Abstract
Formation of colonic diverticula, via herniation of the colonic wall, is responsible for the development of diverticulosis. When diverticulosis becomes symptomatic, it becomes diverticular disease. Diverticular disease is common in Western and industrialized countries, and it is associated with numerous abdominal symptoms (including pain, bloating, nausea, diarrhea, and constipation). Standard medical therapies with antibiotics are currently recommended for patients affected by diverticular disease. However, changing concepts on the pathophysiology of the disease suggest that diverticular disease may share many of the hallmarks of inflammatory bowel diseases. On this basis, the addition of therapies using mesalazine and probiotics may enhance treatment efficacy by shortening the course of the disease and preventing recurrences.
Keywords: Diverticulitis, Antibiotics, 5-Aminosalicylic acid, Mesalazine, Probiotics
INTRODUCTION
Diverticular disease was first described in the early 20th century[1] but was rarely encountered at that time. Since then, the prevalence of this disease increased from 5%-10% in 1930[2] and to 35%-50% in 1969 according to an autopsy series[3]. No subsequent population-based studies have been reported. At this point, the etiology and disease course of this disease have been sufficiently well researched to allow for diagnosis and treatment of this condition. However, due to the lack of research evidence, treatment options are not well defined. For example, many patients presenting with acute diverticulitis are treated conservatively and receive antibiotics as a standard course of care. A recent study, however, has shown that outcomes for patients treated with antibiotics were not significantly different from patients treated with only observation[4]. This suggests that there is considerable room for improvement in our understanding of this disease and optimal treatment plans.
CLINICAL CLASSIFICATION OF DIVERTICULAR DISEASE
Currently, there is no universally accepted classification system for the development of diverticular disease. When patients with diverticulosis become symptomatic, they are said to have diverticular disease. Diverticular disease is currently described as symptomatic uncomplicated disease, recurrent symptomatic disease or complicated disease[5,6] (Table 1).
Table 1.
Current classification of diverticular disease of the colon
| Classification | Description |
| Asymptomatic diverticulosis | Patients with diverticula and the absence of any sign or symptom of diverticular inflammation |
| Symptomatic uncomplicated | Patients with diverticula who experience symptoms, but without signs of diverticular inflammation |
| diverticular diseae | |
| Symptomatic recurrent | Patients with diverticula who experience recurrent symptoms (more than 1 attack per year) but without signs of |
| diverticular disease | diverticular inflammation |
| Complicated | Patients with diverticula who experience symptoms and demonstrate signs of diverticular inflammation with further |
| diverticular disease | complications (hemorrhage, abscess, phlegmon, perforation, purulent and fecal peritonitis, strictures, fistulas) |
Symptomatic uncomplicated diverticular disease
Symptomatic uncomplicated diverticular disease is characterised by non-specific episodes of lower abdominal pain without macroscopic evidence of inflammation[6]. The abdominal pain is usually colicky, but can be constant, and is often relieved by passing flatus or having a bowel movement. Bloating and a change in bowel habits may also occur due to bacterial overgrowth. In these cases, constipation is more common than diarrhea. In addition, fullness or tenderness in the left lower quadrant, or occasionally a tender, palpable loop of sigmoid colon may be noted on physical examination.
Recurrent symptomatic diverticular disease
Recurrent symptomatic diverticular disease is characterized by the reappearance of symptoms, which were previously described, usually several times per year[6].
Complicated diverticular disease
The most common complication of diverticular disease is acute diverticulitis, which is defined as diverticular disease with signs and symptoms of diverticular inflammation. Diverticular hemorrhage is also a frequent complication of diverticular disease, occurring in 5%-15% of patients (severe in 3%-5%)[7,8]. Other, less prevalent, complications include abscess, phlegmon, perforation of the intestinal wall, purulent or fecal peritonitis, stricture, fistula and small-bowel obstruction due to post-inflammatory adhesions[6]. The Hinchey’s grading system[5,9], describes all complications related to perforated diverticular disease.
Although a clinical classification system for acute diverticulitis has not yet been clearly defined, patients generally present with acute abdominal and gastrointestinal symptoms, combined with signs and symptoms of diverticula inflammation with or without complications[10]. Clinical features of acute diverticulitis may, therefore, include constant abdominal pain, localized abdominal tenderness, nausea, vomiting, constipation or diarrhea, fever and leucocytosis. Additionally, patients may occasionally present with urinary symptoms, such as dysuria and increased frequency, related to bladder irritation.
Finally, a causal relationship between diverticulosis and colorectal cancer (CRC) has been suggested[11]. This topic is currently receiving much attention and not all studies support this suggestion[12]. Analysis of this question is beyond the scope of the current paper, however, a summary of this debate can be found in a recent review by Morini et al[11]. The view of Morini and co-workers is that the available data are not yet strong enough to suggest a more aggressive CRC prevention in diverticular as compared with nondiverticular subjects.
MEDICAL TREATMENT OF DIVERTICULAR DISEASE
When a patient develops the symptoms of diverticular disease, medical therapy is generally required. The main objectives of treatment include improving symptoms, resolving any infection or consequences of inflammation, preventing recurrence of symptoms, and preventing or limiting the development of serious complications. While there is consensus on the approach for medical treatment of symptomatic uncomplicated diverticular disease, a clinical challenge is present for the treatment of acute diverticulitis. A surgical approach is traditionally used when treating acute, recurrent diverticulitis, and is usually recommended after two or more prior episodes[13]. However, recent data suggest that surgery may not always be necessary, as diverticula inflammation can be present without other complications[10,14,15]. Moreover, it is quite common in clinical practice to observe patients with raised inflammatory indices (e.g. high erythrosedimentation rate, C-reactive protein and calprotectin) and endoscopic evidence of inflamed diverticula, but with no other disease-related complications. From a diagnostic perspective, clinical, radiological [computerized tomography (CT)], endoscopic and laboratory findings can differentiate between uncomplicated and complicated acute diverticulitis. This is important in clinical practice, as patients with uncomplicated diverticulitis (and indeed patients with uncomplicated diverticular disease) may be managed successfully as outpatients using pharmaceutical therapy, whereas patients with complicated disease typically require urgent hospital admission and surgical intervention[16]. The current therapeutic approach to symptomatic diverticular disease is summarized in Table 2.
