Abstract
The inositol 1,4,5-trisphosphate receptor (InsP3R), an intracellular calcium channel, has three isoforms with >65% sequence homology, yet the isoforms differ in their function and regulation by post-translational modifications. We showed previously that InsP3R-1 is functionally modified by O-linked β-N-acetylglucosamine glycosylation (O-GlcNAcylation) (Rengifo, J., Gibson, C. J., Winkler, E., Collin, T., and Ehrlich, B. E. (2007) J. Neurosci. 27, 13813–13821). We now report the effect of O-GlcNAcylation on InsP3R-2 and InsP3R-3. Analysis of AR4-2J cells, a rat pancreatoma cell line expressing predominantly InsP3R-2, showed no detectable O-GlcNAcylation of InsP3R-2 and no significant functional changes despite the presence of the enzymes for addition (O-β-N-acetylglucosaminyltransferase) and removal (O-β-N-acetylglucosaminidase) of the monosaccharide. In contrast, InsP3R-3 in Mz-ChA-1 cells, a human cholangiocarcinoma cell line expressing predominantly InsP3R-3, was functionally modified by O-GlcNAcylation. Interestingly, the functional impact of O-GlcNAcylation on the InsP3R-3 channel was opposite the effect measured with InsP3R-1. Addition of O-GlcNAc by O-β-N-acetylglucosaminyltransferase increased InsP3R-3 single channel open probability. Incubation of Mz-ChA-1 cells in hyperglycemic medium caused an increase in the InsP3-dependent calcium release from the endoplasmic reticulum. The dynamic and inducible nature of O-GlcNAcylation and the InsP3R isoform specificity suggest that this form of modification of InsP3R and subsequent changes in intracellular calcium transients are important in physiological and pathophysiological processes.
Keywords: Calcium; Calcium Imaging; Calcium Intracellular Release; Glycosylation; Ion Channels; Calcium Signaling; Inositol 1,4,5-Trisphosphate Receptor; Intracellular Calcium Channels; O-GlcNAc Glycosylation
Introduction
Intracellular calcium signaling is vital to a myriad of cellular processes, including cell proliferation, fertilization, secretion, neuronal development and plasticity, light transduction, and muscle contraction (1, 2). The inositol 1,4,5-trisphosphate receptor (InsP3R)6 is an intracellular calcium channel. It is associated primarily with the endoplasmic reticulum, but it is also found in the nuclear envelope and Golgi apparatus (3). The InsP3R is activated when bound with InsP3 produced by stimulation of G protein- or tyrosine kinase-coupled receptors (4) following activation by their agonists, compounds that range from hormones and growth factors to neurotransmitters and odorants (2).
Three mammalian isoforms of the InsP3R have been identified, InsP3R-1, InsP3R-2, InsP3R-3, with a sequence homology of >65% (5). Expression of the isoforms is cell-specific and regulated in response to extracellular stimulation during development and various disease states (6). They differ in many functional aspects such as InsP3 affinity, calcium dependence, and regulation by endogenous compounds, accessory proteins, and post-translational modifications (7). These functional differences and their diverse cell and tissue expression patterns contribute to specialized calcium signals in each cell.
O-Linked β-N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation) is a post-translational modification discovered >25 years ago (8). O-GlcNAcylation is characterized by O-linked attachment of a single β-N-acetylglucosamine (GlcNAc) moiety to serine or threonine residues of proteins. Unlike N-linked glycosylation, this monosaccharide modification is reversible and occurs in both the cytoplasm and nucleus. To date, >1000 proteins have been found to be O-GlcNAcylated (9). The O-GlcNAc proteome includes proteins involved in metabolism, cell structure, stress response, cell cycle, and transcription/translation and nuclear pore proteins involved in cell signaling (10).
Over 600 enzymes are known to regulate O-phosphorylation (11), but only two unique, highly conserved enzymes are directly involved in adding and removing O-GlcNAc from the protein backbone: O-β-N-acetylglucosaminyltransferase (OGT) adds the sugar, and O-β-N-acetylglucosaminidase (OGA) removes it. Loss-of-function mutations of the OGT gene are lethal in embryonic stem cells, and intact OGT alleles are required for completion of embryogenesis (12). O-GlcNAcylation is a major factor in nutrient and stress responses (13, 14). Altered function of these enzymes has been linked to diabetes, cancer, and Alzheimer disease. UDP-GlcNAc, the substrate for O-GlcNAcylation, is one of the end products of the hexosamine biosynthesis pathway (HBP). UDP-GlcNAc is the second most abundant high energy compound within the cell after ATP (10, 15). Normally, 2–5% of total glucose is metabolized through the HBP (10), but this rate can be increased by an augmented supply of glucose or glucosamine (16, 17).
Several lines of evidence indicate that O-GlcNAcylation can act in a manner reciprocal to O-phosphorylation (18). Because O-phosphorylation of the InsP3R is isoform-specific (19), we wanted to determine whether O-GlcNAcylation is also isoform-dependent. Reciprocity between these two forms of protein modification on the InsP3R would expand the functional range of regulation of intracellular calcium signaling.
We showed previously that InsP3R-1 is modified by O-GlcNAcylation (20) and that this biochemical modification has a functional effect at the single channel level and in the whole cell. We also found that O-GlcNAcylation inhibits the ability of InsP3R-1 to diffuse through the endoplasmic reticulum (21). The goal of these experiments was to determine whether InsP3R-2 and InsP3R-3 are modified by O-GlcNAc and, if so, the functional effect of the modification. We found that InsP3R-2 is not modified by O-GlcNAc and that there is no effect of hyperglycemic treatment on calcium signaling. In contrast, InsP3R-3 is functionally modified by O-GlcNAc. Surprisingly, the functional effect on InsP3R-3 is opposite that found for InsP3R-1.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment of Cells
AR4-2J cells were grown in Ham's F-12 (Kaighn's modification) medium (Invitrogen) supplemented with 20% heat-inactivated FBS (Invitrogen) and 1% penicillin/streptomycin stock containing 10,000 μg/ml streptomycin and 10,000 units/ml penicillin (Invitrogen). Mz-ChA-1 cells were cultured in minimum essential medium (Invitrogen) supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. SH-SY5Y cells were grown in a 1:1 mixture of Ham's F-12 medium and minimum essential medium supplemented with 10% FBS, 1% minimum essential medium nonessential amino acids (×100, Invitrogen), and 1% penicillin/streptomycin. Cells were cultured in a water-saturated atmosphere at 37 °C and 5% CO2.
To induce an increase in O-GlcNAcylation, 8 mm N-acetyl-d-glucosamine (GlcNAc; cell culture-tested; Sigma), 8 mm glucosamine (d-glucosamine hydrochloride; cell culture-tested, diluted in H2O, pH 7.4; Sigma), or 20 mm d-glucose (to achieve a final concentration of 25.6 mm for Mz-ChA-1 cells and 27 mm for AR4-2J cells) was added to the growth medium for 72 h (22). 20 mm mannitol was added to the control medium as an osmotic control. The pH of the media was not altered by this supplementation. For drug-induced increases in O-GlcNAcylation, O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate (PUGNAc; 100 or 200 μm as indicated for 24 h; Sigma or Toronto Research Chemicals, Ontario, Canada), an inhibitor of OGA (23), or streptozotocin (10 mm for 60 min; AXXORA, San Diego, CA), an inhibitor of OGA and an agent that induces diabetes in rodents (24), was added to the growth medium.
