Abstract
Chemokines (chemotactic cytokines) are a family of proteins associated with the trafficking and activation of leukocytes and other cell types in immune surveillance and inflammatory response. Besides their roles in the immune system, they play pleiotropic roles in tumor initiation, promotion, and progression. Chemokines can be classified into four subfamilies of chemokines, CXC, CC, C, or CX3C, based on their number and spacing of conserved cysteine residues near the N-terminus. This CXC subfamily can be further subclassified into two groups, depending on the presence or absence of a tripeptide motif glutamic acid–leucine–arginine (ELR) in the N-terminal domain. ELR-CXCL12, which binds to CXCR4 has been frequently implicated in various cancers. Over the past several years, studies have increasingly shown that the CXCR4/CXCL12 axis plays critical roles in tumor progression, such as invasion, angiogenesis, survival, homing to metastatic sites. This review focuses on involvement of CXCR4/CXCL12 interaction in neuroectodermal cancers and their therapeutic potentials. As an attractive therapeutic target of CXCR4/CXCL12 axis for cancer chemotherapy, development history and application of CXCR4 antagonists are described.
Keywords: chemokine, chemokine receptor, CXCR4, CXCL12, SDF-1
1. Introduction
Chemokines are a superfamily of small secreted cytokines that induce cytoskeletal rearrangements and directional migration of several cell types through their interaction with G-protein-coupled receptors [1-3]. They are small peptidic ligands involved in the trafficking of leukocytes and other motile cells [4, 5]. These secreted proteins act in a coordinated fashion with cell-surface proteins, including integrins, to direct the specific homing of various subsets of hematopoietic cells to specific anatomical sites [6-9]. They play a major role in regulating the migration of cells of the immune system, leading to modulation of immune responses[10, 11].
The primary role of chemokines/receptors is to regulate the recruitment and trafficking of leukocyte subsets to inflammatory sites. This action is achieved through chemoattraction by activating leukocyte integrins that bind to their adhesion receptors on endothelial cells [12, 13]. Chemokines are also involved in neuronal cell migration and patterning [14]. Chemokines bind within the extracellular domain of the chemokine receptors, which comprises the N-terminus and three extracellular loops [15]. Upon activation, their intracellular domain, which consists of three loops and the C-terminus, drives dissociation of G-protein heterotrimers into α and βγ subunits. This action leads to events such as inhibition of adenylyl cyclase activity [15, 16]. Activation of these effectors leads to functional outcomes induced by chemokine receptor signaling (Figure 1) [17-19]. Typical cellular consequences of chemokine binding include changes in gene expression, cell polarization, and chemotaxis (directed cell migration) [20]. Their exact role depends on the expression pattern of receptors on specific leukocyte subsets [5] but encompasses the regulation of lymphocyte trafficking, lymphoid tissue development, Th1/Th2 modulation and the effecting of inflammatory reactions. Chemokine receptors are also found on other cell types, and play a part in stem cell recruitment, angiogenesis, development and wound healing [20]. Recent studies suggest that many cancers express an extensive network of chemokines and chemokine receptors [21, 22]. These tumors are characterized by deregulation of chemokines and abnormal chemokine receptor expression.
Figure 1.
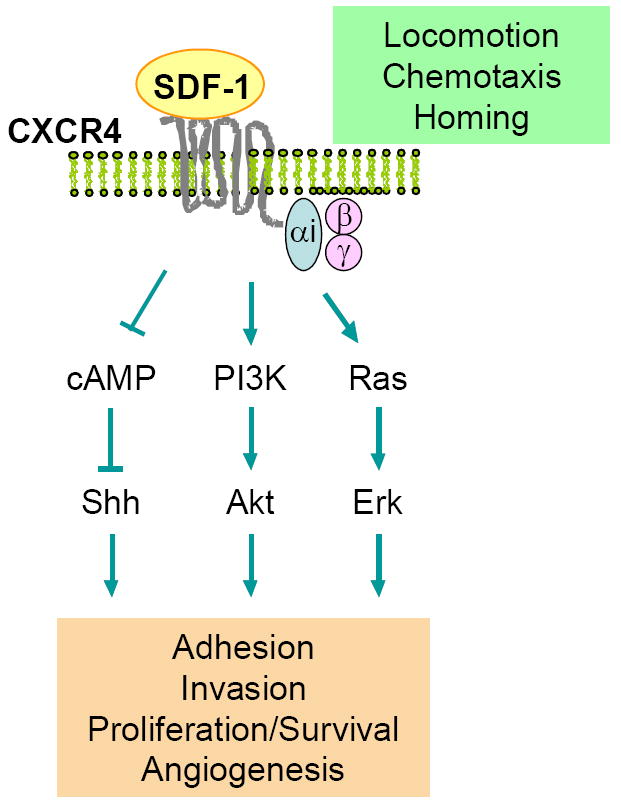
CXCR4 as an example of chemokine receptor signaling. Stromal cell derived factor-1 (SDF-1) interacts with CXCR4. Binding of SDF-1 (CXCL12) to CXCR4 activates Gαi (pertussis toxin-sensitive) signaling pathways through which reduces cAMP signaling molecules in cells, and leads to phosphatidylinositol 3-kinase (PI3K)/Akt and Ras/MAPK signaling pathways. These signaling pathways include locomotion, chemotaxis, homing, adhesion, invasion and angiogenesis in lymphocytes, macrophages, neutrophils, hematopoietic stem cells, and cancerous cells.
Interest in chemokines/chemokine receptors in the neuroectoderm has been rapidly increasing due to their involvement in a diverse range of neurological diseases. Concordantly, the volume of literature pertaining to the involvement of chemokines and chemokine receptors in neuroectoderm development has been growing rapidly in recent years [23]. During neurological diseases, the expression of chemokines can be selectively induced or upregulated in a wide range of cells, including microglia, astrocytes, neurons, and endothelial cells [12, 24]. As such, these molecules—both chemokines and chemokine receptors—represent potential therapeutic targets.
2. Classifications of chemokines and their receptors
Chemokine receptors are G protein-coupled receptors (GPCRs) with seven-transmembrane domains that are highly conserved in evolution [25-27]. By binding to their corresponding receptors, chemokines activate a series of downstream signaling pathways to guide the movement of leukocytes to target tissues or organs [28]. Directional movement of leukocytes through a concentration gradient of chemokine is defined as “chemotaxis”. Chemokines can be classified into four subfamilies of chemokines, CXC, CC, C, or CX3C, based on their number and spacing of conserved cysteine residues near the N-terminus [3, 4, 15] (Figure 2A). CXC, CC, and CX3C chemokines all have four conserved cysteines, whereas C chemokines have only two. CXC and CX3C chemokines are distinguished by the presence of one (CXC) or three (CX3C) amino acids between the first and second cysteines, whereas the first two cysteines of CC chemokines are adjacent. The nomenclature of chemokines (e.g., ‘CXCL1’) is comprised of their subclass (CXC, CC, etc.) followed by “L” for ligand, and a specific number [4, 5]. This CXC subfamily can be further subclassified into two groups, depending on the presence or absence of a tripeptide motif glutamic acid–leucine–arginine (ELR) in the N-terminal domain (Figure 2B). The ELR presence has been proposed to relate to the functional correlation of structural characteristics of CXC chemokines, such as specificity for neutrophil chemotaxis and angiogenesis [29-33]. The ELR-containing chemokines (such as IL-8, GRO, and ENA-78) have a uniform function as neutrophil chemoattractants and activators. They have also been reported to induce angiogenesis and to be chemotactic for endothelial cells. In contrast, the non-ELR-CXC chemokines (such as PF-4, IP-10, MIG, and CXCL12) show disparate activities. They are themselves nonangiogenic and are even known to possess anti-angiogenic properties. One exception is CXCL12, which induces neovascularization [33, 34]. In general, the members of each chemokine subfamily show overlapping specificities. For instance, the CXC-ELR1 chemokines are chemoattractants for neutrophils, but not for monocytes. However, CXC-ELR2 chemokines attract lymphocytes and monocytes but are poor chemoattractants for neutrophils. So far, 53 human chemokines and 23 chemokine receptors have been cloned or characterized (http://cytokine.medic.kumamoto-u.ac.jp/).
Figure 2.
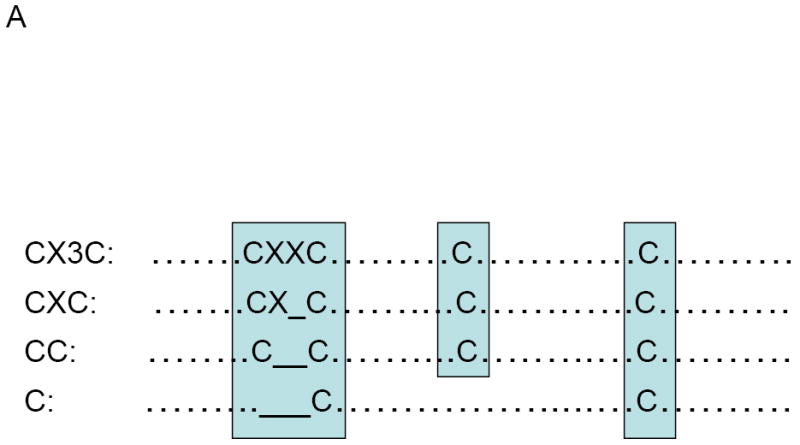
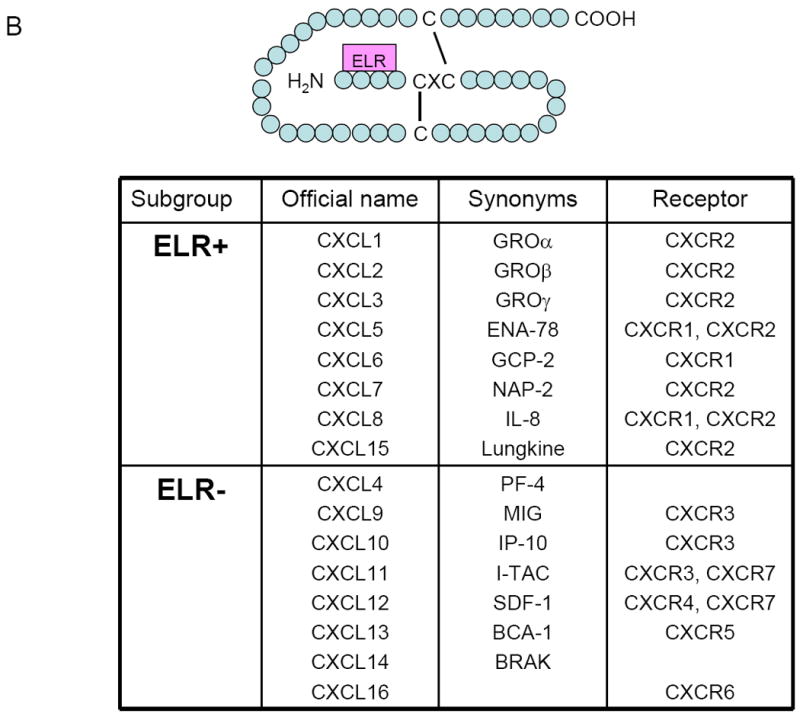
(A) Chemokines are classified into four major subfamilies according to the configuration of cysteine residues (http://cytokine.medic.kumamoto-u.ac.jp/). (B) CXC subfamily is further classified two subgroups depending on the presence or absence of the sequence motif glutamate-leucine-arginine (ELR) at the N-terminus. Individual chemokines can bind more than one chemokine receptors.