Table 2.
Symptomatic diverticular disease: Current therapeutic approach
| Symptomatic uncomplicated | Uncomplicated diverticulitis | Complicated diverticulitis |
| Fibers | Mild + familiar support | Abscess |
| Spasmolytics | Outpatients treatment | Drainage (CT) |
| Anti-cholinergic | Liquid diet | Drainage + surgery |
| Antibiotics | Oral antibiotics | Perforation and fistulas |
| Not recommended (ACG, ASCRS Guidelines) | Improvement within 2/3 d | Surgery |
| Rifaximin | Severe, comorbidity, elderly, immunodepression | Acute obstruction |
| Inpatients treatment | Conservative therapy | |
| "Bowel rest" | Chronic obstruction | |
| I.V. antibiotics | Endoscopic treatment | |
| Oral antibiotics at discharging (ACG, ASCRS Guidelines) | Surgery | |
| Bleeding | ||
| Conservative therapy | ||
| Angiography | ||
| Colonoscopy | ||
| Surgery |
CURRENT PRACTICE IN DIVERTICULAR DISEASE
Asymptomatic diverticulosis
Therapeutic intervention is not usually necessary in patients with uncomplicated asymptomatic diverticulosis. Many physicians advise patients with diverticulosis to adopt a high-fiber, low-fat diet and increase their physical activity, even though controlled clinical trials are lacking and the evidence that this may help to prevent the development of diverticular disease is not conclusive[17-19].
Symptomatic uncomplicated diverticular disease
The initial treatment of diverticular disease is usually pharmaceutical, since the large majority of patients respond very well and emergency surgery is not normally required[20].
The first stage of treatment for mild symptoms may involve diet modification or fiber supplementation, along with antibiotic therapy[20]. However, it may also be necessary to let the bowel rest. It is usually advised that patients with uncomplicated diverticular disease should start a clear-liquid diet, while patients with severe or complicated disease (who may be experiencing nausea and vomiting) should change to parenteral nutrition alone[7,13,21,22]. The second, and usually concomitant step, is to prescribe an appropriate antibiotic to resolve infection and bacterial overgrowth. This treatment is critical for preventing and/or reducing disease complications[6,14]. Ideally, the choice of antibiotic should be based on an individual patient’s fecal flora, since bacteremia is often associated with acute diverticulitis. The most commonly isolated bacteria include coliforms (e.g. Escherichia. coli), Bacteroides spp. (e.g. B. fragilis) and Clostridia spp[6,22,23]. Given the potential involvement of these micro-organisms, it is advisable to use a single broad-spectrum antibiotic with activity against both Gram-negative and anaerobic bacteria. The choice of antibiotic is usually based on clinical experience, since well-controlled, comparative antimicrobial trials are rare in this setting. In contrast, there is new evidence suggesting that the poorly absorbable, broad-spectrum, oral antibiotic, rifaximin, is effective against both Gram-positive and Gram-negative aerobic and anaerobic bacteria[24]. Multiple studies have shown that rifaximin can effectively improve symptoms and maintain periods of remission in patients with uncomplicated diverticular disease, while also being well tolerated[25-29]. Rifaximin may therefore be deemed an appropriate and effective therapy for uncomplicated diverticular disease, especially when used in addition to dietary fiber supplementation[30].
Acute diverticulitis
It is reasonable to start empirical treatment immediately in patients with clinical signs and symptoms that are strongly suggestive of acute diverticulitis. Outpatient therapy is usually appropriate in patients with mild-to-moderate, uncomplicated, non-recurrent disease, who can tolerate oral hydration/administration, who do not have appreciable fever, excessive vomiting, or marked peritonitis, providing there is the opportunity for follow-up[20]. In these patients, oral, broad-spectrum antibiotics are usually given for 7-10 d,
with clinical improvement expected within 2-3 d[21,22]. If opioid analgesics are required, meperidine is the preferred option since morphine causes colonic spasm and may accentuate colonic hyper-segmentation[22]. Hospitalization, with intravenous antibiotic treatment, is usually recommended if the above conditions cannot be met, or if the patient fails to improve using outpatient therapy[20].
Various antibiotics are used in the treatment of acute diverticulitis. Patients with severe or complicated disease are commonly treated with ampicillin, gentamicin, metronidazole, piperacillin, clindamycin, third-generation cephalosporins (e.g. ceftazidime, cefotaxime and ceftriaxone) or tazobactam[31-33]. Use of these antibiotics ensures complete coverage against aerobic, anaerobic and Gram-negative flora, especially E. coli and Bacterioides spp[31-33]. In a North American survey of 373 fellows of The American Society of Colon and Rectal Surgeons, second-generation cephalosporins (27%) and ampicillin–sulbactam (16%) were the most commonly used antibiotics in patients with uncomplicated diverticulitis[34]. While the combination of ciprofloxacin and metronidazole is also a common treatment option for uncomplicated diverticulitis[32], these particular antibiotics may be poorly tolerated by some patients due to their high systemic absorption. In addition, single-agent use of these treatments is not recommended because, when used on their own, ciprofloxacin and metronidazole do not provide coverage against all potentially pathogenic bacteria[10]. Ciprofloxacin and metronidazole monotherapy should, therefore, not be regarded as an adequate first-line treatment option. On the contrary, after resolution of acute diverticulitis, rifaximin can reduce its recurrence[35].
