Abstract
Purpose
We present the results of a randomized, multicenter clinical trial of adjuvant ZA in postmenopausal women with high-risk breast cancer. The primary objective was change in bone mineral density (BMD) at the lumbar spine and femoral neck at 1 year. Secondary objectives included change in calcaneal BMD, disease-free survival (DFS), overall survival (OS), and toxicity.
Patients and Methods
Postmenopausal women with stage II/III breast cancer diagnosed up to five years prior were eligible and randomized to either observation or ZA 4 mg IV every 3 months. BMD testing was performed at 0, 6 and 12 months.
Results
Sixty-eight women were enrolled: 36 (ZA) and 32 women (observation). The population was a median of 2 years from diagnosis and the majority received tamoxifen during study. There was a significant difference in the mean change from baseline to 1 year follow-up for lumbar spine (increased by 4.28±0.62%; p=0.01), total femur (increased by 1.9±0.4%; p=0.03), trochanter (increased by 2.97±0.69%; p=0.03) and calcaneal BMD (increased by 2±0.57%; p=0.01) in favor of the ZA arm. No significant difference in the mean change for the femoral neck was seen. No significant differences in DFS or OS were observed.
Conclusion
ZA significantly improved the BMD at multiple skeletal sites in postmenopausal women largely on tamoxifen. No new safety signals were noted. There were insufficient events to comment on DFS or OS.
Keywords: bone mineral density, zoledronic acid, breast cancer, adjuvant therapy
INTRODUCTION
Osteoporosis represents a major cause of morbidity and health care costs in the United States[1], particularly for the 4.4 million estimated breast cancer survivors.[2] In addition to the skeletal effects of metastatic breast cancer, common adjuvant and metastatic therapies often accelerate bone loss and increase fracture risk. Chemotherapy-induced premature ovarian failure and aromatase inhibitors (AI) increase the risk of osteoporosis and fracture in breast cancer survivors.
ZA is a potent third-generation biphosphonate[3] demonstrating antitumor and anti-metastatic activity in preclinical and early clinical studies.[4] Studies have suggested bisphosphonates reduce the incidence and number of new bony and visceral metastases.[5] Powels et al[6] reported that two years of oral clodronate significantly improved overall survival when compared to placebo in the adjuvant setting. However, the adoption of bisphosphonates as an adjuvant breast cancer therapy awaits the results of several large randomized clinical trials. Trials such as ABSCG-12[7], AZURE[8], ZO-FAST[9, 10], GAIN[11] and NSABP-34[12] address whether adjuvant use of bisphosphonates improve breast cancer-related outcomes (see Table 1).
Table 1.
Design of Recent or Ongoing Adjuvant Bisphosphonate Trials in Breast Cancer Patients
| Trial | Population | Design | Schedule | Bisphosphonate timing | Results |
|---|---|---|---|---|---|
| ABCSG-12[7] | Premenopausal HR+ Stage I–III breast cancer N = 1803 |
ZA vs control | 4 mg IV every 6 mos for 3 yrs | Started at same time as endocrine therapy | HR = 0.64 for DFS in favor of ZA, p = 0.01 |
| AZURE[8] | Stage II–III breast cancer N = 3360 |
ZA vs control | 4 mg IV monthly for 6 mos, then every 3 mos for 8 doses, then every 6 months for 5 doses | Neoadjuvant arm: No more than 30 days after initiation of neoadjuvant therapy Adjuvant arm: No more than 60 days since prior definitive surgery |
Accrual complete, results of neoadjuvant arm presented at SABCS 2008 |
| Z-FAST ZO-FAST[9, 10] E-ZO-FAST |
HR+ Stage I–III breast cancer N = 2193 |
ZA: immediate vs delayed | 4 mg every 6 mos | Immediate arm: Started at same time as endocrine therapy Delayed arm: Started when significant BMD change or fracture |
Accrual complete, 36 month followup presented at SABCS 2008 |
| NSABP-B34[12] | Stage I–II breast cancer N = 3400 |
Clodronate vs placebo | 1600 mg PO daily for 3 yrs | Randomized within 84 days of surgery | Accrual complete |
| GAIN [11] | Stage II–III breast cancer N > 3000 |
Ibandronate vs control | 50 mg PO daily for 2 yrs | Accrual ongoing |
HR+ = hormone-receptor positive
SABCS = San Antonio Breast Cancer Symposium
PO = per orum or by mouth
IV = intravenous
The University of Wisconsin (UWCCC) conducted a clinical trial of adjuvant ZA in postmenopausal women with high-risk breast cancer. The primary endpoints were change in BMD at the lumbar spine and femoral neck. Secondary objectives included change in calcaneal BMD, rates of bone and visceral metastases, all distant metastases, OS, and the toxicity of ZA as dosed on study. Notably, this study included both hormone-receptor negative and HER2 amplified disease and patients could enroll up to five years from diagnosis.
