Abstract
The serine proteinase inhibitor α-1 antitrypsin (AAT) is produced principally by the liver at the rate of 2 g/d. It is secreted into the circulation and provides an antiprotease protective screen throughout the body but most importantly in the lung, where it can neutralise the activity of the serine protease neutrophil elastase. Mutations leading to deficiency in AAT are associated with liver and lung disease. The most notable is the Z AAT mutation, which encodes a misfolded variant of the AAT protein in which the glutamic acid at position 342 is replaced by a lysine. More than 95% of all individuals with AAT deficiency carry at least one Z allele. ZAAT protein is not secreted effectively and accumulates intracellularly in the endoplasmic reticulum (ER) of hepatocytes and other AAT-producing cells. This results in a loss of function associated with decreased circulating and intrapulmonary levels of AAT. However, the misfolded protein acquires a toxic gain of function that impacts on the ER. A major function of the ER is to ensure correct protein folding. ZAAT interferes with this function and promotes ER stress responses and inflammation. Here the signalling pathways activated during ER stress in response to accumulation of ZAAT are described and therapeutic strategies that can potentially relieve ER stress are discussed.
Keywords: α-1 antitrypsin, Unfolded protein response, Endoplasmic reticulum stress, Apoptosis, Autophagy, NFκB
INTRODUCTION
Conformational disorders are characterised by protein misfolding and are dependent on the accumulation or localisation of specific misfolded proteins to various cellular organelles. These disorders are caused by inherited or acquired modifications in protein folding culminating in accumulation of aberrant protein conformers and include metabolic disorders such as diabetes, the neurological disorders Alzheimer’s and Huntington’s diseases as well as a number of pulmonary disorders such as cystic fibrosis and α-1 antichymotrypsin deficiency. α-1 antitrypsin (AAT) deficiency is a genetic conformational disease associated with accumulation of misfolded AAT in the endoplasmic reticulum (ER) of hepatocytes and other AAT-producing cells[1]. It predisposes sufferers to early onset emphysema or liver disease. It is the only known cause of genetic emphysema and is the most common genetic cause of liver disease in children.
α-1 ANTITRYPSIN DEFICIENCY
AAT deficiency is a hereditary disorder associated with mutations in the SERPINA1 gene that can affect the liver and the lungs. The most common disease-causing mutation is a single nucleotide polymorphism encoding a glutamic acid to lysine substitution at position 342 of the mature protein (Glu342Lys) that leads to misfolding and intracellular polymerisation of AAT. This ‘‘Z’’ mutation, as it is called, modifies the protein’s reactive centre loop enabling it to interact with another ZAAT molecule in a process that can continue until large polymers form[2,3]. The normal AAT protein is referred to MAAT; it and all other conformers are named based on their electrophoretic mobility in isoelectric focusing gels and in some cases on the geographical location where they were first identified e.g. MMalton. MAAT is a serine proteinase inhibitor with activity against neutrophil elastase (NE), a powerful neutrophil-derived protease capable of degrading a wide range of soluble proteins and most connective tissue components of the lung. Although up to 100 different AAT alleles have been identified it is the Z allele that is predominantly associated with disease.
In the 1970s the largest unbiased nationwide AAT deficiency screen of newborns was carried out by Laurell and Sveger in Sweden[4]. Of 200 000 individuals studied 127 were identified as being homozygous for the Z allele and have been studied since. Notwithstanding Sveger’s study, globally ZAAT deficiency is an under-diagnosed condition; many being diagnosed as non-responsive asthmatics or chronic obstructive pulmonary disease (COPD) sufferers. Since 1997 the World Health Organization together with the American Thoracic Society and European Respiratory Society have advocated targeted detection programmes for AAT deficiency in all COPD, non-responsive asthma and cryptogenic liver disease patients and also for first degree relatives of AAT deficient individuals[5]. In Ireland emerging data from the national targeted detection programme, the only government funded AAT deficiency targeted detection programme, indicates that AAT deficiency is twice as prevalent as previously estimated with one of the highest frequencies in Europe. These findings support the concept that AAT deficiency is not a rare disease but a disease that is rarely diagnosed.
Pathophysiology
Although ZAAT deficiency is associated with lung and liver manifestations, both organs are rarely affected in the same individual. AAT deficiency is the most common genetic diagnosis in children undergoing liver transplantation[6,7]. It is also an important cause of hepatocellular carcinoma in adults[8]. Even so there is a marked heterogeneity in the liver disease phenotype. The histological hallmark of the disease is the presence of ZAAT-containing globules in some but not all hepatocytes. These glycoprotein-containing globules stain positive with periodic acid-Schiff (PAS) after treatment with diastase[9]. Transgenic ZAAT mice (PiZ mice) can develop hepatic inflammation and carcinomas that are associated with evidence of these characteristic intra-hepatocytic globules[10,11]. Although relatively little is known about the pathogenesis of hepatocellular carcinoma in ZAAT deficiency, based on their observations in ZAAT mouse livers and studies on biopsies from ZZ homozygous individuals Perlmutter et al[12] have proposed a model whereby increased hepatocellular proliferation occurs in resting globule-devoid hepatocytes in response to an unidentified ‘‘trans’’ signal from adjacent globule-containing cells. It is these globule-devoid cells that proliferate and in which adenomas and later carcinomas develop[13,14].