3. History of CXCR4/CXCL12 and the functions in the normal neuroectoderm
A CXCL12 cDNA clone was first isolated by Tashiro et al. from a murine bone marrow stroma cell line by the signal sequence trap method. The gene was originally named stromal cell-derived factor-1 (SDF-1) [35]. A few years later, two groups identified its receptor, an orphan GPCR called LESTR/fusin [36, 37]. LESTR/fusin was also identified as the co-receptor for human immunodeficiency virus (HIV-1) infection of CD4+ lymphocytes [38]. CXCL12 was shown to inhibited infection by T-tropic HIV of HeLa-CD4 cells [36, 37]. Because of its reactivity to CXCL12, the nomenclature was revised and LESTR/fusion was replaced by CXCR4. CXCR4/CXCL12 plays pleiotropic functions in the peripheral immune system. Bleul et al. also reported that CXCL12 is a highly efficacious chemoattractant for lymphocytes and monocytes, but not neutrophils [36]. In addition, Klein et al. reported that CXCR4/CXCL12 not only regulates the development of T and B lymphocytes but also contributes to the survival of mature lymphocytes and to the generation of memory T cells [39]. Later studies have indicated that CXCR4/CXCL12 enhances the inflammatory infiltration of neutrophils or lymphocytes in diverse models and settings involving acute inflammation or fulminant infection [40-42]. Most importantly, CXCL12 is a major regulator for the homing of hematopoietic progenitor cells to the bone marrow microenvironment [43]. Deficient development of blood cells and the heart were also described in CXCL12 knockout mice [44]. Identical phenotypes were also observed in knockout mice for its receptor, CXCR4 [45, 46], suggesting that the interaction between CXCL12 and CXCR4 may be the “key and lock” pair relationship. Only recently, a new receptor, CXCR7, was reported as an alternative non-signaling CXCL12 receptor, suggesting that the CXCR4/CXCL12 relationship is not entirely exclusive [47]. However, CXCR7, unlike CXCR4, is only expressed in limited tissues, and needs further studies to determine its role.
The interplay between CXCL12 and CXCR4 is critical to normal development. Unlike mice deficient in other chemokine/receptors, mice lacking CXCL12 or CXCR4 die in uterus or shortly after birth [4, 44-46, 48]. CXCR4/CXCL12 signaling is required during the development of the hematopoietic, cardiac, vascular, muscular, and nervous systems. Absence of CXCR4/CXCL12 axis in embryonic life leads to defects in bone marrow myeloid cell formation, cardiac function due to impaired ventricular septum formation, and developmental defects in the cerebellum and in the vasculature of the gastrointestinal tract [44-46].
The expression of CXCL12 and its receptor, CXCR4, has been described in neuronal, astroglial, and microglial cells [14, 49]. CXCL12 exerts its chemotactic action to direct organogenesis and tissue structure in the developing brain. This is demonstrated by studies of both CXCL12−/− and CXCR4−/− knockout mice, in which gene deletion resulted in significant abnormalities in cerebellar and hippocampus development such as a misplaced external granule cell layer and clusters of proliferating granule cell precursors [44, 46, 50-54]. Thus, evidence is accumulating that the CXCR4 plays a crucial role in normal development of the cerebellar cortex and in cell cycle control of neuronal precursors within the external granule cell layer.
4. Pleiotropic roles of CXCR4 in various types of cancers
CXCR4 is the most widely expressed chemokine receptor in many different cancers. The effects of CXCL12 on CXCR4-bearing tumor cells include a wide diversity of functions such as angiogenesis, invasion, locomotion, extravasation, directional migration, homing, and cell survival (see reviews: [55-59]). Among these, the function of controlling cell migration and homing, which is the rate-limiting step of multistep processes of metastasis, is unique for CXCR4/CXCL12. The precise mechanisms determining the directional migration and invasion of tumor cells into specific organs remained elusive for a long time [59, 60]. The CXCR4 chemokine receptor mediates the migration of human stem cells to marrow and possibly peripheral blood cells to lymph nodes and spleen by the binding of its ligand, CXCL12 [61-68]. The process of metastasis is similar to leukocyte and stem cell trafficking, processes which utilize the CXCR4/CXCL12 axis [69]. Cancer cells that express CXCR4 exploit the same signaling pathway leading to homing and retention in tissues with enriched CXCL12. When CXCL12 binds to CXCR4, the complex activates Gαi-protein-mediated signaling (pertussis toxin-sensitive) [70], including downstream signal pathways such as Ras/MAP Kinases and phosphatidylinositol 3-kinase (PI3K)/Akt in lymphocyte, megakaryocytes, and hematopoietic stem cells [16, 36, 71-75]. The interaction of CXCR4 and CXCL12 has been shown to induce the activation of the PI3K/Akt signaling pathway. Also, PI3K activation is in turn closely correlated with cell motility and migration. Akt plays a critical role in promoting cell survival by phosphorylating and inactivating components of the apoptotic machinary, such as BAD, caspase-9, focal adhesion kinase (FAK), and FKHRL1.
Müller et al. published a landmark study on the involvement of CXCR4 in breast cancer metastasis [76]. In samples collected from various breast cancer patients, they found that the level of expression of CXCR4 is higher in primary tumors relative to normal mammary glands or mammary epithelial cells. In contrast, CXCL12 is highly expressed in the most common destinations of breast cancer metastasis, including the lymph nodes, lung, liver, and bone marrow. The CXCR4/CXCL12 axis has been demonstrated to play critical roles in the metastasis of various types of cancers as listed in Table 1. Liang et al. demonstrated that silencing CXCR4 by RNA interference technology prevented tumorigenesis in an animal model of breast cancer metastasis [77]. Furthermore, decreasing expression levels of CXCR4 by microRNA against CXCR4 also reduced migration and invasion in vitro and lung metastases in vivo [78]. MicroRNAs have been shown to function as regulatory molecules and to play an important role in cancer progression (for reviews, see: [79, 80]). These data support the possibility that small interfering RNA or microRNA against CXCR4 can serve as an alternative means of lowering CXCR4 expression to block subsequent invasion and metastasis. Taken together, these studies confirm the necessity of CXCR4 in breast cancer metastasis and suggest a novel preventive and therapeutic strategy for cancer management.
Table 1.
Literatures on the involvement of CXCR4 in various cancers
| Cancer type | References |
|---|---|
| Breast cancer | Muller, et al. [76]; Tamamura, et al. [147]; Chen, et al. [198]; Liang, et al. [148]; Li, et al. [199]; Lee, et al. [200]; Liang, et al. [77]; Schmid, et al. [201]; Cabioglu, et al. [202]; Shim, et al. [203]; Liang, et al. [78]; Hao, et al. [92] |
| Esophageal cancer | Gockel, et al. [204]; Kaifi, et al. [205]; Koishi, et al. [206]; Sasaki, et al. [207] |
| Gastrointestinal Cancer | Ottaiano, et al. [97]; Tachibana, et al. [46]; Zeelenberg, et al. [95]; Kim, et al. [208] |
| Gynecological cancer | Kodama, et al. [209]; Jiang, et al. [210]; Pils, et al. [211]; Porcile, et al. [212] |
| Head and neck cancer | Samara, et al. [213]; Yoon, et al. [90] |
| Hepatocellular carcinoma | Begum, et al. [214]; Schimanski, et al. [215]; Shibuta, et al. [216] |
| Leukemia & Lymphoma | Barretina, et al. [217]; Burger, et al. [218]; Dao-Ung, et al. [219]; Fierro, et al. [220]; Ghobrial, et al. [221]; Voermans, et al. [62]; Ishibe, et al. [94]; Jin, et al. [222]; Juarez, et al. [223]; Konoplev, et al. [224]; Mohle, et al. [225]; Monaco, et al. [226]; Scupoli, et al. [227]; Spoo, et al. [228]; Tavor, et al. [229]; Wu, et al. [230]; Bertolini, et al. [231]; Chan, et al. [232]; Piovan, et al. [233] |
| Lung cancer | Kijima, et al. [133]; Spano, et al. [234]; Hartmann, et al. [135]; Burger, et al. [134]; Phillips, et al. [235] |
| Melanoma | Robledo, et al. [125]; Scala, et al. [126]; Murakami, et al. [127]; Takenaga, et al. [128]; Scala, et al. [129]; Longo-Imedio, et al. [130] |
| Multiple myeloma | Alsayed, et al. [236]; Moller, et al. [237] |
| Pancreatic cancer | Koshiba, et al. [151]; Mori, et al. [152]; Marchesi, et al. [238]; Hermann, et al. [104] |
| Prostate cancer | Taichman, et al. [239]; Darash-Yahana, et al. [240]; Sun, et al. [241]; Akashi, et al. [242]; Chinni, et al. [243]; Hart, et al. [244]; Mochizuki, et al. [245]; Wang, et al. [251,246] |
| Renal carcinoma | Jones, et al. [247]; Pan, et al. [248]; Reckamp, et al. [249]; Staller, et al. [250]; Struckmann, et al. [251] |
| Sarcoma | Libura, et al. [252]; Laverdiere, et al. [253]; Oda, et al. [254] |
| Thyroid cancer | Hwang, et al. [255]; De Falco, et al. [256] |
Various studies have shown significant CXCL12 concentrations in the fluid-filled cavities through which many cancers disseminate and at tissue locations in which metastases characteristically develop. Furthermore, CXCL12:CXCR4 can promote cancer dissemination indirectly by enhancing the vascular supply, since the CXCR4/CXCL12 axis may also promote tumour angiogenesis. CXCL12 influences the interaction of CD34+ hematopoietic cells with the hematopoietic microenvironment by regulating their migration and adhesion as well as the secretion of vascular endothelial growth factor (VEGF) [81-84]. On the one hand, CXCL12 has been shown to induce secretion of VEGF in lymphohematopoietic CXCR4+ cell lines [72]. In this study, the authors showed that the VEGF protein levels increased in conditioned medium of cell lines treated with CXCL12. While the other hand, VEGF increased CXCL12 expression in endothelial cells [85], and anti-CXCR4 antibody disrupted extracellular matrix-dependent endothelial cell tube formation in vitro. This morphogenic process is closely associated with CXCR4 expression. Pertussin toxin (inhibitor of Gαi) and neutralizing antibodies of CXCL12 inhibited bFGF (basic fibroblast growth factor) and VEGF-dependent neovascularization in vivo. The fact that blocking either CXCR4/CXCL12 interaction or the major G-protein of the CXCR4/CXCL12 signaling pathway (GαI) inhibits VEGF-dependent neovascularization, strongly suggests that CXCR4/CXCL12 indeed regulates VEGF-dependent angiogenesis. These results indicate that CXCR4/CXCL12 regulates VEGF-regulated autocrine signaling systems, which in turn are essential regulators of endothelial cell morphogenesis and angiogenesis [86-89]. The role of CXCR4/CXCL12 in tumor angiogenesis in vivo was demonstrated in a squamous cell carcinoma of the head and neck (SCCHN) orthotopic animal model using highly metastatic subclones generated via in vivo selection of SCCHN cells through four rounds of serial metastases [90]. They showed that anti-CXCR4 treatment suppressed primary tumor growth by inhibiting tumor angiogenesis. Liang et al. also reported that CXCR4/CXCL12 induced Akt phosphorylation, which resulted in upregulation of VEGF at both the mRNA and protein levels, because blocking the activation of Akt signaling led to a decrease in VEGF protein levels induced by CXCR4/CXCL12 axis [91]. In addition, blocking CXCR4/CXCL12 interaction with a CXCR4 antagonist suppressed tumor angiogenesis and growth in vivo. Furthermore, VEGF mRNA levels correlated well with CXCR4 mRNA levels in patient tumor samples. Thus, their study demonstrates that the CXCR4/CXCL12 signaling axis can induce angiogenesis and progression of tumors by increasing expression of VEGF through the activation of the PI3K/Akt pathway.