Patients and Methods
This randomized, open-label, multicenter trial was opened through the Wisconsin Oncology Network. Local Institutional Review Boards (IRB)s granted approval. Participating clinics included Gundersen Clinic (La Crosse, WI), Green Bay Oncology (Green Bay, WI), and UWCCC (Madison, WI). The UWCCC was the coordinating site. All participants provided written informed consent before study registration in accordance with institution and federal guidelines. The study was funded by Novartis Pharmaceuticals.
Patient Eligibility
Eligible women had histologically-confirmed T4 or node positive adenocarcinoma of the breast. Diagnosis had to have occurred within five years of enrollment. Only postmenopausal women were eligible. At the time of protocol development, menopause was conventionally defined as 1) ≥ 1 year since the last menstrual period and no prior oophorectomy/hysterectomy, 2) prior bilateral oophorectomy or 3) previous hysterectomy, one or both ovaries intact, ≥60 years or FSH level in postmenopausal range. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, age > 18 years, adequate bone marrow reserve (absolute neutrophil count ≥ 1,500/mm3, platelet count ≥ 100,000/mm3); adequate renal function (serum creatinine ≤ 1.5mg/dL; BUN ≤ 30); adequate hepatic function (alkaline phosphatase and SGOT ≤ 1.5 times institutional upper limit of normal and bilirubin ≤ 1.5mg/dL) and normal calcium. Participants had no evidence of breast cancer recurrence, as demonstrated by normal complete blood cell count, liver function tests, chest X-ray and bone scan within 28 days of enrollment. Prior adjuvant chemotherapy was permitted and choice of regimen was decided upon by the treating physician. Use of supplemental calcium and vitamin D was permitted at the discretion of the treating physician, but not routinely assessed or tracked.
Participants with a history of second or other cancers were excluded, if the estimated risk of recurrence for the second malignancy was over 5%. Participants were also excluded for concurrent bisphosphonate use. Women not receiving tamoxifen were excluded for a T score of < −2.0 at the hip or spine.
Study Design
Participants were randomized to observation or ZA 4mg IV every 12 weeks administered over at least 15 minutes for four cycles. Ancillary treatments were allowed, as appropriate for symptom control and cancer therapy management. Participants were stratified based on whether or not they received endocrine therapy at all (tamoxifen or aromatase inhibitors versus none). BMD was measured by dual energy x-ray absorptiometry (DXA) at the lumbar spine, femoral neck and calcaneus at registration, 6 and 12 months. Toxicity evaluations were performed in clinic on day 1 of each cycle and by telephone one week after each cycle of ZA.
Bone Mineral Density Measurement
BMD of the lumbar spine, femoral neck and calcaneus was measured by DXA using a standardized approach. BMD analysis was planned at registration, 6 months, and 12 months. DXA measurements were obtained at the participating locations using local DXA devices. A Bone-fide® calibration phantom was measured by the densitometers at all participating facilities. Quality assurance phantom data from all participating facilities was evaluated. All results were reviewed centrally at the University of Wisconsin; all results were reviewed by a single physician specializing in bone mass measurement.. No densitometer shift or drift occurred during the course of this trial.
