The primary reactions of photosynthesis are mediated by three protein complexes embedded in the thylakoid membranes of chloroplasts. These complexes are PSII, the cytochrome b6f complex (Cytb6f), and PSI, which are connected in series through the photosynthetic electron transport chain. Light energy captured by the light-harvesting systems of PSII (LHCII) and PSI (LHCI) is transferred to the reaction center chlorophylls to create a charge separation across the membrane. This leads to the formation of a strong oxidant on the donor side of PSII capable of splitting water into molecular oxygen, protons, and electrons. The electrons are transferred stepwise from PSII to the plastoquinone pool, Cytb6f, plastocyanin, and PSI where a second charge separation creates a strong reductant capable of reducing ferredoxin that subsequently reduces NADP+ to NADPH. Besides this linear electron pathway there is a cyclic pathway in which electrons are cycled around PSI through the Cytb6f complex. The electron transfer reactions are coupled to proton pumping into the lumen space of the thylakoids and the resulting pH gradient drives the ATP synthase for ATP production. Ultimately NADPH and ATP are used for CO2 assimilation by the Calvin-Benson cycle. Thylakoid membranes of land plants are organized in two domains, the grana stacks, comprising 80% of the membrane, and the stromal nonappressed membranes that connect different grana stacks. The photosynthetic complexes with exception of Cytb6f, are not distributed evenly through the thylakoid membrane system. Whereas PSII is mostly confined to the grana regions, PSI and ATP synthase are located in the stroma lamellae. Electron transfer between these complexes is mediated by the diffusible electron carriers plastoquinone and plastocyanin.
A characteristic feature of the photosynthetic complexes is that they all consist of numerous chloroplast and nucleus-encoded subunits. In the case of PSII, PSI, and Cytb6f these complexes contain in addition pigments such as chlorophylls and xanthophylls as well as hemes, quinones, and iron-sulfur (Fe-S) centers that act as redox cofactors. Hence the biogenesis of the photosynthetic apparatus involves a concerted interplay between the chloroplast and nucleocytosolic genetic systems as well as a tight coordination between protein and pigment synthesis and insertion into the thylakoid membranes. Analysis of numerous mutants affected in photosynthetic activity in Chlamydomonas reinhardtii, Arabidopsis (Arabidopsis thaliana), and Zea mays has provided many new insights into the assembly of the photosynthetic complexes (Barkan and Goldschmidt-Clermont, 2000; Eberhard et al., 2008).
A remarkable feature of photosynthetic organisms is their ability to adapt to changing light conditions, which is reflected in changes in the organization of the photosynthetic complexes. This Update reviews recent advances in our understanding of the assembly of these complexes and for some of them, their remodeling and/or reversible association into supercomplexes that depends on environmental conditions.
ASSEMBLY OF PHOTOSYNTHETIC COMPLEXES
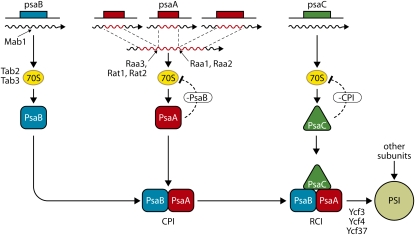
A major problem in the assembly of photosynthetic complexes is how the synthesis and insertion into the membrane of their different subunits and cofactors is coordinated. While the plastid protein synthesizing system has been well characterized and includes components such as RNA polymerases, RNA processing factors, ribosomes, and translation factors that have a general role in gene expression, genetic analysis in C. reinhardtii, Arabidopsis, and Z. mays has revealed a surprisingly large number of nucleus-encoded factors that act on specific plastid genes or RNAs. These factors are required for different posttranscriptional steps including RNA processing, RNA stability, RNA editing, splicing, translation, and assembly of the complexes (Barkan and Goldschmidt-Clermont, 2000; Eberhard et al., 2008). In C. reinhardtii most of these factors act in a gene-specific way whereas in land plants they usually have a more pleiotropic role by acting on more than one gene or its product. In the case of factors involved in RNA processing and translation in C. reinhardtii, the target site is very often located in the 5′-untranslated region of a specific RNA. Many of these factors have been identified, some are well conserved in all photosynthetic organisms while others appear to be organism specific. In C. reinhardtii one of these factors is usually required for RNA processing/stability and one or two others for translation of a given RNA. However, there are exceptions. As an example, maturation of the chloroplast psaA mRNA encoding a reaction center subunit of PSI and that is assembled from three independently transcribed RNAs corresponding to three exons requires at least 14 factors involved in either of the two trans-splicing reactions or in both (Fig. 1; see Barkan and Goldschmidt-Clermont, 2000).
Figure 1.