In colorectal cancer, CXCR4 is abundantly expressed in various colorectal carcinoma cells and elevated CXCR4 expression is associated with disease progression and reduced survival in patients [92-98].
Increasing evidence suggests that stem cells may play a crucial role in cancer progression such as tumor initiation, growth, and metastasis [99-103]. Hermann et al. reported the involvement of cancer stem cells in pancreatic cancer [104]. Pancreatic cancer has a poor prognosis of a 5-year survival rate of 1 – 4 % and a median survival of 4 – 6 months [105]. By using two biomarkers for cancer stem cells, CD133, a well-established marker for cancer stem cell for brain tumors, and CXCR4, they demonstrated that a subpopulation of CD133+CXCR4+ was responsible for metastatic process as well as resistance to standard chemotherapy. Taken together, strategies aiming CXCR4/CXCL12 axis may have important clinical application to inhibit cancer progression in numerous cancer types.
5. CXCR4 and neuroectodermal cancer
5.1. CXCR4 in neuroblastoma
Neuroblastoma is the second most common solid tumor found in children, originating from precursors derived from embryonic neural crest cells that form the peripheral sympathetic nervous system with a high potential to migrate. About one-half of children have localized tumors that can be cured with surgery alone, while the remaining children have widespread metastatic disease or quite large, aggressive, localized tumors. The latter have a poor long-term survival rate of approximately 30%. Despite advances in combined therapies, the survival rate of patients with metastatic neuroblastoma has not significantly improved over the last decade. Therefore, there is an urgent need for genetic and biologic markers for the diverse clinical phenotypes observed in neuroblastoma patients. One of the emerging biomarkers in neuroblastoma is the overexpression of the CXCR4. CXCR4 expression correlates with high-stage disease [106], and the interactions of CXCR4 by CXCL12 was shown to be necessary for the survival of several neuroblastoma cells in vitro [107]. Vasudevan et al. [108] reviewed the prognostically significant molecular biomarkers of high risk neuroblastoma and found that CXCR4 is one such marker. A higher expression of CXCR4 was found in primary neuroblastoma cells from patients with high-stage disease and in patients with bone and bone marrow metastases [106]. Disease-free survival in patients with tumors expressing high levels of CXCR4 is significantly worse than in patients with low CXCR4 tumor expression. Geminder et al. [109] investigated the expression levels of CXCR4 in various NB cell lines. Using CXCR4-expressing SH-SY5Y cells, they found that CXCL12 induces the migration of CXCR4-expressing neuroblastoma cells in CXCR4- and G protein-dependent manners. SH-SY5Y cells interacted at multiple levels with bone marrow components so that bone marrow-derived constituents promote SH-SY5Y cell migration, adhesion to bone marrow stromal cells, and proliferation. These results suggest that SH-SY5Y neuroblastoma cells are capable of homing to the bone marrow and that the ability of neuroblastoma tumors to preferentially form metastases in the bone marrow may be influenced by CXCR4/CXCL12 interactions. Moreover, Zhang et al. [110] screened chemokine/receptor profiles in different neuroblastoma cell lines and investigated the roles of CXCR4 in neuroblastoma tumor growth and progression using mouse xenograft models. They demonstrated the important role of stromal cells in neuroblastoma metastasis and a potential regulatory tumor-host mechanism for CXCR4 in neuroblastoma. Several studies further demonstrated that CXCR4 expression can be regulated positively by cytokines such as TGF-β1, VEGF, and bFGF. CXCR4 expression can also be regulated negatively by cytokines such as IL-5 and IFN-α in leukocytes, endothelial cells, and neural cells [34, 111-114]. In addition, hypoxia was shown to strongly affect the chemokine system in macrophages [115]. Overexpression of CXCR4 promoted neuroblastoma cell migration selectively toward bone marrow stromal cell conditioned medium in vitro [110]. In a mouse xenograft model, bone marrow metastasis could be achieved by CXCR4 overexpression. Furthermore, Chen et al. [116] suggested the regulation of angiogenesis by CXCR4 in neuroblastomas and that the dissemination of CXCR4-overexpressed neuroblastoma cells from primary tumors could result from increased neovasculatures [110].
5.2. CXCR4 in medulloblastoma
Medulloblastomas are the most common malignant brain tumors in childhood with a median age of onset at 9 years [117]. Medulloblastomas are histologically divided into 4 major subgroups. While classic and desmoplastic tumors account for the vast majority of cases, medulloblastomas with extensive nodularity and large cell medulloblastomas are rare [118]. All medulloblastoma subtypes are believed to be originated from neural progenitors of the cerebellum, although, the exact cellular origin remains to be elucidated in most cases.
CXCR4 is strongly expressed in proliferating granule cell precursors [39, 119]. CXCL12, which is segregated by meningeal cells of the leptomeninx, significantly enhances cell proliferation [119]. This effect is reduced by blocking the CXCR4 receptor either by AMD 3100 or pertussis toxin. This indicates coupling of neuronal CXCR4 to Gαi, which has previously been demonstrated to be expressed in granule cell precursors [120, 121]. The involvement of CXCR4 in medulloblastomas was first reported by Rubin et al. [120], who used a desmoplastic medulloblastoma cell line for both in vitro experiments and in vivo tumor models. Their study has shown that CXCR4 mRNA and protein are expressed at high levels in brain tumors of both neuronal and astrocytic lineage. The ligand CXCL12 is expressed in tumor-associated blood vessels and/or tumor cells, suggesting a paracrine relationship for CXCR4 activation in vivo. In vitro, CXCL12 exerts proliferative, antiapoptotic, and chemotactic effects on medulloblastoma cell lines.
Schuller et al. [122] examined the expression pattern of the CXCR4 receptor in 90 cases of tumor specimens, including classic medulloblastoma, desmoplastic medulloblastoma, and medulloblastoma with extensive nodularity. In this study, they found that a small subset of medulloblastomas carry mutations in the gene encoding the CXCR4. While overexpression of CXCR4 is dominant in most cases of cancers, CXCR4 mutation is rarely reported. However, Schuller et al. described 2 cases of medulloblastomas carrying mutations in the CXCR4 gene [122]. The A157C mutation is located in the first transmembrane section and the C414T mutation is located in the second transmembrane region, a part of the receptor which is relatively close to the cell surface and possibly important for binding of its ligand [123]. They speculated that mutations within the first and second transmembrane regions might contribute to pathologic receptor activity or to resistance to inhibitors such as AMD 3100. Moreover, strong expression of CXCR4 mRNA was demonstrated in medulloblastomas that likely derive from the cerebellar external granule cell layer. These data suggest that CXCR4 may be responsible for the development of specific medulloblastoma subtypes. Furthermore, expression of CXCR4 could be an improved detection means of tumors derived from the cerebellar external granule cell layer over classical histology and silver staining.
CXCL12 could also contribute to the pattern of medulloblastoma spread. Medulloblastoma is distinctively different from other brain tumors because it often metastasizes to bone and liver tissues in which CXCL12 are enriched in their stromas. Therefore, CXCR4 may play a critical role in a small subtype of medulloblastomas for their growth and metastasis.
5.3. CXCR4 in other neuroectodermal tumors
Melanoma preferentially metastasizes to the lung, liver and brain, that are sites of breast cancer metastasis[124]. High expression of CXCR4, CCR7 and CCR10 was observed in malignant melanoma cell lines and melanoma cells as compared with normal primary melanocytes[76,125,126]. Transduction of B16 melanoma cells with CXCR4 increased pulmonary metastasis by i.v. and s.c. inoculation of tumor cells[127], indicating that organ-specific metastasis of melanoma is mediated by CXCR4. The increase of pulmonary metastasis was supresses by treatment with CXCR4 antagonists [127,128]. It was demonstrated by two independent groups that primary melanoma expressing CXCR4 is related to a higher incidence of metastases and a highly motality rate[129,130], suggesting CXCR4 could be a useful biomarker for predicting metastatic melanoma in patients as well as the therapeutic target in melanoma.