Toxicities and Dose Modification
There were no planned dose modifications for toxicity due to ZA. Clinical toxicities must have resolved to ≤ grade 1, and any laboratory-based toxicities resolved or be within the study eligibility parameters, prior to administration of subsequent doses. All reporting of toxicity was done based on NCI CTC version 2.0. Participants developing toxicities that failed to meet criteria for the next dose of ZA were taken off study. Participants could also be removed from study for progressive disease or withdrawal of consent. Participants removed from study were followed for toxicity until 4 weeks after the last dose of ZA and until death for survival data.
Pre-Treatment Assessment and Follow-up
Baseline laboratory and imaging studies were performed prior to enrollment. Participants in the ZA arm had toxicity evaluations, serum calcium, BUN and creatinine measurements performed pre- and post-treatment (within 7 days pre and post). All participants had complete blood counts and chemistries, chest X-ray, bone scan and toxicity assessments at the end of study (one year from enrollment). Any additional cancer-directed investigation was performed at the discretion of the patient’s physician. Participants have been followed for relapse and survival every 6 months since the first year by telephone interview of the patient and referring physician. Disease recurrence and death are based on local oncology center records. These data are current through May 2009.
Statistical Analysis
The study was designed as a two arm, randomized trial with a target sample size of 37 participants per arm (74 total). Participants were randomized using permuted blocks of varying sizes, stratified by the status of current endocrine therapy, i.e., endocrine therapy vs. no endocrine therapy. It was anticipated that the mean difference in lumbar spine BMD change between the ZA and observation arm would be at least 1.75%. Based on the results from previous studies, an overall standard deviation of 2.7% for BMD change from baseline was anticipated. Therefore, the proposed sample size of 37 participants per arm provided 80% power to detect the anticipated mean difference in BMD change from baseline between the ZA and observation arm at the two-sided 5% significance level. A sample size re-estimation was performed after the first 38 participants had been accrued and followed for 6 months. The sample size re-estimation was performed in a blinded fashion using the Gould-Shi procedure.[13]
This analysis of BMD parameters and clinical efficacy endpoints was conducted on an intent-to-treat basis. Categorical data were summarized as proportions and percentages. Continuous data were summarized and reported as means, standard deviations and ranges. The comparison of baseline characteristics between two treatment study arms was performed using a chi-square test for the categorical measurements and two-sample t-test for continuous measurements. Changes in BMD were summarized in terms of means and 95% confidence intervals. A two-sample t-test was used to compare mean changes in BMD between study arms. Differences in OS and DFS were explored using the Kaplan-Meier methodology. A z-test was used to compare the 5-year overall and disease-free survival rates between study arms. All data analyses were performed using SAS version 9. A two-sided significance level of 0.05 was used for all statistical tests.
RESULTS
Patient demographics
Between February 2000 and February 2007, 68 women enrolled at three sites. Enrollment closed before the targeted 74 participants were enrolled due to sample size adjustment. Overall, 36 women were entered in the ZA arm and 32 women in the observation arm. One woman in the observation arm withdrew consent in the initial part of the study and was not available for further follow-up. Demographics are summarized in Table 2.
Table 2.