In contrast to the liver disease which is believed to arise due to a toxic gain of function resulting from intracellular accumulation of ZAAT, the lung manifestations occur due to a loss of function. The liver is the body’s major source of AAT. Decreased AAT secretion from the liver, decreases circulating AAT levels and markedly diminishes the lungs’ anti-NE protective defences. This can lead to a number of deleterious events. In addition to causing direct damage to airway epithelial cells, unchecked NE activity can promote goblet cell hyperplasia and enhance mucus secretion. Impaired mucociliary clearance, cleavage of complement, immunoglobulins and cell surface receptors occurring as a direct result of too much NE on the respiratory epithelial surface also has important consequences for innate immunity[15]. Efficient neutrophil killing of microorganisms is also impaired. Clinically, ZAAT-related lung disease is associated with aggressive emphysema in the 4th and 5th decades which is aggravated by cigarette smoking[16].
Modifiers of liver disease
The variable clinical presentation among AAT deficient individuals suggests that genetic and environmental disease modifiers make an important contribution to disease severity and outcome. In Sveger’s study only 8%-10% of ZZ homozygotes developed clinically significant liver disease[17]. A study by Wu et al[18] demonstrated that there is a delay in intracellular disposal of ZAAT after gene transfer into fibroblast cell lines from ZZ homozygotes with liver disease compared to cell lines from homozygotes without liver disease. This suggests that differences exist in the quality control recognition and/or disposal mechanisms for misfolded proteins in cells of ZZ liver disease sufferers. ER mannosidase I (ERManI) is a quality control factor that plays a key role in the sorting and targeting of misfolded glycoproteins for proteosomal degradation. ZAAT is a misfolded asparagine-linked secretory glycoprotein and as such, the removal of mannose units from its N-linked glycans represents a crucial early event in its disposal. ERManI plays a stochastic and rate limiting role in distinguishing and targeting ZAAT for degradation. Pan et al[19] have identified a G/A single nucleotide polymorphism (SNP) at position 4567 of ERManI that mediates translational suppression of ERManI and have proposed that the ‘‘A’’ SNP can accelerate the onset of end stage liver disease in AAT deficiency. They showed that substitution of ‘‘A’’ for ‘‘G’’ disrupts a potential microRNA (miRNA) binding site for miR-205 in ERManI, and suggest that this may facilitate negative regulation by other miRNAs and concomitant translational repression of ERManI. Another potential gene modifier associated with ZAAT-related liver disease is regulator of G signaling 16 (RGS16) which is up regulated in the liver of ZAAT-deficient individuals[20]. Interestingly the degree of up regulation of RGS16 in vivo correlates with hepatic levels of insoluble ZAAT.
ENDOPLASMIC RETICULUM STRESS
A major area of interest in the biology of ZAAT deficiency is ER stress. The ER is an organelle which extends from the nuclear membrane. It is the site of translation, folding, modification and transport of membrane and secreted proteins and consists of an extensive membranous network of tubes and cisternae. Correct folding of newly made proteins is made possible by several ER-resident chaperones, foldases and enzymes including, for example, Hsc proteins, calnexin, calreticulin and protein disulfide isomerases. Properly folded proteins are transported from the ER to the Golgi apparatus via COPII vesicles. Incorrectly folded proteins become complexed with Bip/Grp78 and are targeted to the Sec61/Derlin/p97 VCP channel located in the ER membrane for extrusion into the cytosol and degradation by proteosomal and non-proteosomal mechanisms[21]. Intracellular conditions such as increased temperature, high salt concentrations, unchecked protease activity and the presence of other unfolded proteins can lead to intracellular protein damage. Together with aberrant oxidative, glycation, nitrosylation or deamination events, these conditions promote protein damage and possible misfolding in the ER. Chaperone proteins facilitate the recognition of these incorrectly folded proteins. Misfolded ZAAT protein accumulation in the ER of hepatocytes and other AAT-expressing cells is a hallmark of ZAAT deficiency[6,22,23].
Perturbation of ER homeostasis can induce ER stress and a number of discrete signalling pathways can be activated as a result[1]. These include the unfolded protein response (UPR) which is a conserved signalling pathway that measures unfolded protein levels in the ER and adjusts the production of ER chaperones, foldases and degradation factors appropriately to keep levels of misfolded proteins in the ER lumen acceptably low. ER stress can also lead to apoptotic cell death mediated by the activation of specific caspases. A third signalling pathway activated in response to ER stress in the ER overload pathway (EOR). This is characterised by activation of the transcription factor NFκB which regulates proinflammatory gene expression. Much is now known regarding the effect of ZAAT expression on ER stress responses from studies done with cell culture models, human monocytes and airway epithelial cells, and human and animal liver biopsies[6,20,22-30].
Endoplasmic reticulum overload response
The transcription factor NFκB is activated by a range of diverse stimuli. Many of these engage cell surface receptors and initiate intracellular signal transduction pathways that converge at IκB kinase (IKK). IKK can also be activated in response to accumulation of misfolded proteins in the ER. This enzyme complex phosphorylates the NFκB inhibitory proteins IκB-α, -β and -γ on key serine residues, leading to their ubiquitination and ultimate recognition and degradation by the proteasome. After removal of IκB protein, NFκB is free to translocate to the nucleus due the exposure of its nuclear localisation sequences, where it binds to NFκB recognition elements in the promoter of target genes and promotes their transcription.