Small cell lung cancer (SCLC) is also one of the aggressive and rapidly metastasizing neoplasms. Due to the widespread metastasis and resistance against chemotherapeutic drugs, two-year survival rate of patients is extremely low[131,132]. SCLC cell lines and primary tumor sample from SCLC patients express CXCR4 on the cell surface[133,134]. Kijima et al. reported that functional expression of CXCR4 receptor is involved in the pathogenesis of SCLC in vivo [133]. CXCL12 increased cell motility, adhesion, and formation of filopodia and neurite-like projections of CXCR4-expressing SCLC cells. CXCL12-induced integrin activation mediates adhesion of SCLC cells to extracellular matrix such as VCAM-1, fibronectin and collagen [134,135]. This is mediated by co-operation of CXCR4 and integrin signaling. Increased adhesion of SCLC cells to stromal cells protects from the chemotherapy-induced apoptosis to confer chemoresistance. Accordingly, CXCR4 antagonists would be useful to overcome CXCL12-mediated adhesion survival signals in the microenvironment of SCLC.
6. Potential therapeutics targeting CXCR4
6.1. Peptide-based CXCR4 antagonists
CXCR4 is a major co-receptor for T-cell line-tropic (T-tropic) HIV infection and, thus, numerous compounds targeting CXCR4 have been developed and reported as HIV entry inhibitors in recent years. T22 is the first potent anti-HIV peptide, which was designed from horseshoe crab-derived anti-microbial peptide, polyphemusin II [136]. Although the molecular target of T22 had not been originally identified, Murakami et al reported the anti-HIV activity was derived from the inhibition of CXCR4 [137], which was reported to be a second receptor for HIV-1 infection [36, 37]. T22 specifically inhibits T-tropic HIV-1 infection, but not macrophage-tropic strain. ALX40-4C is also a polycationic anti-HIV peptide, which prevents the viral binding on CXCR4 [138]. Tamamura et al reported a landmark development of a specific CXCR4 inhibitor, T140 [139] through structure-activity relationship study [140] and down-sizing study [141, 142] of T22. T140 is a 14-residue peptide that possessed high levels of anti-HIV activity and antagonism of T cell line-tropic HIV-1 entry as compared to other antagonists of CXCR4 that existed in 1998. This peptide exerts inverse agonistic activity, while AMD3100 is a weak partial agonist [143]. The compound was further improved by amidating the C-terminus of T140 and reducing the total number of positive charges by substituting basic residues with non-basic, polar amino acids to generate TN14003, which is less cytotoxic and more stable in serum compared to T140 [144]. The concentration of TN14003 required for 50% protection of HIV-induced cytopathogenicity in MT-4 cells is 0.6 nM in contrast to 410 μM leading to 50% toxicity. These results reflect an excellent therapeutic index for TN14003 (Safety index, SITN14003=680,000) as well as a compelling therapeutic opportunity for a new inhibitor of CXCR4. Further optimization and N-terminal acylation of TN14003 led to development of the biostable analogue 4F-benzoyl-TN14003 (TF14016), which possess highly potent anti-HIV activity as well as CXCR4 antagonistic activity [145, 146]. Two groups independently evaluated the efficacy of TN14003 in inhibiting metastasis in an animal model [147, 148]. They confirmed that blocking CXCR4 was effective in limiting metastasis of breast cancer. Numerous investigators have used T140 analogs as proof of principal to study the effect of CXCR4 blocade [128, 149-152]. Several labeled T140 derivatives including biotin, fluorescent chromophores [153, 154] and radioisotope [155] have also been developed, which would be applicable to metastatic cancer diagnosis. T140 analogs provide a compelling efficacy benchmark with which to compare future generations of drug candidates.
A new class of peptides and peptidomimetics has been reported that was converted from these T140 analogs with better phamaceutical properties than peptides. Fujii et al. reported that cyclic pentapeptide FC131 (Figure 3, 1) exerts anti-HIV activity as well as CXCR4 antagonist activity equipotent to T140 [156]. Using orthogonal peptide library strategy, pharmacophore residues in the close proximity, which were identified by a structure-activity relathionship study [157, 158] and conformational analysis [159] of T140, are embedded on the cyclic peptide template. It is noteworthy that even one-third molecular-size reduction of T140 could reproduce both CXCL12-binding inhibition to CXCR4 as well as CXCR4-mediated HIV-1 infection. FC131 analogues having retro-inverso sequence [160] or N-methyl amino acid [161] are also potent CXCR4 antagonists. Design of pseudopeptides containing peptide backbone mimetics of FC131 was attempted; however, most of the compounds showed similar or less potent bioactivity compared with FC131 [162-165]. Recently, DeMarco has reported a novel CXCR4 antagonist, POL3026 [166]. POL3026 was designed by head-to-tail cyclization of TC14003 [158] based on the β-hairpin protein epitope mimetic (PEM) design concept, posseting high potency and favorable pharmacokinetic properties [167].
Figure 3.
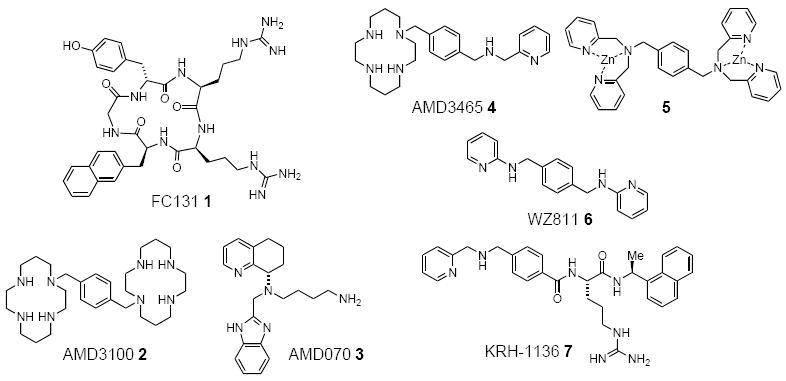
Structures of CXCR4 antagonists.
6.2. Small molecule CXCR4 antagonists
The poor ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of peptide inhibitors typically limit their clinical use due to low bioavailability. To mimic a peptide with a small non-peptide drug remains a major goal of medicinal chemistry programs. To date, a modest number of reports have appeared in the literature describing efforts to develop selective, non-peptidic, small molecule antagonists of CXCR4.
The cyclams and bicyclams are a class of non-peptide molecules that have been shown to demonstrate antagonist activity against the CXCR4 receptor, and these have dominated literature research publications [168]. The most notable compound from this class is AMD3100 (Figure 3, 2), which has a partial agonistic activity [169]. It is a symmetrical molecule that contains two cyclam subunits linked by an aromatic tether and shows a high degree of specificity for CXCR4 as opposed to other chemokine receptors [170]. It exhibits inhibitory activity against several strains of T-tropic HIV, but suffers poor oral bioavailability and must therefore be administered via intravenous or subcutaneous injection [170-172]. AMD3100 was advanced as far as Phase II clinical evaluation, where it demonstrated early evidence of anti-viral activity [170-172]; however, AMD3100 did not reduce viral load in HIV patients, while causing thrombocytopenia in one patient, premature ventricular contractions in two patients, and paresthesias in several patients [173, 174]. The cyclams (a structural class represented by AMD3100) are a well-known metal-chelating moiety and it is reasonable to presume that chelated metals may be playing a key role in the cardiac side effects that were observed with AMD3100 [175]. This presumption is reflected in the exclusion criteria outlined for a second generation tetrahydroquinoline-based compound, AMD070 (Figure 3, 3), which has recently entered Phase I clinical evaluation as an anti-HIV agent [176].
Systemic administration of AMD3100 decreased the growth of GBM and medulloblastoma in an orthotopic animal model [177]. AMD 3100 treatment reduced activation of extracellular signal-regulated kinases 1 and 2 (Erk 1/2) as well as Akt and also increased rates of apoptosis in both tumor types. Their studies suggest CXCR4 signaling is a critical component of brain tumor biology. Based on the abundant implication of CXCL12-CXCR4 axis in many physiological processes as well as the experience of AMD3100 in its HIV trial, safer drugs without potential side-effects are desired for the treatment of brain tumors. Currently, AMD3100 is being tested in the clinic for an indication of stem cell mobilization, which would only require a one time administration of the drug.
Several nonpeptide CXCR4 antagonists, having two aromatic amine moieties connected by a para-xylenediamine group, were reported. AMD3465 (Figure 3, 4) is a monomacrocyclic CXCR4 antagonist, in which one cyclam unit of AMD3100 has been replaced with an N-picolyl group [178]. More effective inhibition of CXCL12-induced chemotaxis by AMD3465 as compared with AMD3100 indicates that eight basic amino groups are not needed for the CXCR4 antagonism. Bis(dipicolylamine)-p-xylene-Zn complex (Figure 3, 5), which was originally designed as a molecular probe to recognize phospholylated peptide sequences [179], showed potent CXCR4 antagonistic activity [180]. A stable complex of the dipicolylamine moieties with zinc ion maintains the active structure. A recent note-worthy report on small molecule CXCR4 antagonists including WZ811 (Figure 3, 6) conducted by Zhan et al. [154]. They designed a series of small molecules based on the structure-activity profile of AMD3100 vs. CXCR4 published by Trent et al. [169] and identified a lead via an affinity binding assay using TN14003 (a peptide–based CXCR4 antagonist) and subsequent functional assays using CXCL12 (cAMP and Matrigel invasion assays).
A series of small molecules containing pharmacophores of T140 and FC131 has been reported. Kureha has developed KRH1636 (Figure 3, 7) based on the N-terminal fragment of T140 [181]. KRH1636 does not contain a cyclam subunit, thereby avoiding the potential liability of metal ion encapsulation. From tripeptide library bearing three pharmacophores of the N-terminal region of 4F-benzoyl-TN14003 and 4F-benzoyl-TE14011, several potent CXCR4 antagonists have been identified [182]. Small molecules containing 3,6-dihydropyridin-2-one [183] and indole scaffolds [184] has been designed by scaffold-based approach using FC131 pharmacophores, which are expected to be lead molecules for structure-activity relationship study.
