGA-Pi ratio, leading to an activation of AGPase also in the face of decreasing levels of substrates and the activator 3PGA and increasing levels of the inhibitor Pi (Tiessen et al., 2002). This allows the rate of starch synthesis to be increased in response to external inputs and independently of any increase in the levels of phosphorylated intermediates. In darkened leaves and roots of Arabidopsis plants, knockout of NTRC almost completely prevented sugar-dependent redox activation of AGPase and the related stimulation of starch synthesis (Michalska et al., 2009). This provides direct evidence for (1) the importance of the NADP-NTRC system for the reduction of AGPase in nonphotosynthetic tissues and (2) the in vivo relevance of redox activation of AGPase to mediate the sugar-dependent stimulation of starch accumulation (Fig. 3). The oxidative pentose phosphate pathway most likely functions in the production of NADPH to activate NTRC under nonphotosynthetic conditions, although more studies are needed to evaluate its contribution to regulate AGPase and starch biosynthesis. External supply of Glc to darkened leaves and heterotrophic potato tubers led to a strong increase in hexose phosphate levels via hexokinase and to a subsequent increase in the reduction state of the NADP system, leading to redox activation of AGPase (Geigenberger et al., 2005; Kolbe et al., 2005). In contrast to this, increased redox activation of AGPase by Suc was not accompanied by substantial changes in the hexose phosphate levels or the NADP reduction state, implying that additional signaling mechanisms are involved.
There is evidence implicating the sugar signaling molecule trehalose-6-phosphate (Tre-6-P) in the signal transduction pathway that mediates Suc-dependent redox activation of AGPase (Kolbe et al., 2005; Lunn et al., 2006; Fig. 3). Tre-6-P is the phosphorylated intermediate of trehalose biosynthesis and has been found as an indispensable regulator of sugar utilization and growth in eukaryotic organisms as different as yeast and plants (Paul et al., 2008). Different lines of evidence have been provided that Tre-6-P promotes redox activation of AGPase in response to Suc. First, Tre-6-P levels showed an accentuated increase in response to increasing Suc levels during the diurnal cycle in leaves or after external feeding of Suc to carbon-starved seedlings, leading to redox activation of AGPase and stimulation of starch synthesis (Lunn et al., 2006). Second, addition of micromolar concentrations of Tre-6-P to isolated intact chloroplasts led to a specific stimulation of reductive activation of AGPase within 15 min (Kolbe et al., 2005). Uptake studies using radiolabeled Tre-6-P provide evidence for a transport system with micromolar affinities for Tre-6-P at the chloroplast envelope (J. Michalska and P. Geigenberger, unpublished data). Third, increased Tre-6-P levels by expression of a heterologous Tre-6-P synthase in the cytosol led to increased redox activation of AGPase and starch in Arabidopsis leaves, while expression of a Tre-6-P phosphatase to decrease Tre-6-P levels showed the opposite effect (Kolbe et al., 2005). Moreover, Tre-6-P phosphatase expression strongly attenuated the increase in AGPase activation in response to external Suc feeding. While this establishes Tre-6-P as an intracellular signal linking Suc in the cytosol with AGPase in the plastid, it remains unclear at the molecular level (1) how Tre-6-P is connected with Suc, (2) how it is transported into the plastid, and (3) by which mechanism(s) it modulates thioredoxin/NTRC-dependent activation of AGPase. In addition to its role in metabolic signaling, Tre-6-P may also be involved in other signaling pathways leading to changes in cell shape, leaf, and branching phenotypes (Satoh-Nagasawa et al., 2006; Chary et al., 2008). More studies are needed to dissect the emerging role of Tre-6-P in the coordination of metabolism with development.