If patients have severe or unresponsive diverticular disease/diverticulitis despite adequate outpatient treatment, or are unsuitable for outpatient treatment, hospitalization may be required[10,21,22]. Patients who are unsuitable for outpatient treatment may include the very elderly or immunosuppressed patients, patients with multiple and/or severe comorbidities and those with no home support or who are unable to tolerate oral hydration/antibiotics. It is usually recommended that hospitalized patients are given broad-spectrum, intravenous antibiotics for 7-10 d[10,22,23]. Furthermore, all hospitalized patients should undergo a multi-slice CT scan of the abdomen and pelvis within 48 h of admission. A CT scan not only confirms the diagnosis, but also helps to assess the risk of imminent complications by measuring the thickness of the colonic wall, small pericolonic or retrocolonic abscesses, collections and localized perforations. CT scan also has therapeutic potential; in the case of a localized pericolonic abscess or contained collection, CT-guided drainage can be performed. In this way, acceleration of the effect of the antibiotics can be achieved and emergency surgery can be avoided[36]. Clinical improvement should be observed within 2-4 d[10,30]. Once the acute episode has resolved, patients should be advised to maintain a high-fiber diet in order to optimize their bowel movements. A 7-10 d course of oral antibiotics is also often given following discharge[10]. Current antibiotics that are advised for acute diverticulitis are summarized in Table 3.
Table 3.
Current antibiotic therapy in acute diverticulitis
| Route | Antibiotic |
| Oral route | Amoxycillin + clavulanic acid |
| Sulphametoxazole-trimethoprim + metronidazole | |
| Chinolone + metronidazole | |
| Intravenous route | Metronidazole + aminoglycosidic (e.g. gentamicin) |
| Clindamycin | |
| Aztreonam | |
| Third generation cephalosporins | |
| Second generation cephalosporins | |
| Association of several β-lactamase inhibitors (e.g. | |
| ampicillin-sulbactam) |
The prognosis for patients following treatment for an acute episode of diverticulitis is generally good, and medical therapy has been shown to resolve the first acute attack in 70%-100% of patients[20]. However, recent data suggest reconsideration of the role of antibiotics in treating acute diverticulitis. In a retrospective audit of 311 patients that were hospitalized for AD, Hjern et al[4] observed patients initially treated conservatively with observation and restriction of oral intake. Patients receiving antibiotics (n = 118) were compared with patients treated with observation and restriction of oral intake only (n = 193). When initially treated with antibiotics, 3 patients (3%) failed to respond to treatment and had to undergo surgery. There were 7 (4%) failures in patients initially treated without antibiotics, and antibiotics were then added. During follow-up, 29% of patients treated with antibiotics had further events (recurrent acute diverticulitis and/or subsequent surgery) compared with 28% (P = NS) among those treated without antibiotics. In a multivariate analysis, the risk of a further event was not influenced by antibiotic treatment (OR : 1.03, 95% CI : 0.61-1.74). These surprising results indicate that antibiotics are not always mandatory in mild acute diverticulitis, and that treatment without antibiotics appears to be safe and seems not to change the rate of occurrence of further events. Another important point to consider is that approximately one-third of patients will experience recurrent diverticulitis, often within 1 year of the first episode[20,21,24] (19%-54% recurrence rate within 5 years[37]). Treatment for repeat episodes may follow the same course, however, a surgical approach is traditionally used when treating acute, recurrent diverticulitis, and is usually recommended after two or more prior episodes[13]. This suggests that current medical therapy could still be improved.
The medical treatment of symptomatic uncomplicated diverticular disease and uncomplicated diverticulitis is evolving. Antibiotics are likely to remain the mainstay of therapy, particularly for inducing remission. However, a greater understanding of the pathophysiology of diverticular disease, in particular the implication of colonic microflora and chronic inflammation, has resulted in the investigation of new medical treatment strategies.
NEW INSIGHTS INTO THE PATHOPHYSIOLOGY OF DIVERTICULAR DISEASE
The pathophysiology of diverticular disease is extremely complicated due to multiple contributing factors, including diet, colonic wall structure intestinal motility and possible genetic predispositions[38].
However, recent observations suggest that the natural history of the disease bears many similarities to that of chronic IBD[6,8]. For example, some of the cellular mechanisms that underlie the development of chronic inflammation and inflammatory complications are common to both diverticulitis and IBD[39]. As in IBD, diverticula inflammation appears to be generated by an increased production of pro-inflammatory cytokines, a reduced production of anti-inflammatory cytokines and enhanced intramucosal synthesis of nitric oxide[40]. Furthermore, many clinical and laboratory findings associated with uncomplicated acute diverticulitis, such as abdominal pain, change in bowel habits, and occasionally fever and leucocytosis with or without increasing inflammatory indices, overlap with those of acute IBD[22].
Other studies have shown that, in addition to promoting diverticula formation, a fiber-deficient diet may also bring about a change in the colonic microflora, resulting in a decrease in healthy flora and an increase in pathogenic bacteria. Indeed, both fiber supplementation with wheat bran and diet composition (Western vs Japanese or UK vs African) have been shown to alter the composition of human fecal flora[41-43]. As in IBD[44], altering the intestinal micro-ecology can lead to reduced influence on the immune response of the host and decreased bacterial production of short-chain fatty acids, which results from degradation of soluble fibre[7,8,45,46]. This may permit chronic inflammation and epithelial cell proliferation to develop in the colonic mucosa in and around the diverticula[6,45,47-49], as observed in acute diverticulitis[8,45]. The degree of inflammation appears to be related to the severity of the disease[49]. If severe and/or persistent, the inflammation may lead to focal necrosis and ultimately micro- or macro-perforation. The altered microflora and diminished immunological tolerance to commensal bacteria may also permit bacterial overgrowth, a phenomenon linked with colonic ischemia, diverticula and peridiverticula inflammation, increased exposure to intraluminal antigens and toxins, and changes in bacterial flora related to stasis[39,50].
These new insights into the pathophysiology of diverticular disease may explain recent data demonstrating the effectiveness of new medical interventions. If, as in IBD, chronic inflammation and bacterial overgrowth occur as consequences of a change in the colonic microflora, it stands to reason that "normalising" the flora or administering an anti-inflammatory agent (with proven effectiveness in IBD) may help to treat the symptoms of diverticular disease, prevent the onset of acute diverticulitis and/or reduce the risk of symptomatic recurrence. This has led to research on the potential of 5-aminosalicylic acid (5-ASA) and probiotics as adjunctive treatments for diverticular disease.
