Abstract
Anti-tumour necrosis factor-α (TNF) therapy has revolutionised the management of chronic inflammatory conditions. With ever increasing numbers of patients being treated with these agents, uncommon adverse reactions will inevitably occur more frequently. Cutaneous manifestations are associated with many of these chronic conditions and can complicate anti-TNF therapy in about 20% of cases. Vasculitic complications are rarely associated with anti-TNF therapy. Henoch-Schönlein purpura (HSP), a small vessel vasculitis, has been described following infliximab and etanercept therapy but never with adalimumab, a fully humanized TNF antibody. The risk of such immune-mediated reactions is theoretically less with adalimumab compared to infliximab but can still occur. Here we report the first case in the literature of HSP that can be attributed to the use of adalimumab in a 19-year-old male with recalcitrant Crohn’s disease.
Keywords: Henoch-Schönlein purpura, Adalimumab, Anti-TNF therapy, Leukocytoclastic vasculitis, Crohn's disease
INTRODUCTION
Anti-tumour necrosis factor-α (TNF) therapies have radically improved the management of chronic inflammatory conditions that are frequently associated with significant morbidity and a substantial burden to health service resources. These biologic agents are efficacious both for induction and long-term maintenance of remission in several conditions including; rheumatoid arthritis[1], ankylosing spondylitis[2], psoriasis and psoriatic arthritis[3], and the idiopathic inflammatory bowel diseases [Crohn’s disease (CD)[4-8], ulcerative colitis[9]].
Anti-TNF agents can be broadly divided into neutralizing antibodies (infliximab, adalimumab, certolizumab) and recombinant receptors (etanercept). The neutralising antibodies are commonly used to treat patients with refractory, steroid-dependent, or fistulising CD. Adalimumab (HUMIRA®, Abbott), marketed in the UK for 6 years, is currently used primarily in those who have lost response to, or are unable to tolerate infliximab.
A number of cutaneous adverse events have been reported with anti-TNF therapy including; infusion and injection site reactions, psoriatic eruptions, lupus-like disorders, vasculitis, granulomatous reactions, and cutaneous infections/ neoplasms[10,11]. Whilst infusion and injection site reactions are unquestionably related to administration of anti-TNF agents, all other events have a varying strength of association and severity, not necessarily requiring drug discontinuation. Eczematous/ psoriatic eruptions and infections are the commonest cutaneous complications with vasculitic manifestations occurring less frequently[12,13]. Henoch-Schönlein purpura (HSP), a small vessel vasculitis, is an extremely rare complication of anti-TNF therapy but has been described following infliximab[14] and etanercept[15,16] therapy. Although adalimumab is associated with various features of HSP there are no formal reports of adalimumab-induced HSP in the literature. The risk of such immune-mediated reactions is theoretically less with adalimumab, a fully humanized TNF antibody, compared to infliximab, a chimeric monoclonal antibody, but can still occur.
CASE REPORT
A 19-year-old male non-smoker with quiescent ileo-colonic and perianal CD on combination therapy with azathioprine (AZA) and fortnightly adalimumab, presented with a 10 d history of a purpuric rash on both legs and diffuse joint pain and swelling. These symptoms were preceded by a short-lived coryzal illness from which he had made a full recovery. There were no gastrointestinal symptoms of note. He had taken his most recent dose of adalimumab 2 d prior to developing the rash.
His CD, initially diagnosed in 1999, was not associated with any extra-gastrointestinal manifestations. Over the decade since diagnosis his CD had failed to respond to various established (5-aminosalicylates, AZA, methotrexate, infliximab) and experimental (natalizumab and leukocytapheresis (adacolumn)) medical therapies, and surgical interventions including a subtotal colectomy, ileal resection and ileostomy formation had been required. Adalimumab therapy was initiated 7 mo previously due to grumbling active disease on AZA monotherapy. The patient received a routine induction regimen with 160 mg at week 0 and 80 mg at week 2, and continued on a fortnightly maintenance dose of 40 mg. This was well tolerated achieving a prolonged remission.