We and others have investigated NFκB activation in the context of ER accumulation of misfolded ZAAT in Chinese hamster ovary (CHO) and 16HBE14o- human bronchial epithelial cell lines and in human monocytes[27,30]. In all cell types studied expression of ZAAT is associated with activation of NFκB. In liver cells in cell culture, animal models of ZAAT deficiency and also in vivo in liver biopsies from ZZ homogygous individuals, NFκB activation has also been detected[26,29]. In liver cells it is feasible that activation of NFκB in response to accumulation of ZAAT could mediate inflammation and neutrophil infiltration via up regulation of interleukin-8. Given that NFκB is involved in inflammation-associated carcinogenesis, EOR is likely to have important role in the pathogenesis of hepatocellular carcinoma in ZAAT-deficient individuals[31].
Apoptosis
Although NFκB is largely cytoprotective, when the balance between the misfolded protein load in the ER and the ability to correct ER homeostasis cannot be restored, cell death may occur via apoptosis. This is the second ER stress-induced response. Apoptosis induced by ER stress differs from conventional apoptosis by the involvement of an ER-resident caspase, caspase-4[32]. Two other mechanisms of ER stress-induced apoptosis are associated with the UPR (see below) and involve PERK-CHOP-GADD34 and IRE1-TRAF2-JNK.
We evaluated ER stress-induced apoptosis in HEK293 cells using the ER stress inducer thapsigargin and MAAT and ZAAT transgenes[28]. Our studies showed that, similar to thapsigargin treatment, expression of ZAAT, but not MAAT, induced cleavage of both procaspase-4 and downstream procaspase-7. However the role of caspase-4 in ER stress-induced apoptosis appears to be stimulus- and cell type- specific. In order to evaluate its role in ZAAT-induced cell death we performed gene knock-down studies using caspase-4 or GAPDH siRNAs. The caspase-4 siRNA resulted in greater than 70% inhibition of caspase-4 protein production. However, surprisingly this did not promote cell survival in ZAAT expressing cells, nor did it inhibit ZAAT-induced caspase-7 activation. We concluded that caspase-4 is not at the apex of the ZAAT/ER-induced apoptotic signalling pathway, nor is it essential for ER-mediated apoptosis in HEK293 cells and it is unlikely to represent a useful target for interfering with ER stress-induced apoptosis by ZAAT.
Using TUNEL staining, terminal apoptosis could be detected in HEK293 cells expressing ZAAT compared to mock-transfected cells[28]. In contrast to these in vitro studies, increased apoptosis has not been detected histologically or by TUNEL staining in the livers of ZAAT mice. This may be related to the robust regenerative and cell survival properties of hepatocytes and emphasizes once again the highly specific stimulus- and cell type-specific nature of ER stress responses. The mechanism by which the caspase pathway is inhibited in globule-containing hepatocytes is not known but it could be related to the known antiapoptotic effects of MAAT and ZAAT[30,33] which, in the case of MAAT, are related to direct binding and inhibition of caspase-3. How ZAAT might gain access to caspase-3 in liver cells and in which cellular compartment this may occur are not known.
Unfolded protein response
The third important pathway activated by ER stress is the unfolded protein response[21,34]. This is an integrated signalling network activated by ER stress. UPR governs repression of translation and results in the synthesis of molecular chaperones, degradation factors and ER-resident enzymes, which co-operate to restore correct protein folding in the ER. If this response fails, UPR activation may lead to induction of ER-associated degradation (ERAD) to effectively remove terminally misfolded proteins.
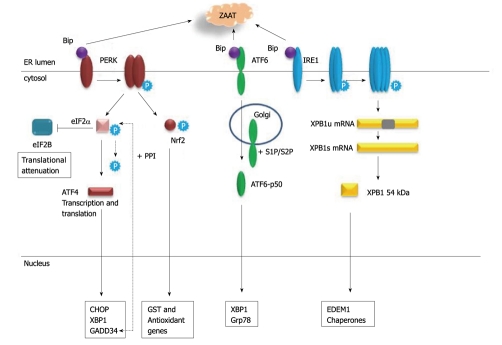
Figure 1 summarises the major events that occur during UPR. Signalling involves three key ER membrane-localised proteins PERK, ATF6 and IRE1 which are normally maintained in an inactive state by association with Bip/Grp78. In the presence of misfolded proteins in the ER lumen, Bip is titrated away enabling oligomerization and activation of each of these factors. Activation of PERK results in phosphorylation and inactivation of the eukaryotic initiation factor eIF2α which interferes with global protein synthesis via inhibiting eIF2B. Paradoxically this event actually enhances transcription and translation of the ATF4 transcription factor[35]. ATF4 induces expression of the transcription factor CHOP (also called GADD153) which can trigger the dephosphorylation of eIF2α via induction of GADD34 which in turn stimulates protein phosphatase-1 (PP1)[36]. Prolonged CHOP activation can also lead to apoptosis characterised by a decrease in Bcl-2 protein and translocation of Bax from the cytosol to the mitochondria[37].