Studies in potato tubers (Tiessen et al., 2003; McKibbin et al., 2006) and Arabidopsis leaves (Jossier et al., 2009) also implicate the highly conserved SNF1-related protein kinase (SnRK1) to be involved in the signaling pathway linking redox activation of AGPase and starch synthesis to sugars. Recent studies provide evidence that Tre-6-P inhibits SnRK1 activity (Zhang et al., 2009), indicating a possible feedback loop that turns down SnRK1 signaling when Tre-6-P is accumulating. However, this depended on the presence of an unidentified component that was present in many growing tissues, but not in mature leaves, indicating that the interactions between Tre-6-P and SnRK1 may be indirect and tissue specific. It will obviously be of great interest to identify and further analyze the relation between Tre-6-P and SnRK1 signaling, which may involve interactions at the transcriptional (Paul et al., 2008) and posttranslational (Harthill et al., 2006) levels. Interestingly, antisense repression of SnRK1 in developing pea embryos led to an inhibition of starch accumulation despite sugar and Tre-6-P levels that were increased, indicating that changes in SnRK1 can override the Tre-6-P-dependent regulation of starch biosynthesis (Radchuk et al., 2010). This suggests SnRK1 to be involved in different signaling pathways acting on starch synthesis. In mammals, AMP-activated protein kinase, which is homologous to SnRK1, has recently been found to be inhibited by glycogen and suggested to act as a glycogen sensor (McBride et al., 2009). Whether there is a similar sensing mechanism in plants that monitors starch availability remains to be determined.
REGULATION OF STARCH BIOSYNTHESIS IN RESPONSE TO CHANGES IN MITOCHONDRIAL METABOLISM
In addition to changes in the carbon status, mitochondrial metabolism has recently been implicated in the regulation of starch accumulation in the plastid (Geigenberger et al., 2010). Mitochondrial respiration is linked to starch due to its predominant role to provide ATP to fuel starch biosynthesis in heterotrophic tissues. In developing tubers and seeds, inhibition of respiration in response to a decrease in internal oxygen concentrations led to a decrease in the cellular energy status and in the rate of starch synthesis (Geigenberger, 2003b). Moreover, starch accumulation was stimulated and the adenylate energy state increased when growing tubers were exposed to superambient oxygen concentrations, indicating that the levels of adenylate pools are colimiting for starch synthesis in growing tubers (A. Langer, J.T. van Dongen, and P. Geigenberger, unpublished data). This conclusion was further strengthened by several independent studies providing genetic and physiological evidence that manipulation of the synthesis (Loef et al., 2001; Oliver et al., 2008), equilibration (Regierer et al., 2002; Oliver et al., 2008; Riewe et al., 2008b), salvaging (Riewe et al., 2008a), or transport (Tjaden et al., 1998; Geigenberger et al., 2001) of adenylates led to corresponding changes in the rate of tuber starch synthesis. The stimulation of starch synthesis was mechanistically linked to an increase in AGPase activity. This suggests a close interaction between ATP availability in the plastid, AGPase activity, and starch biosynthesis. There are two possible explanations. First, AGPase activity is most likely restricted by the plastidial concentration of ATP as a substrate (Geigenberger et al., 2001). This conclusion is supported by studies on subcellular metabolite concentrations in growing potato tubers, showing that the plastidial ATP concentration is close to the Km(ATP) of AGPase (Farré et al., 2001; Tiessen et al., 2002). Second, increased ATP levels and ATP-AMP ratios were closely linked to an increase in the redox activation state of AGPase (Oliver et al., 2008; Riewe et al., 2008b). The underlying mechanism is unclear at the moment, but it may involve changes in the midpoint redox potential of the regulatory Cys of APS1 in response to binding of ATP as substrate. Alternatively, redox regulation of AGPase may be subject to low-energy signaling involving SnRK1 (Baena-González et al., 2007). Although there is no direct activation of SnRK1 by changes in adenylate levels, AMP has been shown to modulate the phosphorylation state of SnRK1, leading to an increase in its activity in vitro (Sugden et al., 1999).
More recently, changes in mitochondrial malate metabolism have been implicated in the regulation of plastidial starch synthesis. In transgenic potato tubers, antisense inhibition of malic enzyme, catalyzing the NAD-dependent conversion of malate to pyruvate in the mitochondrial matrix, led to activation of AGPase and increased accumulation of starch (Jenner et al., 2001). Starch synthesis was also altered in transgenic tomato (Solanum lycopersicum) plants with antisense repression of mitochondrial malate dehydrogenase or fumarase, which was shown to be mechanistically linked to an altered redox status of AGPase in the plastid (Centeno et al., 2011). While the intracellular signals linking mitochondrial malate metabolism to the plastid still have to be resolved, a strong correlation was observed between changes in cellular malate concentration, NADP reduction state, and starch synthesis in the fruit (Centeno et al., 2011). Similar effects were observed after external supply of malate to tomato fruit tissue. It is quite likely that the increase in the reduction state of NADP activates plastidial NTRC, which in turn leads to redox activation of AGPase and starch synthesis. This would suggest that NTRC-related reduction of AGPase can be triggered by a mitochondrially derived metabolite, signaling changes in the mitochondrial redox status to the plastid.