Participant characteristics
| Characteristic | ZA | Observation |
|---|---|---|
| No. of participants | 36 | 32 |
| Median age at study entry (Range) | 54.5 yrs (41–83) | 50.5 yrs (37 – 65) |
| Race | ||
| Caucasian | 35 | 32 |
| Hispanic | 1 | 0 |
| Performance status | ||
| 0 | 31 | 28 |
| 1 | 4 | 3 |
| Unknown | 1 | 1 |
| Tumor size | ||
| ≤2cm | 14 | 2 |
| 2.1–5cm | 11 | 18 |
| >5cm | 9 | 9 |
| Inflammatory | 1 | 3 |
| Unknown | 1 | 0 |
| Lymph node status | ||
| Node Negative | 1 | 1 |
|
Node Positive 1–3 nodes ≥4 nodes |
35 | 31 |
| 15 | 5 | |
| 20 | 26 | |
| Receptor Status | ||
| ER+ and/or PR+ | 29 | 29 |
| ER-/PR- | 7 | 3 |
| HER2Amplified | 3 | 5 |
| HER2Not Amplified/ Unknown | 33 | 27 |
| Adjuvant chemotherapy | 33 | 31 |
| None | 3 | 1 |
| Any adjuvant chemotherapy | 33 | 31 |
| Trastuzumab | 1 | 4 |
| Received Adjuvant Radiation | 24 | 26 |
| Endocrine Therapy During Year 1 on Study | ||
| None | 5 | 3 |
| Tamoxifen (Tam) or other SERM | 23 | 18 |
| Aromatase Inhibitor (AI) | 4 | 5 |
| Tam switched to AI during study year | 1 | 2 |
| Lumbar spine (L1-L4) total at baseline, g/cm3, mean (SD) | 1.148 (0.161) | 1.144 (0.185) |
| Femoral neck (FN) total at baseline, g/cm3, mean (SD) | 0.937 (0.144) | 0.942 (0.146) |
| Total femur (TF) total at baseline, g/cm3, mean (SD) | 0.982 (0.137) | 0.983 (0.143) |
| Trochanter (T) total at baseline, g/cm3, mean (SD) | 0.793 (0.118) | 0.807 (0.131) |
| Calcaneus (OC) total at baseline, g/cm3, mean (SD) | 0.498 (0.094) | 0.498 (0.081) |
| Time from Diagnosis to Enrollment on Trial, median in years (range) | 2.0 (0.8–5.0) | 2.1 (0.8–4.8) |
The majority of participants had undergone mastectomy (79%) and adjuvant radiation (73%), reflecting the locally-advanced disease of enrolled participants. Nearly all the participants (94%) received adjuvant chemotherapy, largely anthracycline (94%) and taxane-based (73%). One woman also underwent autologous stem cell transplant. Only five women received adjuvant trastuzumab. Data on HER2 status were collected retrospectively. Most women (83%) received adjuvant endocrine therapy; the majority (65%) were receiving tamoxifen at enrollment.
Efficacy Evaluation
Fifty-six women completed DXA measurements at 0, 6 and 12 months (ZA 30, observation 26). In the ZA arm, six women did not complete the study: one withdrew consent, one withdrew due to adverse event (grade 2 headache, fatigue and pain), two failed to obtain all three DXAs, and two had progression of disease within the first 12 months of the study. In the observation arm, six participants did not complete the study. Three withdrew consent, one withdrew due to adverse event (grade 1 fatigue and myalgia), one failed to obtain all three DXAs required, and one developed a primary ovarian cancer within the first 12 months of the study.
Bone Mineral Density
