Abstract
The dopamine D2 receptor (D2R) plays an important role in mesencephalic dopaminergic neuronal development, particularly coupled with extracellular signal-regulated kinase (ERK) activation. Wnt5a protein is known to regulate the development of dopaminergic neurons. We analyzed the effect of Wnt5a on dopaminergic neuron development in mesencephalic primary cultures from wild-type (WT) and D2R knock-out (D2R−/−) mice. Treatment with Wnt5a increased the number and neuritic length of dopamine neurons in primary mesencephalic neuronal cultures from WT mice, but not from D2R−/− mice. The effect of Wnt5a was completely blocked by treatment with D2R antagonist or inhibitors of MAPK or EGFR. Wnt5a-mediated ERK activation in mesencephalic neuronal cultures was inhibited by treatment of D2R antagonist and EGFR inhibitors in WT mice. However, these regulations were not observed for D2R−/− mice. Co-immunoprecipitation and displacement of [3H]spiperone from D2R by Wnt5a demonstrated that Wnt5a could bind with D2R. This interaction was confirmed by GST pulldown assays demonstrating that the domain including transmembrane domain 4, second extracellular loop, and transmembrane domain 5 of D2R binds to Wnt5a. These results suggest that the interaction between D2R and Wnt5a has an important role in dopamine neuron development in association with EGFR and the ERK pathway.
Keywords: ERK, Neurotransmitter Receptors, Receptor Tyrosine Kinase, Signal Transduction, Wnt Pathway, EGFR, Dopamine Receptor, Dopaminergic Neuronal Development
Introduction
Dopaminergic pathways in the midbrain have been closely associated with serious neurological disorders. Thus, clarification of the mechanisms underlying dopaminergic neuronal development has important implications for the elucidation of novel treatments for disorders involving dopaminergic circuitry. Dopamine-producing cells are generated within the embryonic ventral midbrain. This developmental programming has been shown to require a complex network of transcription factors and signaling pathways (1–6).
We have previously reported that the dopamine D2 receptor (D2R)4 plays a crucial role in the development of dopaminergic neurons as an autoreceptor expressed in midbrain (7, 8). We determined that in the absence of D2R, the later stages of dopaminergic neuronal development, including terminal differentiation, were blunted in association with altered expression of Nurr1 and Ptx3, which are prerequisite for development of dopaminergic neurons. Thus, D2R expressed in the midbrain during the embryonic stage may function as a key regulatory factor, facilitating maintenance of the secure development of dopaminergic neurons during this critical period.
Wnt family proteins are known to be important for neural development. Particularly, it has been suggested that Wnt5a regulates dopaminergic neuron differentiation (9, 10). Recent reports showed that during development, Wnt5a protein is maximally expressed in the midbrain the day that dopaminergic neurons are born. Wnt5a expression increases differentiation of Nurr1+ precursors into DA (dopamine) neurons (9, 10). However, the mechanism by which Wnt5a promotes dopaminergic neuronal differentiation remains to be elucidated.
In situ hybridization for Wnt5a in mice revealed that Wnt5a expression overlaps with tyrosine hydroxylase (TH)-positive cells in the midbrain during embryonic days E9.5–E18.5. Wnt5a expression is then down-regulated in the adult (11). D2R expression in TH neurons begins in the mesencephalon at a similar period, and it appears that D2R functions as a dopaminotropic factor in assuring the homeostatic regulation of terminal differentiation in dopaminergic neuronal development. Thus, it is important to understand whether D2R might cross-talk with Wnt5a in mesencephalic dopaminergic neuron development.
In this study, we examined the effects of Wnt5a on dopaminergic neuronal development in wild-type (WT) and D2R−/− mice and analyzed the interaction between the two systems. Our results reveal an interaction between Wnt5a and D2R on dopaminergic neuron development through Wnt5a binding to D2R. The D2R in turn is coupled to extracellular signal-regulated kinase (ERK) activation, providing a novel network regulating dopamine neuron development that selectively works in a specific time window during dopaminergic neuronal development.
EXPERIMENTAL PROCEDURES
Animal Preparation and Primary Mesencephalic Neuronal Cell Culture
All experiments were performed with D2R−/− and wild-type mice. Mice were produced from heterozygous D2R+/− mice (Jackson Laboratory). Primary mesencephalic neuronal cultures were prepared as described previously (7, 8). To visualize morphological features immunohistochemically, DA neurons were treated from days 2 to 4 with 1 μm quinpirole (Tocris) every 12 h and with 1 ml of Wnt5a concentration conditioned medium (12) or rm (recombinant mouse) Wnt5a (100 ng/ml) or rmWnt9b (50, 100, 200 ng/ml) (R&D Systems) every 24 h. DA neurons were treated in the presence or absence of pretreated 1 μm haloperidol (Sigma) for 10 min, pretreated 50 μm PD98059 (Tocris) for 30 min, or pretreated 10 μm AG1478, pretreated 1 μm PD168393 (EGFR inhibitor; Calbiochem) for 1 h during the experiment. For Western blot analysis, at the 4th day in vitro, DA neurons were incubated with Neurobasal medium without B27 supplement (Invitrogen). DA neurons were then treated with various experimental reagents for the time periods indicated, except PD168393 (10 μm).
Immunocytochemistry
DA neurons were fixed and incubated with rabbit polyclonal anti-TH (1:7500; Pel-Freez). Neurons were then stained according to avidin-biotin immunohistochemical procedures (Vector Laboratories). The number of cells, number of neurites, and neurite length were analyzed as described previously (7, 8).
Immunoprecipitation and Western Blotting
HEK 293T cells were transfected with p3×FLAG-myc CMVTM-26-D2R and pLNCX-HA-Wnt5a using jetPEI transfection reagent (QBiogene) in 100-mm dishes. Cells were treated in lysis buffer, and equal protein aliquots of cell lysates (300 μg) were incubated with 7.5 μg/ml anti-FLAG® M2 monoclonal antibody or HA probe (Santa Cruz Biotechnology; 4 h at 4 °C) followed by the addition of 20 μl of 50% slurry Sepharose A or G (4 h at 4 °C). The complexes were washed three times with washing buffer. Samples were then denatured (3 min at 95 °C). The immunoprecipitated proteins were separated with a 10% SDS-polyacrylamide gel. Western blotting was performed using monoclonal anti-FLAG (1:4000), anti-HA (1:500).