CONCLUSION
There have been recent advances in our understanding of the regulation of starch synthesis in response to environmental and metabolic signals. However, our knowledge of the signal transduction cascades remains far from complete. Specifically, there is a lack of knowledge on the molecular identity of the sensors, the intracellular signaling pathways, and the integration between photosynthetic and metabolic signals. Work in the last years also extended our understanding of the role of posttranslational protein modifications and protein-protein interaction in the regulation of starch synthesis. Evidence is emerging that starch synthesis is regulated by reversible protein phosphorylation and protein complex formation. However, it remains unclear whether these mechanisms are significant in vivo and whether their roles can be generalized for different plant species. More work will be needed to achieve a better understanding of these important aspects of the regulation of starch synthesis and to apply this knowledge for crop improvement.
Acknowledgments
I am very grateful to Alisdair R. Fernie (Max-Planck Institute of Molecular Plant Physiology, Golm, Germany) for critical reading of the manuscript.
References
- ap Rees T, Hill SA. (1994) Metabolic control analysis of plant metabolism. Plant Cell Environ 17: 587–599 [Google Scholar]
- Arsova B, Hoja U, Wimmelbacher M, Greiner E, Ustün S, Melzer M, Petersen K, Lein W, Börnke F. (2010) Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. Plant Cell 22: 1498–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Baginsky S, Gruissem W. (2009) The chloroplast kinase network: new insights from large-scale phosphoproteome profiling. Mol Plant 2: 1141–1153 [DOI] [PubMed] [Google Scholar]
- Ball SG, Morell MK. (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 [DOI] [PubMed] [Google Scholar]
- Ballicora MA, Frueauf JB, Fu Y, Schürmann P, Preiss J. (2000) Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem 275: 1315–1320 [DOI] [PubMed] [Google Scholar]
- Balmer Y, Vensel WH, Cai N, Manieri W, Schürmann P, Hurkman WJ, Buchanan BB. (2006) A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts. Proc Natl Acad Sci USA 103: 2988–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Pozueta-Romero J. (2005) Response to Neuhaus et al.: no need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends Plant Sci 10: 156–158 [DOI] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Zandueta-Criado A, Morán-Zorzano MT, Viale AM, Alonso-Casajús N, Pozueta-Romero J. (2004) Most of ADP x glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc Natl Acad Sci USA 101: 13080–13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM. (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106: 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D. (2009) Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 106: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M. (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher CG, Scrase-Field EFAL, Esposito S, Emes MJ, Tetlow IJ. (2007) Characterization of ADP-glucose transport across the cereal endosperm amyloplast envelope. J Exp Bot 58: 1321–1332 [DOI] [PubMed] [Google Scholar]
- Bräutigam K, Dietzel L, Kleine T, Ströher E, Wormuth D, Dietz K-J, Radke D, Wirtz M, Hell R, Dörmann P, et al. (2009) Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell 21: 2715–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C. (1986) Alterations in growth, photosynthesis and respiration in a starch deficient mutant of Arabidopsis thaliana (L.) Heynh deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno DC, Osorio S, Nunes-Nesi A, Bertolo ALF, Carneiro RT, Araújo WL, Steinhauser MC, Michalska J, Rohrmann J, Geigenberger P, et al. (2011) On the crucial role of malate in transitory starch metabolism, ripening, total soluble solid content at harvest and post-harvest softening in tomato fruit. Plant Cell 23: 162–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary SN, Hicks GR, Choi YG, Carter D, Raikhel NV. (2008) Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol 146: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevillén P, Ballicora MA, Mérida A, Preiss J, Romero JM. (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278: 28508–28515 [DOI] [PubMed] [Google Scholar]
- Cross JM, Clancy M, Shaw JR, Greene TW, Schmidt RR, Okita TW, Hannah LC. (2004) Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol 135: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan DNP, Rudi H, Olsen O-A. (1999) The allosterically unregulated isoform of ADP-glucose pyrophosphorylase from barley endosperm is the most likely source of ADP-glucose incorporated into endosperm starch. Plant Physiol 121: 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L. (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127: 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettke J, Malinova I, Albrecht T, Hejazi M, Steup M. (2011) Glucose 1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis thaliana. Plant Physiol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu FF, Xue HW. (2010) Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol 154: 927–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Ballicora MA, Leykam JF, Preiss J. (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273: 25045–25052 [DOI] [PubMed] [Google Scholar]