Figure 1.
Unfolded protein response. Accumulation of misfolded proteins in the endoplasmic reticulum lumen (e.g. Z α-1 antitrypsin) titrates Bip/Grp78 away from PERK, ATF6 and IRE1. PERK dimerises, becomes phosphorylated and inactivates eIF2α by phosphorylation which blocks translation via eIF2B. ATF4 gene and protein expression is induced leading to ATF4-regulated gene expression, including GADD34 which stimulates protein phosphatase-1 (PP1) and triggers dephosphorylation of eIF2α. Nrf2 is phosphorylated and activated by PERK, translocates to the nucleus and induces antioxidant gene expression. ATF6 is transported to the Golgi and cleaved by the proteases S1P and S2P to generate the active ATF6p50 transcription factor which can activate expression of XBP1 and chaperone genes. IRE1 is phosphorylated, assembles into multimers and via its RNAse activity uses unspliced XBP1 (uXBP1) mRNA as a substrate to generate spliced XBP1 (XBP1s) mRNA which encodes a transcription factor that regulates expression of EDEM1 and chaperones.
PERK can also directly phosphorylate Nrf2[38], a transcription factor that together with Maf binds to antioxidant response elements in the promoters of many antioxidative genes, and promotes their transcription[39]. Nrf2 also has a role in regulating the proteasome system that degrades damaged and misfolded proteins, in particular the proteosomal subunit PSMB6[40]. Impaired Nrf2 signaling can significantly impair proteosomal activity and heighten ER stress responses in the airways. Malhotra et al[40] suggest that pharmacological approaches which augment Nrf2 activity may up-regulate antioxidant defenses, relieve ER stress, and could be important for treatment of COPD. How this might affect the liver manifestations of ER stress in the context of intracellular accumulation of ZAAT remains unclear.
Another cascade activated in the UPR involves ATF6 (Figure 1). Full length ATF6p90 is transported to the Golgi apparatus where it is cleaved by two proteases S1P and S2P[41] to generate a basic leucine zipper transcription factor ATF6p50 that migrates to the nucleus and activates transcription of genes under the control of ER stress response elements (ERSE) e.g. Grp78, XBP-1.
IRE1 is an endoribonuclease that becomes activated by phosphorylation when it forms a dimer. Following its subsequent assembly into multimers IRE1 acquires RNAse activity and acts on XBP1 mRNA as a substrate[42]. The resulting alternatively spliced transcript encodes a transcriptionally active 54kDa XBP1 protein which regulates the transcriptional induction of important factors involved in degradation of misfolded proteins e.g. ER degradation-enhancing α-mannosidase-like protein (EDEM) 1. Mulimerisation of IRE1 can also lead to JNK activation via TRAF2 and ASK1 and potentially promote apoptosis[34].
Although we know that all three arms of the UPR can be activated in response to overexpression of ZAAT in CHO, HEK293, HepG2 and 16HBE14o- cells[27-30] and basally in human peripheral blood monocytes isolated from ZZ homozygous individuals[23], these pathways do not appear to be activated in inducible models of ZAAT deficiency liver disease or in liver cells in vivo. Indeed a number of studies have failed to detect activation of the UPR in cell culture and animal liver models of ZAAT deficiency[20,26] and it has been speculated that the absence of UPR signalling permits the survival of cells that have accumulated high levels of ZAAT i.e. globule-containing hepatocytes.
Recently it has emerged that ZAAT deficiency is also associated with aberrant immune cell function[23]. Peripheral blood monocytes express AAT. Carroll et al[23] have generated evidence for UPR activation in monocytes from individuals with ZAAT deficiency and link this phenomenon to an altered inflammatory response. Specifically ATF4, XBP-1 and a subset of genes involved in the UPR are all increased in monocytes from ZZ compared to MM individuals. Expression of these genes could be induced in MM monocytes by treatment with the ER stress inducer thapsigargin, linking the observed ZZ monocyte changes to ER stress. Confocal microscopy demonstrated that not only is ZAAT retained in the ER in ZZ monocytes but that GRP78 is also increased even in resting cells. These altered gene expression patterns contribute to enhanced basal and agonist-induced cytokine production by ZZ monocytes and activation, again, of the NFκB pathway. Thus our current understanding of the mechanisms regulating ZAAT-related lung and liver disease should now be expanded to include of a role for exaggerated inflammatory responses by circulating blood cells.
REMOVAL OF MISFOLDED α-1 ANTITRYPSIN
There are at least two pathways for degradation of ZAAT that accumulates in the ER, the proteosomal and autophagic degradative pathways. Soluble ZAAT is degraded by the proteasome whilst polymerized ZAAT is degraded by autophagy[9].