Cell Lysis and Immunoprecipitation
Cells were plated on 35-mm cell culture dishes and incubated in the respective media. After sugar treatment, cells were harvested by incubation with 100 μl of M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA) supplemented with 2 mm Na3VO4 (Sigma), 50 mm GlcNAc or 5 μm PUGNAc (protection against enzymatic cleavage of the O-GlcNAc moiety for O-GlcNAcylation experiments), and protease inhibitors (protease inhibitor mixture, lyophilized powder; Sigma) on ice for 2.5 min. After harvesting and centrifuging the lysate, the supernatant was saved and immediately used for gel electrophoresis or immunoprecipitation. For immunoprecipitation, 100 or 200 μg of protein of the whole cell lysate was precleared with 10 μl of protein G-Sepharose beads (Amersham Biosciences AB, Uppsala, Sweden) and incubated with the primary antibody for 2 h at 4 °C on a rotating wheel. For experiments examining InsP3R-3, either 4 μl of anti-InsP3R-3 antibody (labeling the N-terminal region; BD Transduction Laboratories) or 4 μl of anti-O-GlcNAc antibody (RL2, isotype IgG1; Affinity BioReagents, Golden, CO) (25) was used. For experiments examining InsP3R-2, either 5 μl of anti-InsP3R-2 antibody (C-terminal site; kindly provided by Dr. Richard J. Wojcikiewicz, State University of New York at Syracuse, Syracuse, NY) (26) or 5 μl of antibody RL2 was used. This incubation was followed by an additional 1.5 h of incubation with 30 μl of washed protein G-Sepharose beads. Washing steps with wash buffer (M-PER supplemented with protease inhibitors, 2 mm sodium orthovanadate, and 50 mm GlcNAc or 5 μm PUGNAc) were subsequently performed. The final pellet of beads was resuspended in 35 μl of 5× sample buffer (20% glycerol, 20% SDS, 2% 2-mercaptoethanol, 0.5 m Tris-HCl (pH 6.8), and bromphenol blue), and the whole sample was analyzed by gel electrophoresis and Western blotting. In the case of immunoprecipitation with anti-InsP3R-3 antibody, samples were boiled for 2 min before gel electrophoresis.
Gel Electrophoresis and Western Blotting
Protein concentration was measured with a BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of protein (25 or 30 μg) were resuspended in 5× sample buffer and resolved by SDS-PAGE. For resolution of protein bands, Tris-HCl-5% precast polyacrylamide gels (Bio-Rad) were used for O-GlcNAcylation analysis of InsP3R-1, InsP3R-2, and InsP3R-3. The proteins were transferred to a PVDF membrane (Bio-Rad). The membrane was incubated in blocking solution A (5% dry milk powder and 0.05% Tween 20 in PBS) for 2 h at room temperature and then with the primary antibody in blocking solution A or B (3% BSA supplemented with 0.05% Tween 20 in PBS) either overnight at 4 °C or for 2 h at room temperature. Antibodies were used at the following dilutions: mouse anti-β-actin (AC-15), 1:8000 for 2 h (Abcam, Cambridge, MA); rabbit anti-InsP3R-1, 1:2000 overnight (affinity-purified from rabbit polyclonal antiserum directed against the 19 C-terminal residues of mouse InsP3R-1) (27); rabbit anti-InsP3R-2, 1:2000 overnight; mouse anti-InsP3R-3, 1:1000 for 2 h; rabbit anti-glutamine:fructose-6-phosphate amidotransferase (GFAT), 1:3000 overnight (kindly provided by Dr. Erwin Schleicher, University of Tubingen, Tubingen, Germany); mouse anti-Na,K-ATPase, 1:1000 overnight (kindly provided by Dr. M. J. Caplan, Yale University); mouse anti-O-GlcNAc, 1:1500 overnight; rabbit anti-OGA, 1:2000 overnight (kindly provided by Dr. Sidney W. Whiteheart, University of Kentucky, Lexington, KY); and rabbit anti-OGT (H-300), 1:1000 2 h (Santa Cruz Biotechnology, Santa Cruz, CA). All incubations for O-GlcNAcylation detection using antibody RL2 were conducted using blocking solution B. Bands were visualized using ECL SuperSignal West Dura extended duration substrate (Thermo Fisher Scientific). To prepare for reprobing, the blot was stripped using a ReBlot Plus kit (Millipore, Temecula, CA) according to the manufacturer's protocol. Each blot was probed twice, once for the InsP3R and once for O-GlcNAcylation.
Western blots were analyzed by scanning densitometry comparisons of non-saturated bands using UN-SCAN-IT (Silk Scientific, Orem, UT). Total pixels were measured, and values were normalized to densitometry values of the loading control.
Calcium Imaging
Mz-ChA-1 cells were plated on 22 × 22-mm glass coverslips (Thermo Fisher Scientific) and grown in sugar supplements for 72 h to a confluence of ∼70%. On the day of imaging, coverslips were washed with HEPES-buffered saline (HBS; 130 mm NaCl, 4.7 mm KCl, 1.25 mm CaCl2, 1.2 mm KH2PO4, 1 mm MgSO4, 19.7 mm HEPES, and 5 mm glucose (pH 7.4) with NaOH) and incubated in HBS supplemented with 6 μm Fluo-4/AM (Invitrogen) dissolved in 0.1% Pluronic F-127 (Invitrogen) for 30 min at 37 °C, followed by a 10-min de-esterification period in imaging solution. The coverslip was transferred into an open superfusion chamber (Warner Instruments, Hamden, CT) and mounted onto the stage of a Diaphot fluorescence microscope (Nikon Instruments, Melville, NY). Fluo-4 was illuminated through a ×40 air objective from a mercury arc lamp, and emission was detected through a GFP filter cube to a cooled CCD camera (NeuroCCD-SM256, RedShirt Imaging, Decatur, GA).
AR4-2J cells were grown overnight on 22 × 22-mm glass coverslips coated with poly-d-lysine hydrobromide (Sigma). After sugar incubation, cells were washed and incubated in HBS supplemented with 3 μm Fluo-4/AM and 1.0 mm probenecid (water-soluble; Invitrogen) for 30 min, followed by a 15-min de-esterification period in HBS supplemented with 1.0 mm probenecid. The coverslip was transferred to a custom-built imaging perfusion chamber and mounted onto the stage of an LSM 510 NLO inverted confocal laser scanning microscope (Axiovert 100, Carl Zeiss, Thornwood, NY) equipped with a Plan-Apochromat 63 × 1.4 oil objective. Fluo-4 was excited with laser light at 488 nm, and emission was detected with a 500/550-nm band-pass filter.
Imaging experiments were conducted in calcium-free HBS (with CaCl2 replaced with 1.25 mm MgCl2 and 0.1 mm EGTA). All solutions throughout dye loading and perfusion were supplemented with the indicated sugar. Cells were continuously superfused. After acquiring a base line (60 s), cells were stimulated with Mg-ATP (Mz-ChA-1 cells; Sigma) or carbachol (AR4-2J cells; Sigma) (120 s), followed by a washout period (30 s) and a second stimulation to determine the maximum release. Images were acquired at 1 Hz. There was no change in size, shape, or location of cells.
Whole cell fluorescence was measured by defining each cell as one region of interest. Data were further analyzed using Microsoft Excel. The base line was defined as the average of the 10 pictures prior to the addition of agonist. Drift in the base line due to stage drift was mathematically corrected using a linear regression model. 15 images prior to the first stimulus and 15 images prior to the second stimulus were used to form a linear function. The difference between the y value y1 of this function for a certain point of time x and the base line defined as y0 = 0 for all x was added to the corresponding value y2 to define ΔF/F0 of the calcium curve. Background fluorescence was subtracted from all measurements. Increases in calcium were expressed as the ratio of the change in fluorescence intensity to base-line fluorescence (ΔF/F0). Cells were scored as responders if the peak height after stimulation was 0.1 arbitrary unit greater than the base line. Cells that had an abnormal morphological appearance or cells that did not respond to stimulation with high concentrations of agonist (10 μm Mg-ATP or 100 nm carbachol) were excluded from the evaluation.