Biosynthesis and CES cascade for the assembly of PSI in Chlamydomonas. The core PSI subunits are encoded by the chloroplast genes psaA, psaB, and psaC, indicated by colored boxes. The corresponding RNAs are shown as wavy lines. The psaA gene is fragmented into three exons whose transcripts are assembled into the mature psaA mRNA through two trans-splicing reactions mediated in part by the nucleus-encoded factors Raa1, Raa2, Raa3, Rat1, and Rat2. Only those factors whose genes have been identified are shown. 70S represents chloroplast ribosomes. Mab1 is specifically required for the processing/stability of the psaB mRNA and Tab2 and Tab3 are necessary for its translation. PsaB is the first PSI subunit to be inserted into the membrane. In its absence the CES subunit cannot assemble and inhibits directly or indirectly its own synthesis. Once CPI consisting of PsaA and PsaB has been formed, the next subunit PsaC can assemble to form RCI. In the absence of CPI, PsaC inhibits its own synthesis. The other PSI subunits are integrated subsequently into the complex. Ycf3, Ycf4, and Ycf37 are specific PSI assembly factors that act at early stages of assembly.
The trans-acting factors involved in chloroplast gene expression fall into several classes. The first includes RNA-binding proteins derived from enzymes involved in RNA metabolism such as for example pseudouridine synthase (Perron et al., 1999) and peptidyl-tRNA hydrolase (Jenkins and Barkan, 2001). These proteins appear to have been recruited for novel roles in chloroplast gene expression during evolution. A second class comprises factors containing degenerate repeats of 34 to 38 amino acids. Repeat-containing proteins are widespread in nature and are involved in a broad range of cellular activities. The atomic structure of the 34 amino acid repeats, called tetratricopeptide repeats (TPRs) has revealed that each repeat consists of a pair of antiparallel α-helices with adjacent repeats packed together and forming a superhelix (Das et al., 1998). Although TPR motifs are generally involved in protein-protein interactions, in some cases they may also act as RNA-binding sites. In the case of the helical repeat Pumilio protein from Drosophila that contains Puf repeats, it was shown that the internal side of the superhelix acts as RNA-binding site (Edwards et al., 2001). Unfortunately the chloroplast TPR proteins are highly insoluble and attempts to test for RNA binding in vitro have failed. In contrast specific RNA binding could be demonstrated with pentatricopeptide repeat (PPR) proteins, which are structurally similar to TPR proteins except that the repeats contain 35 residues (Small and Peeters, 2000). The PPR family contains 450 members in Arabidopsis that are involved in many steps of RNA metabolism, especially RNA editing and translation (Schmitz-Linneweber and Small, 2008). This large set of proteins may provide an explanation for the specificity of the chloroplast RNA targets and suggests the existence of a PPR motif code in which specific RNA sequences are recognized by specific PPR repeats. In C. reinhardtii a large group of repeat-containing proteins are the PPPEW/OPR (octatricopeptide repeats) proteins. They were first identified in Tbc2, a factor required for the translation of the psbC mRNA (Auchincloss et al., 2002). These repeats were subsequently found in other nucleus-encoded factors involved in chloroplast gene expression. In contrast to the TPR and PPR proteins, the PPPEW/OPR family appears to be restricted to Chlamydomonas among photosynthetic organisms but proteins of this type are also present in protozoans such as Plasmodium and Theileria.
The existence of a large set of nucleus-encoded factors required for proper chloroplast gene expression raises the question whether these factors are constitutively required or whether they also play a regulatory role. This problem was addressed with two nucleus-encoded factors MCA1and TCA1 required for the stable accumulation and translation, respectively, of the chloroplast petA mRNA encoding cytochrome f (Raynaud et al., 2007). Accumulation of petA mRNA and translation of cytochrome f was reduced in transgenic strains producing decreased amounts of MCA1 or TCA1, indicating that these factors are limiting. Moreover because MCA1 is a short-lived protein, its abundance varies rapidly under physiological conditions where the demand for Cytb6f changes. Such a case occurs under nitrogen starvation or in aging cultures where a decrease of both factors can be correlated with a loss of petA mRNA and the disappearance of Cytb6f (Raynaud et al., 2007).
The assembly of the photosynthetic complexes within the thylakoid membrane requires both general and complex-specific proteins. In vascular plants and Chlamydomonas, the ALB3 protein is required for the integration of the LHC proteins into the thylakoid membrane (Sundberg et al., 1997; Bellafiore et al., 2002). Mutants deficient in ALB3 are albino in Arabidopsis. This protein is related to the mitochondrial Oxa1p and YidC proteins from Escherichia coli that are essential components for inserting proteins into membranes. There are two ALB3-related genes in Chlamydomonas Alb3.1 and Alb3.2. Mutants deficient in Alb3.1 accumulate reduced levels of LHCII. Moreover the insertion of D1 into functional PSII complexes is retarded and Alb3.1 is associated with D1 upon its insertion into membranes. As Alb3.1, Alb3.2 is also located in the thylakoids. Coimmunoprecipitation experiments reveal that Alb3.2 interacts with Alb3.1 and with the reaction center polypeptides of PSI and PSII, and also with VIPP1, a protein involved in thylakoid formation (Göhre et al., 2006). Although Alb3.1 and Alb3.2 are related, with 37% sequence identity and 57% sequence similarity, the phenotypes of the respective mutants differ drastically. A decrease of Alb3.2 to 25% to 40% of wild-type levels leads to a reduction in PSII and PSI and several other photosynthetic proteins, indicating that Alb3.2 is limiting for the assembly and/or maintenance of these complexes in thylakoids. However other proteins such as VIPP1 and the chloroplast chaperone pair Hsp70/Cdj2 are overproduced under the same conditions. Moreover the size of the vacuoles increases considerably and, after a prolonged period, cell death occurs, indicating that in contrast to Alb3.1, Alb 3.2 has an essential function probably related to thylakoid biogenesis and/or function (Göhre et al., 2006). This raises new questions on the role of thylakoids in cell growth and survival.