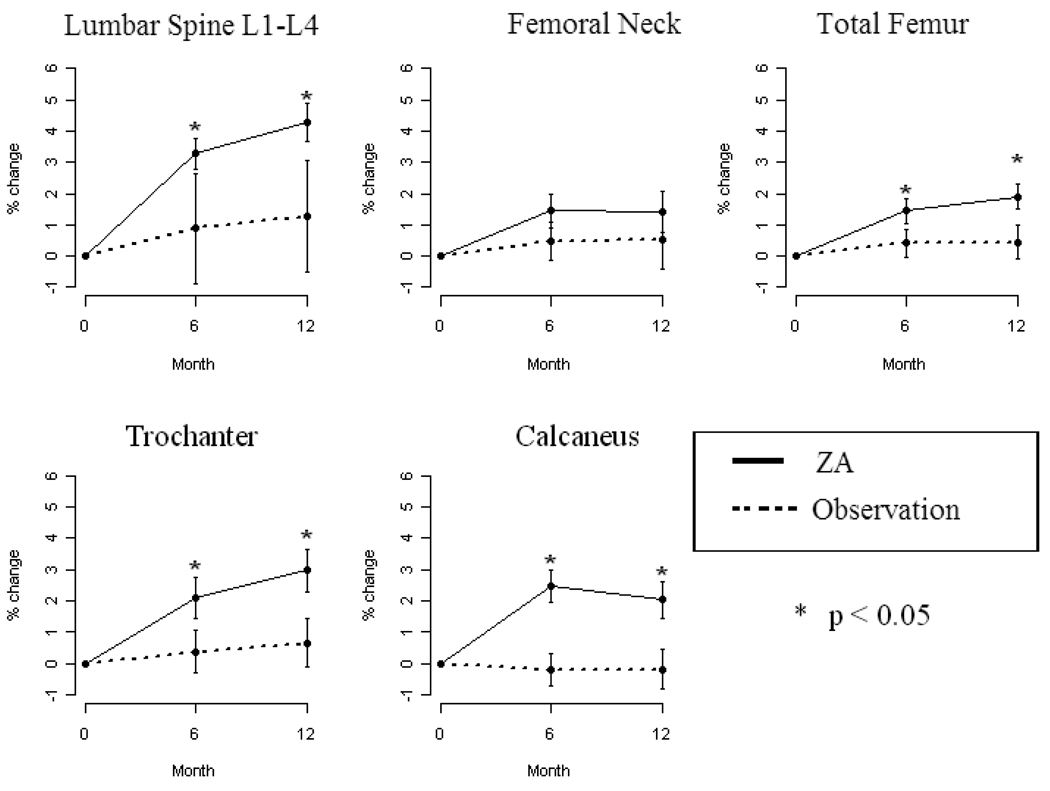
The primary objective was percentage change in BMD at the lumbar spine and femoral neck. Fifty-six women were evaluable based on completing DXAs at 0, 6 and 12 months. There was a significant difference in the mean change (from baseline to 1 year follow-up) for lumbar spine (increased by 4.28±0.62%; p=0.01), total femur (increased by 1.9±0.4%; p=0.03), trochanter (increased by 2.97±0.69%; p=0.03) and calcaneal BMD (increased by 2±0.57%; p=0.01) in favor of the ZA arm. No significant difference in the mean change for the femoral neck was seen. See Figure 1 and Table 3. The improvement in BMD persisted, even when the population was limited to women who received only tamoxifen during the study year (see Table 4). However, since the latter is a subgroup analysis, the results should be interpreted with caution.
Figure 1.
Percent change in BMD at the lumbar spine, trochanter, femoral neck, total femur and calcaneus
Table 3.
Change in BMD from baseline to 1 year
| ZA | Observation | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean(SD) | 95% CI | N | Mean (SD) | 95% CI | p-value | |
| L1-L4, g/cm3 | 29 | 0.048 (0.038) | 0.034–0.062 | 26 | 0.007 (0.071) | −0.020–0.034 | 0.01 |
| FN, g/cm3 | 30 | 0.014 (0.035) | 0.002–0.027 | 26 | 0.005 (0.046) | −0.012–0.023 | 0.42 |
| TF, g/cm3 | 30 | 0.019 (0.021) | 0.011–0.026 | 26 | 0.004 (0.027) | −0.006–0.015 | 0.02 |
| T, g/cm3 | 30 | 0.023 (0.029) | 0.013–0.034 | 26 | 0.005 (0.032) | −0.007–0.017 | 0.02 |
| OC, g/cm3 | 27 | 0.010 (0.015) | 0.004–0.015 | 24 | −0.001 (0.015) | −0.007–0.005 | 0.01 |
Two-sample t-test, two-tailed
Table 4.
Subgroup analysis: Change in BMD from baseline to 1 year for patients treated with tamoxifen
| ZA | Observation | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean(SD) | 95% CI | N | Mean (SD) | 95% CI | p-value* | |
| L1-L4, g/cm3 | 16 | 0.047 (0.037) | 0.029–0.065 | 16 | 0.015 (0.085) | −0.027–0.057 | 0.19 |
| FN, g/cm3 | 17 | 0.022 (0.038) | 0.005–0.04 | 16 | 0.008 (0.031) | −0.007–0.023 | 0.24 |
| TF, g/cm3 | 17 | 0.024 (0.021 | 0.014–0.035 | 16 | 0.006 (0.028) | −0.007–0.02 | 0.04 |
| T, g/cm3 | 17 | 0.03 (0.034) | 0.014–0.046 | 16 | 0.004 (0.035) | −0.013–0.021 | 0.04 |
| OC, g/cm3 | 16 | 0.011 (0.014) | 0.004–0.017 | 15 | −0.002 (0.015) | −0.010–0.006 | 0.02 |
Two-sample t-test, two-tailed
Clinical Toxicities
ZA was well tolerated; side effects were mild and transient. The most common side effects were fatigue, myalgia, arthralgia, pain and headache. Rare, reversible grade 3 events occurred. One woman withdrew due to subjectively intolerable events (grade 2 headache, fatigue and pain). No clinically significant changes in creatinine or calcium occurred. We retrospectively assessed charts for any events consistent with osteonecrosis of the jaw; none were identified.