Motility changes in diverticular disease are well documented and have given rise to research on the potential involvement of neurotransmitter disorders. Indeed, an increased presence of serotonin-producing cells has been identified in resected colonic specimens with diverticulitis[51]. However, it is unknown if this is a causative association. An additional study has identified increased serotonin expression in symptomatic patients with diverticula[52]. A third group has attempted to implicate hypersensitivity to acetyl-choline, caused by decreased activity of choline acetyltransferase[53]. Additionally, decreased nitric oxide activity in the longitudinal muscle of diverticular colon could account for decreased relaxation and increased muscle spasm in this condition[54]. While these observations provide insufficient evidence that the pathogenetic mechanisms of diverticular disease operate at a neurotransmitter level, they suggest that further research in this area may be productive.
NEW THERAPEUTIC APPROACHES TO DIVERTICULAR DISEASE
New therapeutic approaches for treating diverticular disease are now under investigation. In particular, 5-aminosalicylic acid (5-ASA) and probiotics are currently being investigated. However, some interesting data are available and the rationale for treating diverticular disease with 5-ASA and/or probiotics is summarized in Figure 1.
Figure 1.
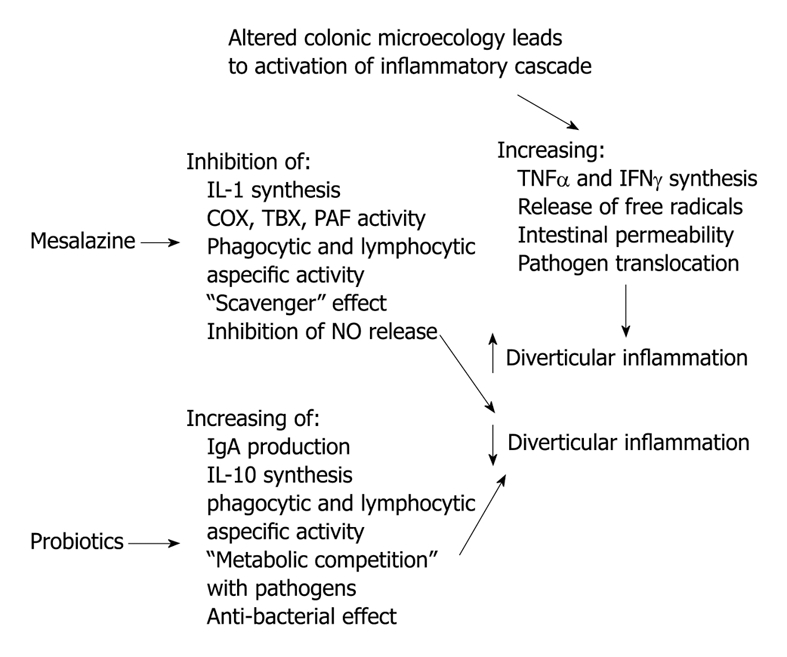
Rationale for treating diverticular disease with mesalazine and/or probiotics. IgA: Immunoglobulin A; IFN: Interferon; IL: Interleukin; TNF: Tumour necrosis factor; COX: Cyclooxygenase; TBX: Thromboxane; PAF: Platelet activating factor.
5-ASA in diverticular disease
5-ASA is the primary therapy used for induction and maintenance of remission of mild-to-moderately severe inflammatory bowel diseases, particularly ulcerative colitis (UC)[55,56]. This treatment acts topically, rather than systemically, on the colonic mucosa to reduce inflammation[57,58]. Various oral and rectal 5-ASA formulations are available with proven efficacy in treating symptoms and maintaining periods of remission in patients with UC, while also displaying excellent tolerability[57,59]. In general, newer non-sulphur-containing therapies are preferred over the traditional 5-ASA pro-drug, sulphasalazine, because of their improved side-effect profiles[55,56,59]. Newer 5-ASA therapies include mesalazine (of which there are multiple formulations with different release and delivery characteristics) and the 5-ASA pro-drugs olsalazine and balsalazide. The mechanisms of action of 5-ASA and its active metabolite N-acetyl-5-ASA are not well understood. However, there is some evidence that 5-ASA therapies may have both anti-inflammatory and immunomodulatory properties[58,60].
The influence of mesalazine on the signs and symptoms of diverticular disease has been investigated in a number of studies[61-70]. Overall, the results of these studies suggested that mesalazine, given either continuously or in cycles, may be effective at reducing symptoms (including abdominal pain, gastrointestinal symptoms and rectal bleeding) and improving health-related quality of life in patients with symptomatic, uncomplicated diverticular disease[70]. Furthermore, maintenance therapy with mesalazine may also help to maintain disease quiescence[70]. Evidence suggests that combining mesalazine with a broad-spectrum antibiotic may result in complementary and possibly synergistic effects[66]. Indeed, the combination of mesalazine and rifaximin has been shown to be significantly more effective than rifaximin alone at preventing disease recurrence and improving symptoms in patients with symptomatic uncomplicated diverticulitis and mild-to-moderate colonic obstruction[66]. This complementary effect was probably mediated via the respective influences of rifaximin and mesalazine on the colonic microflora (which seems to play a key role in determining both disease-related symptoms and diverticula inflammation[8]) and the inflammatory cascade[6]. Notably, in patients suffering from recurrent attacks of symptomatic uncomplicated diverticular disease, continuous administration of mesalazine appeared to be more effective than cyclical administration at maintaining remission[69]. While the clinical studies discussed thus far have shown a consistent benefit of mesalamine, they all utilized an open-label design.