Besides these general assembly factors, a large number of complex-specific assembly factors have been identified. They include Ycf3, Ycf4, and Ycf37 in the case of PSI (Fig. 1). In mutants deficient in these proteins, the PSI proteins are still synthesized, but they do not accumulate presumably because of a defect in the assembly of the complex. These proteins are also present in cyanobacteria. However in several cases their role appears to have changed during evolution as the loss of these proteins generally has more dramatic effects in plants and algae than in cyanobacteria. It is not clear how these factors act. Ycf3 contains TPR repeats and was shown to interact with PsaA and PsaD (Naver et al., 2001). Ycf4 of C. reinhardtii is part of a high molecular complex of more than 1,500 kD that associates with newly synthesized PSI subunits partially assembled as a chlorophyll-containing subcomplex, suggesting that the Ycf4 complex may act as a scaffold for PSI assembly (Ozawa et al., 2009). Ycf37 is essential for PSI assembly in land plants whereas in cyanobacteria PSI still accumulates, although in lower amounts, in mutants lacking Ycf37 (Dühring et al., 2007).
During biogenesis of the photosynthetic complexes, the first step in the assembly is the insertion of an anchor protein that acts as a scaffold for the following assembly steps. These anchor proteins (also called dominant subunits) are D2 for PSII, PetB for Cytb6f, and PsaB for PSI (Fig. 1). In the absence of these proteins translation of the next protein to be inserted in the complex, called a control by epistasy of synthesis (CES) subunit, is inhibited (see Choquet and Vallon, 2000). In this CES control, the unassembled CES subunit inhibits its own translation through a negative-feedback mechanism. Thus translation of the CES subunits only occurs if the previous step in the assembly pathway has been accomplished. In the case of PSI a CES cascade has been identified that proceeds first by the insertion of the PsaB subunit into the membrane (Wostrikoff et al., 2004). The PsaA subunit that forms the PSI core together with PsaA is translated as long as the partner of its assembly, PsaB is present. Similarly, the following subunit to be inserted, PsaC is only translated in the presence of PsaA and PsaB, but not in their absence (Fig. 1). Similar CES cascades have been found for PSII, the Cytb6f complex, and ATP synthase.
PSII ASSEMBLY, DEGRADATION, AND REPAIR
PSII exists mainly in dimeric form with the monomer containing at least 27 to 28 subunits (Dekker and Boekema, 2005). The core includes the D1 and D2 reaction center proteins that bind the redox cofactors, the α- and β-subunits of cytochrome b559, PsbI, CP43, and CP47 that coordinate chlorophyll a molecules and constitute the inner antenna, and numerous small Mr subunits. The products of the PsbO, PsbP, and PsbQ genes form the oxygen-evolving complex on the lumen side. Assembly of PSII starts with the formation of the reaction center complex followed by the association of the intrinsic inner antenna proteins CP43/CP47, the integration of the small subunits, and the assembly of the water-splitting complex and finally the dimerization of PSII monomers. Several PSII assembly factors have been identified such as HCF136, LPA1, LPA2, and LPA3. In the absence of HCF136, PSII proteins are normally synthesized but they do not assemble into stable PSII complexes (Meurer et al., 1998). In the lpa1 mutant of Arabidopsis, assembly of the newly synthesized PSII proteins is less efficient than in wild-type plants (Peng et al., 2006). LPA1 appears to be a membrane chaperone that interacts specifically with D1. LPA2 and LPA3 have overlapping functions and interact with ALB3. Together these proteins appear to be specifically involved in the folding and assembly of CP43 within PSII (Cai et al., 2010). In the absence of LPA2 newly synthesized CP43 is rapidly degraded and there is a defect in PSII supercomplex formation (Ma et al., 2007). LPA2 is only present in plants and absent in Chlamydomonas and cyanobacteria, suggesting that it evolved after the divergence of the land plant lineage or that it was lost in the other lineages. FKBP-2, a lumen-localized immunophilin, appears to play a role in the formation of PSII supercomplexes as in its absence the levels of PSII monomers and dimers are increased whereas accumulation of PSII supercomplexes is diminished (Lima et al., 2006). Thus a large number of factors participate in the assembly of a functional PSII complex. How they act mechanistically is still largely unknown.