6.3. Miscellaneous
Chemokine fragment peptides reproduce the binding affinity to chemokine receptors as ligands and/or inhibitors. Crump et al. demonstrated the substitution of Pro2 of CXCL12 with Gly resulted in complete loss of receptor activation ability of CXCR4 with slightly weaker binding affinity with CXCR4 [185]. Loetscher et al. reported that N-terminal 9 residues of CXCL12 (1-9), and the dimeric peptide induce chemotactic and calcium responses at high concentration, while the P2G analogues show antagonistic activity for CXCR4 [186]. CTCE-9908 is a 17-mer peptide CXCR4 antagonist, which is a dimeric analogue of CXCL12 (1-8) having P2G substitution [187]. Treatment with CTCT-9908 decreased migration, invasion and the growth rate of osteosarcoma in vitro, and reduced the development of pulmonary metastases of osteosarcoma and melanoma cells in vivo [188]. CTCE-0214, a 31-mer analogue of CXCL12, in which the intervening sequence of CXCL12 is deleted and α-helix inducible cyclic structure is introduced, reproduces intracellular calcium mobilization induced by CXCL12 [189]. This agonistic peptide mobilizes human colony-forming cells (CFC) to spleen and peripheral blood [190], and synergizes with other cytokines on survival of stem and progenitor cells [191].
vMIP-II is a viral chemokine encoded by Kaposi’s sarcoma–associated herpesvirus. vMIP-II binds CXCR4 with high affinity, and inhibits the calcium mobilization elicited by CXCL12 as well as HIV-1 infection [192]. The full length of vMIP-II is recognized both by CXCR4 and CCR5, N-terminal 21-mer fragment, vMIP-II (1-21), displays the antagonistic activity against CXCR4 [193]. Dimerized peptide of vMIP-II N-terminal 11-residue, named vMIP-II (1-11) dimer, is also a weak antagonist for CXCR4 [194]. Dong et al. reported that synthetically and modularly modified chemokine, in which N-terminal 8 residues of CXCL12 is replaced with all-D-isomer of N-terminal 10-mer fragment of vMIP-II, does not activate CXCR4, but is effective to block HIV-1 infection [195, 196].
7. Concluding remarks
Vast evidence indicates that cancer cells express the CXCR4 chemokine receptor and that its interaction with CXCL12 is crucial for tumor proliferation, migration, and angiogenesis. CXCR4/CXCL12 plays a central role in Paget’s famous hypothesis of “seed-and-soil” [197], and thus modulating this CXCR4/CXCL12 axis may provide an alternative target for cancer therapy by blocking cancer progression. In addition, CXCR4 expression profiles can be utilized to determine different stages of malignancy in various cancers. This may lead to alternative prognostic markers for cancers and a strategy to enhance both diagnostic and therapeutic strategies. Numerous research articles validate the possibility of targeting CXCR4/CXCL12 interaction in tumor progression as not only a therapeutic approach, but also a chemopreventive strategy, blocking the cancer progression or malignant transformation.
Acknowledgments
This study was financially supported by a Research Grant from NIH NCI (1R01CA109366. H.S.), a Grant-in-Aid for Scientific Research and Molecular Imaging Research Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (S.O. and N.F.). The authors thank Dr. Joann B. Powell and Mr. Eric Armstrong (Emory University), and Ms. Tomoko Kurose (Kyoto University) for proof-reading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 3.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 4.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 5.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 6.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 7.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin- homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–5. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor- 2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–70. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 9.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 10.Krieg C, Boyman O. The role of chemokines in cancer immune surveillance by the adaptive immune system. Semin Cancer Biol. doi: 10.1016/j.semcancer.2008.10.011. this issue. [DOI] [PubMed] [Google Scholar]
- 11.Navarini-Meury AA, Conrad C. Melanoma and innate immunity - active inflammation or just erroneous attraction? (Melanoma as the source of leukocyte-attracting chemokines) Semin Cancer Biol. doi: 10.1016/j.semcancer.2008.10.012. this issue. [DOI] [PubMed] [Google Scholar]
- 12.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 13.Laudanna C, Alon R. Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb Haemost. 2006;95:5–11. [PubMed] [Google Scholar]
- 14.Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 15.Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 16.Rubin J. Chemokine Signaling in Cancer: One Hump or Two? Semin Cancer Biol. doi: 10.1016/j.semcancer.2008.10.001. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–8. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 19.Sozzani S, Molino M, Locati M, Luini W, Cerletti C, Vecchi A, et al. Receptor-activated calcium influx in human monocytes exposed to monocyte chemotactic protein-1 and related cytokines. J Immunol. 1993;150:1544–53. [PubMed] [Google Scholar]
- 20.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 21.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 22.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–54. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 23.Civenni G, Sommer L. Development Chemokines in neuroectodermal development and cancer stem cells. Semin Cancer Biol. doi: 10.1016/j.semcancer.2008.11.003. this issue. [DOI] [PubMed] [Google Scholar]
- 24.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 26.Kawasawa Y, McKenzie LM, Hill DP, Bono H, Yanagisawa M. G protein-coupled receptor genes in the FANTOM2 database. Genome Res. 2003;13:1466–77. doi: 10.1101/gr.1087603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–15. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 28.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 29.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 30.Hebert CA, Vitangcol RV, Baker JB. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991;266:18989–94. [PubMed] [Google Scholar]
- 31.Moore BB, Keane MP, Addison CL, Arenberg DA, Strieter RM. CXC chemokine modulation of angiogenesis: the importance of balance between angiogenic and angiostatic members of the family. J Investig Med. 1998;46:113–20. [PubMed] [Google Scholar]
- 32.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Ransohoff RM. The roles of chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin Cancer Biol. 2009;19:111–115. doi: 10.1016/j.semcancer.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1α. Am J Pathol. 1999;154:1125–35. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–3. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 36.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 37.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 39.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, et al. SDF-1α induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–81. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 40.Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, et al. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–74. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- 41.Ding Z, Jia SH, Marshall JC, Downey GP, Waddell TK. Up-regulation of functional CXCR4 expression on human lymphocytes in sepsis. Crit Care Med. 2006;34:3011–7. doi: 10.1097/01.CCM.0000247719.37793.43. [DOI] [PubMed] [Google Scholar]
- 42.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 43.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 44.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–9. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 46.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 47.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, et al. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, et al. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249:163–6. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- 50.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–60. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 52.Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–20. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006 doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 56.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 57.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–56. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 59.Raffaghello L, Cocco C, Corrias MV, Airoldi I, Pistoia V. Chemokines in neuroectodermal tumour progression and metastasis. Semin Cancer Biol. doi: 10.1016/j.semcancer.2008.10.003. this issue. [DOI] [PubMed] [Google Scholar]
- 60.Nicolson GL. Paracrine/autocrine growth mechanisms in tumor metastasis. Oncol Res. 1992;4:389–99. [PubMed] [Google Scholar]
- 61.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voermans C, van Heese WP, de Jong I, Gerritsen WR, van Der Schoot CE. Migratory behavior of leukemic cells from acute myeloid leukemia patients. Leukemia. 2002;16:650–7. doi: 10.1038/sj.leu.2402431. [DOI] [PubMed] [Google Scholar]
- 63.Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–9. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- 64.Spencer A, Jackson J, Baulch-Brown C. Enumeration of bone marrow ‘homing’ haemopoietic stem cells from G-CSF- mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transplant. 2001;28:1019–22. doi: 10.1038/sj.bmt.1703289. [DOI] [PubMed] [Google Scholar]
- 65.Vainchenker W. Hematopoietic stem cells. Therapie. 2001;56:379–81. [PubMed] [Google Scholar]
- 66.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 67.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 68.Blades MC, Manzo A, Ingegnoli F, Taylor PR, Panayi GS, Irjala H, et al. Stromal cell-derived factor 1 (CXCL12) induces human cell migration into human lymph nodes transplanted into SCID mice. J Immunol. 2002;168:4308–17. doi: 10.4049/jimmunol.168.9.4308. [DOI] [PubMed] [Google Scholar]
- 69.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 70.Chen WJ, Jayawickreme C, Watson C, Wolfe L, Holmes W, Ferris R, et al. Recombinant human CXC-chemokine receptor-4 in melanophores are linked to Gi protein: seven transmembrane coreceptors for human immunodeficiency virus entry into cells. Mol Pharmacol. 1998;53:177–81. doi: 10.1124/mol.53.2.177. [DOI] [PubMed] [Google Scholar]
- 71.Deng HK, Unutmaz D, KewalRamani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 72.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, et al. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–66. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 73.Majka M, Ratajczak J, Baj-Krzyworzek M, Kijowski J, Reca R, Machalinski B, et al. Biological significance of chemokine receptor expression by normal human megakaryoblasts. Folia Histochem Cytobiol. 2001;39:235–44. [PubMed] [Google Scholar]
- 74.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol. 1999;163:5954–63. [PubMed] [Google Scholar]
- 75.Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–54. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 76.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 77.Liang Z, Yoon Y, Votaw J, Goodman M, William L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–71. [PMC free article] [PubMed] [Google Scholar]