Bacterial Protein Expression and Purification
Five domain fragments of D2R (amino acids 1–71, 72–151, 131–210, 211–374, 375–444) were cloned into the pGEX-4T-3 vector (GE Healthcare) for expression with glutathione S-transferase (GST). Wnt5a was cloned into the pET28-a vector (Novagen) for expression with an N-terminal His tag. GST alone and various fragments of D2R were transformed into Escherichia coli BL21 (DE3). Cultures were harvested by centrifugation and resuspended in 5% of original volume with PBS containing 1 mm DTT, 10 μg/ml lysozyme, and protease inhibitors (1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mm PMSF) for GST fusion proteins or 20 mm Tris-HCl (pH 7.5) containing protease inhibitors for a His-tagged protein. After sonication, the lysate were clarified by centrifugation, and GST fusion proteins were purified on glutathione-Sepharose 4B (GE Healthcare), and His-tagged protein was purified on nickel-nitrilotriacetic acid-agarose (Qiagen).
GST Pulldown Assay
GST fusion proteins were immobilized 2 h with GST-Sepharose beads. Beads were then washed three times with PBS containing 1 mm DTT, 10 μg/ml lysozyme, and protease inhibitors. Subsequently, His-tagged Wnt5a was added to each batch with Tris-HCl (pH 7.5), 1 mm EDTA, 50 mm NaCl, 1% Triton X-100, 1% Nonidet P-40, and protease inhibitors. Unbound proteins were washed three times with the same buffer, and then bound proteins were eluted.
Ligand Binding Assay
To perform Wnt5a and Wnt9b binding to D2R-transfected HEK 293T cell membranes, cells were first harvested in PBS, and membranes were isolated (13). Membrane protein (40 μg) was used for ligand binding assays with [3H]spiperone (specific activity 90 Ci/mmol; PerkinElmer Life Sciences). (+)-Butaclamol (5 μm) was used to define nonspecific binding (14, 15). For displacement experiments, we used haloperidol and Wnt5a at concentrations ranging from 10−11 to 10−6 m. All samples were incubated with 0.5 nm [3H]spiperone. All binding data were analyzed with GraphPad Prism version 4.0 using a one-site binding model.
In Situ Hybridization
Embryonic 14.5-day-old (E14.5) WT and D2R−/− mice were killed. Whole heads were immediately extracted from bodies and mounted in a cryostat maintaining −20 °C and serially sectioned into 10-μm slices. Sections were placed on glass slides, coated with 0.5% gelatin and 0.5% chromium potassium sulfate, and baked at 150 °C. The Wnt5a template was prepared from pLNCX-Wnt5a by PCR with the forward primer 5′-AATGGATCCATGAAGAAGCCCATTGGA-3′ and the reverse primer 5′-CGAAAGCTTCTATTTGCACACACGAACTG-3′. The amplified fragments were gel-purified and subcloned into BamHI and HindIII sites of pBluescript (Stratagene). Antisense and sense Wnt5a were prepared by linearizing the plasmid pBluescript-Wnt5a with BamHI and HindIII. The 35S-labeled cRNA probe was prepared by transcribing 1 μg of linearized DNA with T7 (antisense) and T3 (sense) polymerase for 1.5 h at 37 °C. The reaction mixture contained 35S-labeled CTP (1250 Ci/mmol; PerkinElmer Life Sciences). The reaction was performed using a Riboprobe® Combination System T3/T7 (Promega). Hybridization was completed as described previously (16). Darkfield images were processed using an Imaging Technology image digitalizer to quantify the in situ hybridization. Digitized images were analyzed on a Metamorph image analysis system (Universal Imaging Corp.).
Oligomeric siRNA Transfection and Drug Treatment
A pair of oligonucleotides, 5′-AACAUAGACUUGAAGAAGAACTT-3′ (antisense) and 5′-GUUCUUCUUCAAGUCUAUGUUTT-3′ (sense) (17), was prepared to silence disheveled3 (Dvl3) (Invitrogen). A second pair of oligonucleotides was designed to silence green fluorescence protein (GFP): 5′-GUUCAUCGUGUCCGGCGAGTT-3′ (sense) and 5′-CUCGCCGGACACGCUGAACTT-3′ (antisense), and this pair of oligonucleotides was used as a negative control for siRNA transfection (Samchully Pharm). Transfection was performed as described previously (8). After 72 h, cells were treated with 100 ng/ml rmWnt5a for 10 min for analysis of p-ERK activation by D2R. To determine morphological features of DA neurons using immunocytochemistry, neurons were treated with 100 ng/ml rmWnt5a every 24 h for the duration of the experiment from 24 to 72 h.
Western Blot Analysis of Dvls and p-ERK
After treatment with rmWnt5a (100 ng/ml) or rmWnt9b (100, 200 ng/ml) for 10 min in the presence or absence of pertussis toxin (100 ng/ml for 12 h; Calbiochem), haloperidol (10 μm for 5 min), AG1478 (10 μm for 1 h), PD168393 (10 μm for 1 h), or PD98059 (50 μm for 30 min), DA neurons were washed with ice-cold PBS and treated in lysis buffer on ice. Cells were followed by centrifugation at 13,000 × g for 10 min at 4 °C. Protein (∼30 μg) was separated on a 10% SDS-PAGE for p-ERK and an 8% SDS-PAGE for Dvl1, Dvl2, and Dvl3. Protein was blotted onto pre-wetted polyvinylidene difluoride nitrocellulose membranes. Mouse monoclonal anti-p-ERK (1:3000; Cell Signaling), rabbit monoclonal anti-ERK (1:5000; Santa Cruz Biotechnology), and mouse monoclonal anti-Dvl1, anti-Dvl2, and anti-Dvl3 (1:500; Santa Cruz Biotechnology) were used as primary antibodies. Specific bands were detected by enhanced chemiluminescence (ECL; Amersham Biosciences) and analyzed using a LAS3000 image analysis system (Fuji).
Statistical Analysis
For statistical analyses, two-sample comparisons were performed using the Student's t test. Multiple comparisons were made by one-way ANOVA followed by appropriate post hoc comparisons.
RESULTS
Effect of Wnt5a on Dopaminergic Neuronal Development in WT and D2R−/− Mice
To explore the interaction of Wnt5a and D2R on dopaminergic neuronal development, we first investigated the effects of Wnt5a on the development of dopaminergic neurons in primary mesencephalic cultures from WT and D2R−/− mice. Previously, we demonstrated that in D2R−/− mice, the number of mesencephalic TH cells is significantly low during ontogeny compared with WT mice, indicating an alteration in dopaminergic neuronal development in the absence of D2R (7, 8).
Primary mesencephalic neuronal cell cultures from WT and D2R−/− mice were prepared and treated with Wnt5a-conditioned medium for 48 h. After 48 h of culture, cells were fixed, and TH neurons were counted. In WT mice, Wnt5a treatment was shown to increase the number of TH neurons by 130%. Wnt5a treatment also increased the number and length of neurites, showing that Wnt5a enhances dopaminergic neuronal development. However, in D2R−/− mice, the effect of Wnt5a was absent (Fig. 1).