- Geigenberger P. (2003a) Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot 54: 457–465 [DOI] [PubMed] [Google Scholar]
- Geigenberger P. (2003b) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247–256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR. (2006) Starch synthesis in the potato tuber. Hui YH, Corke H, DeLeyn I, Nip W-K, Cross N, , Food Biochemistry and Food Processing. Blackwell Publishing, Ames, IA, 253–270 [Google Scholar]
- Geigenberger P, Geiger M, Stitt M. (1998) High-temperature perturbation of starch synthesis is attributable to inhibition of ADP-glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiol 117: 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Kolbe A, Tiessen A. (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56: 1469–1479 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Riewe D, Fernie AR. (2010) The central regulation of plant physiology by adenylates. Trends Plant Sci 15: 98–105 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stamme C, Tjaden J, Schulz A, Quick PW, Betsche T, Kersting HJ, Neuhaus HE. (2001) Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol 125: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. (2000) Diurnal changes in sucrose, nucleotides, starch synthesis and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J 23: 795–806 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M, Fernie AR. (2004) Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27: 655–673 [Google Scholar]
- Geiger DR, Servaites JC. (1994) Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annu Rev Plant Physiol Plant Mol Biol 45: 235–256 [Google Scholar]
- Gelhaye E, Rouhier N, Navrot N, Jacquot JP. (2005) The plant thioredoxin system. Cell Mol Life Sci 62: 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. (1987) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in non-aqueous media. Plant Physiol 75: 542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HP, Preiss J. (1966) Adenosine diphosphate glucose pyrophosphorylase: a regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem 241: 4491–4504 [PubMed] [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M. (2004a) A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M. (2004b) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Pyl E-T, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM. (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaud F, Rogniaux H, James MG, Myers AM, Planchot V. (2008) Proteome and phosphoproteome analysis of starch granule-associated proteins from normal maize and mutants affected in starch biosynthesis. J Exp Bot 59: 3395–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC, James M. (2008) The complexities of starch biosynthesis in cereal endosperms. Curr Opin Biotechnol 19: 160–165 [DOI] [PubMed] [Google Scholar]
- Harmer S. (2010) Plant biology in the fourth dimension. Plant Physiol 154: 467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harthill JE, Meek SE, Morrice N, Peggie MW, Borch J, Wong BH, Mackintosh C. (2006) Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J 47: 211–223 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Durek P, Hummel J, Selbig J, Weckwerth W, Walther D, Schulze WX. (2008) PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res 36: D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P. (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen TA, Lin Q, Grimaud F, Planchot V, Keeling PL, James MG, Myers AM. (2009) Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiol 149: 1541–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen TA, Liu F, Marsh RS, Kim S, Gan Q, Tetlow IJ, Emes MJ, James MG, Myers AM. (2008) Starch biosynthetic enzymes from developing maize endosperm associate in multisubunit complexes. Plant Physiol 146: 1892–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias AA, Barry GF, Meyer C, Bloksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TW, Kishore GM, Preiss J. (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268: 1081–1086 [PubMed] [Google Scholar]
- James MG, Denyer K, Myers AM. (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6: 215–222 [DOI] [PubMed] [Google Scholar]
- Jenner HL, Winning BM, Millar AH, Tomlinson KL, Leaver CJ, Hill SA. (2001) NAD malic enzyme and the control of carbohydrate metabolism in potato tubers. Plant Physiol 126: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y. (2010) Starch biosynthesis in cereal endosperm. Plant Physiol Biochem 48: 383–392 [DOI] [PubMed] [Google Scholar]
- Jossier M, Bouly J-P, Meimoun P, Arjmand A, Lessard P, Hawley S, Grahame Hardie D, Thomas M. (2009) SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J 59: 316–328 [DOI] [PubMed] [Google Scholar]
- Kacser H, Burns JA. (1973) The control of flux. Symp Soc Exp Biol 27: 65–104 [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U-I. (1998) Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell 10: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger S, Leroch M, Huynen MA, Wahl M, Neuhaus HE, Tjaden J. (2007) Molecular and biochemical analysis of the plastidic ADP-glucose transporter (ZmBT1) from Zea mays. J Biol Chem 282: 22481–22491 [DOI] [PubMed] [Google Scholar]