Preparation of InsP3R-1 and InsP3R-3 Microsomes
InsP3R-1 microsomes from mouse cerebella (Pel-Freez Biologicals, Rogers, AR) were prepared as described previously (20). To prepare InsP3R-3 microsomes, Mz-ChA-1 cells were grown to confluence in T-75 flasks and harvested. Cells from three flasks were pooled, washed with PBS, and centrifuged. The pellet was resuspended in 1–2 ml of buffer H (250 mm sucrose, 5 mm HEPES/KOH (pH 7.4), 1 mm EGTA, 1 mm DTT, and protease inhibitors) and sonicated (2 × 10 s). Sonication was repeated after the addition of 9 ml of buffer H. Sonicated cells were centrifuged at 8000 × g for 10 min at 4 °C, and the supernatant was saved and centrifuged again at 100,000 × g for 1 h at 4 °C. The pellet was resuspended in 300 μl of buffer H without EGTA, aliquoted, flash-frozen, and stored at −80 °C.
Preparation of Mouse Cerebellar Cytosol
Isolation of OGT from rat brain cytosol was adapted for mouse cerebella using previously described methods (28).
Single Channel Recordings
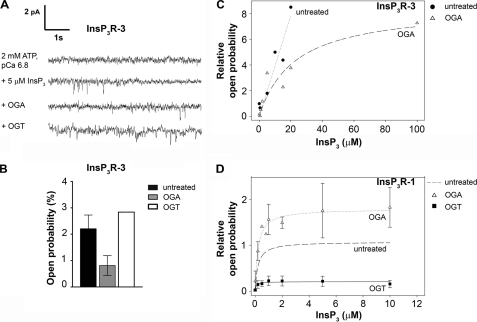
Cerebellar microsomes containing the InsP3R were incorporated into planar lipid bilayers and recorded as described previously (20, 29, 30). The buffer on the cis-side of the bilayer (corresponding to the cytosolic side of the endoplasmic reticulum) contained 250 mm HEPES and 110 mm Tris (pH 7.35). The buffer on the trans-side (corresponding to the luminal side of the endoplasmic reticulum) contained 250 mm HEPES and 53 mm Ba(OH)2 (pH 7.35). After fusion, the single channel activity of InsP3R-1 or InsP3R-3 was recorded in the presence of 0.5 mm ATP or 2 mm ATP, respectively (as indicated in Fig. 3), and 8 μm ruthenium red at pCa 6.8 on the cytoplasmic side. InsP3 dependence was examined for both subtypes by application of increasing concentrations of InsP3 (Calbiochem) to the cis-compartment. Channel de-O-GlcNAcylation was accomplished by the addition of a mixture of 4 μl of OGA (Sigma) plus 1 μl of 5× manufacturer reaction solution directly over the membrane on the cytoplasmic side without stirring. To add O-GlcNAc to the channel, a mixture of 1 μl of a 76 μm UDP-GlcNAc solution plus 4 μl of desalted cerebellar cytosol was added directly over the membrane on the cytoplasmic side without stirring. After 1 min, the reactions were halted by stirring for 30 s. Experiments were recorded under voltage-clamp conditions, and the data were amplified (BC-525C, Warner Instruments), filtered at 1 kHz by a low-pass 8-pole Bessel filter, digitized at 10 kHz, and directly transferred to a computer. Data were acquired and analyzed. At least 2 min of recordings of each condition in each experiment were used for calculations of open probability. Single channel data were collected with Clampfit (Axon Instruments, Foster City, CA), filtered digitally at 500 Hz, and prepared for analysis. The representative traces shown in Fig. 3A were filtered at 200 Hz for the figure. Po was determined with automatic event detection applying the half-threshold crossing criteria (t > 2 ms) with pCLAMP 9 (Axon Instruments) or analyzed manually.
FIGURE 3.
Addition and removal of O-GlcNAc has subtype-specific effects on channel open probability of the InsP3R. A, the removal of O-GlcNAc decreases and addition increases the channel open probability of InsP3R-3 inserted into planar lipid bilayers. All channel recordings were performed in the presence of 2 mm ATP and 8 μm ruthenium red at pCa 6.8. Four representative traces of recordings of one InsP3R-3 from the same experiment are shown. First trace, in the absence of InsP3, no channel activity was measured. Second trace, the addition of 5 μm InsP3 evoked channel activity. Third trace, the addition of OGA to the cis-side of the channel and subsequent de-O-GlcNAcylation decreased channel open probabilities. Fourth trace, the addition of OGT to the cis-side of the channel, causing O-GlcNAcylation, reversed the effect of OGA and increased channel open probabilities. Channel openings are defined as downward deflections from the base line. B, the removal and addition of O-GlcNAc has reciprocal effects on the channel open probability of InsP3R-3. Recordings were performed in the presence of 5 μm InsP3, 2 mm ATP, and 8 μm ruthenium red at pCa 6.8. The open probability of untreated channels incorporated in lipid membranes was measured (mean ± S.E., n = 4). Treatment with OGA, causing removal of O-GlcNAc, decreased open probability (mean ± S.E., n = 4), whereas following OGT treatment, the addition of O-GlcNAc increased channel activity (mean, n = 2). C, InsP3-dependent measurement of channel open probability. The channel open probabilities of untreated and OGA-treated (deglycosylated) InsP3R-3 are shown for different InsP3 concentrations. Data points were normalized to averaged values of untreated channels stimulated with 5 μm InsP3. D, the channel open probabilities of OGA-treated (deglycosylated) and OGT-treated (O-GlcNAcylated) InsP3R-1 compared with previously published data for untreated WT InsP3R-1 (67) are shown for different InsP3 concentrations. Data points were normalized to averaged values of channels stimulated with 2 μm InsP3. Data were taken from at least three independent experiments for each [InsP3].
Statistical Analysis
Data are displayed as means ± S.E. Statistical differences between two groups were analyzed using Student's t test (InStat, GraphPad, San Diego, CA). Analysis of more than two groups was performed using one-way analysis of variance for multiple groups, followed by a Bonferroni post-test (InStat). Responding rates of cells in calcium imaging were tested using a χ2 test (SPSS, Chicago, IL). p < 0.05 was considered statistically significant and is indicated with asterisks on Fig. 4. Fitting curves in Fig. 3 (C and D) are single rectangular hyperbolas with two parameters fitted with SigmaPlot 10 (Systat Software Inc., San Jose, CA).
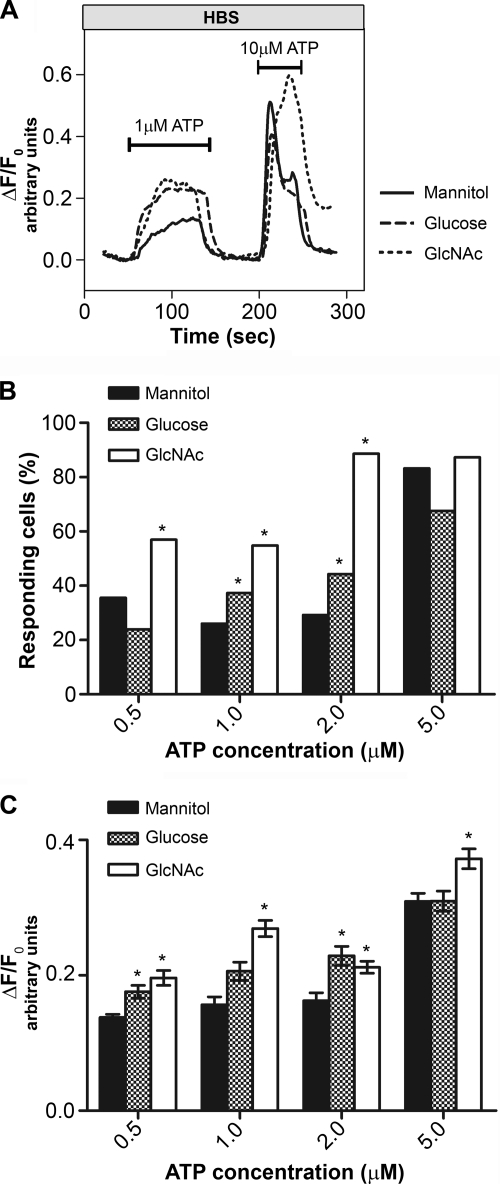
FIGURE 4.