- 78.Liang Z, Wu H, Reddy S, Zhu A, Wang S, Blevins D, et al. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363:542–6. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Gartel AL, Kandel ES. miRNAs: Little known mediators of oncogenesis. Semin Cancer Biol. 2008;18:103–10. doi: 10.1016/j.semcancer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Ma L, Weinberg RA. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–2. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- 81.Majka M, Janowska-Wieczorek A, Ratajczak J, Kowalska MA, Vilaire G, Pan ZK, et al. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96:4142–51. [PubMed] [Google Scholar]
- 82.Majka M, Ratajczak J, Kowalska MA, Ratajczak MZ. Binding of stromal derived factor-1α (SDF-1α) to CXCR4 chemokine receptor in normal human megakaryoblasts but not in platelets induces phosphorylation of mitogen-activated protein kinase p42/44 (MAPK), ELK-1 transcription factor and serine/threonine kinase AKT. Eur J Haematol. 2000;64:164–72. doi: 10.1034/j.1600-0609.2000.90112.x. [DOI] [PubMed] [Google Scholar]
- 83.Majka M, Ratajczak J, Lee B, Honczarenko M, Douglas R, Kowalska MA, et al. The role of HIV-related chemokine receptors and chemokines in human erythropoiesis in vitro. Stem Cells. 2000;18:128–38. doi: 10.1634/stemcells.18-2-128. [DOI] [PubMed] [Google Scholar]
- 84.Richard CL, Tan EY, Blay J. Adenosine upregulates CXCR4 and enhances the proliferative and migratory responses of human carcinoma cells to CXCL12/SDF-1α. Int J Cancer. 2006;119:2044–53. doi: 10.1002/ijc.22084. [DOI] [PubMed] [Google Scholar]
- 85.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–11. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 86.Gontero P, Banisadr S, Frea B, Brausi M. Metastasis markers in bladder cancer: a review of the literature and clinical considerations. Eur Urol. 2004;46:296–311. doi: 10.1016/j.eururo.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Kortylewski M, Jove R, Yu H, Ribatti D, Marimpietri D, Pastorino F, et al. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–27. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 88.Ribatti D, Marimpietri D, Pastorino F, Brignole C, Nico B, Vacca A, et al. Angiogenesis in neuroblastoma. Ann N Y Acad Sci. 2004;1028:133–42. doi: 10.1196/annals.1322.014. [DOI] [PubMed] [Google Scholar]
- 89.Turner HE, Harris AL, Melmed S, Wass JA. Angiogenesis in endocrine tumors. Endocr Rev. 2003;24:600–32. doi: 10.1210/er.2002-0008. [DOI] [PubMed] [Google Scholar]
- 90.Yoon Y, Liang Z, Zhang X, Choe M, Cho HT, Shin DM, et al. CXCR4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–24. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 91.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–22. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin M, et al. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett. 2007;253:34–42. doi: 10.1016/j.canlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Alix-Panabieres C, Brouillet JP, Fabbro M, Yssel H, Rousset T, Maudelonde T, et al. Characterization and enumeration of cells secreting tumor markers in the peripheral blood of breast cancer patients. J Immunol Methods. 2005;299:177–88. doi: 10.1016/j.jim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Ishibe N, Albitar M, Jilani IB, Goldin LR, Marti GE, Caporaso NE. CXCR4 expression is associated with survival in familial chronic lymphocytic leukemia, but CD38 expression is not. Blood. 2002;100:1100–1. doi: 10.1182/blood-2002-03-0938. [DOI] [PubMed] [Google Scholar]
- 95.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. [PubMed] [Google Scholar]
- 96.Fukunaga S, Maeda K, Noda E, Inoue T, Wada K, Hirakawa K. Association between expression of vascular endothelial growth factor C, chemokine receptor CXCR4 and lymph node metastasis in colorectal cancer. Oncology. 2006;71:204–11. doi: 10.1159/000106070. [DOI] [PubMed] [Google Scholar]
- 97.Ottaiano A, di Palma A, Napolitano M, Pisano C, Pignata S, Tatangelo F, et al. Inhibitory effects of anti-CXCR4 antibodies on human colon cancer cells. Cancer Immunol Immunother. 2005;54:781–91. doi: 10.1007/s00262-004-0636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–50. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 99.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 101.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 102.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 103.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 104.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 105.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 106.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:1506–11. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 107.Meier R, Muhlethaler-Mottet A, Flahaut M, Coulon A, Fusco C, Louache F, et al. The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS ONE. 2007;2:e1016. doi: 10.1371/journal.pone.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vasudevan SA, Nuchtern JG, Shohet JM. Gene profiling of high risk neuroblastoma. World J Surg. 2005;29:317–24. doi: 10.1007/s00268-004-7820-7. [DOI] [PubMed] [Google Scholar]
- 109.Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–57. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L, Yeger H, Das B, Irwin MS, Baruchel S. Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9:36–46. doi: 10.1593/neo.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franitza S, Kollet O, Brill A, Vaday GG, Petit I, Lapidot T, et al. TGF-β1 enhances SDF-1α-induced chemotaxis and homing of naive T cells by up-regulating CXCR4 expression and downstream cytoskeletal effector molecules. Eur J Immunol. 2002;32:193–202. doi: 10.1002/1521-4141(200201)32:1<193::AID-IMMU193>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 112.Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, et al. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol. 2001;70:113–20. [PubMed] [Google Scholar]
- 113.Rostasy K, Gorgun G, Kleyner Y, Garcia A, Kramer M, Melanson SM, et al. Tumor necrosis factor α leads to increased cell surface expression of CXCR4 in SK-N-MC cells. J Neurovirol. 2005;11:247–55. doi: 10.1080/13550280590952763. [DOI] [PubMed] [Google Scholar]
- 114.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shirazi Y, Pitha PM. Interferon downregulates CXCR4 (fusin) gene expression in peripheral blood mononuclear cells. J Hum Virol. 1998;1:69–76. [PubMed] [Google Scholar]
- 116.Chen GS, Yu HS, Lan CC, Chow KC, Lin TY, Kok LF, et al. CXC chemokine receptor CXCR4 expression enhances tumorigenesis and angiogenesis of basal cell carcinoma. Br J Dermatol. 2006;154:910–8. doi: 10.1111/j.1365-2133.2006.07150.x. [DOI] [PubMed] [Google Scholar]
- 117.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17:2127–36. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 118.Giangasparo F, Bigner SH, Kleinhues PK, Pietsch T, Trojanowsk JQ. Pathology and genetics of tumors of the nervous system. In: Kleihues P, Cavenee WK, editors. Medulloblastoma. Lyon: IARC; 2000. pp. 129–37. [Google Scholar]
- 119.Zhao Q, Kho A, Kenney AM, Yuk Di DI, Kohane I, Rowitch DH. Identification of genes expressed with temporal-spatial restriction to developing cerebellar neuron precursors by a functional genomic approach. Proc Natl Acad Sci U S A. 2002;99:5704–9. doi: 10.1073/pnas.082092399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schuller U, Lamp EC, Schilling K. Developmental expression of heterotrimeric G-proteins in the murine cerebellar cortex. Histochem Cell Biol. 2001;116:149–59. doi: 10.1007/s004180100303. [DOI] [PubMed] [Google Scholar]
- 122.Schuller U, Koch A, Hartmann W, Garre ML, Goodyer CG, Cama A, et al. Subtype-specific expression and genetic alterations of the chemokinereceptor gene CXCR4 in medulloblastomas. Int J Cancer. 2005;117:82–9. doi: 10.1002/ijc.21116. [DOI] [PubMed] [Google Scholar]
- 123.Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275:23736–44. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- 124.Cohn-Cedermark G, Måsson-Brahme E, Rutqvist LE, Larsson O, Singnomklao T, Ringborg U. Metastatic patterns, clinical outcome, and malignant phenotype in malignant cutaneous melanoma. Acta Oncol. 1999;38:549–57. doi: 10.1080/028418699431122. [DOI] [PubMed] [Google Scholar]
- 125.Robledo MM, Bartolome RA, Longo N, Rodríguez-Frade JM, Mellado M, Longo I, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 126.Scala S, Giuliano P, Ascierto PA, Ieranò C, Franco R, Napolitano M, et al. Human melanoma metastases express functional CXCR4. Clin Cancer Res. 2006;12:2427–33. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- 127.Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–34. [PubMed] [Google Scholar]
- 128.Takenaga M, Tamamura H, Hiramatsu K, Nakamura N, Yamaguchi Y, Kitagawa A, et al. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem Biophys Res Commun. 2004;320:226–32. doi: 10.1016/j.bbrc.2004.05.155. [DOI] [PubMed] [Google Scholar]
- 129.Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–41. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 130.Longo-Imedio MI, Longo N, Treviño I, Lázaro P, Sánchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–5. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- 131.Ihde DC. Chemotherapy of lung cancer. N Engl J Med. 1992;327(20):1434–41. doi: 10.1056/NEJM199211123272006. [DOI] [PubMed] [Google Scholar]
- 132.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–85. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 133.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–11. [PubMed] [Google Scholar]
- 134.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 135.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 136.Nakashima H, Masuda M, Murakami T, Koyanagi Y, Matsumoto A, Fujii N, et al. Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob Agents Chemother. 1992;36:1249–55. doi: 10.1128/aac.36.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, et al. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–93. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Doranz BJ, Grovit-Ferbas K, Sharron MP, Mao SH, Goetz MB, Daar ES, et al. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J Exp Med. 1997;186:1395–400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tamamura H, Xu Y, Hattori T, Zhang X, Arakaki R, Kanbara K, et al. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem Biophys Res Commun. 1998;253:877–82. doi: 10.1006/bbrc.1998.9871. [DOI] [PubMed] [Google Scholar]
- 140.Tamamura H, Imai M, Ishihara T, Masuda M, Funakoshi H, Oyake H, et al. Pharmacophore identification of a chemokine receptor (CXCR4) antagonist, T22 ([Tyr(5,12),Lys7]-polyphemusin II), which specifically blocks T cell-line-tropic HIV-1 infection. Bioorg Med Chem. 1998;6:1033–41. doi: 10.1016/s0968-0896(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 141.Tamamura H, Arakaki R, Funakoshi H, Imai M, Otaka A, Ibuka T, et al. Effective lowly cytotoxic analogs of an HIV-cell fusion inhibitor, T22 ([Tyr5,12, Lys7]-polyphemusin II) Bioorg Med Chem. 1998;6:231–8. doi: 10.1016/s0968-0896(97)10037-2. [DOI] [PubMed] [Google Scholar]
- 142.Tamamura H, Waki M, Imai M, Otaka A, Ibuka T, Waki K, et al. Downsizing of an HIV-cell fusion inhibitor, T22 ([Tyr5,12, Lys7]-polyphemusin II), with the maintenance of anti-HIV activity and solution structure. Bioorg Med Chem. 1998;6:473–9. doi: 10.1016/s0968-0896(97)10055-4. [DOI] [PubMed] [Google Scholar]