- Kloosterman B, De Koeyer D, Griffiths R, Flinn B, Steuernagel B, Scholz U, Sonnewald S, Sonnewald U, Bryan GJ, Prat S, et al. (2008) Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Funct Integr Genomics 8: 329–340 [DOI] [PubMed] [Google Scholar]
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P. (2005) Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 102: 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O, Kossman J, Zeeman SC, Lloyd JR. (2010) Regulation of starch metabolism: the age of enlightment. Curr Opin Plant Biol 13: 320–328 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Häusler RE, Fettke J, Herbst K, Niewiadomski P, Gierth M, Bell K, Steup M, Flügge U-I, Schneider A. (2010) The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol (Stuttg) (Suppl 1) 12: 115–128 [DOI] [PubMed] [Google Scholar]
- Liu F, Makhmoudova A, Lee EA, Wait R, Emes MJ, Tetlow IJ. (2009) The amylose extender mutant of maize conditions novel protein-protein interactions between starch biosynthetic enzymes in amyloplasts. J Exp Bot 60: 4423–4440 [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P. (2001) Increased adenine nucleotide levels modify the interaction between respiration and starch synthesis when adenine is fed to discs of growing potato tubers. Planta 212: 782–791 [DOI] [PubMed] [Google Scholar]
- Lohrig K, Müller B, Davydova J, Leister D, Wolters DA. (2009) Phosphorylation site mapping of soluble proteins: bioinformatical filtering reveals potential plastidic phosphoproteins in Arabidopsis thaliana. Planta 229: 1123–1134 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M. (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf PC, Jones DF. (1926) The expression of mendelian factors in the gametophyte of maize. Genetics 11: 423–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsen E, Wanke D, Kilian J, Sundberg E, Harter K, Jansson C. (2010) Significance of light, sugar, and amino acid supply for diurnal gene regulation in developing barley caryopses. Plant Physiol 153: 14–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A, Hardie DG. (2009) The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab 9: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin RS, Muttucumaru N, Paul MJ, Powers SJ, Burrell MM, Coates S, Purcell PC, Tiessen A, Geigenberger P, Halford NG. (2006) Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnol J 4: 409–418 [DOI] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. (2009) NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 106: 9908–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber BT, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U. (1990) One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224: 136–146 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Baroja-Fernández E, Morán-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajús N, Pozueta-Romero J. (2005) Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol 46: 1366–1376 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Zorzano MTM, Alonso-Casajus N, Baroja-Fernandez E, Etxeberria E, Pozueta-Romero J. (2006) New enzymes, new pathways and an alternative view on starch biosynthesis in both photosynthetic and heterotrophic tissues of plants. Biocatalysis Biotransform 24: 63–76 [Google Scholar]
- Neuhaus HE, Häusler RE, Sonnewald U. (2005) No need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends Plant Sci 10: 154–156; author reply 156–158 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Stitt M. (1990) Control analysis of photosynthate partitioning: impact of reduced activity of ADP glucose pyrophosphorylase and plastid phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana L. Heynh. Planta 182: 445–454 [DOI] [PubMed] [Google Scholar]
- Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M. (1998) The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ 21: 443–454 [Google Scholar]
- Obana Y, Omoto D, Kato C, Matsumoto K, Nagai Y, Kavakli H, Hamada S, Edwards GE, Okita TW, Matsui H, et al. (2006) Enhanced turnover of transitory starch by expression of up-regulated ADP-glucose pyrophosphorylases in Arabidopsis thaliana. Plant Sci 170: 1–11 [Google Scholar]
- Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J. (1990) The subunit structure of potato tuber ADP-glucose pyrophosphorylase. Plant Physiol 93: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SN, Tiessen A, Fernie AR, Geigenberger P. (2008) Decreased expression of plastidial adenylate kinase in potato tubers results in an enhanced rate of respiration and a stimulation of starch synthesis that is attributable to post-translational redox-activation of ADP-glucose pyrophosphorylase. J Exp Bot 59: 315–325 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y. (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59: 417–441 [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. (2006) Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18: 2356–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. (1988) Biosynthesis of starch and its regulation. Preiss J, , The Biochemistry of Plants, Vol 14. Academic Press, San Diego, 181–254 [Google Scholar]