Among photosynthetic complexes PSII is particularly prone to photooxidative damage because the water-splitting reaction catalyzed by this complex inevitably leads to the formation of reactive oxygen species that damage the complex. Thus PSII is constantly damaged and needs to be repaired. Indeed a very efficient repair system has evolved in which the D1 subunit of photodamaged PSII is predominantly degraded and the complex moves from the grana to the stromal region for repair (see Nixon et al., 2010). Possibly, chlorophyll released during this process is transiently stored in small CAB-like proteins of cyanobacteria containing a single transmembrane helix with high similarity to some transmembrane domains of the light-harvesting proteins (Kufryk et al., 2008). Newly synthesized D1 precursor with a C-terminal extension is inserted into PSII in the stromal thylakoid region followed by cleavage of the extension by the C-terminal peptidase CtpA and assembly of the catalytic manganese cluster (see Nixon et al., 2010). The reconstituted PSII complex moves then back to the grana. Several factors have been identified that play a role in this repair cycle among which the ATP-dependent zinc metalloprotease FtsH present in all species from cyanobacteria to plants (see Nixon et al., 2010). FtsH forms a multisubunit complex exposed to the outer surface of thylakoid membranes and is enriched in stroma thylakoids and grana margins. Deg proteases also appear to be involved in D1 degradation (Sun et al., 2010). Interestingly the lumenally exposed thylakoid Deg1 protease is involved both in D1 degradation and in PSII assembly presumably through its direct interaction with D2. Besides proteases, other factors have been discovered such as LPA19 that facilitates D1 protein precursor processing and interacts with the C-terminal region of the mature and precursor form of D1 (Wei et al., 2010). LPA19 is related to Psb27 present in both cyanobacteria and plants that appears to function in the assembly of the manganese cluster (Roose and Pakrasi, 2004). A second Psb27 homolog of Arabidopsis was identified as a PSII component and it is required for efficient repair of photodamaged PSII but is nonessential for PSII accumulation (Chen et al., 2006).
COFACTOR ASSEMBLY IN PHOTOSYNTHETIC COMPLEXES
An important step in the assembly of photosynthetic complexes is the insertion of chlorophyll, carotenoids, cytochromes, and Fe-S centers that need to be assembled in a coordinate way with the proteins of these complexes. It is not possible within this short Update to cover all these aspects. One area that has progressed rapidly in recent years is the identification of several novel factors required for the maturation of c-type cytochromes in which heme is covalently attached to the protein most often by two thioether bonds between the heme vinyl groups and the thiols of two Cys residues in a conserved CXXCH motif in which His acts as one of the axial ligands to heme Fe. Covalent heme binding plays a major role during assembly of the Cytb6f complex that consists of four large subunits (cytochrome b6, subunit IV, cytochrome f, and the Rieske protein) and four small subunits (PetG, PetL, PetM, and PetN). Additionally it contains a chlorophyll a molecule, β-carotene, one Fe2S2 cluster, two b-hemes, and two c-hemes. Two maturation pathways for heme c attachment are operating in the chloroplast (see de Vitry and Kuras, 2009). The first, system II (c-type cytochrome synthesis), comprises one chloroplast- and six nucleus-encoded proteins required for heme attachment to the apoforms of cytochrome f and cytochrome c6 on the lumen side of the thylakoid membrane. The latter replaces plastocyanin under copper deprivation for transferring electrons from the Cytb6f complex to PSI. In Arabidopsis the thioredoxin-like protein HCF164 and a protein related to the bacterial thiol disulfide transporter CCDA are involved in cytochrome f maturation (Lennartz et al., 2001; Page et al., 2004). They have been proposed to be part of a redox relay system that shuttles thiol-reducing equivalents across the thylakoid membrane. The second, system IV (cofactor assembly on complex C subunit B [CCB]), comprises at least four factors, CCB1 to CCB4, identified through genetic screens in Chlamydomonas that are required for the attachment of heme ci’ to apocyochrome b6 of the Cytb6f complex on the stromal face of the thylakoid membrane (see de Vitry and Kuras, 2009). Heme ci’ is only covalently bound to a single Cys residue close to the Qi site where quinone is reduced during the Q cycle. The CCB factors are conserved in all photosynthetic organisms performing oxygenic photosynthesis.
Fe-S clusters play a key role in photosynthetic electron transport, mostly at the level of the Cytb6f complex and of PSI that contain one 2Fe-2S and three 4Fe-4S centers, respectively. Although it is known that isolated chloroplasts are able to form Fe-S clusters, it is only recently that components of the plastid Fe-S assembly machinery have been identified (see Balk and Lobréaux, 2005). The finding of six SUF-like proteins in chloroplasts suggest the existence of a plastid system related to the bacterial SUF system that is required in E. coli under Fe-limiting and oxidative stress conditions. The SUF machinery is known to be more robust to oxidative stress than the common ISC system of bacteria and appears therefore to be the appropriate system in the plastid environment (Balk and Lobréaux, 2005). Only few of the plastid SUF components have been characterized and the regulation of this machinery in response to oxidative stress, Fe status, and light is still poorly understood. Another key player in plastid Fe homeostasis is ferritin, which is able to store large amounts of Fe. Interestingly in response to reduced Fe availability in C. reinhardtii, PSI is degraded, its LHC is remodeled, and ferritin levels increase in contrast of what is observed in animal or plant systems in which ferritin is repressed (Busch et al., 2008). In this way ferritin is used to buffer the Fe released by PSI during its degradation and the Fe can be reutilized efficiently upon Fe repletion. The adaptation to Fe deficiency involves also a remodelling of the LHCI antenna with N-terminal truncation of the Lhca3 subunit and the specific depletion of some LHCI subunits coupled to the up-regulation of others, resulting in the disconnection of LHCI from PSI and thus diminished energy transfer to PSI (Moseley et al., 2002; Naumann et al., 2005).