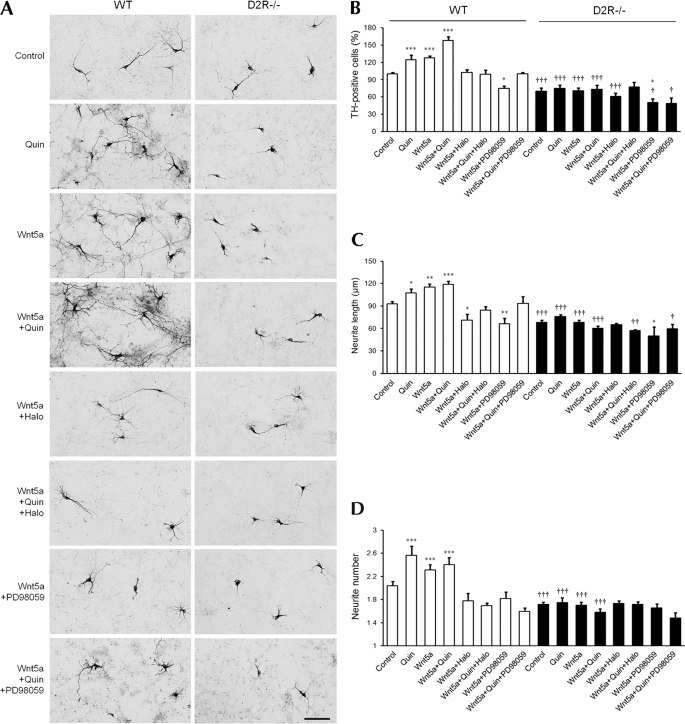
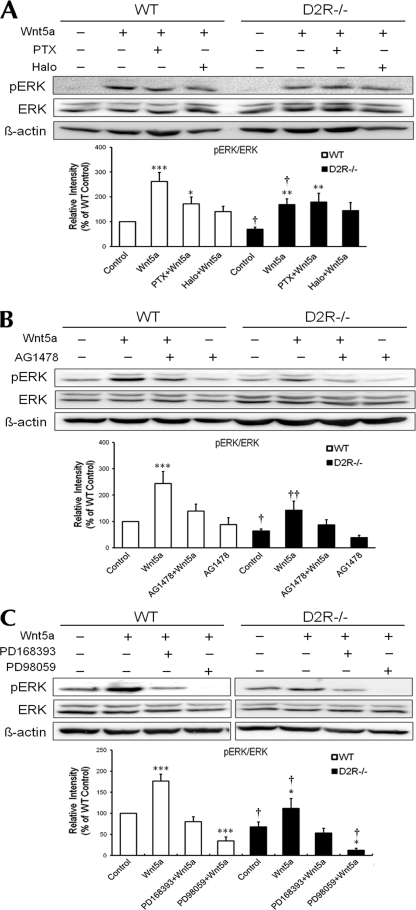
FIGURE 1.
Effect of Wnt5a on number of TH neurons and morphological changes in mesencephalic neuronal cultures from WT and D2R−/− mice. A, effect of treatment with quinpirole (1 μm), Wnt5a (100 ng/ml), Wnt5a plus quinpirole (1 μm), Wnt5a plus haloperidol (1 μm), Wnt5a plus quinpirole plus haloperidol, Wnt5a plus PD98059 (50 μm), Wnt5a plus quinpirole plus PD98059 with a control group on the mesencephalic neuronal cultures from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 100 μm. B–D, effect of treatments on the numbers of TH neurons (B), neurite length (C), and neurite number (D) on mesencephalic neuronal cultures from WT and D2R−/− mice. Mean values ± S.E. are shown for WT (n ≥ 5) and D2−/− (n ≥ 5). *, p < 0.05; **, p < 0.01; ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. †, p < 0.05; ††, p < 0.01; †††, p < 0.001, unpaired t test for WT and D2R−/− mice. Quin, quinpirole; Halo, haloperidol.
It has been shown that treatment with the dopamine D2R agonist quinpirole promotes development of TH neurons, increasing both the number of TH neurons and the length of neurites (7). We examined the effect of quinpirole and co-treatment with quinpirole and Wnt5a on mesencephalic neuronal cultures. Quinpirole and Wnt5a co-treatment resulted in a increased number of TH neurons, up to 160%, accompanied with increases in neuritic length and the number of neuronal process. Again, these effects were not detected in cultures from D2R−/− mice (Fig. 1). Treatment with haloperidol, a D2R antagonist, completely blocked the effect produced by Wnt5a alone and blocked the effects of co-treatment of Wnt5a and quinpirole in mesencephalic neurons from WT mice. However, haloperidol did not have an effect on TH neurons from D2R−/− mice, indicating that this effect is dependent on D2R (Fig. 1).
We previously showed that D2R-mediated enhancement of dopaminergic neuronal development involves the ERK pathway (7, 8). We then attempted to test whether this effect of Wnt5a and D2R includes ERK signaling. We treated mesencephalic neurons with the MEK (MAP kinase kinase) inhibitor PD98059. PD98059 treatment suppressed the effect of Wnt5a and the synergistic effect by Wnt5a and quinpirole on the increase of TH-positive neurons in WT mice (Fig. 1, A and B). Additionally, the average length of neurites and numbers of neurites were reduced in PD98059 treatment in WT neurons. However, no such significant differences were observed in the number and morphology of TH neurons from D2R−/− mice (Fig. 1). Therefore, Wnt5a-mediated enhancement of dopaminergic neuronal development is dependent on both dopamine D2R and ERK signaling.
Wnt5a Expression in Embryo Brain of WT and D2R−/− Mice
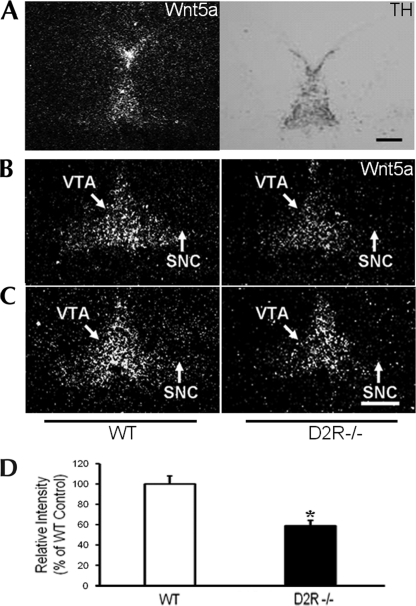
It has been reported that Wnt5a is expressed in midbrain during midbrain development. We determined the expression levels of Wnt5a in the midbrain of WT and D2R−/− mouse embryos. The expression pattern of Wnt5a was analyzed using in situ hybridization; TH-positive neurons were stained by immunocytochemistry at E14.5. As shown in Fig. 2A, the localization of Wnt5a staining signal overlapped with the region of TH expression in the mesencephalon at E14.5. In the substantia nigra (SN) and ventral tegmental area (VTA), expression levels of Wnt5a mRNA were 40% lower in D2R−/− mice than in WT mice (Fig. 2B). Our results indicate that loss of the D2R may affect the expression of Wnt5a in the SN and VTA, which can result in the alteration of development of midbrain dopaminergic neurons in D2R−/− mice.