Disease Free Survival and Overall Survival
With a median follow-up of eight years, 10 (15%) participants are deceased, 8 from breast disease recurrence, and one patient each arm died of other causes. No significant differences in DFS or OS were observed.
DISCUSSION
This study showed that ZA administered every 12 weeks for four doses leads to a statistically significant improvement in BMD at the lumbar spine, total femur, trochanter and calcaneus. These results are consistent in trend and magnitude with previous studies, which have shown that bisphosphonates can slow or reverse bone loss in women with breast cancer.[9, 14, 15]. Recently, Ellis et al published an article about the impact of denosumab on BMD in adjuvant breast cancer.[16] At 12 months, they report an increase in lumbar spine BMD of 5.5% for denosumab. This contrasts with an improvement of 4.28% in our study. Such BMD changes could be expected to reduce fracture risk by approximately 35–50% in women with osteoporosis.[17, 18] Our regimen (ZA 4 mg IV every 3 months) was safe and well-tolerated.
ZO-FAST[9, 10] demonstrated a statistically significant improvement in BMD at the spine and hip whether ZA was administered immediately or in a delayed fashion. Our study supports this finding, showing a BMD benefit to initiating ZA even up to five years after breast cancer diagnosis in a population of women largely receiving tamoxifen. To our knowledge, this also represents the first time that the impact of ZA on calcaneal BMD has been assessed. Our results suggest that ZA also influences calcaneal BMD, making this site a possible surrogate for other BMD measurement sites when using bisphosphonate therapy. Calcaneal BMD measurement is simple and used in parts of the world where spine and hip BMD measurements are not practicable or cost-effective[19].
However, limitations were imposed on this study because of its long accrual period. For instance, at the time of the initial protocol development, only tamoxifen was commonly used for hormone-receptor positive breast cancer. Thus, the definitions of menopausal status do not reflect current concerns regarding the impact of adjuvant chemotherapy on menses. In addition, use of calcium and vitamin D was not routinely assessed or controlled for. Furthermore, the study did not prospectively follow patients for subsequent fractures, the ultimate endpoint of any study in BMD. Additionally, later use of bisphosphonate was not tracked, thus potentially clouding recurrence and survival data. Lastly, the ZA schedule used (4 mg every 3 months) was more frequent than the schedules used in ABSCG-12 and Z-FAST but less frequent than that employed in AZURE. The overall duration of ZA was a shorter period than has been employed in other studies (see Table 1). This makes it more difficult to compare and contrast our results.
Relapse does remain a significant concern in the node-positive breast cancer population. Analysis of annual hazard rates of recurrence for breast cancer patients entered onto seven ECOG adjuvant trials by Saphner et al demonstrated that patients 5 years post-surgery for breast cancer appeared to have a very slow decreasing hazard of recurrence with a mean 5–12 years post-surgery of 4.3% per year.[20] Although small, our study did examine the impact of ZA on DFS and OS in the adjuvant setting. Despite our small numbers, we included these results given the ongoing debate over the impact of bisphosphonates on breast cancer DFS and OS. The large, randomized phase III trials assessing adjuvant bisphosphonate use in breast cancer are assigning patients to bisphosphonate therapy within a short time frame from diagnosis (see Table 1). Only ZO-FAST includes patients starting bisphosphonate therapy in a delayed fashion,[9] albeit in relatively small number, with 20% of women in the delayed group receiving ZA at the 36-month follow-up. Even if the findings of ABSCG-12 are confirmed, the question of benefit to a delayed start for adjuvant ZA will remain unanswered. In the wake of positive results from other clinical trials, the temptation may exist to initiate adjuvant bisphosphonates for any adjuvant breast cancer patient, even if such a patient is relatively distant from diagnosis. Our study was not adequately powered to detect significant differences in DFS or OS, and given the small numbers must be regarded as purely exploratory. However, there is no suggestion that the addition of ZA altered the rate of DFS or OS. There was also no suggestion that adjuvant ZA delayed or slowed the onset of recurrence.