On examination he was haemodynamically stable with a non-blanching purpuric rash on his lower limbs (Figure 1), marked synovitis and swelling of both hands and a tender swollen right elbow. There were no signs of meningism. The full blood count was normal apart from a mildly elevated eosinophil count of 0.44 × 109/L (normal range 0-0.4 × 109/L). The C-reactive protein (CRP) was 15.1 mg/L (normal range 0-5 mg/L) and erythrocyte sedimentation rate (ESR) 13 mm/h (normal range 1-5 mm/h). Urea and electrolytes and liver function tests were within normal limits and a chest x-ray and x-rays of the swollen joints were unremarkable. The total IgA was elevated at 4.38 g/L (normal range 0.5-3.5 g/L) with normal IgG and IgM levels. The results of other blood tests that were performed are shown in Table 1. Urinalysis showed proteinuria and numerous amorphous deposits but no casts, red or white cells. The total urine protein was 0.2. An ultrasound scan of the renal tract was normal. A skin biopsy was also taken under local anesthetic.
Figure 1.
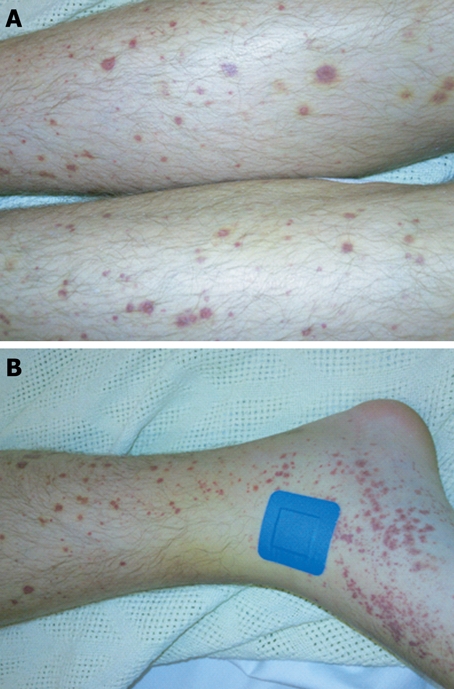
Vasculitic skin rash of Henoch-Schönlein purpura. A: Typical palpable purpuric lesions on the legs; B: Site of skin punch biopsy.
Table 1.
Blood tests
| Blood cultures: No growth |
| ASO titre: Negative |
| HSV 1/2, CMV antibodies: Not detected |
| EBV IgM: Not detected |
| EBV IgG: Positive consistent with past infection |
| Parvovirus IgM: Not detected |
| Parvovirus IgG positive consistent with past infection |
| HBV/HCV serology negative |
| ANA, ANCA, Rheumatoid factor: negative |
| C3, C4 levels: Normal |
| Cryoglobulins: Not detected |
| Serum protein electrophoresis: Normal |
The patient remained systemically well and was managed conservatively in conjunction with the rheumatologists and nephrologists. After a few days the pain and joint swelling improved dramatically and the rash began to fade. The skin biopsy confirmed a leukocytoclastic vasculitis (Figure 2) consistent with the clinical diagnosis of HSP, although the precise aetiology was unclear (recent viral illness vs adalimumab). A multi-disciplinary decision was taken to commence a further trial of adalimumab, given the favorable response of his previously recalcitrant CD to treatment and a paucity of evidence linking it to HSP. After a repeat dose of adalimumab on the ward, which was well tolerated, he was discharged with outpatient follow-up.
Figure 2.