To address this, a recent, randomized, double-blind, placebo-controlled, multicenter, 6 wk study was carried out to assess the efficacy and tolerability of mesalamine granules (Salofalk Granustix®, Dr. Falk Pharma GmbH, Freiburg, Germany) in 117 patients suffering from painful, uncomplicated diverticular disease[65]. All patients were experiencing moderate-to-severe abdominal pain caused by diverticular disease. The intention-to-treat results have been summarized in a recent abstract and showed a trend towards improved pain relief (in terms of pain intensity score, time to complete pain relief and patients with complete pain relief) with mesalamine compared with placebo, although this did not reach statistical significance. Patients receiving mesalamine reported a greater improvement in their combined median symptom score over the 6 wk compared with patients receiving placebo (P = 0.06), and patients treated with mesalamine expressed a greater satisfaction with treatment compared with those on placebo (P = 0.03). When the per-protocol population was analyzed, patients receiving mesalamine experienced a statistically significant improvement in most endpoints compared with patients receiving placebo. Currently, two other double-blind, placebo-controlled studies are being conducted. The first is comparing mesalazine 3 g/d vs placebo in order to investigate the effect of mesalazine on symptoms, inflammation and neurological alteration in adult patients suffering from symptomatic diverticular disease[71]. The second, subdivided into two different trials including the USA and the rest of the world, is comparing SPD476 MMX mesalazine 1.2 g extended release tablets, provided in three groups under treatment with different doses of mesalazine (1.2 g/d, 2.4 g/d, 4.8 g/d, respectively), in order to assess whether mesalazine is able to reduce the recurrence rate of diverticulitis compared to placebo[72,73].
There is a need for further, well-powered, controlled studies to clarify the role of single-agent mesalamine in the treatment of diverticular disease. Additionally, research to identify optimal drugs and dosing schedules in specific patient populations is required.
Probiotics in diverticular disease
Probiotics are living micro-organisms that, if consumed in sufficient numbers, can alter the host microflora and exert specific health benefits without increasing the risk of antibiotic resistance[74]. These micro-organisms have been investigated for the treatment of various digestive disorders and prevention of antibiotic-related gastrointestinal side effects[50,75-78]. Probiotics commonly include the bacteria Bifidobacterium spp., Lactobacillus spp. and certain strains of E. coli, and the budding yeast Saccharomyces cerevisiae[38]. Probiotic micro-organisms appear to have multiple modes of action, including inhibition of pathogen adherence, stimulation of immunoglobulin A secretion in Peyer’s patches and enhancement of immune system activity by controlling the balance of pro- and anti-inflammatory cytokines[75]. Moreover, probiotics may also interfere with pathogen metabolism[75].
Following early demonstration of activity against post-diverticulitic stenoses and other complications of diverticulosis[38,78], three subsequent open-label studies have investigated the efficacy of probiotics, given alone or in combination with a broad-spectrum antibiotic or 5-ASA, in preventing recurrence of symptomatic, uncomplicated diverticular disease. In the first study, the addition of non-pathogenic E. coli (Nissle strain) to antibiotic therapy (dichlorchinolinol) and an intestinal absorbent (active coal tablets) resulted in greater symptomatic improvement and longer periods of disease quiescence than with the antibiotic/absorbent regimen alone[79].
The remaining two studies investigated the efficacy of Lactobacillus casei DG (Enterolactis®, SOFAR S.p.A., Trezzano Rosa, Italy) or the high-potency, probiotic mixture VSL#3® (VSL Pharmaceuticals, Inc., Townsend, MD, USA) in combination with 5-ASA [a pH-dependent formulation of mesalazine (Pentacol®, SOFAR S.p.A., Trezzano Rosa, Italy) or balsalazide (Balzide®, Menarini Industrie Farmaceutiche S.p.A, Firenze, Italy), respectively] in patients with symptomatic uncomplicated diverticular disease in remission[67] or in patients with acute uncomplicated diverticulitis in remission, respectively[68]. The rationale for this approach is that inflammation is targeted in two ways: 5-ASA acts on the inflammatory cascade to inhibit pro-inflammatory factors; and probiotics maintain a balanced colonic microflora that prevent bacterial overgrowth and pathogen metabolism, and reduce pro-inflammatory cytokines[6,58,80]. In both studies, the probiotic/5-ASA combination performed better at preventing disease relapses and improving symptoms than the single-agent regimens[67].
CONCLUSION
Recent data strongly implicate chronic inflammation and abnormal colonic microflora in the pathogenesis of diverticular disease. This new pathogenic theory suggests that diverticular disease is predominantly an inflammatory mucosal disease, similar to IBD.
Currently, the treatment for diverticular disease requires dietary modification with antibiotic therapy and it is likely that antibiotic therapy will remain the principal component of medical therapy for diverticular disease.
The pivotal role of inflammation in the pathogenesis of diverticular disease suggests that new therapeutic tools may be required. If we consider diverticular disease to be a chronic inflammatory disease, then we need to understand how to optimally treat all aspects of the condition. As such, the final results of several ongoing, randomized, double-blind, placebo-controlled, phase III studies will be awaited with interest.
New studies are required to further investigate the classification of diverticular disease. Moreover, further large studies are required to investigate new treatment options. Once this information becomes available, the classification and medical treatment of diverticular disease may be transformed.