O-GlcNAcylation alters intracellular calcium transients due to extracellular stimulation. Shown are imaging data of Mz-ChA-1 cells grown in different sugar media and stimulated with Mg-ATP. A, representative single traces showing the time course for single cells stimulated with 1 μm Mg-ATP and 10 μm for the second stimulus. B, percentage of cells responding to stimulation with 0.5, 1.0, 2.0, and 5.0 μm Mg-ATP after incubation in different media as indicated. C, peak heights of cell calcium response to Mg-ATP. Cells were grown in different media as indicated. *, p < 0.05.
RESULTS
InsP3R-3 Is O-GlcNAcylated
To determine whether InsP3R-3 is modified by O-GlcNAc, the human cholangiocarcinoma cell line Mz-ChA-1 was used because this cell line expresses predominantly InsP3R-3 (supplemental Fig. S1) (31). For comparison, we used SH-SY5Y cells, which express predominantly InsP3R-1, and AR4-2J cells, which express predominantly InsP3R-2 (supplemental Fig. S1) (26).
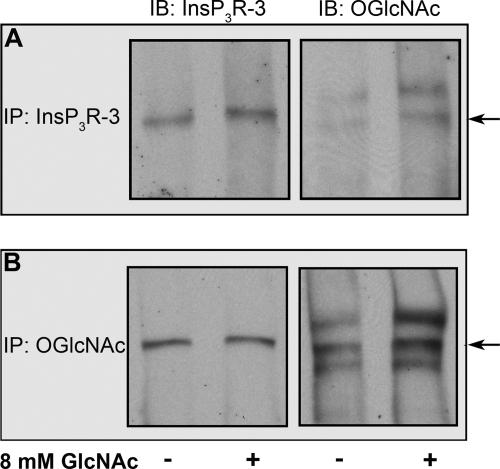
Mz-ChA-1 cells were grown in normal medium (containing 5.6 mm glucose) or in normal medium supplemented with 8 mm GlcNAc. InsP3R-3 was immunoprecipitated from whole cell lysate using an antibody recognizing InsP3R-3 (Fig. 1A) or O-GlcNAc (RL2) (Fig. 1B). The sample immunoprecipitated with anti-InsP3R-3 antibody showed a single InsP3R-3 band on the immunoblot. Probing the same membrane with antibody RL2 detected a band at the same location as InsP3R-3 (Fig. 1A), indicating that InsP3R-3 is O-GlcNAcylated and that the ratio of the intensity of the O-GlcNAc band to that of the corresponding InsP3R-3 band as determined by densitometry is higher in GlcNAc-treated cells. As in previous experiments in which O-GlcNAcylation of InsP3R-1 was examined (20), multiple high molecular mass bands were present when probing the anti-InsP3R-3 antibody-immunoprecipitated samples with anti-O-GlcNAc antibody RL2, and they likely represent additional O-GlcNAcylated proteins pulled down with the InsP3R. When probing the sample immunoprecipitated with anti-O-GlcNAc antibody, several O-GlcNAcylated proteins were detected; probing with anti-InsP3R-3 antibody correspondingly showed one band (Fig. 1B). This increase in O-GlcNAcylation of the InsP3R could have led to an increase in InsP3R-3 from samples immunoprecipitated with anti-O-GlcNAc antibody; however, similar levels of InsP3R-3 were detected in O-GlcNAc-treated and control samples. There are several possibilities to explain this observation (inefficient binding of anti-O-GlcNAc antibody, greater pulldown of other O-GlcNAcylated proteins, or multiple O-GlcNAc sites on each InsP3R-3 monomer), but the explanation remains for future studies. Regardless of the explanation, the amount of O-GlcNAcylation of InsP3R-3 immunoprecipitated from O-GlcNAc-treated samples was greater than that of the control.
FIGURE 1.
InsP3R-3 is modified by O-GlcNAcylation. A and B, Western blot analysis of InsP3R-3 immunoprecipitated (IP) from Mz-ChA-1 cell lysates using anti-InsP3R-3 antibody or anti-O-GlcNAc antibody RL2, respectively. Cells were grown for 72 h in normal medium (5.6 mm glucose) or in medium supplemented with 8 mm GlcNAc as indicated. Membranes were probed with anti-InsP3R-3 or anti-O-GlcNAc antibody, respectively. The arrows indicate the InsP3R-3 band. Quantitation of bands was done as described under “Experimental Procedures.” O-GlcNAcylation after treatment with 8 mm GlcNAc was twice the amount in immunoprecipitates from untreated cell lysates (A). O-GlcNAcylation after treatment with 8 mm GlcNAc was enhanced by 1.6-fold in comparison with untreated InsP3R-3 (B). IB, immunoblot.
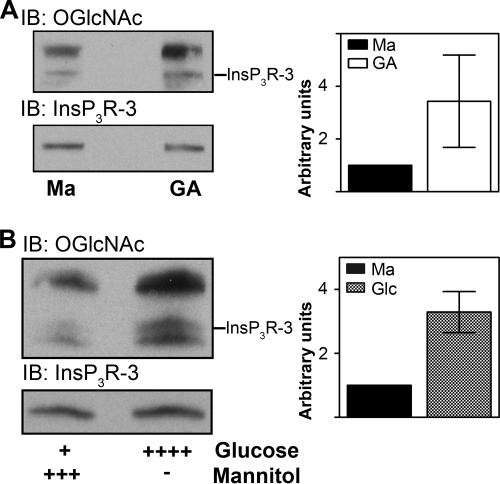
Next, we compared the effect of different treatments on O-GlcNAcylation of InsP3R-3 (Fig. 2). Because of the lability of the O-GlcNAc linkage, we used full cell lysates rather than immunoprecipitated samples for subsequent experiments to minimize the delay between treatment and measurement. When assessed in conjunction with the immunoprecipitation data, this method provided a reliable estimate of the degree of InsP3R-3 O-GlcNAcylation. Cells were grown for 72 h in normal medium supplemented with 20 mm mannitol or 8 mm glucosamine (Fig. 2A). Mannitol was used as an osmotic control. Membranes were probed with antibody RL2 or anti-InsP3R-3 antibody to confirm the identity of InsP3R-3 among the O-GlcNAcylated bands. Results are shown as the ratio of the density of the O-GlcNAc band to the density of the corresponding InsP3R-3 band. Values were normalized to the ratio obtained for mannitol treatment. An increase in O-GlcNAcylation of InsP3R-3 was observed after incubation with glucosamine (3.43 ± 1.75, n = 3) (Fig. 2A). When cells were treated with medium supplemented with 20 mm glucose (total concentration of 25.6 mm), O-GlcNAcylation of InsP3R-3 also increased (3.29 ± 0.64, n = 4) (Fig. 2B). A similar increase in O-GlcNAcylation of InsP3R-3 was seen in cells incubated in medium supplemented with 8 mm GlcNAc for 72 h (data not shown). These results show that InsP3R-3 is O-GlcNAcylated and that the level of O-GlcNAcylation can be modulated by the addition of extracellular sugars.