FIGURE 2.
In situ hybridization of Wnt5a gene expression in WT and D2R−/− mice. A, immunohistochemical image of TH staining and in situ hybridization image of Wnt5a in the mesencephalon of an E15.5 embryo brain are shown. B and C, In the middle (B) and end (C) of the SNC and VTA, expression levels of mesencephalic Wnt5a mRNA were assessed by in situ hybridization in WT and D2R−/− E14.5 mouse brains. Scale bars, 500 μm. D, levels of mRNA are expressed as percentage of WT controls. All values are expressed as means ± S.E. *, p < 0.05, n = 3 each.
Interaction between Wnt5a and Dopamine D2R
Because the effect of Wnt5a on dopaminergic neurons was absent in D2R−/− mice and the effect of Wnt5a and quinpirole was suppressed by D2R antagonist haloperidol, we investigated whether Wnt5a can interact with D2R. To address this question, immunoprecipitation was conducted to determine whether there is an interaction between D2R and Wnt5a. HEK 293T cells were transfected with FLAG-tagged D2R and HA-tagged Wnt5a. Cell lysates from the transfected cells were subjected to immunoprecipitation with an anti-FLAG antibody, followed by Western blotting for HA. As shown in Fig. 3A (left panel), the D2 receptor interacts with Wnt5a. A similar result was obtained when immunoprecipitation was performed using HA antibody, followed by Western blotting, for HA and FLAG (Fig. 3A, middle panel).
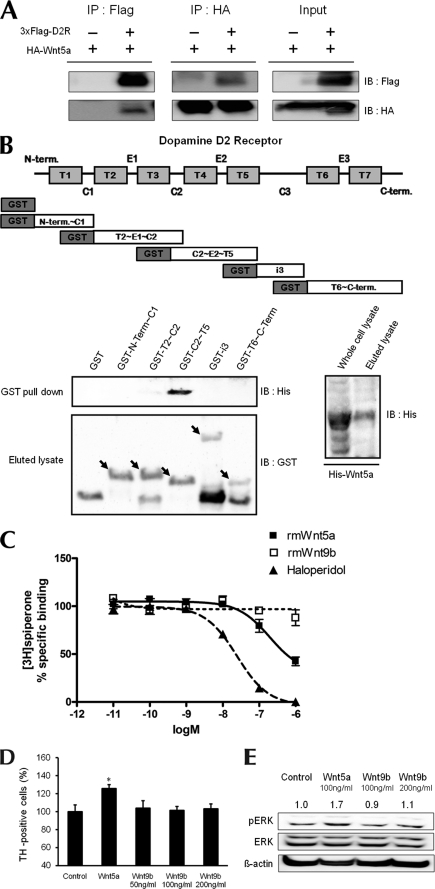
FIGURE 3.
Interaction of Wnt5a with D2R. A, immunoprecipitation showing an association between D2R and Wnt5a. Protein samples were obtained from transfecting p3×FLAG-myc-CMVTM-26-D2R and pLNCX-HA-Wnt5a into HEK293T cells. B, schematic diagram of five domain fragments of D2R (amino acids 1–71, 72–151, 131–210, 211–374, 375–444). Domain fragments are represented as GST-N-Term∼C1, GST-T2∼C2, GST-C2∼T5, GST-i3, GST-T6∼C-Term, respectively. GST pulldown assays used bacterially expressed GST-D2R mutants and His-Wnt5a. Pulldown proteins were analyzed by immunoblotting with His antibody. The left bottom panel shows GST and GST-D2R mutants eluted with glutathione-Sepharose 4B and immunoblotted with GST antibody. The right bottom panel shows His-Wnt5a purified on nickel-nitrilotriacetic acid-agarose and immunoblotted with His antibody. C, displacement of spiperone biding by haloperidol or by Wnt5a. The binding capacities of rmWnt5a, rmWnt9b, and haloperidol were evaluated by inhibition of [3H]spiperone binding to the D2R following treatment with rmWnt5a and haloperidol. Total binding was assessed in the absence of compounds; nonspecific binding was measured in the presence of 5 μm butaclamol. Values are mean ± S.E. of four independent experiments, each conducted in duplicate. In each of the experiments, the curves were fitted via nonlinear regression analysis using the one-site competition model (GraphPad Prism program). D, effect of treatment with Wnt5a (100 ng/ml), Wnt9b (50, 100, 200 ng/ml) on the numbers of TH neurons analyzed in mesencephalic neuronal cultures from WT mice. The mean values ± S.E. are shown for WT, n = 3. *, p < 0.05, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells of WT mice. E, cells treated with 100 ng/ml Wnt5a and 100 ng/ml or 200 ng/ml Wnt9b. *, p < 0.05; **, p < 0.01; ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. †, p < 0.05; ††, p < 0.01; †††, p < 0.001, unpaired Student's t test for WT and D2R−/− mice. Halo, haloperidol.
To examine the detail of the interaction between D2R and Wnt5a, we constructed five domain fragments of D2R (amino acids 1–71, 72–151, 131–210, 211–374, 375–444) and expressed them as GST fusion proteins. The interaction of each purified GST fusion protein with recombinant His-Wnt5a was analyzed by using the GST pulldown assay. As shown in Fig. 3B, Wnt5a binds specifically to the domain containing residues 131–210 of D2R, which includes transmembrane domain 4, the second extracellular loop, and transmembrane domain 5 of D2R.
We also hypothesized that Wnt5a would be able to bind to D2R as a ligand. To address this question, we attempted to determine the binding capacities of Wnt5a to D2R by evaluating the ability to compete with [3H]spiperone, a specific antagonist of the dopamine D2R. As a control, we performed same experiment with haloperidol, a D2R-specific antagonist. Haloperidol displayed a highly selective affinity for the D2R with 20.8 nm of Ki for the D2R, consistent with previous findings (18). The affinity of Wnt5a for the D2R was 165 nm Ki (Fig. 3C). These results show that Wnt5a could bind to D2R with an 8-fold lower affinity compared with haloperidol, suggesting that Wnt5a may function as a ligand of D2R in vivo.