- 143.Zhang H, Issekutz AC. Down-modulation of monocyte transendothelial migration and endothelial adhesion molecule expression by fibroblast growth factor: reversal by the anti-angiogenic agent SU6668. Am J Pathol. 2002;160:2219–30. doi: 10.1016/S0002-9440(10)61169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tamamura H, Omagari A, Hiramatsu K, Gotoh K, Kanamoto T, Xu Y, et al. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett. 2001;11:1897–902. doi: 10.1016/s0960-894x(01)00323-7. [DOI] [PubMed] [Google Scholar]
- 145.Tamamura H, Hiramatsu K, Kusano S, Terakubo S, Yamamoto N, Trent JO, et al. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org Biomol Chem. 2003;1:3656–62. doi: 10.1039/b306473p. [DOI] [PubMed] [Google Scholar]
- 146.Tamamura H, Hiramatsu K, Mizumoto M, Ueda S, Kusano S, Terakubo S, et al. Enhancement of the T140-based pharmacophores leads to the development of more potent and bio-stable CXCR4 antagonists. Org Biomol Chem. 2003;1:3663–9. doi: 10.1039/b306613b. [DOI] [PubMed] [Google Scholar]
- 147.Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, et al. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003;550:79–83. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]
- 148.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 149.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 150.Driessen WH, Fujii N, Tamamura H, Sullivan SM. Development of peptide-targeted lipoplexes to CXCR4-expressing rat glioma cells and rat proliferating endothelial cells. Mol Ther. 2008;16:516–24. doi: 10.1038/sj.mt.6300388. [DOI] [PubMed] [Google Scholar]
- 151.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–5. [PubMed] [Google Scholar]
- 152.Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 153.Oishi S, Masuda R, Evans B, Ueda S, Goto Y, Ohno H, et al. Synthesis and application of fluorescein- and biotin-labeled molecular probes for the chemokine receptor CXCR4. Chembiochem. 2008;9:1154–8. doi: 10.1002/cbic.200700761. [DOI] [PubMed] [Google Scholar]
- 154.Zhan W, Liang Z, Zhu A, Kurtkaya S, Shim H, Snyder JP, et al. Discovery of small molecule CXCR4 antagonists. J Med Chem. 2007;50:5655–64. doi: 10.1021/jm070679i. [DOI] [PubMed] [Google Scholar]
- 155.Hanaoka H, Mukai T, Tamamura H, Mori T, Ishino S, Ogawa K, et al. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006;33:489–94. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 156.Fujii N, Oishi S, Hiramatsu K, Araki T, Ueda S, Tamamura H, et al. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation- and sequence-based libraries. Angew Chem Int Ed Engl. 2003;42:3251–3. doi: 10.1002/anie.200351024. [DOI] [PubMed] [Google Scholar]
- 157.Tamamura H, Omagari A, Hiramatsu K, Oishi S, Habashita H, Kanamoto T, et al. Certification of the critical importance of L-3-(2-naphthyl)alanine at position 3 of a specific CXCR4 inhibitor, T140, leads to an exploratory performance of its downsizing study. Bioorg Med Chem. 2002;10:1417–26. doi: 10.1016/s0968-0896(01)00419-9. [DOI] [PubMed] [Google Scholar]
- 158.Tamamura H, Omagari A, Oishi S, Kanamoto T, Yamamoto N, Peiper SC, et al. Pharmacophore identification of a specific CXCR4 inhibitor, T140, leads to development of effective anti-HIV agents with very high selectivity indexes. Bioorg Med Chem Lett. 2000;10:2633–7. doi: 10.1016/s0960-894x(00)00535-7. [DOI] [PubMed] [Google Scholar]
- 159.Tamamura H, Sugioka M, Odagaki Y, Omagari A, Kan Y, Oishi S, et al. Conformational study of a highly specific CXCR4 inhibitor, T140, disclosing the close proximity of its intrinsic pharmacophores associated with strong anti-HIV activity. Bioorg Med Chem Lett. 2001;11:359–62. doi: 10.1016/s0960-894x(00)00664-8. [DOI] [PubMed] [Google Scholar]
- 160.Tamamura H, Mizumoto M, Hiramatsu K, Kusano S, Terakubo S, Yamamoto N, et al. Topochemical exploration of potent compounds using retro-enantiomer libraries of cyclic pentapeptides. Org Biomol Chem. 2004;2:1255–7. doi: 10.1039/b401485p. [DOI] [PubMed] [Google Scholar]
- 161.Ueda S, Oishi S, Wang ZX, Araki T, Tamamura H, Cluzeau J, et al. Structure-activity relationships of cyclic peptide-based chemokine receptor CXCR4 antagonists: disclosing the importance of side-chain and backbone functionalities. J Med Chem. 2007;50:192–8. doi: 10.1021/jm0607350. [DOI] [PubMed] [Google Scholar]
- 162.Cluzeau J, Oishi S, Ohno H, Wang Z, Evans B, Peiper SC, et al. Design and synthesis of all diastereomers of cyclic pseudo-dipeptides as mimics of cyclic CXCR4 pentapeptide antagonists. Org Biomol Chem. 2007;5:1915–23. doi: 10.1039/b702649h. [DOI] [PubMed] [Google Scholar]
- 163.Tamamura H, Araki T, Ueda S, Wang Z, Oishi S, Esaka A, et al. Identification of novel low molecular weight CXCR4 antagonists by structural tuning of cyclic tetrapeptide scaffolds. J Med Chem. 2005;48:3280–9. doi: 10.1021/jm050009h. [DOI] [PubMed] [Google Scholar]
- 164.Tamamura H, Hiramatsu K, Ueda S, Wang Z, Kusano S, Terakubo S, et al. Stereoselective synthesis of [L-Arg-L/D-3-(2-naphthyl)alanine]-type (E)-alkene dipeptide isosteres and its application to the synthesis and biological evaluation of pseudopeptide analogues of the CXCR4 antagonist FC131. J Med Chem. 2005;48:380–91. doi: 10.1021/jm049429h. [DOI] [PubMed] [Google Scholar]
- 165.Tamamura H, Esaka A, Ogawa T, Araki T, Ueda S, Wang Z, et al. Structure-activity relationship studies on CXCR4 antagonists having cyclic pentapeptide scaffolds. Org Biomol Chem. 2005;3:4392–4. doi: 10.1039/b513145f. [DOI] [PubMed] [Google Scholar]
- 166.DeMarco SJ, Henze H, Lederer A, Moehle K, Mukherjee R, Romagnoli B, et al. Discovery of novel, highly potent and selective β-hairpin mimetic CXCR4 inhibitors with excellent anti-HIV activity and pharmacokinetic profiles. Bioorg Med Chem. 2006;14:8396–404. doi: 10.1016/j.bmc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 167.Moncunill G, Armand-Ugon M, Clotet-Codina I, Pauls E, Ballana E, Llano A, et al. Anti-HIV activity and resistance profile of the CXC chemokine receptor 4 antagonist POL3026. Mol Pharmacol. 2008;73:1264–73. doi: 10.1124/mol.107.042911. [DOI] [PubMed] [Google Scholar]
- 168.Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23:459–67. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- 169.Trent JO, Wang ZX, Murray JL, Shao W, Tamamura H, Fujii N, et al. Lipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonists. J Biol Chem. 2003;278:47136–44. doi: 10.1074/jbc.M307850200. [DOI] [PubMed] [Google Scholar]
- 170.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 171.Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–7. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 172.Schols D, Este JA, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res. 1997;35:147–56. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 173.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–7. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 174.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37:1253–62. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 175.Scozzafava A, Mastrolorenzo A, Supuran CT. Non-peptidic chemokine receptors antagonists as emerging anti-HIV agents. J Enzyme Inhib Med Chem. 2002;17:69–76. doi: 10.1080/14756360290024227. [DOI] [PubMed] [Google Scholar]
- 176.Stone ND, Dunaway SB, Flexner C, Tierney C, Calandra GB, Becker S, et al. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother. 2007;51:2351–8. doi: 10.1128/AAC.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem. 2001;276:14153–60. doi: 10.1074/jbc.M010429200. [DOI] [PubMed] [Google Scholar]
- 178.Hatse S, Princen K, De Clercq E, Rosenkilde MM, Schwartz TW, Hernandez-Abad PE, et al. AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor. Biochem Pharmacol. 2005;70:752–61. doi: 10.1016/j.bcp.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 179.Ojida A, Mito-Oka Y, Inoue MA, Hamachi I. First artificial receptors and chemosensors toward phosphorylated peptide in aqueous solution. J Am Chem Soc. 2002;124:6256–8. doi: 10.1021/ja025761b. [DOI] [PubMed] [Google Scholar]
- 180.Tamamura H, Ojida A, Ogawa T, Tsutsumi H, Masuno H, Nakashima H, et al. Identification of a new class of low molecular weight antagonists against the chemokine receptor CXCR4 having the dipicolylamine-zinc(II) complex structure. J Med Chem. 2006;49:3412–5. doi: 10.1021/jm060025u. [DOI] [PubMed] [Google Scholar]
- 181.Ichiyama K, Yokoyama-Kumakura S, Tanaka Y, Tanaka R, Hirose K, Bannai K, et al. A duodenally absorbable CXC chemokine receptor 4 antagonist, KRH-1636, exhibits a potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 2003;100:4185–90. doi: 10.1073/pnas.0630420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tamamura H, Tsutsumi H, Masuno H, Mizokami S, Hiramatsu K, Wang Z, et al. Development of a linear type of low molecular weight CXCR4 antagonists based on T140 analogs. Org Biomol Chem. 2006;4:2354–7. doi: 10.1039/b603818b. [DOI] [PubMed] [Google Scholar]
- 183.Niida A, Tanigaki H, Inokuchi E, Sasaki Y, Oishi S, Ohno H, et al. Stereoselective synthesis of 3,6-disubstituted-3,6-dihydropyridin-2-ones as potential diketopiperazine mimetics using organocopper-mediated anti-SN2’ reactions and their use in the preparation of low-molecule CXCR4 antagonists. J Org Chem. 2006;71:3942–51. doi: 10.1021/jo060390t. [DOI] [PubMed] [Google Scholar]
- 184.Ueda S, Kato M, Inuki S, Ohno H, Evans B, Wang ZX, et al. Identification of novel non-peptide CXCR4 antagonists by ligand-based design approach. Bioorg Med Chem Lett. 2008;18:4124–9. doi: 10.1016/j.bmcl.2008.05.092. [DOI] [PubMed] [Google Scholar]
- 185.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem. 1998;273:22279–83. doi: 10.1074/jbc.273.35.22279. [DOI] [PubMed] [Google Scholar]
- 187.Faber A, Roderburg C, Wein F, Saffrich R, Seckinger A, Horsch K, et al. The Many Facets of SDF-1α, CXCR4 Agonists and Antagonists on Hematopoietic Progenitor Cells. J Biomed Biotechnol. 2007;2007:26065. doi: 10.1155/2007/26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25:201–11. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tudan C, Willick GE, Chahal S, Arab L, Law P, Salari H, et al. C-terminal cyclization of an SDF-1 small peptide analogue dramatically increases receptor affinity and activation of the CXCR4 receptor. J Med Chem. 2002;45:2024–31. doi: 10.1021/jm0104015. [DOI] [PubMed] [Google Scholar]
- 190.Perez LE, Alpdogan O, Shieh JH, Wong D, Merzouk A, Salari H, et al. Increased plasma levels of stromal-derived factor-1 (SDF-1/CXCL12) enhance human thrombopoiesis and mobilize human colony-forming cells (CFC) in NOD/SCID mice. Exp Hematol. 2004;32:300–7. doi: 10.1016/j.exphem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 191.Li K, Chuen CK, Lee SM, Law P, Fok TF, Ng PC, et al. Small peptide analogue of SDF-1α supports survival of cord blood CD34+ cells in synergy with other cytokines and enhances their ex vivo expansion and engraftment into nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2006;24:55–64. doi: 10.1634/stemcells.2005-0082. [DOI] [PubMed] [Google Scholar]
- 192.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 193.Zhou N, Luo Z, Luo J, Hall JW, Huang Z. A novel peptide antagonist of CXCR4 derived from the N-terminus of viral chemokine vMIP-II. Biochemistry. 2000;39:3782–7. doi: 10.1021/bi992750v. [DOI] [PubMed] [Google Scholar]