FIGURE 2.
O-GlcNAcylation of InsP3R-3 in Mz-ChA-1 cells occurs in vivo. A, Mz-ChA-1 cells were grown in medium supplemented with either 20 mm mannitol (Ma; control; n = 4) or 8 mm glucosamine (GA; n = 3) for 72 h and lysed, and 30 μg of protein was analyzed by Western blotting. B, to examine O-GlcNAcylation under physiological conditions, Mz-ChA-1 cells were grown for 72 h in medium supplemented with 20 mm mannitol or the physiological sugar glucose at a concentration of 20 mm (total concentration of 25.6 mm), and the whole cell lysate was analyzed by Western blotting (n = 4). Membranes were probed with anti-InsP3R-3 or anti-O-GlcNAc antibody, respectively. Results are shown as averaged densitometry values normalized to control values (20 mm mannitol) per blot; graphs are means ± S.E. IP, immunoprecipitation; IB, immunoblot.
O-GlcNAcylation Has a Functional Impact on Single Channel Open Probability
To determine whether the addition of O-GlcNAc also had functional effects on InsP3R-3 channels, we incorporated microsomes isolated from Mz-ChA-1 cells in planar lipid bilayers (Fig. 3A). Ruthenium red was added to inhibit any ryanodine receptors (RyRs) that may have been present. However, even if RyRs were present, previous studies found a lack of functional changes associated with O-GlcNAcylation of the RyR (20). No channel activity was observed under control conditions (in the absence of InsP3 but in the presence of 2 mm ATP and 8 μm ruthenium red at pCa 6.8 on the cytoplasmic side of the channel) (Fig. 3A, first trace). The addition of 5 μm InsP3 evoked channel activity (Fig. 3A, second trace) with an open probability of 2.2 ± 0.5% (n = 4) (Fig. 3B). To study the functional effect of de-O-GlcNAcylation of InsP3R-3, we applied OGA directly to the bilayer on the cytoplasmic side. This treatment induced a decrease in the open probability (Fig. 3A, third trace) to 0.8 ± 0.4% (n = 4) (Fig. 3B). To reverse the effects of OGA, cerebellar cytosolic extract containing high levels of OGT and the GlcNAc donor UDP-GlcNAc were added to the cytoplasmic side of the membrane. This treatment induced an increase in channel activity (Fig. 3A, fourth trace). In channels pretreated with OGA, channel activity was increased after OGT addition to a value higher than in OGA-treated channels (2.4 and 3.3%, n = 2) (Fig. 3B), showing that OGT addition reversed the effect of OGA. These values indicate that there is already a certain amount of O-GlcNAc modification under basal conditions of untreated InsP3R-3. Similar observations were made in previous O-GlcNAcylation (20) and PKA phosphorylation (32) experiments. The current amplitude was unchanged by O-GlcNAcylation; at a holding potential of 0 mV, the single channel current remained at 2 pA. These results show that O-GlcNAc alters the activity of InsP3R-3 and that the modulation is opposite that observed previously with InsP3R-1 (20).
To further characterize InsP3R-3 O-GlcNAcylation, the effect of O-GlcNAc on the InsP3 dependence was measured (Fig. 3C). As expected, a higher InsP3 concentration was required to maximally activate InsP3R-3 than to maximally activate InsP3R-1 under control conditions (33). When InsP3R-3 was treated with OGA to remove the O-GlcNAc modification, channel activity was decreased (Fig. 3C). A similar series of experiments was done with InsP3R-1 isolated from cerebellar microsomes. In this case, the addition of O-GlcNAcylation decreased channel activation over the entire concentration range tested, and treatment with OGA caused channel activity to be increased at all InsP3 concentrations used (Fig. 3D).
O-GlcNAcylation of InsP3R-3 Has Functional Effects on Intracellular Calcium Transients
We next examined whether there were any effects of O-GlcNAcylation of InsP3R-3 on calcium transients in intact cells (Fig. 4) using Mz-ChA-1 cells loaded with the calcium-sensitive dye Fluo-4/AM. After establishing a base line, cells were stimulated by the addition of 0.5, 1.0, 2.0, or 5.0 μm Mg-ATP, an agonist that increases intracellular calcium by binding to P2Y receptors on the cell surface (34). After a brief washout period, a final stimulation with 10 μm Mg-ATP was applied, serving as a viability control. Representative traces of single cells after stimulation with 1 μm Mg-ATP followed by stimulation with 10 μm Mg-ATP are shown for each treatment group (Fig. 4A).
Cells were scored as responding when the change in fluorescence in relation to base-line fluorescence was ΔF/F0 > 0.1 arbitrary unit. We compared the percentage of responding cells grown in medium with 20 mm mannitol or 8 mm GlcNAc (Fig. 4B). With the addition of 0.5 μm Mg-ATP to GlcNAc-treated cells, 57% responded (179 cells, n = 4, where n indicates the number of independent cultures) in comparison with 36% cells in the control group (246 cells, n = 6). The same effect was observed for 1.0 μm Mg-ATP stimulation (55% of GlcNAc-treated cells (278 cells, n = 6) versus 26% of control cells (198 cells, n = 6)) and for 2.0 μm Mg-ATP stimulation (89% of GlcNAc-treated cells (176 cells, n = 4) versus 29% of control cells (155 cells, n = 4)). Stimulation with 5.0 μm Mg-ATP showed a similar level of responding cells for both groups (87% of GlcNAc-treated cultures (370 cells, n = 9) versus 83% of mannitol-treated cells (364 cells, n = 10)), indicating that the maximum response frequency was reached. When comparing mannitol- and GlcNAc-treated cells, a difference in the magnitude of calcium release was seen at all ATP concentrations tested (Fig. 4C).
To test a more physiological stimulus of the HBP and subsequent production of the substrate of O-GlcNAcylation, UDP-GlcNAc, cells were incubated in medium supplemented with 20 mm glucose (total concentration of 25.6 mm). As shown above for GlcNAc-treated cells, the peak height of the transient was higher in cells grown in glucose-supplemented medium than in cells grown in mannitol-supplemented medium (Fig. 4C). The percentage of responding cells increased with increasing Mg-ATP concentrations and was higher in glucose- than in mannitol-treated cells, but with stimulation of 0.5 and 5.0 μm Mg-ATP, this parameter did not differ significantly from the value obtained with cells pretreated with mannitol (Fig. 4B).
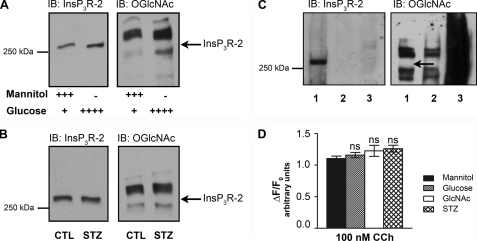
InsP3R-2 Is Not O-GlcNAcylated, and Its Function Is Unchanged by Incubation with GlcNAc
We next examined whether there were any effects of O-GlcNAcylation of InsP3R-2 on calcium transients in intact cells (Fig. 5). Experiments were performed as described above for InsP3R-3. When probing for InsP3R-2, a band was detected at the expected molecular mass (Fig. 5A, left panel), but probing for O-GlcNAc did not show a band at the corresponding molecular mass even though other proteins were O-GlcNAcylated (right panel). The same result was obtained when cells were treated with 10 mm streptozotocin for 60 min (Fig. 5B). An additional test was done by incubating AR4-2J cells with 100 μm PUGNAc, followed by immunoprecipitation using anti-InsP3R-2 or anti-O-GlcNAc antibody (Fig. 5C). When InsP3R-2 was immunoprecipitated, InsP3R-2 was detected, whereas no corresponding O-GlcNAcylation band was identified. In contrast, when O-GlcNAc was immunoprecipitated, several O-GlcNAcylated bands were detected, but there was no corresponding band detected when probed for InsP3R-2.