To assess the specificity of Wnt5a in the effects and in the binding to the dopamine D2R, we examined amino acids of different 19 subtypes of Wnt proteins and compared them with Wnt5a using the ClustalW2 multiple sequence alignment program. We found that Wnt9b displayed low level similarity with Wnt5a, and we attempted then to determine the binding capacities of Wnt9b by evaluating its competition with [3H]spiperone. Wnt9b had virtually no affinity for the D2R (Fig. 3C). Thus, the binding of Wnt5a to D2R appears to be specific among Wnt protein family.
We also examined the effect of Wnt9b on the dopaminergic neuronal development and the ability to induce ERK activation by Wnt9b, in mesencephalic cultures. As shown in Fig. 3, treatment of Wnt9b induced no changes with regard to both the number of dopaminergic neurons (Fig. 3D) and ERK activation (Fig. 3E) in primary mesencephalic neuronal culture. These data revealed that interaction of Wnt5a to D2R is specific, and this interaction is important for the effect of Wnt5a on the regulation of the dopaminergic neuronal development.
Effect of D2R Agonist and Wnt5a on Dvl3 Phosphorylation via Dopamine D2R
We then examined how Wnt5a can regulate dopaminergic neuronal development via the D2R. It has been reported that Wnt5a treatment induces phosphorylation of Dvl in SN4741 immortalized cell lines downstream signaling of Wnt5a (19). Likewise, immunoprecipitation using protein isolated from rat prefrontal cortex showed that only Dvl3 is associated specifically with the dopamine D2R, but not Dvl1 or Dvl2 (20). Treatment with Wnt5a in SN4741 cells induces phosphorylation of Dvl3 (19, 21).
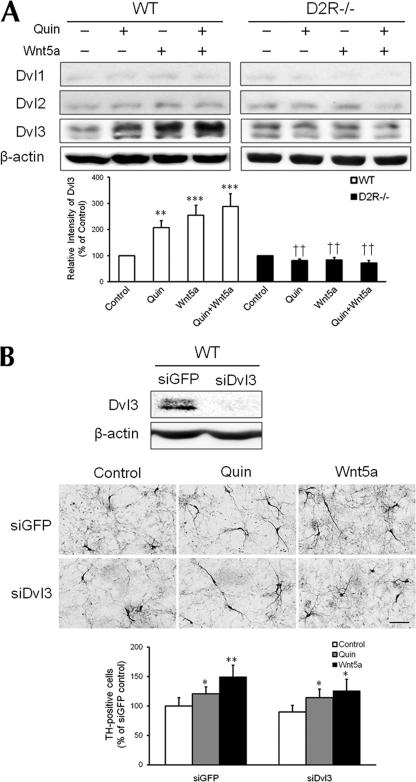
First of all, we examined the expression level of three Dvls and we found that Dvl3 was most abundant Dvl in the mesencephalic neuronal culture (Fig. 4A). We investigated whether quinpirole or Wnt5a treatment can induce phosphorylation of different Dvls in dopaminergic mesencephalic neurons. As shown in Fig. 4A, quinpirole treatment alone induced an increase in phosphorylation of Dvl3 by 207%. Wnt5a treatment increased phosphor-Dvl3 by ∼255% compared with control cells. Co-treatment with Wnt5a and quinpirole also increased phosphorylation of Dvl3 up to 288% (Fig. 4A). However, these treatments had no effect on the other Dvl isoforms. On the other hand, the induction of phosphorylation of Dvl3 was not observed in D2R−/− mice (Fig. 4A). These results show that Dvl3 activation occurs in response to the D2R agonist and Wnt5a via dopamine D2R in mesencephalic dopaminergic neurons.
FIGURE 4.
Dvl3 phosphorylation induced by stimulation of the D2R and Wnt5a in mesencephalic dopaminergic neurons. A, representative Western blot and quantitative relative intensity analyses from Western blot of phosphor-Dvl1, Dvl2, Dvl3, and β-actin levels of mesencephalic neuronal cells of WT and D2R−/− mice. The cells were treated with 10 μm quinpirole, 100 ng/ml rmWnt5a, or both. Mean values ± S.E. are shown for WT (n = 6) and D2R−/− (n = 6). **, p < 0.01; ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. ††, p < 0.01, unpaired Student's t test for WT and D2R−/− mice. B, representative immunoblots of Dvl3 from cell lysates of WT mesencephalic neuronal cultures transfected with 20 nm oligomeric siRNA of GFP (siGFP) or Dvl3 (siDvl3). β-Actin blots were used as an internal standard. Knockdown of Dvl3 expression in mesencephalic neurons did not reduce the increase of TH-positive neurons induced by quinpirole (1 μm) or rmWnt5a (100 ng/ml). The TH neurons were visualized via immunocytochemistry. Scale bars, 100 μm. Mean values ± S.E. are shown for n = 3. *, p < 0.05; **, p < 0.01, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. Quin, quinpirole.
We then attempted to determine whether knockdown of Dvl3 expression modulates the increase in TH-positive neurons induced by the D2R agonist and Wnt5a. The effects of a D2R agonist and Wnt5a on dopaminergic neurons after transfection of siGFP or siDvl3 (17) were determined (Fig. 4B, upper panel). In WT mice, siDvl3 transfection did not significantly affect the number of TH-positive neurons compared with a siGFP-transfected group, upon treatment with either the D2R agonist quinpirole or Wnt5a (Fig. 4B, middle and lower panels). Alternatively, knockdown of Dvl3 did not affect the ERK activation induced by Wnt5a between the siGFP and siDvl3 groups (data not shown). These data suggest that D2R regulates phosphorylation of Dvl3, but phosphorylation of Dvl3 via D2R is not involved in the regulation of dopaminergic neuronal development.
ERK Activation Induced by Wnt5a
Given that ERK signaling induced by Wnt5a plays a critical role in dopaminergic neuronal development and that the effect of Wnt5a is dependent on D2R, we investigated how Wnt5a mediates ERK activation more closely. We also examined whether this Wnt5a-mediated ERK activation was dependent on the D2R.
Wnt5a-mediated ERK activation has been described (22, 23). It has been suggested that Wnt5a induces ERK activation via transactivation of ErbB1. ErbB1 belongs to the EGF family of receptor tyrosine kinases (22). Thus, we investigated how Wnt5a-mediated ERK activation is associated with EGFR signaling and whether this regulation can affect dopaminergic neuronal development.