DYNAMICS OF PSII-LHCII COMPLEXES MEDIATED BY PROTEIN PHOSPHORYLATION
PSII and LHCII form large supercomplexes in the thylakoid membranes. The peripheral antenna system of PSII consists of six different complexes that coordinate Chl a, Chl b, and several xanthophylls (Jansson, 1999). Whereas the major LHCII complex of land plants is organized in heterotrimers containing the products of the Lhcb1-3 genes, the other three minor LHCIIs, CP29, CP26, and CP24, are present as monomers (Dekker and Boekema, 2005). The structure of these complexes has been intensively studied by electron microscopy and single-particle analysis after mild solubilization of membranes (Dekker and Boekema, 2005). The larger supercomplex of Arabidopsis contains a dimeric PSII core (C2), two LHCII trimers (trimer S) strongly bound to the complex on the side of CP43 and CP26, and two additional trimers moderately bound (trimer M) to CP29 and CP24 (C2S2M2 complex). A series of PSII supercomplexes with different antenna sizes, ranging from the core complex to C2S2M2 was isolated and functionally characterized (Caffarri et al., 2009). The study of Lhcb-deficient mutants provided insights into the assembly steps and into the role of the individual subunits in the supramolecular organization. Thus CP24 binds the M trimer through interaction with Lhcb3. Interestingly CP24 and Lhcb3 are only present in land plants where they could have been responsible for the increase in the PSII antenna size. In marked contrast only C2S2 complexes were found in green algae (Minagawa, 2010). CP26 is important for the stable binding of trimer S while CP29 is mostly involved in stabilizing the PSII dimer (Caffarri et al., 2009).
Phosphorylation of PSII and LHCII profoundly affects both the formation/dissociation of PSII-LHCII supercomplexes in the grana and the association of LHCII to PSI in the stromal lamellae. The two protein kinases STN7/Stt7 and STN8/Stl1 that are tightly associated with thylakoid membranes and conserved in oxygenic photosynthetic organisms play a major role in these phosphorylations (see Lemeille and Rochaix, 2010). The former is involved in LHCII phosphorylation whereas the latter phosphorylates the PSII core. As STN7, STN8 is associated with thylakoid membranes and activated by moderate light. The availability of mutants of Arabidopsis and Chlamydomonas lacking either STN7, STN8, or both kinases and therefore deficient in LHCII and PSII core protein phosphorylation has made it possible to test to what extent surface charges are responsible for thylakoid membrane folding. Earlier studies indicated that unappressed regions carry a higher negative surface charge than the appressed regions and that destacking of thylakoid membranes can be induced in vitro through depletion of cations presumably because of the unmasking of repulsive negative charges (Barber, 1982). Analysis of the membranes from the stn8 mutant deficient in PSII core protein phosphorylation revealed a more compact thylakoid organization with increased thylakoid membrane folding as compared to wild-type plants (Fristedt et al., 2009). The size of the stacked thylakoid membranes was significantly larger. This phenotype was only detected in the absence of the STN8 kinase but not in plants lacking STN7. Hence PSII core protein phosphorylation plays a major role in the folding of thylakoid membranes whereas the phosphorylation status of LHCII appears to be less important in this respect.
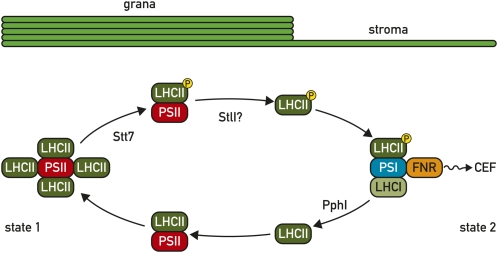
The phosphorylation of LHCII by the STN7/Stt7 kinase affects its association with PSII and PSI within the thylakoid membrane. The activity of this kinase is sensitive to changes in light quality and quantity that can lead to unequal excitation of the two photosystems because of differences in the light absorption properties of their corresponding antennae. This kinase is at the heart of state transitions, a process that rebalances the absorbed light energy between two photosystems by adjusting the size of their LHCs (Wollman, 2001; Lemeille and Rochaix, 2010). Upon excess excitation of PSII and reduction of the plastoquinone pool, this kinase induces, directly or indirectly, the phosphorylation of LHCII that leads to the detachment of the mobile antenna from PSII and its movement and binding to PSI (state 2). The process is reversible as preferential excitation of PSI with far-red light inactivates the kinase and the phosphatase PPH1/TAP38 dephosphorylates LHCII that then moves back to PSII (state 1; Fig. 2; see Lemeille and Rochaix, 2010). At high light, when the acceptor side of PSI is reduced, the LHCII kinase is inactivated through the ferredoxin/thioredoxin system (Rintamäki et al., 1997). Two conserved Cys residues in the N-terminal domain of the kinase are obvious candidate targets for the high-light-mediated inactivation of the kinase. In this respect the trans-thylakoid thiol-reducing pathway mediated by the thiol disulfide transporter CcdA and thioredoxin-like Hcf164 could be involved in this process (see Lemeille and Rochaix, 2010). Site-directed mutagenesis in C. reinhardtii showed that these two Cys are essential for kinase activity (Lemeille and Rochaix, 2010).