Footnotes
Peer reviewer: Nanne KH de Boer, MD, PhD, Department of Gastroenterology and Hepatology, VU University Medical Center, PO Box 7057, 1007 MB, Amsterdam, Netherlands
S- Editor Li LF L- Editor Lutze M E- Editor Yang C
References
- 1.Telling WHM. Discussion on diverticulitis. Proc R Soc Med. 1920;13:55–64. [PMC free article] [PubMed] [Google Scholar]
- 2.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450–454. doi: 10.1136/bmj.2.5759.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes LE. Postmortem survey of diverticular disease of the colon. I. Diverticulosis and diverticulitis. Gut. 1969;10:336–344. doi: 10.1136/gut.10.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjern F, Josephson T, Altman D, Holmström B, Mellgren A, Pollack J, Johansson C. Conservative treatment of acute colonic diverticulitis: are antibiotics always mandatory? Scand J Gastroenterol. 2007;42:41–47. doi: 10.1080/00365520600780650. [DOI] [PubMed] [Google Scholar]
- 5.Köhler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13:430–436. doi: 10.1007/s004649901007. [DOI] [PubMed] [Google Scholar]
- 6.Tursi A. New physiopathological and therapeutic approaches to diverticular disease of the colon. Expert Opin Pharmacother. 2007;8:299–307. doi: 10.1517/14656566.8.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Bogardus ST Jr. What do we know about diverticular disease? A brief overview. J Clin Gastroenterol. 2006;40 Suppl 3:S108–S111. doi: 10.1097/01.mcg.0000212603.28595.5c. [DOI] [PubMed] [Google Scholar]
- 8.Floch MH, White JA. Management of diverticular disease is changing. World J Gastroenterol. 2006;12:3225–3228. doi: 10.3748/wjg.v12.i20.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85–109. [PubMed] [Google Scholar]
- 10.Salzman H, Lillie D. Diverticular disease: diagnosis and treatment. Am Fam Physician. 2005;72:1229–1234. [PubMed] [Google Scholar]
- 11.Morini S, Zullo A, Hassan C, Tomao S, Campo SM. Diverticulosis and colorectal cancer: between lights and shadows. J Clin Gastroenterol. 2008;42:763–770. doi: 10.1097/MCG.0b013e31816200fb. [DOI] [PubMed] [Google Scholar]
- 12.Meurs-Szojda MM, Terhaar sive Droste JS, Kuik DJ, Mulder CJ, Felt-Bersma RJ. Diverticulosis and diverticulitis form no risk for polyps and colorectal neoplasia in 4,241 colonoscopies. Int J Colorectal Dis. 2008;23:979–984. doi: 10.1007/s00384-008-0510-4. [DOI] [PubMed] [Google Scholar]
- 13.Aydin HN, Remzi FH. Diverticulitis: when and how to operate? Dig Liver Dis. 2004;36:435–445. doi: 10.1016/j.dld.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Comparato G, Pilotto A, Franzè A, Franceschi M, Di Mario F. Diverticular disease in the elderly. Dig Dis. 2007;25:151–159. doi: 10.1159/000099480. [DOI] [PubMed] [Google Scholar]
- 15.Ghorai S, Ulbright TM, Rex DK. Endoscopic findings of diverticular inflammation in colonoscopy patients without clinical acute diverticulitis: prevalence and endoscopic spectrum. Am J Gastroenterol. 2003;98:802–806. doi: 10.1111/j.1572-0241.2003.07383.x. [DOI] [PubMed] [Google Scholar]
- 16.Tursi A, Brandimarte G, Giorgetti G, Elisei W, Maiorano M, Aiello F. The clinical picture of uncomplicated versus complicated diverticulitis of the colon. Dig Dis Sci. 2008;53:2474–2479. doi: 10.1007/s10620-007-0161-2. [DOI] [PubMed] [Google Scholar]
- 17.Aldoori W, Ryan-Harshman M. Preventing diverticular disease. Review of recent evidence on high-fibre diets. Can Fam Physician. 2002;48:1632–1637. [PMC free article] [PubMed] [Google Scholar]
- 18.Brodribb AJ. Treatment of symptomatic diverticular disease with a high-fibre diet. Lancet. 1977;1:664–666. doi: 10.1016/s0140-6736(77)92112-2. [DOI] [PubMed] [Google Scholar]
- 19.Painter NS. The treatment of uncomplicated diverticular disease of the colon with a high fibre diet. Acta Chir Belg. 1979;78:359–368. [PubMed] [Google Scholar]
- 20.Rafferty J, Shellito P, Hyman NH, Buie WD. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939–944. doi: 10.1007/s10350-006-0578-2. [DOI] [PubMed] [Google Scholar]
- 21.Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110–3121. doi: 10.1111/j.1572-0241.1999.01501.x. [DOI] [PubMed] [Google Scholar]
- 22.World Gastroenterology Organisation (WGO) Practice Guidelines 2007. Diverticular disease. 2007 Accessed 4 February 2009. Available from: http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/07_diverticular_disease pdf. [Google Scholar]
- 23.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363:631–639. doi: 10.1016/S0140-6736(04)15597-9. [DOI] [PubMed] [Google Scholar]
- 24.Lamanna A, Orsi A. In vitro activity of rifaximin and rifampicin against some anaerobic bacteria. Chemioterapia. 1984;3:365–367. [PubMed] [Google Scholar]
- 25.Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, Pistoia F, Brandimarte G, Festi D. Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol. 2007;13:264–269. doi: 10.3748/wjg.v13.i2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Incà R, Pomerri F, Vettorato MG, Dal Pont E, Di Leo V, Ferronato A, Medici V, Sturniolo GC. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther. 2007;25:771–779. doi: 10.1111/j.1365-2036.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 27.Latella G, Pimpo MT, Sottili S, Zippi M, Viscido A, Chiaramonte M, Frieri G. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis. 2003;18:55–62. doi: 10.1007/s00384-002-0396-5. [DOI] [PubMed] [Google Scholar]
- 28.Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. A pilot multicentre open trial. Diverticular Disease Study Group. Ital J Gastroenterol. 1992;24:452–456. [PubMed] [Google Scholar]