- Radchuk R, Emery RJ, Weier D, Vigeolas H, Geigenberger P, Lunn JE, Feil R, Weschke W, Weber H. (2010) Sucrose non-fermenting kinase 1 (SnRK1) coordinates metabolic and hormonal signals during pea cotyledon growth and differentiation. Plant J 61: 324–338 [DOI] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J. (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W, Baginsky S. (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol 150: 889–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Fernie AR, Zauber H, Wucke C, Geigenberger P. (2008a) A cell wall-bound adenosine nucleosidase is involved in the salvage of extracellular ATP in Solanum tuberosum. Plant Cell Physiol 49: 1572–1579 [DOI] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Zauber H, Wucke C, Fernie AR, Geigenberger P. (2008b) Metabolic and developmental adaptations of growing potato tubers in response to specific manipulations of the adenylate energy status. Plant Physiol 146: 1579–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulsingharoj C, Choi SB, Hwang SK, Edwards GE, Bork J, Meyer CR, Preiss J, Okita TW. (2004) Engineering starch biosynthesis for increasing rice seed weight: the role of the cytoplasmic ADP-glucose pyrophosphorylase. Plant Sci 167: 1323–1333 [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D. (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441: 227–230 [DOI] [PubMed] [Google Scholar]
- Scheibe R. (1991) Redox-modulation of chloroplast enzymes: a common principle for individual control. Plant Physiol 96: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Gonzalez-Fontes A, Lauerer M, Muller-Rober B, Caboche M, Stitt M. (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9: 783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebner I, Pribil M, Zühlke J, Dietzmann A, Leister D. (2008) A survey of chloroplast protein kinases and phosphatases in Arabidopsis thaliana. Curr Genomics 9: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P, Buchanan BB. (2008) The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal 10: 1235–1274 [DOI] [PubMed] [Google Scholar]
- Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ. (2002) Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA 99: 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ. (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216: 656–664 [DOI] [PubMed] [Google Scholar]
- Smith AM. (2008) Prospects for increasing starch and sucrose yields for bioethanol production. Plant J 54: 546–558 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman SC, Smith AM. (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov LN, Déjardin A, Kleczkowski LA. (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336: 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. (1992) Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science 258: 287–292 [DOI] [PubMed] [Google Scholar]
- Stitt M, Huber S, Kerr P. (1987) Control of photosynthetic sucrose synthesis. Hatch MD, Boardman NK, , The Biochemistry of Plants, Vol 10. Academic Press, New York, 327–409 [Google Scholar]
- Stitt M, Lunn J, Usadel B. (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Streb S, Egli B, Eicke S, Zeeman SC. (2009) The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiol 151: 1769–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. (1999) Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J 19: 433–439 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl E-T, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C. (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15: 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Raynaud S, Lucas M, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21: 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow IJ, Beisel KG, Cameron S, Makhmoudova A, Liu F, Bresolin NS, Wait R, Morell MK, Emes MJ. (2008) Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol 146: 1878–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow IJ, Morell MK, Emes MJ. (2004a) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55: 2131–2145 [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ. (2004b) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16: 694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P. (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. (2003) Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J 35: 490–500 [DOI] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE. (1998) Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of starch. Plant J 16: 531–540 [Google Scholar]
- Vriet C, Welham T, Brachmann A, Pike M, Pike J, Perry J, Parniske M, Sato S, Tabata S, Smith AM, et al. (2010) A suite of Lotus japonicus starch mutants reveals both conserved and novel features of starch metabolism. Plant Physiol 154: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen X, Wang J, Liu T, Liu Y, Zhao L, Wang G. (2007) Increasing maize seed weight by enhancing the cytoplasmic ADP-glucose pyrophosphorylase activity in transgenic plants. Plant Cell Tissue Organ Cult 88: 83–92 [Google Scholar]
- Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF, Wei W, Wu HJ, Chen LJ, Chen HW, et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21: 1473–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol 61: 209–234 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]