FIGURE 5.
InsP3R-2 in AR4-2J cells is not O-GlcNAcylated. A, Western blot analysis of AR4-2J cell lysates incubated for 72 h in 20 mm mannitol or 20 mm glucose as indicated. Membranes were probed for O-GlcNAc and InsP3R-2, respectively. The arrow indicates the height of the InsP3R-2 band, which was not recognized by antibody RL2, showing that InsP3R-2 in AR4-2J cells is not glycosylated. B, Western blot analysis of AR4-2J cell lysates incubated for 72 h in 20 mm mannitol (control (CTL)) or grown in normal medium and incubated with 10 mm streptozotocin (STZ) for 60 min. C, immunoprecipitation of InsP3R-2 with anti-InsP3R-2 antibody (lane 1), the control (CTL) with anti-HSP90 antibody (lane 2), and O-GlcNAcylated proteins with anti-O-GlcNAc antibody RL2 (lane 3) from AR4-2J lysates from cells incubated in 100 μm PUGNAc for 24 h ahead of lysis. Western blot analysis was performed, and membranes were probed with anti-InsP3R-2 and anti-O-GlcNAc antibodies, respectively. The arrow indicates the expected height of the InsP3R-2 band. D, AR4-2J cells were grown on coverslips and incubated in different sugar-containing media for 72 h as described. Cells were stimulated with 100 nm carbachol, and single calcium transients were measured. ns, not significant; IB, immunoblot.
Calcium transients were monitored to determine whether treating AR4-2J cells with 20 mm mannitol, 20 mm glucose (total concentration of 27 mm), 8 mm GlcNAc, or 10 mm streptozotocin cells had any functional effects. There was no significant difference in the peak height (Fig. 5D) or the number of cells responding (data not shown) among the different treatment groups.
DISCUSSION
In this work, we have shown that modification of the InsP3R by O-linked addition of N-acetylglucosamine is isoform-specific. We showed previously that the single channel activity of InsP3R-1 is functionally altered by this new type of post-translational modification (20) and that O-GlcNAcylation of InsP3R-1 decreases the receptor's mobility in the membrane (21). In this study, we found that InsP3R-3 but not InsP3R-2 is O-GlcNAcylated and that modification of InsP3R-3 induces functional changes that are opposite those found for InsP3R-1. Functional data of the InsP3R obtained with planar lipid bilayer experiments and whole cell calcium imaging complemented each other. In bilayer experiments, we showed that O-GlcNAc modification is intrinsic to the receptor; in whole cell calcium imaging, we observed the same functional effect on the InsP3R under more physiological conditions. Although it is possible that other components of the calcium signaling pathway are O-GlcNAc-modified as well (35) and may contribute to the change in the calcium signal, the changes in the single channel properties appear to be sufficient to account for the changes in intact cells. With these results, it becomes evident that O-GlcNAcylation is an important form of modification of the InsP3R that links calcium signaling with glucose and energy metabolism.
O-GlcNAcylation of the InsP3R Is Subtype-specific
In addition to the InsP3R, there are other types of intracellular calcium channels, the RyR and polycystin-2 (PC2). We found that the RyR is modified by O-GlcNAc but there was no functional effect (20). Similarly, we found PC2 to be modified by O-GlcNAc (supplemental Fig. S2), but no changes in function related to modification of PC2 were observed. Although PC2 is present in AR4-2J cells (supplemental Fig. S2), there was no change in calcium signaling due to sugar treatment (Fig. 5), and sugar treatment of cells had no effect on the mobility of PC2 but did alter InsP3R-1 mobility (21). These results suggest that functional consequences of O-GlcNAc modification are specific for the InsP3R family of intracellular calcium channels.
For InsP3R-1, the addition of O-GlcNAc decreases the open probability of its single channel currents and decreases agonist-stimulated calcium transients in intact cells (20). O-GlcNAc modification of InsP3R-3 was detected, and the functional response was opposite that of O-GlcNAc modification of InsP3R-1 (Fig. 3, A–D). In contrast, InsP3R-2 from AR4-2J cells was not modified by O-GlcNAcylation, and there were no functional changes after sugar treatment (Fig. 5), even though AR4-2J cells contain both OGA and OGT, the enzymes necessary for O-GlcNAcylation (supplemental Fig. S3B). At similar levels of OGT and OGA, InsP3R-1 and InsP3R-3 could be detected as O-GlcNAcylated proteins (supplemental Fig. S4). It is possible that the lack of O-GlcNAc modification of InsP3R-2 is specific for a splice variant of InsP3R-2 found in the cell line AR4-2J. Support for this suggestion includes the observation that the shortening rate in cardiomyocytes, a tissue that expresses predominantly InsP3R-2 (36), is altered after expression of the enzymes for O-GlcNAcylation (22). Also, several splice variants of InsP3R-2 were identified in a number of tissues, including the submandibular gland, thymus, and liver (37, 38).
The InsP3R is post-translationally modified through a variety of other pathways, including phosphorylation (19), N-glycosylation (39), and ubiquitination (40). Ubiquitination was shown to occur on all InsP3R subtypes at approximately the same site and with a similar functional consequence (40, 41), whereas phosphorylation of the InsP3R is isoform-specific (19). It is now clear that O-GlcNAc modification is an additional and complementary subtype-specific regulation of the InsP3R.
Functional Consequences of O-GlcNAcylation
InsP3R-1 and InsP3R-3 were each biochemically modified by O-GlcNAcylation, and channel function was altered in response to elevation of extracellular sugar as demonstrated by single channel activity (Fig. 3) or InsP3-induced calcium release in intact cells (Fig. 4). On the other hand, InsP3R-2 was not modified and had no functional changes when sugar levels were modified. Even more interesting, the effect of O-GlcNAcylation on InsP3R-1 was opposite that for InsP3R-3 (compare Figs. 3 and 4). This finding is reminiscent of the reciprocal response of InsP3R-1 and InsP3R-3 to PKA phosphorylation. When a protein is O-phosphorylated, a negative charge is added to the protein, potentially changing its electrostatic character. This change in electrostatics could result in a conformational change in the protein that would affect intradomain and protein-protein interactions (42). In contrast O-GlcNAcylation is the addition of a neutral moiety and is therefore more likely to alter the accessibility of a regulatory site. For example, when many proteins are O-GlcNAcylated, it is not possible to subsequently phosphorylate the protein (43).
Our results suggest that the InsP3 dependence of channel opening is altered by O-GlcNAcylation and that these changes could define the threshold at which a cell responds to an InsP3-generating stimulus. This is similar to the effect of adding ATP, which enhances the channel activity but does not open the channel itself (33, 44, 45), or phosphorylation by PKA, which increases the sensitivity of InsP3R-1 to InsP3 without shifting its bell-shaped calcium dependence (46–48). Turnover of InsP3 and nucleotides like ATP is very fast (49), whereas O-GlcNAc modification occurs on a slower time scale (50). In this way, O-GlcNAcylation could provide a dynamic, albeit more long-lasting, way to modulate the InsP3R.