- 29.Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther. 1995;9:33–39. doi: 10.1111/j.1365-2036.1995.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Simpson J, Spiller R. Colonic diverticular disease. Clin Evid. 2005:543–550. [PubMed] [Google Scholar]
- 31.Chow AW. Appendicitis and diverticulitis. In: Hoeprich PD, Jordan MC, Ronald AR, editors. Infectious diseases: A treatise of infectious processes. Philadelphia: JB Lippincott; 1994. pp. 878–881. [Google Scholar]
- 32.Ferzoco LB, Raptopoulos V, Silen W. Acute diverticulitis. N Engl J Med. 1998;338:1521–1526. doi: 10.1056/NEJM199805213382107. [DOI] [PubMed] [Google Scholar]
- 33.Kellum JM, Sugerman HJ, Coppa GF, Way LR, Fine R, Herz B, Speck EL, Jackson D, Duma RJ. Randomized, prospective comparison of cefoxitin and gentamicin-clindamycin in the treatment of acute colonic diverticulitis. Clin Ther. 1992;14:376–384. [PubMed] [Google Scholar]
- 34.Schechter S, Mulvey J, Eisenstat TE. Management of uncomplicated acute diverticulitis: results of a survey. Dis Colon Rectum. 1999;42:470–475; discussion 475-476. doi: 10.1007/BF02234169. [DOI] [PubMed] [Google Scholar]
- 35.Frieri G, Pimpo MT, Scarpignato C. Management of colonic diverticular disease. Digestion. 2006;73 Suppl 1:58–66. doi: 10.1159/000089780. [DOI] [PubMed] [Google Scholar]
- 36.Brandt D, Gervaz P, Durmishi Y, Platon A, Morel P, Poletti PA. Percutaneous CT scan-guided drainage vs. antibiotherapy alone for Hinchey II diverticulitis: a case-control study. Dis Colon Rectum. 2006;49:1533–1538. doi: 10.1007/s10350-006-0613-3. [DOI] [PubMed] [Google Scholar]
- 37.Chautems RC, Ambrosetti P, Ludwig A, Mermillod B, Morel P, Soravia C. Long-term follow-up after first acute episode of sigmoid diverticulitis: is surgery mandatory?: a prospective study of 118 patients. Dis Colon Rectum. 2002;45:962–966. doi: 10.1007/s10350-004-6336-4. [DOI] [PubMed] [Google Scholar]
- 38.Petruzziello L, Iacopini F, Bulajic M, Shah S, Costamagna G. Review article: uncomplicated diverticular disease of the colon. Aliment Pharmacol Ther. 2006;23:1379–1391. doi: 10.1111/j.1365-2036.2006.02896.x. [DOI] [PubMed] [Google Scholar]
- 39.Peppercorn MA. The overlap of inflammatory bowel disease and diverticular disease. J Clin Gastroenterol. 2004;38:S8–S10. doi: 10.1097/01.mcg.0000123993.13937.ec. [DOI] [PubMed] [Google Scholar]
- 40.Di Mario F, Comparato G, Fanigliulo L, Aragona G, Cavallaro LG, Cavestro GM, Franzé A. Use of mesalazine in diverticular disease. J Clin Gastroenterol. 2006;40 Suppl 3:S155–S159. doi: 10.1097/01.mcg.0000225509.98041.4b. [DOI] [PubMed] [Google Scholar]
- 41.Tomkins AM, Bradley AK, Oswald S, Drasar BS. Diet and the faecal microflora of infants, children and adults in rural Nigeria and urban U.K. J Hyg (Lond) 1981;86:285–293. doi: 10.1017/s0022172400069035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finegold SM, Attebery HR, Sutter VL. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27:1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- 43.Floch MH, Fuchs HM. Modification of stool content by increased bran intake. Am J Clin Nutr. 1978;31:S185–S189. doi: 10.1093/ajcn/31.10.S185. [DOI] [PubMed] [Google Scholar]
- 44.Gionchetti P, Rizzello F, Lammers KM, Morselli C, Sollazzi L, Davies S, Tambasco R, Calabrese C, Campieri M. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12:3306–3313. doi: 10.3748/wjg.v12.i21.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floch MH. A hypothesis: is diverticulitis a type of inflammatory bowel disease? J Clin Gastroenterol. 2006;40 Suppl 3:S121–S125. doi: 10.1097/01.mcg.0000225502.29498.ba. [DOI] [PubMed] [Google Scholar]
- 46.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444S–450S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 47.Morini S, Hassan C, Zullo A, De Francesco V, Burattini O, Margiotta M, Panella C, Ierardi E. Epithelial cell proliferation of the colonic mucosa in diverticular disease: a case-control study. Aliment Pharmacol Ther. 2005;21:1385–1390. doi: 10.1111/j.1365-2036.2005.02492.x. [DOI] [PubMed] [Google Scholar]
- 48.Tursi A, Brandimarte G, Elisei W, Inchingolo CD, Aiello F. Epithelial cell proliferation of the colonic mucosa in different degrees of colonic diverticular disease. J Clin Gastroenterol. 2006;40:306–311. doi: 10.1097/01.mcg.0000210093.54425.72. [DOI] [PubMed] [Google Scholar]
- 49.Tursi A, Brandimarte G, Elisei W, Giorgetti GM, Inchingolo CD, Danese S, Aiello F. Assessment and grading of mucosal inflammation in colonic diverticular disease. J Clin Gastroenterol. 2008;42:699–703. doi: 10.1097/MCG.0b013e3180653ca2. [DOI] [PubMed] [Google Scholar]
- 50.Ludeman L, Warren BF, Shepherd NA. The pathology of diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:543–562. doi: 10.1053/bega.2002.0297. [DOI] [PubMed] [Google Scholar]
- 51.Banerjee S, Akbar N, Moorhead J, Rennie JA, Leather AJ, Cooper D, Papagrigoriadis S. Increased presence of serotonin-producing cells in colons with diverticular disease may indicate involvement in the pathophysiology of the condition. Int J Colorectal Dis. 2007;22:643–649. doi: 10.1007/s00384-006-0216-4. [DOI] [PubMed] [Google Scholar]
- 52.Costedio MM, Coates MD, Danielson AB, Buttolph TR 3rd, Blaszyk HJ, Mawe GM, Hyman NH. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golder M, Burleigh DE, Belai A, Ghali L, Ashby D, Lunniss PJ, Navsaria HA, Williams NS. Smooth muscle cholinergic denervation hypersensitivity in diverticular disease. Lancet. 2003;361:1945–1951. doi: 10.1016/S0140-6736(03)13583-0. [DOI] [PubMed] [Google Scholar]