Figure 2.
LHCII cycle during state transitions. Under state 1 conditions PSII and LHCII form megacomplexes within the thylakoid grana. Upon phosphorylation of the major LHCII by the Stt7 kinase, the megacomplex dissociates into PSII-LHCII supercomplexes. After further phosphorylation of the minor monomeric LHCII (CP26 and CP29) and of PSII core proteins possibly mediated by the Stl1 kinase, phosphorylated LHCII is released from PSII and migrates to the stroma region of the thylakoids where it associates with PSI (state 2; see Minagawa, 2010). Upon deactivation of the STN7 kinase, LHCII is dephosphorylated by the PPH1 phosphatase and released from PSI, it then migrates back to PSII in the grana region.
A key feature of state transitions is the movement of the mobile part of the LHC from PSII to PSI. This movement was first documented in C. reinhardtii through spectroscopic measurements that indicated that 80% of the LHCII antenna is mobile (Wollman, 2001). Biochemical evidence for this antenna mobility was provided through the isolation of a PSI-LHCI-LHCII supercomplex in state 2 from Arabidopsis (Zhang and Scheller, 2004). This supercomplex consists of one PSI-LHCI complex and one LHCII trimer (Kouril et al., 2005). Cross-linking studies further revealed that the docking site of LHCII on PSI is formed by the PsaH, PsaI, and PsaO subunits and is consistent with the finding that Arabidopsis mutants lacking any of these subunits are deficient in state transitions (Lunde et al., 2000). In C. reinhardtii a PSI-LHCI-LHCII supercomplex could also be isolated that includes the three LHCII proteins CP29, CP26, and Lhcbm5 (Minagawa, 2010). Lhcbm5 appears to have a similar role as CP24 in land plants as it is present in similar amounts as CP29 and CP26 but at a lower level than the major LHCII proteins. These three monomeric LHCII proteins migrate to the PsaH side of PSI in state 2 where they form a binding site for the LHCII trimers on PSI. Interestingly, the crystal structure of the PSI-LHCI complex indicates that four Lhca subunits form a belt on the opposite side of PsaH (Ben-Shem et al., 2003). The fact that phosphorylated CP29 and Lhcbm5 were found associated with PSI in state 2 suggests that the affinities of CP29 and Lhcbm5 for PSII and PSI are modulated by phosphorylation (Minagawa, 2010). Remarkably, four sites of CP29 are phosphorylated in state 2. Taken together these results show that reversible phosphorylation at the interface between the PSII core and LHCII play an important role in state transitions. More recently the large PSI supercomplex was characterized further and shown to comprise in addition to LHCI, the major trimeric LHCII and the minor monomeric LHCII CP29 and CP26, Cytb6f, including PetO, a subunit that is specific to C. reinhardtii, and the proteins PGRL1 and FNR (Minagawa, 2010). Spectroscopic measurements showed that upon illumination reducing equivalents generated by PSI are transferred to Cytb6f and that oxidized PSI can be reduced by reducing equivalents from Cytb6f, indicating that this complex is capable of driving cyclic electron flow. The location of this PSI supercomplex is still unknown within the thylakoid membrane system. However it is expected to localize with a fraction of the mobile electron carriers plastoquinone, plastocyanin, and ferredoxin in its vicinity in such a way that cyclic and linear electron flow can operate independently from each other to avoid disturbance of the redox poise of cyclic by reduced linear electron flow components.
The dissociation of LHCII from PSII that occurs during a transition from state 1 to state 2 was examined by purifying PSII-containing complexes through a His tag inserted in CP47 by nickel-affinity chromatography (Minagawa, 2010). In this way a PSII core complex, a PSII-LHCII supercomplex, and a multimer of the PSII supercomplex called PSII megacomplex were identified. Moreover the megacomplex was predominant in state 1 whereas the core complex was detected in state 2, indicating a dissociation of LHCII from PSII during a state 1 to state 2 transition. Phosphorylated LHCII was mostly associated with the supercomplex and less with the megacomplex while phosphoryated CP26 and CP29 were only detected in unbound form. A model was derived from these observations in which PSII remodeling during a transition from state 1 to state 2 proceeds in two steps. First, as a result of LHCII phosphorylation, the megacomplex dissociates, giving rise to supercomplexes, and second, LHCII is released from the supercomplex by phosphorylation of the monomeric complexes CP29 and CP26 and the PSII core proteins. According to this model CP29 and CP26 play an essential role because their phosphorylation leads to the release of the entire peripheral antenna during a transition from state 1 to state 2. The released LHCII will then reassociate with the PSI-LHCI complex. The role of CP29 and CP26 was further tested by RNA interference experiments in C. reinhardtii (Minagawa, 2010). These studies showed that in the absence of CP29 the mobile LHCII antenna is still phosphorylated and detached from PSII, but it is unable to bind to the PSI-LHCI complex. Therefore state transitions no longer occur in the absence of CP29. However they are not affected by the loss of CP26. Thus CP29 plays a key role in the docking of LHCII to PSI.