Endoplasmic reticulum associated degradation
ERAD is the process which monitors the production of mutant glycosylated secretory proteins and directs them to the ubiquitin-proteasome system to be degraded[43]. The ER luminal and transmembrane proteins EDEM1, EDEM2 and EDEM 3 extract misfolded AAT from ER chaperones and facilitate enzymatic mannose trimming of its carbohydrate side-chain by ERManI[9]. The misfolded proteins are retro-translocated to the cytosol, modified with polyubiquitin and degraded by the proteasome. Up to 30% of all newly synthesised proteins undergo degradation by this process within minutes of formation most likely because they are products of unsuccessful folding or failures of multimer assembly. When the level of abnormal proteins accumulating surpasses the ability of a cell to degrade and dispose of them, ER accumulation and ER stress ensue. This is classically seen in ZAAT deficiency where terminally misfolded ZAAT proteins and unassembled complexes are eliminated by ERAD.
Autophagy
The proteosomal pathway does not fully account for disposal of all ZAAT. In addition to ERAD ZAAT can also be degraded by autophagy[25], a cellular mechanism for the degradation of cytoplasmic constituents within lysosomes. Autophagy is the process by which cytoplasmic constituents and intracellular organelles are sequestered within double membraned vesicles called autophagosomes that fuse with lysosomes to form autophagolysosomes, inside which their constituents are degraded. Three autophagy gene products ATG5, ATG6 and ATG16 are particularly important for this process and are necessary for the piece-by-piece digestion of aggregated ZAAT[44]. The presence of aggregated rather than soluble ZAAT appears to specifically activate autophagy. Expansion and dilatation of the ER and increased numbers of autophagosomes, two morphological changes characteristic of autophagy, are evident in fibroblasts overexpressing ZAAT, in ZAAT mouse liver cells and liver cells from ZAAT individuals[6,22,24,25]. Autophagy also plays a role in the removal of senescent or damaged mitochondria in a process termed ‘mitophagy’. This is likely to be an important process in the ZAAT deficient liver given that mitochondrial dysfunction and mitophagy have been observed in patient liver samples and mouse models[24].
Thus the current dogma regarding degradation of ZAAT involves two major mechanisms, ERAD and autophagy. ERAD deals with removal of soluble ZAAT that accumulates in the ER presumably bound to chaperones whilst autophagy is specialised for the polymerised and aggregated forms of ZAAT that accumulate constitutively but that become more abundant during the acute phase response when expression of AAT is induced.
POTENTIAL THERAPIES
Notwithstanding the fact that a small percentage of ZAAT can be secreted from hepatocytes and fulfils a functional role as an antiprotease in the body[45], there is a pressing need to design strategies to impair or prevent expression of mutant ZAAT to counteract its deleterious effects on ER accumulation in hepatocytes and monocytes in particular where ZAAT ER accumulation has known effects. This is particularly important during episodes of elevated AAT expression such as during infection or fever. A number of potential interventions likely to have therapeutic potential for the liver disease in ZAAT deficiency are under investigation. These include a variety of approaches.
Surgical intervention
Liver transplantation for ZAAT deficiency is confounded by a number of factors including donor availability and poor, but improving, survival rates. Hepatocyte transplantation now offers hope for some ZAAT deficient liver patients and has been developed as an alternative to whole liver transplantation. Successful hepatocellular transplantation requires transplantation of up to 5% of the liver mass and can achieve 1%-5% repopulation of the liver[9].
Endoplasmic reticulum stress relieving agents and inducers of apoptosis
A selection of compounds that have the ability to alleviate ER stress by a variety of different mechanisms may be worth considering for the treatment of ZAAT-related liver disease and to reverse the hyperinflammatory state of ZAAT monocytes. Selenium is a trace mineral that can relieve a number of indices of ER stress in a HepG2 liver cells model over expressing ZAAT[29]. It mediates its effect in two ways; via inhibition of NFκB activation through up regulated expression of 15deoxy-prostaglandin J2 and PPARγ, and by enhancing expression of selenoproteins such as selenoprotein S which can participate in ERAD.
α-linolenic acid and palmitoleate are agents that inhibit UPR signals[46,47]. α-linolenic acid, for example can inhibit eIF2α phosphorylation and expression of CHOP and GRP78 in rat renal cells. These agents are likely to be useful for treating the immune cell defects in ZZ individuals. However, since UPR is not activated in vivo in ZAAT deficient liver disease patients these agents are unlikely to have therapeutic benefit. Similarly, agents such as tauroursodeoxycholic acid (TUDCA) and salubrinal may prove to have more use in modulating ER stress in monocytes and possibly airway epithelial cells rather than liver cells. TUDCA is a bile acid that can inhibit ER stress-induced apoptosis[28]. Salubrinal can block cell death in response to UPR induction by preventing GADD34/PP1-mediated dephosphorylation of eIF2α[48]. Given that it is desirable to induce rather than inhibit apoptosis in globule-containing hepatocytes, TUDCA and salubrinal have little relevance to liver disease in this context. However, at lower doses than are used for induction of apoptosis they may have other desirable ER stress relieving properties.
Gene therapies
Ribozymes, peptide nucleic acid (PNA) and small-interfering RNAs (siRNA) are all potential tools to inhibit gene expression in ZAAT deficient individuals with liver disease[49]. Ribozymes are naturally occurring catalytic RNA molecules that can cleave target RNA molecules with high specificity[50]. Synthetic ribozymes targetting AAT have been designed[51]. These contain a catalytic RNA domain that cleaves mRNA and a substrate-binding domain specific for AAT mRNA. Ozaki et al[51] successfully transduced human hepatoma cells with ribozymes to inhibit the expression of a mutant AAT gene and co-express a modified AAT gene that was not sensitive to the ribozyme. This gene therapy approach led to inhibition of the mutant gene and expression of the modified AAT gene; unfortunately the overall efficiency of this method was low. This is a major problem associated with ribozyme use in vivo; their activity rarely achieves the desired effect.