O-GlcNAcylation of the InsP3R, Nutrient Sensing, and Diabetes
Several recent reviews have focused on the role of O-GlcNAcylation as a nutrient-sensing mechanism (13, 14). The synthesis of UDP-GlcNAc as the substrate for OGT through the HBP and subsequent O-GlcNAc cycling through OGT and OGA were termed the hexosamine signaling pathway (14). The enzymes of the hexosamine signaling pathway are highly regulated (supplemental Fig. S3, A, C, and D). GFAT is the rate-limiting enzyme of the HBP (51), and it can be inhibited by high levels of UDP-GlcNAc (52). Glucose has to be metabolized to glucosamine 6-phosphate by GFAT to enter the HBP, whereas phosphorylated glucosamine can enter the HBP directly, bypassing this enzymatic step. In our experiments, O-GlcNAcylation and its functional effects as seen through calcium imaging were more pronounced in cells incubated in medium containing glucosamine than in those incubated in glucose-containing medium. This observation is consistent with previous studies of the regulation of the HBP pathway (53, 54). Blocking GFAT inhibited the effects of glucose incubation, whereas glucosamine was active because it was able to bypass this enzyme (55). We also found that expression of GFAT and OGT was down-regulated in cells incubated in glucose or glucosamine (supplemental Fig. S3D). This down-regulation may reflect a mechanism to protect the cell from glucose overload or glucose toxicity, where high concentrations of glucose induce tissue damage as a consequence of the accumulation of advanced glycation end products (15).
InsP3R-3 is the most abundant subtype in tissues involved in secretion, including bile duct cells (>80%) (56), pancreatic islets, and β-cell lines (57). The recent observation that InsP3R-2/3 double knock-out mice are deficient in saliva and gastric juice secretion (58) supports this conclusion. As a sensor of extracellular glucose concentration through the hexosamine signaling pathway, O-GlcNAcylation of the receptor could lower the threshold for initiation of InsP3-induced calcium release and therefore enhance InsP3R-3 activity. This could be an important mechanism for adjusting the secretion of gastric juices to match the supply of nutrients.
The InsP3R is involved in insulin secretion (59), and pancreatic β-cells express much more OGT than any other known cell type (24), suggesting that our findings could be relevant for regulation of insulin signaling. Pancreatic β-cells respond to high glucose, the primary stimulus for insulin secretion, by an alteration in the InsP3R pathway. This may occur, at least in part, through InsP3R O-GlcNAcylation. Incubation of rat pancreatic islet cells for short periods in high glucose enhanced transcription and protein expression of InsP3R-3 (60). Chronic elevation of glucose instead caused a down-regulation of InsP3R-3 mRNA and protein levels accompanied by an up-regulation of InsP3R-2 (61). Similarly, the expression level of InsP3R-3 was decreased in pancreatic acini of diabetic rats compared with normal acini (62). Thus, a response of the β-cell to high glucose would involve two possible regulatory mechanisms depending upon the InsP3R: a change in channel activity through direct post-translational modification by O-GlcNAc and a change in protein expression through the effect of O-GlcNAcylation on the activity of transcription factors and transcription (63–65). Thus, O-GlcNAc modification of InsP3R-3 adds an important piece to the puzzle of how to fine-tune β-cell insulin secretion in response to a variety of nutrient stimuli.
In addition to its role in nutrient sensing, O-GlcNAc is a global player in a multitude of physiological and pathophysiological processes, including growth, metabolism, cellular stress, circadian rhythms, and immune reactions (14). The InsP3R itself plays a physiological role in many of these processes (6), often in an isoform-specific manner. At the same time, glucose has an impact on the specificity of InsP3R isoform expression (60–62, 66) and on the mobility of InsP3R-1 (21), all processes that influence subcellular levels of the InsP3R and spatial calcium signaling. We have shown here that glucose also has an isoform-specific functional impact on InsP3R-1 and InsP3R-3 through O-GlcNAc modification. The subtype-specific modulation of the InsP3R by O-GlcNAcylation can be seen as an additional mechanism for fine-tuning InsP3-mediated calcium release and for providing an intersection of the hexosamine signaling pathway and intracellular calcium signaling.
Supplementary Material
Acknowledgments
We thank Natasha Zachara and Gerald Hart for teaching us about the GlcNAc pathway and its importance in cellular regulation. Rabbit anti-InsP3R-2 antibody was a kind gift of Dr. Richard J. Wojcikiewicz, rabbit anti-GFAT antibody was a kind gift of Dr. Erwin Schleicher, mouse anti-Na,K-ATPase antibody was a kind gift of Dr. M. J. Caplan, and rabbit anti-OGA antibody was a kind gift of Dr. Sidney W. Whiteheart. We thank Ivana Kuo and Colleen Feriod for assistance with the experiments on PC2 and Brenda DeGray, Christin Schulze, Michelle Mo, Xiaoyong Yang, and Andjelka Celic for discussions and comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK57751 and DK61747. This work was also supported by scholarships from the German National Merit Foundation (to P. B., S. S., and W. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- InsP3R
- inositol 1,4,5-trisphosphate receptor
- OGT
- O-β-N-acetylglucosaminyltransferase
- OGA
- O-β-N-acetylglucos-aminidase
- HBP
- hexosamine biosynthesis pathway
- PUGNAc
- O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate
- GFAT
- glutamine:fructose-6-phosphate amidotransferase
- HBS
- HEPES-buffered saline
- RyR
- ryanodine receptor
- PC2
- polycystin-2.
REFERENCES
- 1. Berridge M. J. (1993) Nature 361, 315–325 [DOI] [PubMed] [Google Scholar]
- 2. Mikoshiba K. (2007) J. Neurochem. 102, 1426–1446 [DOI] [PubMed] [Google Scholar]
- 3. Vermassen E., Parys J. B., Mauger J. P. (2004) Biol. Cell 96, 3–17 [DOI] [PubMed] [Google Scholar]
- 4. Furuichi T., Mikoshiba K. (1995) J. Neurochem. 64, 953–960 [DOI] [PubMed] [Google Scholar]
- 5. Patel S., Joseph S. K., Thomas A. P. (1999) Cell Calcium 25, 247–264 [DOI] [PubMed] [Google Scholar]
- 6. Taylor C. W., Genazzani A. A., Morris S. A. (1999) Cell Calcium 26, 237–251 [DOI] [PubMed] [Google Scholar]
- 7. Bezprozvanny I. (2005) Cell Calcium 38, 261–272 [DOI] [PubMed] [Google Scholar]
- 8. Torres C. R., Hart G. W. (1984) J. Biol. Chem. 259, 3308–3317 [PubMed] [Google Scholar]
- 9. Zachara N. E., Hart G. W. (2004) Biochim. Biophys. Acta 1673, 13–28 [DOI] [PubMed] [Google Scholar]
- 10. Love D. C., Hanover J. A. (2005) Sci. STKE 2005, re13. [DOI] [PubMed] [Google Scholar]