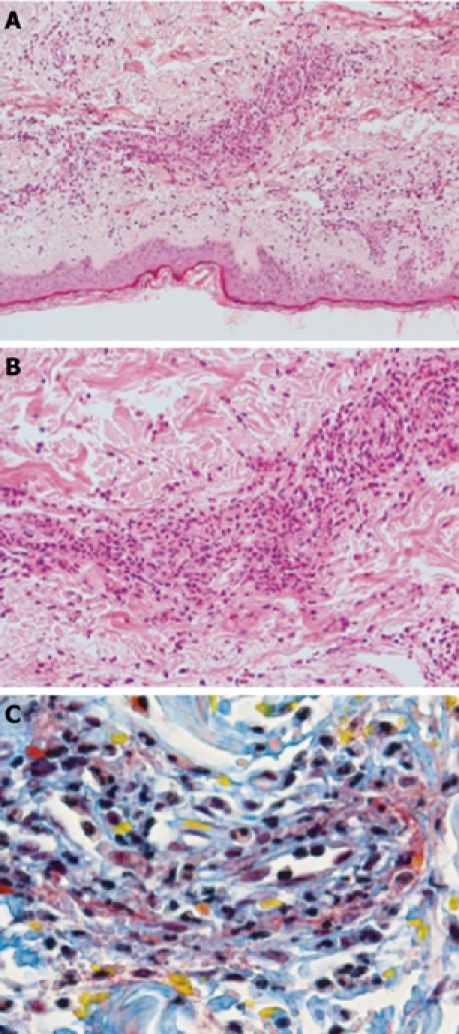
Punch biopsy of skin showing leukocytoclastic vasculitis. A: Low (× 20); B: High (× 100) power views (H&E); C: MSB stain highlighting fibrinoid necrosis (red) and extravasation of red blood cells (yellow) in leukocytoclastic vasculitis.
He re-presented 3 d later with polyarthropathy and a florid vasculitic rash on his legs. Although he remained systemically well he was finding it difficult to weight bear due to pain in his knees and right ankle. Baseline investigations were again unremarkable apart from raised inflammatory markers (CRP 22.2 mg/L, ESR 8 mm/h) and serum IgA level. Urinalysis revealed proteinuria and microscopic haematuria. The right ankle was aspirated excluding septic and crystal arthropathy. He was treated with intra-articular (right ankle) and systemic steroids. His symptoms rapidly improved and he was discharged home on a reducing dose of prednisolone. Adalimumab therapy was discontinued. His HSP-related symptoms completely resolved over the next few weeks and did not recur on completion of the course of steroids. Seven months later his CD is mildly active again on AZA monotherapy and he is being considered for a trial of a novel biological agent (anti-IL-12/23, ustekinumab) in CD.
DISCUSSION
HSP is a multi-system small vessel vasculitis, usually affecting children, which can be defined according to multiple classifications[17]. It commonly manifests with palpable purpura along with acute arthritis (typically involving the ankles, knees and elbows), enteritis and nephritis (causing haematuria and/or proteinuria). Neurological, pulmonary, cardiac and genitourinary complications occur rarely. The prognosis is generally favorable in children, where symptoms tend to last about 4 wk, resolving spontaneously. Adult onset is rare but associated with more severe manifestations and a poor prognosis.
Although the cause is unknown, HSP can develop after various viral and bacteria infections and as an idiosyncratic reaction to several drugs. IgA is thought to play central role in the immunopathogenesis. The rare association between HSP and CD is recognized but poorly understood[18,19].
The diagnosis is based on the combination of symptoms, as very few other diseases cause the same symptoms together. There are no diagnostic laboratory investigations although platelet count, urea, creatinine, IgA (50%), CRP and ESR may be elevated. Histology typically shows a hypersensitivity vasculitis and immunofluorescence demonstrates IgA and C3 (complement system protein) in the blood vessel wall. Treatment is usually supportive and directed against the precipitating cause. Immunosuppressants and immunoglobulin infusions are occasionally required in serious cases.
There have been several prior reports of localised cutaneous adverse events, including necrotizing and leukocytoclastic vasculitis, with infliximab and etanercept[20]. Most cases of cutaneous vasculitis develop within 3 mo of initiating anti-TNF therapy. HSP complicating anti-TNF therapy appears to be rare. The Medicines and Healthcare products Regulatory Agency (MHRA) has received one report each for etanercept and infliximab since 1999. In the literature there are two reports of HSP following etanercept[15,16] and one following infliximab[14]. It has not yet been associated with adalimumab although features of it such as vasculitic rash, arthralgia and glomerulonephritis have. Since January 2003 the MHRA has received 7 reports of vasculitic rash, 27 of arthralgia and 4 of glomerulonephritis for adalimumab. There is one case report of a cutaneous small vessel vasculitis and necrotizing crescentic glomerulonephritis in an anti-neutrophil cytoplasmic antibody positive patient that resolved on withdrawal of adalimumab and immunosuppressive therapy[21]. It is possible that some of these reactions may have been manifestations of undiagnosed HSP.