PNA are synthetic DNA analogues comprising repetitive units of the pseudo-peptide polymer N-(2-aminoethyl) glycine to which purine and pyrimidine bases are attached by a methyl carbonyl linker[52]. PNAs hybridize to complementary DNA or RNA in a sequence-dependent manner leading to anti-gene or antisense inhibition, and blockage of transcription or translation, respectively. As PNA oligomers are stable and have high binding affinities they constitute potentially efficient compounds for gene therapy. Moreover they are non-toxic, even at relatively high concentrations although their uptake by living cells is slow. Modification with cell penetrating peptides can significantly improve their uptake. AAT-directed PNAs can inhibit MAAT expression in HepG2 cells and human MM monocytes (Greene C, unpublished data). They are also capable of interfering with ZAAT transgene expression in HEK293 cells or endogenous ZAAT gene expression in peripheral blood monocytes isolated from ZZ homozygous individuals. Unfortunately only partial inhibition can be achieved and the approach is considerably less effective than using siRNA.
We have effectively knocked down expression of MAAT and ZAAT genes in model systems over-expressing these genes and also in human monocytes from MM and ZZ homozygous individuals[53]. Others have similarly reported successful knockdown of AAT in cell lines, and have taken the technology further by successfully knocking down AAT expression in ZAAT overexpressing transgenic mice[54].
Inhibition of polymerisation and enhanced secretion
Peptides designed to block polymerization of ZAAT represent important therapies for the treatment ZAAT-related liver disease. Mahadeva et al[55] designed a 6-mer peptide Phe-Leu-Glu-Ala-Ile-Gly (FLEAIG) that selectively targets the reactive centre loop of ZAAT and blocks its polymerization. A second generation peptide which has the N-acyl terminus removed is as effective at inhibiting polymerisation and yields an active inhibitor[56]. Two additional peptides Ac-FLAAIG-OH and Ac-FLEAAG-OH and their daughter 4-mers Ac-FLEAA-NH(2) and Ac-FLAA-NH(2) can also bind avidly to ZAAT and prevent polymerization of the protein[57]. In further study using a combinatorial approach based on the inhibitory mechanism of A1AT, another peptide developed by Chang et al[58], Ac-TTAI-NH(2), acts as a tight-binding ligand for ZA1AT. It effectively blocks ZAAT polymerization but can also promote dissociation of the oligomerized serpin.
4-phenylbutyric acid (4PBA) and glycerol are reagents that that can potentially reverse misfolding and enhance secretion of ZAAT. These so-called chemical chaperone reagents have potential for targeting ZAAT. 4PBA can increase secretion of ZAAT although the mechanism remains unclear[59], whilst glycerol (and erythritol, trehalose and glucose) can interfere with polymerisation of ZAAT, by slowing down conformational transitions of the protein. Unfortunately, refolding of the misfolded conformer is usually not possible[60]. Imino sugar compounds including castanospermine, kifunensine and deoxymannojirimycin have also been suggested to be useful for enhancing ZAAT secretion from cells[61].
CONCLUSION
Accumulation of ZAAT in the ER has the potential to induce multiple signalling events related to ER stress. Interestingly, although perturbation of the ER can be induced by ZAAT expression in most cells, important differences exist in the responses induced in different cell types. The next steps in enhancing our understanding of the mechanisms of ER stress will be to identify these stimulus- and cell-specific responses that occur. Research within the next decade is also likely to advance our understanding of the role of gene modifiers in determining the susceptibility to and pathogenesis of ZAAT-related liver and lung disease. This knowledge we will bring us closer to a point where we can tailor make specific therapeutics for ZAAT deficient individuals.
Acknowledgments
The authors would like to thank Dr. Tomás Carroll for constructive discussion on the topic.
Footnotes
Supported by The U.S. Alpha One Foundation, the Health Research Board of Ireland, the Medical Research Charities Group, the Programmes for Research in Third Level Institutes administered by the Higher Education Authority and the Children’s Medical and Research Centre, Crumlin Hospital.