PERSPECTIVES
The picture that emerges from these studies is a dynamic thylakoid membrane network in which the assembly of photosynthetic complexes is mediated by numerous nucleus- and a few chloroplast-encoded factors. The dynamics of this system is influenced to a large extent by environmental factors such as light and nutrients. Some of these factors such as ALB3 appear to have a general role in thylakoid membrane biogenesis whereas others are specifically required for the efficient assembly of the different photosynthetic complexes. Other factors function mainly during the repair of photodamaged complexes. We still know very little how these factors act mechanistically and how their activity is influenced by environmental conditions. This will undoubtedly form an important part of future research. A more ambitious goal will be to reconstitute the assembly process in vitro, a daunting task given the fact that most of the components and the redox cofactors are highly hydrophobic. Once they have been assembled, several of the photosynthetic complexes can form supercomplexes or even megacomplexes. Some of these complexes occur only transiently or are unstable and are therefore difficult to study. Posttranslational protein modifications such as phosphorylations mediated by the thylakoid membrane protein kinases STN7/Stt7 and STN8/Stl1 appear to play a major role in these associations/dissociations. Furthermore some of these phosphorylations also affect the folding of the thylakoid membrane and the movement of the complexes. Additional factors are likely to be involved in these processes and their identification represents a further challenge.
Acknowledgments
I thank N. Roggli for drawings and M. Goldschmidt-Clermont for helpful comments.
References
- Auchincloss AH, Zerges W, Perron K, Girard-Bascou J, Rochaix JD. (2002) Characterization of Tbc2, a nucleus-encoded factor specifically required for translation of the chloroplast psbC mRNA in Chlamydomonas reinhardtii. J Cell Biol 157: 953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Lobréaux S. (2005) Biogenesis of iron-sulfur proteins in plants. Trends Plant Sci 10: 324–331 [DOI] [PubMed] [Google Scholar]
- Barber J. (1982) The control of membrane organization by electrostatic forces. Biosci Rep 2: 1–13 [DOI] [PubMed] [Google Scholar]
- Barkan A, Goldschmidt-Clermont M. (2000) Participation of nuclear genes in chloroplast gene expression. Biochimie 82: 559–572 [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Ferris P, Naver H, Göhre V, Rochaix JD. (2002) Loss of Albino3 leads to the specific depletion of the light-harvesting system. Plant Cell 14: 2303–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Frolow F, Nelson N. (2003) Crystal structure of plant photosystem I. Nature 426: 630–635 [DOI] [PubMed] [Google Scholar]
- Busch A, Rimbauld B, Naumann B, Rensch S, Hippler M. (2008) Ferritin is required for rapid remodeling of the photosynthetic apparatus and minimizes photo-oxidative stress in response to iron availability in Chlamydomonas reinhardtii. Plant J 55: 201–211 [DOI] [PubMed] [Google Scholar]
- Caffarri S, Kouril R, Kereïche S, Boekema EJ, Croce R. (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J 28: 3052–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ma J, Chi W, Zou M, Guo J, Lu C, Zhang L. (2010) Cooperation of LPA3 and LPA2 is essential for photosystem II assembly in Arabidopsis. Plant Physiol 154: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen H, Zhang D, Guo J, Wu H, Jin M, Lu Q, Lu C, Zhang L. (2006) A Psb27 homologue in Arabidopsis thaliana is required for efficient repair of photodamaged photosystem II. Plant Mol Biol 61: 567–575 [DOI] [PubMed] [Google Scholar]
- Choquet Y, Vallon O. (2000) Synthesis, assembly and degradation of thylakoid membrane proteins. Biochimie 82: 615–634 [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PW, Barford D. (1998) The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J 17: 1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C, Kuras R. (2009) The cytochrome b6f complex. Stern DB, , The Chlamydomonas Sourcebook, Organellar and Metabolic Processes. Elsevier, Amsterdam, pp 603–629 [Google Scholar]
- Dekker JP, Boekema EJ. (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706: 12–39 [DOI] [PubMed] [Google Scholar]
- Dühring U, Ossenbühl F, Wilde A. (2007) Late assembly steps and dynamics of the cyanobacterial photosystem I. J Biol Chem 282: 10915–10921 [DOI] [PubMed] [Google Scholar]
- Eberhard S, Finazzi G, Wollman FA. (2008) The dynamics of photosynthesis. Annu Rev Genet 42: 463–515 [DOI] [PubMed] [Google Scholar]
- Edwards TA, Pyle SE, Wharton RP, Aggarwal AK. (2001) Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell 105: 281–289 [DOI] [PubMed] [Google Scholar]
- Fristedt R, Willig A, Granath P, Crèvecoeur M, Rochaix JD, Vener A. (2009) Phosphorylation of photosystem II controls functional macroscopic folding of plant photosynthetic membranes in Arabidopsis. Plant Cell 21: 3950–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhre V, Ossenbühl F, Crèvecoeur M, Eichacker LA, Rochaix JD. (2006) One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 18: 1454–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S. (1999) A guide to the Lhc genes and their relatives in Arabidopsis/IT> Trends Plant Sci 4: 236–240 [DOI] [PubMed] [Google Scholar]