- 194.Crump MP, Elisseeva E, Gong J, Clark-Lewis I, Sykes BD. Structure/function of human herpesvirus-8 MIP-II (1-71) and the antagonist N-terminal segment (1-10) FEBS Lett. 2001;489:171–5. doi: 10.1016/s0014-5793(00)02393-0. [DOI] [PubMed] [Google Scholar]
- 195.Dong CZ, Kumar S, Choi WT, Madani N, Tian S, An J, et al. Different stereochemical requirements for CXCR4 binding and signaling functions as revealed by an anti-HIV, D-amino acid-containing SMM-chemokine ligand. J Med Chem. 2005;48:7923–4. doi: 10.1021/jm050829u. [DOI] [PubMed] [Google Scholar]
- 196.Choi WT, Tian S, Dong CZ, Kumar S, Liu D, Madani N, et al. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors. J Virol. 2005;79:15398–404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 198.Chen Y, Stamatoyannopoulos G, Song CZ. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res. 2003;63:4801–4. [PubMed] [Google Scholar]
- 199.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 200.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38. [PubMed] [Google Scholar]
- 201.Schmid BC, Rudas M, Rezniczek GA, Leodolter S, Zeillinger R. CXCR4 is expressed in ductal carcinoma in situ of the breast and in atypical ductal hyperplasia. Breast Cancer Res Treat. 2004;84:247–50. doi: 10.1023/B:BREA.0000019962.18922.87. [DOI] [PubMed] [Google Scholar]
- 202.Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–93. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 203.Shim H, Lau SK, Devi S, Yoon Y, Cho HT, Liang Z. Lower expression of CXCR4 in lymph node metastases than in primary breast cancers: potential regulation by ligand-dependent degradation and HIF-1α. Biochem Biophys Res Commun. 2006;346:252–8. doi: 10.1016/j.bbrc.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 204.Gockel I, Schimanski CC, Moehler M, Junginger T. Novel therapeutic targets in esophageal cancer: impact of chemokine receptor CXCR4. Future Oncol. 2007;3:119–22. doi: 10.2217/14796694.3.2.119. [DOI] [PubMed] [Google Scholar]
- 205.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. 2005;97:1840–7. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 206.Koishi K, Yoshikawa R, Tsujimura T, Hashimoto-Tamaoki T, Kojima S, Yanagi H, et al. Persistent CXCR4 expression after preoperative chemoradiotherapy predicts early recurrence and poor prognosis in esophageal cancer. World J Gastroenterol. 2006;12:7585–90. doi: 10.3748/wjg.v12.i47.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sasaki K, Natsugoe S, Ishigami S, Matsumoto M, Okumura H, Setoyama T, et al. Expression of CXCL12 and its receptor CXCR4 correlates with lymph node metastasis in submucosal esophageal cancer. J Surg Oncol. 2008;97:433–8. doi: 10.1002/jso.20976. [DOI] [PubMed] [Google Scholar]
- 208.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 209.Kodama J, Hasengaowa, Kusumoto T, Seki N, Matsuo T, Ojima Y, et al. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2007;18:70–6. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 210.Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226–33. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 211.Pils D, Pinter A, Reibenwein J, Alfanz A, Horak P, Schmid BC, et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer. 2007;96:485–91. doi: 10.1038/sj.bjc.6603581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Porcile C, Bajetto A, Barbero S, Pirani P, Schettini G. CXCR4 activation induces epidermal growth factor receptor transactivation in an ovarian cancer cell line. Ann N Y Acad Sci. 2004;1030:162–9. doi: 10.1196/annals.1329.021. [DOI] [PubMed] [Google Scholar]
- 213.Samara GJ, Lawrence DM, Chiarelli CJ, Valentino MD, Lyubsky S, Zucker S, et al. CXCR4-mediated adhesion and MMP-9 secretion in head and neck squamous cell carcinoma. Cancer Lett. 2004;214:231–41. doi: 10.1016/j.canlet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 214.Begum NA, Shibuta K, Mori M, Barnard GF. Reduced expression of the CXCR4 receptor mRNA in hepatocellular carcinoma and lack of inducibility of its ligand α -chemokine hIRH/SDF1α/PBSF in vitro. Int J Oncol. 1999;14:927–34. doi: 10.3892/ijo.14.5.927. [DOI] [PubMed] [Google Scholar]
- 215.Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–7. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002;93:789–97. doi: 10.1111/j.1349-7006.2002.tb01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barretina J, Junca J, Llano A, Gutierrez A, Flores A, Blanco J, et al. CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Ann Hematol. 2003;82:500–5. doi: 10.1007/s00277-003-0679-0. [DOI] [PubMed] [Google Scholar]
- 218.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–67. [PubMed] [Google Scholar]
- 219.Dao-Ung LP, Sluyter R, Fuller SJ, Taper J, Wiley JS. CXCR4 but not CXCR3 expression correlates with lymphocyte counts in B-cell chronic lymphocytic leukemia. Ann Hematol. 2004;83:326–7. doi: 10.1007/s00277-004-0846-y. [DOI] [PubMed] [Google Scholar]
- 220.Fierro FA, Brenner S, Oelschlaegel U, Jacobi A, Knoth H, Ehninger G, et al. Combining SDF-1/CXCR4 antagonism and chemotherapy in relapsed acute myeloid leukemia. Leukemia. 2008 doi: 10.1038/leu.2008.182. in press. [DOI] [PubMed] [Google Scholar]
- 221.Ghobrial IM, Bone ND, Stenson MJ, Novak A, Hedin KE, Kay NE, et al. Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B-cell chronic lymphocytic leukemia/ small lymphocytic lymphoma. Mayo Clin Proc. 2004;79:318–25. doi: 10.4065/79.3.318. [DOI] [PubMed] [Google Scholar]
- 222.Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- 223.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 224.Konoplev S, Rassidakis GZ, Estey E, Kantarjian H, Liakou CI, Huang X, et al. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109:1152–6. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- 225.Mohle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1) Leukemia. 1999;13:1954–9. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 226.Monaco G, Belmont JW, Konopleva M, Andreeff M, Tavor S, Petit I, et al. Correlation between CXCR4 and homing or engraftment of acute myelogenous leukemia. Cancer Res. 2004;64:6832. doi: 10.1158/0008-5472.CAN-04-1936. [DOI] [PubMed] [Google Scholar]
- 227.Scupoli MT, Donadelli M, Cioffi F, Rossi M, Perbellini O, Malpeli G, et al. Bone marrow stromal cells and the upregulation of interleukin-8 production in human T-cell acute lymphoblastic leukemia through the CXCL12/CXCR4 axis and the NF-κB and JNK/AP-1 pathways. Haematologica. 2008;93:524–32. doi: 10.3324/haematol.12098. [DOI] [PubMed] [Google Scholar]
- 228.Spoo AC, Lubbert M, Wierda WG, Burger JA. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood. 2007;109:786–91. doi: 10.1182/blood-2006-05-024844. [DOI] [PubMed] [Google Scholar]
- 229.Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:2817–24. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 230.Wu S, Gessner R, Taube T, Korte A, von Stackelberg A, Kirchner R, et al. Chemokine IL-8 and chemokine receptor CXCR3 and CXCR4 gene expression in childhood acute lymphoblastic leukemia at first relapse. J Pediatr Hematol Oncol. 2006;28:216–20. doi: 10.1097/01.mph.0000212908.14642.a5. [DOI] [PubMed] [Google Scholar]
- 231.Bertolini F, Dell’Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin’s lymphoma. Cancer Res. 2002;62:3106–12. [PubMed] [Google Scholar]
- 232.Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110:421–6. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- 233.Piovan E, Tosello V, Indraccolo S, Cabrelle A, Baesso I, Trentin L, et al. Chemokine receptor expression in EBV-associated lymphoproliferation in hu/SCID mice: implications for CXCL12/CXCR4 axis in lymphoma generation. Blood. 2005;105:931–9. doi: 10.1182/blood-2004-03-0799. [DOI] [PubMed] [Google Scholar]
- 234.Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–7. doi: 10.1093/annonc/mdh136. [DOI] [PubMed] [Google Scholar]
- 235.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J Biol Chem. 2005;280:22473–81. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 236.Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–17. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Moller C, Stromberg T, Juremalm M, Nilsson K, Nilsson G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia. 2003;17:203–10. doi: 10.1038/sj.leu.2402717. [DOI] [PubMed] [Google Scholar]
- 238.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 239.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 240.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. Faseb J. 2004;18:1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 241.Sun Y, Schneider A, Jung Y, Wang J, Dia J, Cook K, et al. Skeletal localization and neutralization of the SDF-1/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Mineral Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 242.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 244.Hart CA, Brown M, Bagley S, Sharrard M, Clarke NW. Invasive characteristics of human prostatic epithelial cells: understanding the metastatic process. Br J Cancer. 2005;92:503–12. doi: 10.1038/sj.bjc.6602325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mochizuki H, Matsubara A, Teishima J, Mutaguchi K, Yasumoto H, Dahiya R, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–63. doi: 10.1016/j.bbrc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 246.Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY, et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17:1578–92. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 247.Jones J, Marian D, Weich E, Engl T, Wedel S, Relja B, et al. CXCR4 chemokine receptor engagement modifies integrin dependent adhesion of renal carcinoma cells. Exp Cell Res. 2007;313:4051–65. doi: 10.1016/j.yexcr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 248.Pan J, Mestas J, Burdick MD, Phillips RJ, Thomas GV, Reckamp K, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Reckamp KL, Strieter RM, Figlin RA. Chemokines as therapeutic targets in renal cell carcinoma. Expert Rev Anticancer Ther. 2008;8:887–93. doi: 10.1586/14737140.8.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 251.Struckmann K, Mertz K, Steu S, Storz M, Staller P, Krek W, et al. pVHL co-ordinately regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell renal cell carcinoma. J Pathol. 2008;214:464–71. doi: 10.1002/path.2310. [DOI] [PubMed] [Google Scholar]
- 252.Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597–606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 253.Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, et al. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561–7. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 254.Oda Y, Yamamoto H, Tamiya S, Matsuda S, Tanaka K, Yokoyama R, et al. CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: analysis within a group of patients, all of whom developed lung metastasis. Mod Pathol. 2006;19:738–45. doi: 10.1038/modpathol.3800587. [DOI] [PubMed] [Google Scholar]
- 255.Hwang JH, Hwang JH, Chung HK, Kim DW, Hwang ES, Suh JM, et al. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88:408–16. doi: 10.1210/jc.2002-021381. [DOI] [PubMed] [Google Scholar]
- 256.De Falco V, Guarino V, Avilla E, Castellone MD, Salerno P, Salvatore G, et al. Biological role and potential therapeutic targeting of the chemokine receptor CXCR4 in undifferentiated thyroid cancer. Cancer Res. 2007;67:11821–9. doi: 10.1158/0008-5472.CAN-07-0899. [DOI] [PubMed] [Google Scholar]