- 11. Forrest A. R., Ravasi T., Taylor D., Huber T., Hume D. A., Grimmond S. (2003) Genome Res. 13, 1443–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shafi R., Iyer S. P., Ellies L. G., O'Donnell N., Marek K. W., Chui D., Hart G. W., Marth J. D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butkinaree C., Park K., Hart G. W. (2010) Biochim. Biophys. Acta 1800, 96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanover J. A., Krause M. W., Love D. C. (2010) Biochim. Biophys. Acta 1800, 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClain D. A., Crook E. D. (1996) Diabetes 45, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 16. Robinson K. A., Weinstein M. L., Lindenmayer G. E., Buse M. G. (1995) Diabetes 44, 1438–1446 [DOI] [PubMed] [Google Scholar]
- 17. Akimoto Y., Hart G. W., Hirano H., Kawakami H. (2005) Med. Mol. Morphol. 38, 84–91 [DOI] [PubMed] [Google Scholar]
- 18. Yang X., Ongusaha P. P., Miles P. D., Havstad J. C., Zhang F., So W. V., Kudlow J. E., Michell R. H., Olefsky J. M., Field S. J., Evans R. M. (2008) Nature 451, 964–969 [DOI] [PubMed] [Google Scholar]
- 19. Vanderheyden V., Devogelaere B., Missiaen L., De Smedt H., Bultynck G., Parys J. B. (2009) Biochim. Biophys. Acta 1793, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rengifo J., Gibson C. J., Winkler E., Collin T., Ehrlich B. E. (2007) J. Neurosci. 27, 13813–13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson C. J., Ehrlich B. E. (2008) Cell Calcium 43, 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark R. J., McDonough P. M., Swanson E., Trost S. U., Suzuki M., Fukuda M., Dillmann W. H. (2003) J. Biol. Chem. 278, 44230–44237 [DOI] [PubMed] [Google Scholar]
- 23. Haltiwanger R. S., Grove K., Philipsberg G. A. (1998) J. Biol. Chem. 273, 3611–3617 [DOI] [PubMed] [Google Scholar]
- 24. Hanover J. A., Lai Z., Lee G., Lubas W. A., Sato S. M. (1999) Arch. Biochem. Biophys. 362, 38–45 [DOI] [PubMed] [Google Scholar]
- 25. Snow C. M., Senior A., Gerace L. (1987) J. Cell Biol. 104, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wojcikiewicz R. J. (1995) J. Biol. Chem. 270, 11678–11683 [DOI] [PubMed] [Google Scholar]
- 27. Wojcikiewicz R. J., Furuichi T., Nakade S., Mikoshiba K., Nahorski S. R. (1994) J. Biol. Chem. 269, 7963–7969 [PubMed] [Google Scholar]
- 28. Marshall S., Duong T., Orbus R. J., Rumberger J. M., Okuyama R. (2003) Anal. Biochem. 314, 169–179 [DOI] [PubMed] [Google Scholar]
- 29. Bezprozvanny I., Watras J., Ehrlich B. E. (1991) Nature 351, 751–754 [DOI] [PubMed] [Google Scholar]
- 30. Hagar R. E., Burgstahler A. D., Nathanson M. H., Ehrlich B. E. (1998) Nature 396, 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minagawa N., Kruglov E. A., Dranoff J. A., Robert M. E., Gores G. J., Nathanson M. H. (2005) J. Biol. Chem. 280, 33637–33644 [DOI] [PubMed] [Google Scholar]
- 32. Straub S. V., Giovannucci D. R., Bruce J. I., Yule D. I. (2002) J. Biol. Chem. 277, 31949–31956 [DOI] [PubMed] [Google Scholar]
- 33. Hagar R. E., Ehrlich B. E. (2000) Biophys. J. 79, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dubyak G. R., el-Moatassim C. (1993) Am. J. Physiol. 265, C577–C606 [DOI] [PubMed] [Google Scholar]
- 35. Dias W. B., Cheung W. D., Wang Z., Hart G. W. (2009) J. Biol. Chem. 284, 21327–21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez P. J., Ramos-Franco J., Fill M., Mignery G. A. (1997) J. Biol. Chem. 272, 23961–23969 [DOI] [PubMed] [Google Scholar]
- 37. Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. (2005) J. Biol. Chem. 280, 10305–10317 [DOI] [PubMed] [Google Scholar]
- 38. Futatsugi A., Kuwajima G., Mikoshiba K. (1998) Biochem. J. 334, 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michikawa T., Hamanaka H., Otsu H., Yamamoto A., Miyawaki A., Furuichi T., Tashiro Y., Mikoshiba K. (1994) J. Biol. Chem. 269, 9184–9189 [PubMed] [Google Scholar]
- 40. Sliter D. A., Kubota K., Kirkpatrick D. S., Alzayady K. J., Gygi S. P., Wojcikiewicz R. J. (2008) J. Biol. Chem. 283, 35319–35328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sliter D. A., Aguiar M., Gygi S. P., Wojcikiewicz R. J. (2011) J. Biol. Chem. 286, 1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wagner L. E., 2nd, Joseph S. K., Yule D. I. (2008) J. Physiol. 586, 3577–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slawson C., Hart G. W. (2003) Curr. Opin. Struct. Biol. 13, 631–636 [DOI] [PubMed] [Google Scholar]
- 44. Bezprozvanny I., Ehrlich B. E. (1993) Neuron 10, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 45. Kaznacheyeva E., Lupu V. D., Bezprozvanny I. (1998) J. Gen. Physiol. 111, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang T. S., Tu H., Wang Z., Bezprozvanny I. (2003) J. Neurosci. 23, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagner L. E., 2nd, Li W. H., Yule D. I. (2003) J. Biol. Chem. 278, 45811–45817 [DOI] [PubMed] [Google Scholar]
- 48. Wagner L. E., 2nd, Li W. H., Joseph S. K., Yule D. I. (2004) J. Biol. Chem. 279, 46242–46252 [DOI] [PubMed] [Google Scholar]
- 49. Allbritton N. L., Meyer T., Stryer L. (1992) Science 258, 1812–1815 [DOI] [PubMed] [Google Scholar]
- 50. Yu Z., Quamme G. A., McNeill J. H. (1994) Am. J. Physiol. 266, H2334–H2342 [DOI] [PubMed] [Google Scholar]
- 51. Buse M. G. (2006) Am. J. Physiol. Endocrinol. Metab. 290, E1–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kornfeld R. (1967) J. Biol. Chem. 242, 3135–3141 [PubMed] [Google Scholar]
- 53. Marshall S., Bacote V., Traxinger R. R. (1991) J. Biol. Chem. 266, 4706–4712 [PubMed] [Google Scholar]
- 54. Daniels M. C., Kansal P., Smith T. M., Paterson A. J., Kudlow J. E., McClain D. A. (1993) Mol. Endocrinol. 7, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 55. Liu K., Paterson A. J., Chin E., Kudlow J. E. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2820–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nathanson M. H., Fallon M. B., Padfield P. J., Maranto A. R. (1994) J. Biol. Chem. 269, 4693–4696 [PubMed] [Google Scholar]
- 57. Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. (1993) J. Biol. Chem. 268, 11356–11363 [PubMed] [Google Scholar]
- 58. Futatsugi A., Nakamura T., Yamada M. K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., Mikoshiba K. (2005) Science 309, 2232–2234 [DOI] [PubMed] [Google Scholar]
- 59. Yamazaki H., Zawalich K. C., Zawalich W. S. (2010) J. Nutr. Sci. Vitaminol. 56, 1–8 [DOI] [PubMed] [Google Scholar]
- 60. Lee B., Bradford P. G., Laychock S. G. (1998) J. Mol. Endocrinol. 21, 31–39 [DOI] [PubMed] [Google Scholar]
- 61. Lee B., Jonas J. C., Weir G. C., Laychock S. G. (1999) Endocrinology 140, 2173–2182 [DOI] [PubMed] [Google Scholar]
- 62. Ryu G. R., Sung C. H., Kim M. J., Sung J. H., Lee K. H., Park D. W., Sim S. S., Min D. S., Rhie D. J., Yoon S. H., Hahn S. J., Kim M. S., Jo A. Y. (2004) Pancreas 29, e106–112 [DOI] [PubMed] [Google Scholar]
- 63. Zachara N. E., Hart G. W. (2006) Biochim. Biophys. Acta 1761, 599–617 [DOI] [PubMed] [Google Scholar]
- 64. Yang X., Su K., Roos M. D., Chang Q., Paterson A. J., Kudlow J. E. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang X., Zhang F., Kudlow J. E. (2002) Cell 110, 69–80 [DOI] [PubMed] [Google Scholar]
- 66. Sharma K., Wang L., Zhu Y., DeGuzman A., Cao G. Y., Lynn R. B., Joseph S. K. (1999) Am. J. Physiol. 276, F54–F61 [DOI] [PubMed] [Google Scholar]
- 67. Watras J., Bezprozvanny I., Ehrlich B. E. (1991) J. Neurosci. 11, 3239–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.