- Jenkins BD, Barkan A. (2001) Recruitment of a peptidyl-tRNA hydrolase as a facilitator of group II intron splicing in chloroplasts. EMBO J 20: 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouril R, Zygadlo A, Arteni AA, de Wit CD, Dekker JP, Jensen PE, Scheller HV, Boekema EJ. (2005) Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44: 10935–10940 [DOI] [PubMed] [Google Scholar]
- Kufryk G, Hernandez-Prieto MA, Kieselbach T, Miranda H, Vermaas W, Funk C. (2008) Association of small CAB-like proteins (SCPs) of Synechocystis sp. PCC 6803 with photosystem II. Photosynth Res 95: 135–145 [DOI] [PubMed] [Google Scholar]
- Lemeille S, Rochaix JD. (2010) State transitions at the crossroad of thylakoid signalling pathways. Photosynth Res 106: 33–46 [DOI] [PubMed] [Google Scholar]
- Lennartz K, Plücken H, Seidler A, Westhoff P, Bechtold N, Meierhoff K. (2001) HCF164 encodes a thioredoxin-like protein involved in the biogenesis of the cytochrome b(6)f complex in Arabidopsis. Plant Cell 13: 2539–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A, Lima S, Wong JH, Phillips RS, Buchanan BB, Luan S. (2006) A redox-active FKBP-type immunophilin functions in accumulation of the photosystem II supercomplex in Arabidopsis thaliana. Proc Natl Acad Sci USA 103: 12631–12636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV. (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408: 613–615 [DOI] [PubMed] [Google Scholar]
- Ma J, Peng L, Guo J, Lu Q, Lu C, Zhang L. (2007) LPA2 is required for efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell 19: 1980–1993 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Meurer J, Plücken H, Kowallik KV, Westhoff P. (1998) A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J 17: 5286–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa J. (November 23, 2010) State transitions: the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim Biophys Acta http://dx.doi.org/10.1016/j.bbabio.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, Hippler M. (2002) Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J 21: 6709–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann B, Stauber EJ, Busch A, Sommer F, Hippler M. (2005) N-terminal processing of Lhca3 is a key step in remodeling of the photosystem I-light-harvesting complex under iron deficiency in Chlamydomonas reinhardtii. J Biol Chem 280: 20431–20441 [DOI] [PubMed] [Google Scholar]
- Naver H, Boudreau E, Rochaix JD. (2001) Functional studies of Ycf3: its role in assembly of photosystem I and interactions with some of its subunits. Plant Cell 13: 2731–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J. (2010) Recent advances in understanding the assembly and repair of photosystem II. Ann Bot (Lond) 106: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Nield J, Terao A, Stauber EJ, Hippler M, Koike H, Rochaix JD, Takahashi Y. (2009) Biochemical and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21: 2424–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ML, Hamel PP, Gabilly ST, Zegzouti H, Perea JV, Alonso JM, Ecker JR, Theg SM, Christensen SK, Merchant S. (2004) A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J Biol Chem 279: 32474–32482 [DOI] [PubMed] [Google Scholar]
- Peng L, Ma J, Chi W, Guo J, Zhu S, Lu Q, Lu C, Zhang L. (2006) LOW PSII ACCUMULATION1 is involved in efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell 18: 955–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron K, Goldschmidt-Clermont M, Rochaix JD. (1999) A factor related to pseudouridine synthases is required for chloroplast group II intron trans-splicing in Chlamydomonas reinhardtii. EMBO J 18: 6481–6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud C, Loiselay C, Wostrikoff K, Kuras R, Girard-Bascou J, Wollman FA, Choquet Y. (2007) Evidence for regulatory function of nucleus-encoded factors on mRNA stabilization and translation in the chloroplast. Proc Natl Acad Sci USA 104: 9093–9098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro EM. (1997) Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo: application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem 272: 30476–30482 [DOI] [PubMed] [Google Scholar]
- Roose JL, Pakrasi HB. (2004) Evidence that D1 processing is required for manganese binding and extrinsic protein assembly into photosystem II. J Biol Chem 279: 45417–45422 [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Small I. (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Small ID, Peeters N. (2000) The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Sun X, Ouyang M, Guo J, Ma J, Lu C, Adam Z, Zhang L. (2010) The thylakoid protease Deg1 is involved in photosystem-II assembly in Arabidopsis thaliana. Plant J 62: 240–249 [DOI] [PubMed] [Google Scholar]
- Sundberg E, Slagter JG, Fridborg I, Cleary SP, Robinson C, Coupland G. (1997) ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. Plant Cell 9: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Guo J, Ouyang M, Sun X, Ma J, Chi W, Lu C, Zhang L. (2010) LPA19, a Psb27 homolog in Arabidopsis thaliana, facilitates D1 protein precursor processing during PSII biogenesis. J Biol Chem 285: 21391–21398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman FA. (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostrikoff K, Girard-Bascou J, Wollman FA, Choquet Y. (2004) Biogenesis of PSI involves a cascade of translational autoregulation in the chloroplast of Chlamydomonas. EMBO J 23: 2696–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Scheller HV. (2004) Light-harvesting complex II binds to several small subunits of photosystem I. J Biol Chem 279: 3180–3187 [DOI] [PubMed] [Google Scholar]