In conclusion, our study showed ZA significantly improved the BMD at the lumbar spine, total femur, trochanter and calcaneus. This is the first time that ZA has been shown to improve BMD at the calcaneus. The improvement in BMD persisted even when the study population was limited to women who received tamoxifen during the study period.
ACKNOWLEDGEMENTS
This study was conducted by the University of Wisconsin. It was supported by Novartis Pharmaceuticals. The authors would like to thank the patients who participated, as well as the physicians, nurses and research specialists of the Wisconsin Oncology Network (WON) and the UWCCC Research Program who assisted with this trial.
Funding Source: Novartis
REFERENCES
- 1.Keen RW. Burden of osteoporosis and fractures. Curr Osteoporos Rep. 2003;1:66–70. doi: 10.1007/s11914-003-0011-x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 4.Ottewell PD, Lefley DV, Coleman RE, Holen I. Evaluation of the molecular mechanisms for the sequence-dependent, synergistic anti-tumour effects of doxorubicin and zoledronic acid in breast cancer. 31st Annual San Antonio Breast Cancer Symposium; 2008. Abstr 2151. [Google Scholar]
- 5.Diel IJ, Solomayer EF, Costa SD, et al. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 6.Powles T, Paterson S, Kanis JA, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 8.Winter MC, Thorpe HC, Burkinshaw R, et al. The addition of zoledronic acid to neoadjuvant chemotherapy may influence pathological response exploratory evidence for direct anti-tumor activity in breast cancer; 31st Annual San Antonio Breast Cancer Symposium; 2008. Abstr 5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundred NJ, Campbell ID, Davidson N, et al. Effective Inhibition of Aromatase Inhibitor-associated Bone Loss by Zoledronic Acid in Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole ZO-FAST Study Results. Cancer. 2008;5 doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 10.Eidtmann H, Bundred NJ, DeBoer R, et al. The effect of zoledronic acid on aromatase inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36 months follow-up of ZO-FAST. 31st Annual San Antonio Breast Cancer Symposium; 2008. Abstr 44. [DOI] [PubMed] [Google Scholar]
- 11.Möbus V, Conrad B, Schneeweis A, et al. Gain study: A phase III trial to compare ETC versus EC-TX and ibandronate versus observation in patients with node-positive primary breast cancer. J Clin Oncol. 2009;27 doi: 10.1200/JCO.2012.47.2167. abst 568. [DOI] [PubMed] [Google Scholar]
- 12.Mamounas EP. NSABP Breast Cancer Clinical Trials: Recent Results and Future Directions. Clin Med Res. 2003;1:309–326. doi: 10.3121/cmr.1.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould AL, Shih WJ. Sample size re-estimation without unblinding for normally distributed outcomes with unknown variance. Communications in statistics. Theory and methods. 1992;21:2833–2853. [Google Scholar]
- 14.Safra T, Bernstein Molho R, Stephansky I, et al. Effect of zoledronic acid on bone loss in postmenopausal women with early breast cancer treated with sequential tamoxifen and letrozole. J Clin Onc. 2009;27 doi: 10.1159/000334456. Abstr 599. [DOI] [PubMed] [Google Scholar]
- 15.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 16.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 17.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 18.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. N Engl J Med. 2007;357 doi: 10.1056/NEJMoa074941. nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Klerk G, van der Velde D, van der Palen J, et al. The usefulness of dual energy X-ray and laser absorptiometry of the calcaneus versus dual energy X-ray absorptiometry of hip and spine in diagnosing manifest osteoporosis. Arch Orthop Trauma Surg. 2009;129:251–257. doi: 10.1007/s00402-008-0755-y. [DOI] [PubMed] [Google Scholar]
- 20.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]