Treatment of Wnt5a induced a strong ERK activation in mesencephalic neuronal cultures from WT mice (by 260%) and a slightly weaker ERK activation in cultures from D2R−/− mice (170%). This Wnt5a-induced ERK activation was inhibited by treatment with pertussis toxin (Gαi inhibitor) to the Wnt5a-mediated ERK activation level of D2R−/− mice. There was no change in D2R−/− mice upon pertussis toxin treatment, indicating the involvement of Gαi and D2R-mediated signaling in Wnt5a-induced ERK activation in mesencephalic neuronal cultures (Fig. 5A). Pretreatment with haloperidol, a D2R antagonist, before Wnt5a treatment significantly suppressed ERK activation in mesencephalic cultures from WT mice. In contrast, there was no effect of haloperidol pretreatment in D2R−/− mice, suggesting that ERK activation induced by Wnt5a is dependent on the D2R (Fig. 5A).
FIGURE 5.
ERK activation induced by Wnt5a in mesencephalic dopaminergic neurons from WT and D2R−/− mice. Representative Western blot and quantitative relative intensity analysis from Western blot of phospho-ERK and ERK levels of mesencephalic neuronal cells from WT and D2R−/− mice are shown. A, cells were treated with 100 ng/ml Wnt5a, with or without 100 ng/ml pertussis toxin (PTX) or 1 μm haloperidol (Halo). Mean values ± S.E. are shown for WT (n = 8) and D2R−/− (n = 6). B, cells were treated with 100 ng/ml Wnt5a, with or without 10 μm AG1478. Mean values ± S.E. are shown for WT (n = 5) and D2R−/− (n = 4). C, cells were treated with 100 ng/ml Wnt5a, with or without 10 μm PD168393 or 50 μm PD98059. Mean values ± S.E. are shown for WT (n = 7) and D2R−/− (n = 6). *, p < 0.05; **, p < 0.01; ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. †, p < 0.05; ††, p < 0.01; †††, p < 0.001, unpaired Student's t test for WT and D2R−/− mice.
Role of EGFR Signaling in Wnt5a-mediated ERK Activation and in Dopaminergic Neuronal Development
We also investigated the role of the EFGR signaling in modulation of Wnt5a-mediatd ERK activation. Mesencephalic neurons from WT and D2R−/− mice were pretreated by the EGFR inhibitor AG1478. Wnt5a-mediated ERK activation was evaluated. Treatment with AG1478 suppressed Wnt5a-mediated ERK activation in WT mice, producing similar levels of Wnt5a-mediated ERK activation as seen in D2R−/− mice. Upon treatment of AG1478, Wnt5a-mediated ERK activation was also slightly decreased in D2R−/− mice (Fig. 5B). AG1478 alone completely suppressed ERK activation in WT and D2R−/− mice, showing that EGFR signaling is essential for ERK activation in mesencephalic cells in both mice genotypes.
We also determined the effect of another EGFR inhibitor, PD168393, and the MAPK inhibitor PD98059 on Wnt5a-mediated ERK activation. Treatment with PD168393 suppressed Wnt5a-mediated ERK activation in WT and D2R−/− mice in a manner similar to AG1478 (Fig. 5C). Similar but stronger effects were seen when PD98059 was pretreated in WT and D2R−/− mice (Fig. 5C).
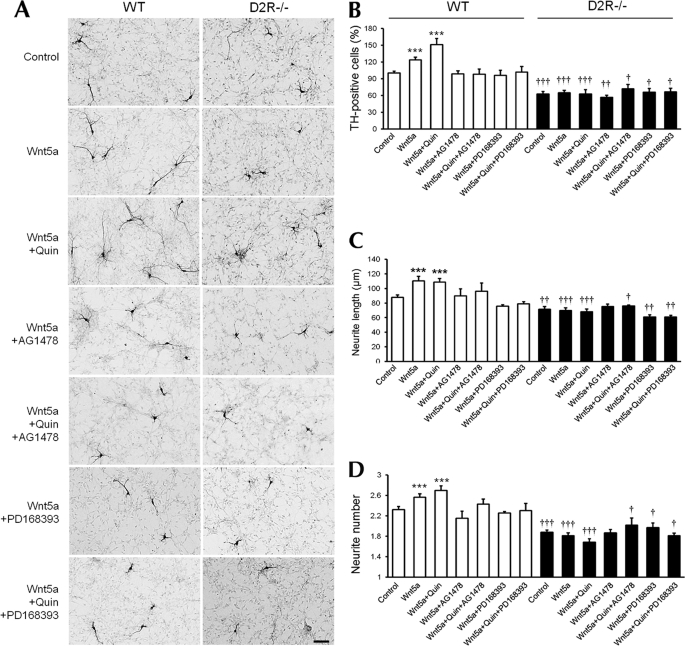
Finally, we investigated whether these modulators of Wnt5a-induced ERK activation indeed regulated dopaminergic neuronal development in mesencephalic neurons. Treatment with the EGFR inhibitors AG1478 or PD168393 completely suppressed the effects of Wnt5a alone, as well as the combined effect of Wnt5a and quinpirole on the number of TH-positive cells by reducing to the basal level of cells in the control group in WT mice (Fig. 6, A and B). However, AG1478 or PD168393 had no observable effect on mesencephalic neuronal cultures from D2R−/− mice (Fig. 6, A and B).
FIGURE 6.
EGFR inhibitors prevent the changes in the number of TH neurons and morphology in cultured mesencephalic neurons induced by D2R agonist/Wnt5a. A, treatment with Wnt5a (100 ng/ml), Wnt5a plus quinpirole (1 μm), Wnt5a plus AG1478 (10 μm), Wnt5a plus quinpirole plus AG1478 (10 μm), Wnt5a plus PD168393 (1 μm), Wnt5a plus quinpirole plus PD168393 (1 μm), control, in mesencephalic neuronal cultures from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 100 μm. B–D, effect of treatment with combination of drugs on the numbers of TH neurons (B), neurite length (C), and neurite number (D) analyzed in mesencephalic neuronal cultures from WT and D2R−/− mice. Mean values ± S.E. are shown for WT (n = 6) and D2R−/− (n = 6). ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells of WT mice. †, p < 0.05; ††, p < 0.01; †††, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells of D2R−/− mice. Quin, quinpirole.
Treatment with AG1478 or PD168393 produced similar effects on the average neurite length and numbers of TH-positive neurons in mesencephalic neuronal cultures from WT mice. AG1478 or PD168393 induced no significant morphologic changes of TH-positive neurons in the cultures from D2R−/− mice (Fig. 6, A, C, and D). These data strongly suggest that the effect of Wnt5a and EGFR signaling on dopaminergic neuronal development is dependent on the presence of D2R.
Taken together, these data show that along with modulating the Wnt5a-induced ERK signaling via ERGR signaling, Wnt5a regulates the TH-positive cell numbers and morphogenic properties in mesencephalic neuronal cultures through ERK activation, which is D2R-dependent within dopaminergic neuronal cells.