Here we describe the first case of adalimumab-associated HSP occurring after 7 mo of treatment for CD. Discontinuation of the drug and treatment with systemic steroids led to the complete resolution of the vasculitis and polyarthropathy. HSP has now been described with all three commonly used anti-TNF agents- and occurred after several months of anti-TNF therapy in all cases. Thus HSP or features of it, occurring during chronic use of these biologics must be considered as possibly related to the therapy. HSP manifests more severely and is associated with a worse prognosis in adults. Renal involvement, a key discriminator of long-term outcome, is more common in this group. It has been reported that up to one third of HSP patients with renal involvement eventually progress to end stage renal failure[22]. Given that the use of anti-TNF therapies is likely to increase further in the future, prescribers need to be aware of this potentially serious complication.
Footnotes
Peer reviewers: Won Ho Kim, MD, Professor, Department of Internal Medicine, Yonsei Uiversity College of Medicine, 134 Shinchon-dong Seodaemun-ku, Seoul 120-752, South Korea; Shmuel Odes, Professor, MD, Department of Gastroenterology and Hepatology, Soroka Medical Center, POBox 151, Beer Sheva 84101, Israel
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
References
- 1.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:iii–iv, xi-xiii, 1-229. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- 2.McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, Hill RA, Jones A, Mujica Mota R, Walley T. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–158, iii-iv. doi: 10.3310/hta11280. [DOI] [PubMed] [Google Scholar]
- 3.Tobin AM, Kirby B. TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs. 2005;19:47–57. doi: 10.2165/00063030-200519010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 5.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 10.Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, Henckaerts L, Van Assche G, Vermeire S, Rutgeerts P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 11.Lee HH, Song IH, Friedrich M, Gauliard A, Detert J, Röwert J, Audring H, Kary S, Burmester GR, Sterry W, et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol. 2007;156:486–491. doi: 10.1111/j.1365-2133.2007.07682.x. [DOI] [PubMed] [Google Scholar]
- 12.Saint Marcoux B, De Bandt M. Vasculitides induced by TNFalpha antagonists: a study in 39 patients in France. Joint Bone Spine. 2006;73:710–713. doi: 10.1016/j.jbspin.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Casals M, Brito-Zerón P, Cuadrado MJ, Khamashta MA. Vasculitis induced by tumor necrosis factor-targeted therapies. Curr Rheumatol Rep. 2008;10:442–448. doi: 10.1007/s11926-008-0072-z. [DOI] [PubMed] [Google Scholar]
- 14.Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101. doi: 10.1097/RHU.0b013e31819bca9e. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Kasama R, Evangelisto A, Elfenbein B, Falasca G. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249–251. doi: 10.1097/01.rhu.0000239901.34561.5e. [DOI] [PubMed] [Google Scholar]
- 16.Duffy TN, Genta M, Moll S, Martin PY, Gabay C. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:S106. [PubMed] [Google Scholar]
- 17.Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35–40. doi: 10.1097/00002281-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cassater D, Gambaro G, Fabris A, Cena C, Calabria S, Capelluto S, Lupo A. Henoch-Schönlein purpura and Crohn's disease in a family. J Nephrol. 2006;19:387–390. [PubMed] [Google Scholar]
- 19.Saulsbury FT, Hart MH. Crohn's disease presenting with Henoch-Schönlein purpura. J Pediatr Gastroenterol Nutr. 2000;31:173–175. doi: 10.1097/00005176-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Mohan N, Edwards ET, Cupps TR, Slifman N, Lee JH, Siegel JN, Braun MM. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955–1958. [PubMed] [Google Scholar]
- 21.Simms R, Kipgen D, Dahill S, Marshall D, Rodger RS. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11–e14. doi: 10.1053/j.ajkd.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet. 2007;369:976–978. doi: 10.1016/S0140-6736(07)60474-7. [DOI] [PubMed] [Google Scholar]