- 54.Golder M, Burleigh DE, Ghali L, Feakins RM, Lunniss PJ, Williams NS, Navsaria HA. Longitudinal muscle shows abnormal relaxation responses to nitric oxide and contains altered levels of NOS1 and elastin in uncomplicated diverticular disease. Colorectal Dis. 2007;9:218–228. doi: 10.1111/j.1463-1318.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 55.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 57.Cohen RD. Review article: evolutionary advances in the delivery of aminosalicylates for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;24:465–474. doi: 10.1111/j.1365-2036.2006.03010.x. [DOI] [PubMed] [Google Scholar]
- 58.Qureshi AI, Cohen RD. Mesalamine delivery systems: do they really make much difference? Adv Drug Deliv Rev. 2005;57:281–302. doi: 10.1016/j.addr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006:CD000543. doi: 10.1002/14651858.CD000543.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Cohen HD, Das KM. The metabolism of mesalamine and its possible use in colonic diverticulitis as an anti-inflammatory agent. J Clin Gastroenterol. 2006;40 Suppl 3:S150–S154. doi: 10.1097/01.mcg.0000212654.28527.d0. [DOI] [PubMed] [Google Scholar]
- 61.Brandimarte G, Tursi A. Rifaximin plus mesalazine followed by mesalazine alone is highly effective in obtaining remission of symptomatic uncomplicated diverticular disease. Med Sci Monit. 2004;10:PI70–PI73. [PubMed] [Google Scholar]
- 62.Comparato G, Fanigliulo L, Cavallaro LG, Aragona G, Cavestro GM, Iori V, Maino M, Mazzocchi G, Muzzetto P, Colla G, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12-month follow-up. Dig Dis Sci. 2007;52:2934–2941. doi: 10.1007/s10620-007-9766-8. [DOI] [PubMed] [Google Scholar]
- 63.Comparato G, Fanigliulo L, Aragona G, Cavestro GM, Cavallaro LG, Leandro G, Pilotto A, Nervi G, Soliani P, Sianesi M, et al. Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment? Dig Dis. 2007;25:252–259. doi: 10.1159/000103896. [DOI] [PubMed] [Google Scholar]
- 64.Di Mario F, Aragona G, Leandro G, Comparato G, Fanigliulo L, Cavallaro LG, Cavestro GM, Iori V, Maino M, Moussa AM, et al. Efficacy of mesalazine in the treatment of symptomatic diverticular disease. Dig Dis Sci. 2005;50:581–586. doi: 10.1007/s10620-005-2478-z. [DOI] [PubMed] [Google Scholar]
- 65.Kruis W, Meier E, Schumacher M, Mickisch O, Schneider W, Greinwald R, Mueller R. Treatment of painful diverticular disease of the colon with mesalamine: a placebo-controlled study. Gastroenterology. 2007;132:A–191 (Abstract S1187). [Google Scholar]
- 66.Tursi A, Brandimarte G, Daffinà R. Long-term treatment with mesalazine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of colon. Dig Liver Dis. 2002;34:510–515. doi: 10.1016/s1590-8658(02)80110-4. [DOI] [PubMed] [Google Scholar]
- 67.Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: a prospective, randomized, open-label study. J Clin Gastroenterol. 2006;40:312–316. doi: 10.1097/01.mcg.0000210092.77296.6d. [DOI] [PubMed] [Google Scholar]
- 68.Tursi A, Brandimarte G, Giorgetti GM, Elisei W, Aiello F. Balsalazide and/or high-potency probiotic mixture (VSL#3) in maintaining remission after attack of acute, uncomplicated diverticulitis of the colon. Int J Colorectal Dis. 2007;22:1103–1108. doi: 10.1007/s00384-007-0299-6. [DOI] [PubMed] [Google Scholar]
- 69.Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Continuous versus cyclic mesalazine therapy for patients affected by recurrent symptomatic uncomplicated diverticular disease of the colon. Dig Dis Sci. 2007;52:671–674. doi: 10.1007/s10620-006-9551-0. [DOI] [PubMed] [Google Scholar]
- 70.Trepsi E, Colla C, Panizza P, Polino MG, Venturini A, Bottani G, De Vecchi P, Matti C. [Therapeutic and prophylactic role of mesalazine (5-ASA) in symptomatic diverticular disease of the large intestine. 4 year follow-up results] Minerva Gastroenterol Dietol. 1999;45:245–252. [PubMed] [Google Scholar]
- 71.Mesalazine Granules vs. Placebo for the Prevention of Recurrence of Diverticulitis ( NCT00695643) 2009. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00695643.
- 72.Prevention of Recurrence of Diverticulitis (PREVENT 1) ( NCT00545740) 2009. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00545740.
- 73.Prevention of Recurrence of Diverticulitis (PREVENT 2) ( NCT00545103) 2009. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00545103.
- 74.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 75.Gionchetti P, Amadini C, Rizzello F, Venturi A, Palmonari V, Morselli C, Romagnoli R, Campieri M. Probiotics--role in inflammatory bowel disease. Dig Liver Dis. 2002;34 Suppl 2:S58–S62. doi: 10.1016/s1590-8658(02)80166-9. [DOI] [PubMed] [Google Scholar]
- 76.Quigley EM. Probiotics in the management of colonic disorders. Curr Gastroenterol Rep. 2007;9:434–440. doi: 10.1007/s11894-007-0055-7. [DOI] [PubMed] [Google Scholar]
- 77.Sanders ME. Probiotics. Food Technology. 1999;53:66–77. [Google Scholar]
- 78.Giaccari S, Tronci S, Falconieri M, Ferrieri A. Long-term treatment with rifaximin and lactobacilli in post-diverticulitic stenoses of the colon. Riv Eur Sci Med Farmacol. 1993;15:29–34. [PubMed] [Google Scholar]
- 79.Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol. 2003;15:313–315. doi: 10.1097/01.meg.0000049998.68425.e2. [DOI] [PubMed] [Google Scholar]
- 80.Borruel N, Casellas F, Antolín M, Llopis M, Carol M, Espíin E, Naval J, Guarner F, Malagelada JR. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am J Gastroenterol. 2003;98:865–870. doi: 10.1111/j.1572-0241.2003.07384.x. [DOI] [PubMed] [Google Scholar]