DISCUSSION
Although there have been many indications that Wnt5a is an important mediator of development of dopaminergic neurons, the precise molecular mechanisms by which Wnt5a regulate the mesencephalic dopaminergic neurons are still poorly understood. Moreover, Wnt5a−/− mice exhibit only a mild and transient TH neuron differentiation phenotype in vivo (11), showing that Wnt5a is only transiently required for the differentiation of endogenous midbrain dopaminergic precursors in vivo. In the present study, we investigated the interaction between D2R and Wnt5a in the development of dopaminergic neurons. We identified D2R as an important regulator of Wnt5a-mediated dopaminergic neuronal development.
Wnt5a is preferentially expressed in the developing rat ventral midbrain, and it has been demonstrated that Wnt5a selectively promotes a dopaminergic phenotype of Nurr+ neuronal progenitor cells derived from embryonic day 14.5 rat ventral midbrain (9, 10). As described here and elsewhere, Wnt5a expression coincides with that of TH at day E14 (10, 11).
Given that Wnt5a expression overlaps with TH-positive cells in the midbrain during the embryonic period E9.5–E18.5, Wnt5a may be required for the differentiation of dopaminergic neurons transiently for this time period. We have observed that the effect of Wnt5a on promotion of TH-positive neurons was absent in mesencephalic cultures from D2R−/− mice, which underscores the requirement of D2R for proper function of Wnt5a in this period. These findings argue for an additional explanation of why Wnt5a−/− mice exhibit only a mild and transient altered TH neurons differentiation phenotype (11), suggesting that D2R and other factors implicated in TH neuron development can largely compensate in the absence of Wnt5a. The absence of D2R results in more serious and enduring alterations in number of TH-positive neurons (7), indicating that D2R influences several sets of factors, including Wnt5a and other factors implicated in midbrain TH-neuronal development. The D2R seems to offer a center of homeostatic regulation of TH-neuronal development in a time- and space-specific manner.
The dopamine D2-like receptors have been suggested to be involved in a variety of important modulations of mesencephalic dopaminergic cell function (24–30). It has been reported that the dopamine D2-like receptor plays a crucial role in dopaminergic neuron development (7, 31, 32). Therefore, it appears that the onset of dopamine D2R expression in the midbrain during the embryonic stage may function as a dopaminotrophic factor that facilitates maintenance of the secure development of dopaminergic neurons during this critical period.
Another key finding of this study is the direct interaction between Wnt5a and D2R, as shown by co-immunoprecipitation, GST pulldown assay, and a ligand binding displacement assay with a D2R-specific ligand. This striking feature of theWnt5a-D2R interaction might not represent a unique case, but rather a general mode d'emploi of many of Wnt proteins to perform their physiological function in different time- and space-specific windows. For instance, the Wnt expression pattern in adults differs from that in the embryonic period. Thus, it is unclear how the proteins can exert their function with such great dynamic variation in expression and signaling together with different classes of receptors. We and others identified Wnt5a expression in the adult brain. It appears that basal expression of Wnt5a is low throughout the brain, except in some specific regions such as hippocampus and some cortical areas (33). In this context, the mechanisms of the interaction between Wnt5a and D2R in the adult are intriguing and invite further investigation.
Another interesting finding in our study is the involvement of EGFR signaling in the D2R-Wnt5a interaction for dopaminergic neuronal development. It has been demonstrated that EGF and EGFR are expressed in midbrain (34–37). It is believed that EGF and EGFR signaling can contribute to dopaminergic neuronal survival and dopamine-mediated neurogenesis (38–40).
Interestingly, O'Keeffe et al. (40) demonstrated that in vivo dopamine depletion leads to a decrease in precursor cell proliferation in the subventricular zone concomitant with a reduction in local EGF production. EGFR+ cells are significantly depleted in the subventricular zone of individuals with Parkinson's disease, indicating a role of the dopamine-EGFR signaling loop in the regulation of neurogenesis of dopaminergic neurons.
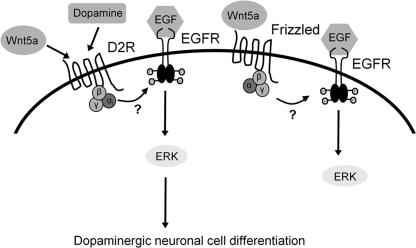
In our study, we have shown that Wnt5a-mediated ERK activation is important in D2R-mediated dopaminergic neuronal development. This Wnt5a-mediated ERK activation is regulated under the control of not only D2R but also EGFR signaling. As a consequence, these signaling events influence each other's role in dopaminergic neuronal development. This indicates that D2R acts as a dopamine-sensing platform, providing a homeostatic environment for regulation of dopaminergic cell development. A speculative model proposing a role of D2R as a dopaminotrophic factor in dopaminergic neurons is depicted in Fig. 7.
FIGURE 7.
Proposed mechanism for D2R-dependent dopaminergic neuronal development, involving Wnt5a and EGFR signaling coupled with ERK activation. A dopamine agonist and Wnt5a can activate the D2R, resulting in the phosphorylation of ERK through EGFR transactivation. This signaling can promote in dopaminergic neuronal development.
Taken together, these findings suggest that stimulation of the D2R by Wnt5a activates ERK phosphorylation via EGFR and promotes dopaminergic neuronal development. Given that EGFR signaling serves as a final transducer for D2R and Wnt5a in the induction of ERK activation, further studies should explore and clarify the role of EGFR signaling in dopamine-associated regulation of ERK signaling in different conditions, including in the adult brain.
Acknowledgments
We thank Dr. Eek-Hoon Jho (University of Seoul, South Korea) and Jang-Soo Chun (Gwangju Institute of Science and Technology, Gwangju, South Korea) for providing Wnt5a cDNAs and stable cell lines expressing Wnt5a. We thank all members of our laboratory for assistance and helpful discussions.
This work was supported in part by a research grant from the Korea Healthcare Technology R&D Project A084139, Grants 2009K001254 and 2010K000814 from the Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, the Republic of Korea, and by a Korea University grant.