Peer reviewers: Omar Mohamed Abdel-Salam, Professor, Department of Toxicology and Narcotics, Medical Division-National Research Centre, Tahrir Street, Dokki, Cairo 1231, Egypt
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
References
- 1.Greene CM, Miller SD, Carroll T, McLean C, O'Mahony M, Lawless MW, O'Neill SJ, Taggart CC, McElvaney NG. Alpha-1 antitrypsin deficiency: a conformational disease associated with lung and liver manifestations. J Inherit Metab Dis. 2008;31:21–34. doi: 10.1007/s10545-007-0748-y. [DOI] [PubMed] [Google Scholar]
- 2.McCracken AA, Kruse KB, Brown JL. Molecular basis for defective secretion of the Z variant of human alpha-1-proteinase inhibitor: secretion of variants having altered potential for salt bridge formation between amino acids 290 and 342. Mol Cell Biol. 1989;9:1406–1414. doi: 10.1128/mcb.9.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomas DA, Mahadeva R. Alpha1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest. 2002;110:1585–1590. doi: 10.1172/JCI16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurell CB, Sveger T. Mass screening of newborn Swedish infants for alpha antitrypsin deficiency. Am J Hum Genet. 1975;27:213–217. [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 6.Perlmutter DH. Liver injury in alpha1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest. 2002;110:1579–1583. doi: 10.1172/JCI16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res. 2006;60:233–238. doi: 10.1203/01.pdr.0000228350.61496.90. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter DH, Brodsky JL, Balistreri WF, Trapnell BC. Molecular pathogenesis of alpha-1-antitrypsin deficiency-associated liver disease: a meeting review. Hepatology. 2007;45:1313–1323. doi: 10.1002/hep.21628. [DOI] [PubMed] [Google Scholar]
- 10.Dycaico MJ, Grant SG, Felts K, Nichols WS, Geller SA, Hager JH, Pollard AJ, Kohler SW, Short HP, Jirik FR. Neonatal hepatitis induced by alpha 1-antitrypsin: a transgenic mouse model. Science. 1988;242:1409–1412. doi: 10.1126/science.3264419. [DOI] [PubMed] [Google Scholar]
- 11.Carlson JA, Rogers BB, Sifers RN, Finegold MJ, Clift SM, DeMayo FJ, Bullock DW, Woo SL. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlmutter DH. Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in alpha-1-antitrypsin deficiency. Cell Death Differ. 2009;16:39–45. doi: 10.1038/cdd.2008.103. [DOI] [PubMed] [Google Scholar]
- 13.Rudnick DA, Liao Y, An JK, Muglia LJ, Perlmutter DH, Teckman JH. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology. 2004;39:1048–1055. doi: 10.1002/hep.20118. [DOI] [PubMed] [Google Scholar]
- 14.Geller SA, Nichols WS, Kim S, Tolmachoff T, Lee S, Dycaico MJ, Felts K, Sorge JA. Hepatocarcinogenesis is the sequel to hepatitis in Z#2 alpha 1-antitrypsin transgenic mice: histopathological and DNA ploidy studies. Hepatology. 1994;19:389–397. [PubMed] [Google Scholar]
- 15.Taggart CC, Greene CM, Carroll TP, O'Neill SJ, McElvaney NG. Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. Am J Respir Crit Care Med. 2005;171:1070–1076. doi: 10.1164/rccm.200407-881PP. [DOI] [PubMed] [Google Scholar]
- 16.Taggart CC, Lowe GJ, Greene CM, Mulgrew AT, O'Neill SJ, Levine RL, McElvaney NG. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 17.Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter DH. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc Natl Acad Sci USA. 1994;91:9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan S, Huang L, McPherson J, Muzny D, Rouhani F, Brantly M, Gibbs R, Sifers RN. Single nucleotide polymorphism-mediated translational suppression of endoplasmic reticulum mannosidase I modifies the onset of end-stage liver disease in alpha1-antitrypsin deficiency. Hepatology. 2009;50:275–281. doi: 10.1002/hep.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidvegi T, Mirnics K, Hale P, Ewing M, Beckett C, Perlmutter DH. Regulator of G Signaling 16 is a marker for the distinct endoplasmic reticulum stress state associated with aggregated mutant alpha1-antitrypsin Z in the classical form of alpha1-antitrypsin deficiency. J Biol Chem. 2007;282:27769–27780. doi: 10.1074/jbc.M704330200. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teckman JH, An JK, Loethen S, Perlmutter DH. Fasting in alpha1-antitrypsin deficient liver: constitutive [correction of consultative] activation of autophagy. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1156–G1165. doi: 10.1152/ajpgi.00041.2002. [DOI] [PubMed] [Google Scholar]
- 23.Carroll TP, Greene CM, O'Connor CA, Nolan AM, O'Neill SJ, McElvaney NG. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol. 2010;184:4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 24.Teckman JH, An JK, Blomenkamp K, Schmidt B, Perlmutter D. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286:G851–G862. doi: 10.1152/ajpgi.00175.2003. [DOI] [PubMed] [Google Scholar]
- 25.Teckman JH, Perlmutter DH. Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G961–G974. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- 26.Hidvegi T, Schmidt BZ, Hale P, Perlmutter DH. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J Biol Chem. 2005;280:39002–39015. doi: 10.1074/jbc.M508652200. [DOI] [PubMed] [Google Scholar]