- D2R
- dopamine D2 receptor
- Dvl
- disheveled
- SN
- substantia nigra
- TH
- tyrosine hydroxylase
- VTA
- ventral tegmental area
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1. Lindvall O., Kokaia Z. (2009) Trends Pharmacol. Sci. 30, 260–267 [DOI] [PubMed] [Google Scholar]
- 2. Smidt M. P., Burbach J. P. (2007) Nat. Rev. Neurosci. 8, 21–32 [DOI] [PubMed] [Google Scholar]
- 3. Perrone-Capano C., Da Pozzo P., di Porzio U. (2000) Neurosci. Biobehav. Rev. 24, 119–124 [DOI] [PubMed] [Google Scholar]
- 4. Simon H. H., Bhatt L., Gherbassi D., Sgadó P., Alberí L. (2003) Ann. N.Y. Acad. Sci. 991, 36–47 [PubMed] [Google Scholar]
- 5. Riddle R., Pollock J. D. (2003) Brain Res. Dev. Brain Res. 147, 3–21 [DOI] [PubMed] [Google Scholar]
- 6. Perlmann T., Wallén-Mackenzie A. (2004) Cell Tissue Res. 318, 45–52 [DOI] [PubMed] [Google Scholar]
- 7. Kim S. Y., Choi K. C., Chang M. S., Kim M. H., Kim S. Y., Na Y. S., Lee J. E., Jin B. K., Lee B. H., Baik J. H. (2006) J. Neurosci. 26, 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S. Y., Lee H. J., Kim Y. N., Yoon S., Lee J. E., Sun W., Choi E. J., Baik J. H. (2008) Exp. Neurol. 214, 69–77 [DOI] [PubMed] [Google Scholar]
- 9. Arenas E. (2005) Ann. N.Y. Acad. Sci. 1049, 51–66 [DOI] [PubMed] [Google Scholar]
- 10. Castelo-Branco G., Wagner J., Rodriguez F. J., Kele J., Sousa K., Rawal N., Pasolli H. A., Fuchs E., Kitajewski J., Arenas E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12747–12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersson E. R., Prakash N., Cajanek L., Minina E., Bryja V., Bryjova L., Yamaguchi T. P., Hall A. C., Wurst W., Arenas E. (2008) PLoS One 3, e3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu J. H., Chun J. S. (2006) J. Biol. Chem. 281, 22039–22047 [DOI] [PubMed] [Google Scholar]
- 13. An J. J., Cho S. R., Jeong D. W., Park K. W., Ahn Y. S., Baik J. H. (2003) Mol. Cell. Endocrinol. 206, 49–62 [DOI] [PubMed] [Google Scholar]
- 14. Choi E. Y., Jeong D., Park K. W., Baik J. H. (1999) Biochem. Biophys. Res. Commun. 256, 33–40 [DOI] [PubMed] [Google Scholar]
- 15. Kim S. J., Kim M. Y., Lee E. J., Ahn Y. S., Baik J. H. (2004) Mol. Endocrinol. 18, 640–652 [DOI] [PubMed] [Google Scholar]
- 16. Kim K. S., Yoon Y. R., Lee H. J., Yoon S., Kim S. Y., Shin S. W., An J. J., Kim M. S., Choi S. Y., Sun W., Baik J. H. (2010) J. Biol. Chem. 285, 8905–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu W., Yamamoto V., Ortega B., Baltimore D. (2004) Cell 119, 97–108 [DOI] [PubMed] [Google Scholar]
- 18. Kim H. S., Song M., Yumkham S., Choi J. H., Lee T., Kwon J., Lee S. J., Kim J. I., Lee K. W., Han P. L., Shin S. W., Baik J. H., Kim Y. S., Ryu S. H., Suh P. G. (2006) J. Neurochem. 99, 458–469 [DOI] [PubMed] [Google Scholar]
- 19. Schulte G., Bryja V., Rawal N., Castelo-Branco G., Sousa K. M., Arenas E. (2005) J. Neurochem. 92, 1550–1553 [DOI] [PubMed] [Google Scholar]
- 20. Sutton L. P., Honardoust D., Mouyal J., Rajakumar N., Rushlow W. J. (2007) J. Neurochem. 102, 153–169 [DOI] [PubMed] [Google Scholar]
- 21. Bryja V., Schulte G., Rawal N., Grahn A., Arenas E. (2007) J. Cell Sci. 120, 586–595 [DOI] [PubMed] [Google Scholar]
- 22. Civenni G., Holbro T., Hynes N. E. (2003) EMBO Rep. 4, 166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. (2005) J. Biol. Chem. 280, 41342–41351 [DOI] [PubMed] [Google Scholar]
- 24. Groves P. M., Wilson C. J., Young S. J., Rebec G. V. (1975) Science 190, 522–528 [DOI] [PubMed] [Google Scholar]
- 25. Meiergerd S. M., Patterson T. A., Schenk J. O. (1993) J. Neurochem. 61, 764–767 [DOI] [PubMed] [Google Scholar]
- 26. Swarzenski B. C., Tang L., Oh Y. J., O'Malley K. L., Todd R. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 649–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cragg S. J., Greenfield S. A. (1997) J. Neurosci. 17, 5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Kampen J. M., Hagg T., Robertson H. A. (2004) Eur. J. Neurosci. 19, 2377–2387 [DOI] [PubMed] [Google Scholar]
- 29. Van Kampen J. M., Robertson H. A. (2005) Neuroscience 136, 381–386 [DOI] [PubMed] [Google Scholar]
- 30. Winner B., Desplats P., Hagl C., Klucken J., Aigner R., Ploetz S., Laemke J., Karl A., Aigner L., Masliah E., Buerger E., Winkler J. (2009) Exp. Neurol. 219, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Kampen J. M., Eckman C. B. (2006) J. Neurosci. 26, 7272–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collo G., Zanetti S., Missale C., Spano P. (2008) Eur. J. Neurosci. 28, 1231–1240 [DOI] [PubMed] [Google Scholar]
- 33. Shimogori T., VanSant J., Paik E., Grove E. A. (2004) J. Comp. Neurol. 473, 496–510 [DOI] [PubMed] [Google Scholar]
- 34. Casper D., Mytilineou C., Blum M. (1991) J. Neurosci. Res. 30, 372–381 [DOI] [PubMed] [Google Scholar]
- 35. Casper D., Roboz G. J., Blum M. (1994) J. Neurochem. 62, 2166–2177 [DOI] [PubMed] [Google Scholar]
- 36. Yamada M., Ikeuchi T., Hatanaka H. (1997) Prog. Neurobiol. 51, 19–37 [DOI] [PubMed] [Google Scholar]
- 37. Abe Y., Namba H., Zheng Y., Nawa H. (2009) Neuroscience 161, 95–110 [DOI] [PubMed] [Google Scholar]
- 38. Moses D., Teper Y., Gantois I., Finkelstein D. I., Horne M. K., Drago J. (2006) Exp. Neurol. 199, 209–221 [DOI] [PubMed] [Google Scholar]
- 39. Inoue H., Lin L., Lee X., Shao Z., Mendes S., Snodgrass-Belt P., Sweigard H., Engber T., Pepinsky B., Yang L., Beal M. F., Mi S., Isacson O. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14430–14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Keeffe G. C., Tyers P., Aarsland D., Dalley J. W., Barker R. A., Caldwell M. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8754–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]