- 27.Lawless MW, Greene CM, Mulgrew A, Taggart CC, O'Neill SJ, McElvaney NG. Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z alpha 1-antitrypsin deficiency. J Immunol. 2004;172:5722–5726. doi: 10.4049/jimmunol.172.9.5722. [DOI] [PubMed] [Google Scholar]
- 28.Miller SD, Greene CM, McLean C, Lawless MW, Taggart CC, O'Neill SJ, McElvaney NG. Tauroursodeoxycholic acid inhibits apoptosis induced by Z alpha-1 antitrypsin via inhibition of Bad. Hepatology. 2007;46:496–503. doi: 10.1002/hep.21689. [DOI] [PubMed] [Google Scholar]
- 29.Kelly E, Greene CM, Carroll TP, McElvaney NG, O'Neill SJ. Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant alpha1-antitrypsin deficiency. J Biol Chem. 2009;284:16891–16897. doi: 10.1074/jbc.M109.006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greene CM, Miller SD, Carroll TP, Oglesby IK, Ahmed F, O'Mahony M, Taggart CC, McElvaney NG, O'Neill SJ. Anti-apoptotic effects of Z alpha1-antitrypsin in human bronchial epithelial cells. Eur Respir J. 2010;35:1155–1163. doi: 10.1183/09031936.00191908. [DOI] [PubMed] [Google Scholar]
- 31.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiseman RL, Haynes CM, Ron D. SnapShot: The unfolded protein response. Cell. 2010;140:590–590.e2. doi: 10.1016/j.cell.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 36.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. 2009;180:1196–1207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 43.Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- 44.Perlmutter DH. The role of autophagy in alpha-1-antitrypsin deficiency: a specific cellular response in genetic diseases associated with aggregation-prone proteins. Autophagy. 2006;2:258–263. doi: 10.4161/auto.2882. [DOI] [PubMed] [Google Scholar]
- 45.Bathurst IC, Travis J, George PM, Carrell RW. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 1984;177:179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- 46.Katsoulieris E, Mabley JG, Samai M, Green IC, Chatterjee PK. alpha-Linolenic acid protects renal cells against palmitic acid lipotoxicity via inhibition of endoplasmic reticulum stress. Eur J Pharmacol. 2009;623:107–112. doi: 10.1016/j.ejphar.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Diakogiannaki E, Welters HJ, Morgan NG. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J Endocrinol. 2008;197:553–563. doi: 10.1677/JOE-08-0041. [DOI] [PubMed] [Google Scholar]
- 48.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 49.McLean C, Greene CM, McElvaney NG. Gene targeted therapeutics for liver disease in alpha-1 antitrypsin deficiency. Biologics. 2009;3:63–75. [PMC free article] [PubMed] [Google Scholar]
- 50.Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 51.Ozaki I, Zern MA, Liu S, Wei DL, Pomerantz RJ, Duan L. Ribozyme-mediated specific gene replacement of the alpha1-antitrypsin gene in human hepatoma cells. J Hepatol. 1999;31:53–60. doi: 10.1016/s0168-8278(99)80163-9. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 53.McLean C. Gene targeted inhibition in alpha-1 antitrypsin deficiency. Ph.D. Thesis. Dublin: Royal College of Surgeons; Ireland; 2009. [Google Scholar]
- 54.Cruz PE, Mueller C, Cossette TL, Golant A, Tang Q, Beattie SG, Brantly M, Campbell-Thompson M, Blomenkamp KS, Teckman JH, et al. In vivo post-transcriptional gene silencing of alpha-1 antitrypsin by adeno-associated virus vectors expressing siRNA. Lab Invest. 2007;87:893–902. doi: 10.1038/labinvest.3700629. [DOI] [PubMed] [Google Scholar]
- 55.Mahadeva R, Dafforn TR, Carrell RW, Lomas DA. 6-mer peptide selectively anneals to a pathogenic serpin conformation and blocks polymerization. Implications for the prevention of Z alpha(1)-antitrypsin-related cirrhosis. J Biol Chem. 2002;277:6771–6774. doi: 10.1074/jbc.C100722200. [DOI] [PubMed] [Google Scholar]
- 56.Parfrey H, Dafforn TR, Belorgey D, Lomas DA, Mahadeva R. Inhibiting polymerization: new therapeutic strategies for Z alpha1-antitrypsin-related emphysema. Am J Respir Cell Mol Biol. 2004;31:133–139. doi: 10.1165/rcmb.2003-0276OC. [DOI] [PubMed] [Google Scholar]
- 57.Chang YP, Mahadeva R, Chang WS, Shukla A, Dafforn TR, Chu YH. Identification of a 4-mer peptide inhibitor that effectively blocks the polymerization of pathogenic Z alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2006;35:540–548. doi: 10.1165/rcmb.2005-0207OC. [DOI] [PubMed] [Google Scholar]
- 58.Chang YP, Mahadeva R, Chang WS, Lin SC, Chu YH. Small-molecule peptides inhibit Z alpha1-antitrypsin polymerization. J Cell Mol Med. 2009;13:2304–2316. doi: 10.1111/j.1582-4934.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharp LK, Mallya M, Kinghorn KJ, Wang Z, Crowther DC, Huntington JA, Belorgey D, Lomas DA. Sugar and alcohol molecules provide a therapeutic strategy for the serpinopathies that cause dementia and cirrhosis. FEBS J. 2006;273:2540–2552. doi: 10.1111/j.1742-4658.2006.05262.x. [DOI] [PubMed] [Google Scholar]
- 60.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcus NY, Perlmutter DH. Glucosidase and mannosidase inhibitors mediate increased secretion of mutant alpha1 antitrypsin Z. J Biol Chem. 2000;275:1987–1992. doi: 10.1074/jbc.275.3.1987. [DOI] [PubMed] [Google Scholar]