Abstract
Xenopus egg extracts execute spontaneous apoptosis without the requirement of transcription and translation, and this intrinsic mechanism is supposed to be involved in the physiological elimination of aged eggs. Although apoptosis in this system is carried out by maternally stockpiled materials, the endogenous apoptosis regulators present in egg extracts are still poorly characterized. Here we examined the mRNA expression profiles and apoptosis-regulating functions of 13 Xenopus Bcl-2 family proteins in egg extracts. Among these, we found that endogenous Xenopus Mcl-1 (xMcl-1) physiologically inhibited apoptosis by counteracting the pro-apoptotic activity of endogenous Xenopus Bid in egg extracts. Exogenously added recombinant xMcl-1 was rapidly degraded by proteasome in egg extracts, and we identified the destabilizing region in the N terminus of xMcl-1. Our results suggest that the proteolytic decay of xMcl-1 may change the functional balance between pro- and anti-apoptotic activities of Bcl-2 family proteins, thereby regulating the timing of cytochrome c release in egg extracts.
Keywords: Apoptosis, Caspase, Cell Death, Proteasome, Xenopus, Xenopus Egg Extracts, Bcl-2 Family Proteins, Mcl-1
Introduction
Bcl-2 family proteins are evolutionarily conserved apoptosis regulators that control the release of cytochrome c from mitochondria (reviewed in Refs. 1 and 2). They are classified as either anti-apoptotic or pro-apoptotic, and the functional balance between these two groups controls the mitochondria-mediated intrinsic pathway of apoptosis. Although more than 10 members of the Bcl-2 family of proteins have been identified in Xenopus laevis (simply referred to as Xenopus hereafter), their functional characterizations are not yet complete (3–19).
Ovulated Xenopus eggs aged without successful fertilization are eventually eliminated by apoptosis, and egg extracts also execute spontaneous apoptosis after incubation for several h at room temperature (reviewed in Refs. 20 and 21). Egg extracts, as well as the mitochondria isolated from them, are frequently used for biochemical experiments of apoptosis (17, 22–41). Apoptosis in egg extracts is triggered solely by the cytoplasmic release of cytochrome c from mitochondria, and neither damage-inducing treatment nor death receptor-ligand signaling is required. Therefore, Bcl-2 family proteins are expected to be functionally involved in the apoptosis-initiating process. Apoptosis in egg extracts is carried out without the requirement of transcription and translation, and all of the components required for apoptotic execution are already stockpiled as maternal products. However, in most studies, exogenously added mammalian proteins or peptides have been employed. For this reason, endogenous Bcl-2 family proteins present in Xenopus egg extracts or mitochondria are still poorly characterized. We have been studying the functions and contributions of apoptosis regulators present in Xenopus egg extracts (17, 33, 36, 39). We recently reported the pro-apoptotic activity of endogenous Xenopus Bid (xBid), a BH3-only member of the Bcl-2 family of proteins, in egg extracts (17). To further extend this finding, here we systematically examined the mRNA expression profiles and molecular functions of 13 Xenopus Bcl-2 family proteins. Especially, we demonstrated the physiological anti-apoptotic activity of endogenous Xenopus Mcl-1 (xMcl-1) in egg extracts.
EXPERIMENTAL PROCEDURES
RT-PCR and cDNA Cloning of Xenopus Bcl-2 Family Proteins
Total RNAs from the eggs, intestine, liver, ovary, and stomach of Xenopus female were purified using the acid guanidinium-phenol chloroform method and LiCl precipitation. Reverse transcription from oligo(dT) primer was carried out using ReverTra Ace (TOYOBO). For known Xenopus Bcl-2 family proteins, the following GenBankTM entries were used for the design of primers to amplify each open reading frame (ORF): xBcl-B (BC142549), xBcl2L12 (BP676787 and BP686551), xBcl-G (NM_001093988), xBcl-w/xR1 (BC073259), xBcl-xL/xR11 (X82461), xMcl-1 and its N-terminal deletion mutants (BX855270), xBax (AF288809), xBak (BC099018), xBid (AY518731), xBim (BC106460), xBmf (BJ032144), and xNoxa (CF519813). Nucleotide sequences of xBcl-2 (AB427203) and xBok (AB427204) were deposited to DDBJ. The primer sequences used in this study are summarized in the supplemental material. PCR was performed for 35 cycles using ExTaq (TaKaRa) as follows: 95 °C for 30 s, 60 °C for 30 s for xBim, and 55 °C for 30 s for the others and 72 °C for 1 min. Amplified fragments were cloned into pGEM-T Easy (Promega), and nucleotide sequences were verified by Bio Matrix Research Inc.
Preparation of Xenopus Egg Extracts
Animal care and use of female frogs was approved by the Animal Research Committee for Animal Experimentation of Toho University. Cytostatic factor (CSF)2-arrested metaphase egg extracts were prepared as described previously (17, 33, 36, 39). Interphase egg extracts were prepared by the addition of 0.4 mm CaCl2 and 0.1 mg/ml cycloheximide. These extracts were incubated at room temperature (∼23 °C) to induce spontaneous apoptosis. Where indicated, a caspase inhibitor, 100 μm Z-VAD-fmk (Peptide Institute); a proteasome inhibitor, 100 μm MG-132 (Peptide Institute); a protein kinase inhibitor, 10 μm staurosporine (Sigma); or a protein phosphatase inhibitor, 10 μm okadaic acid (Wako), was added to egg extracts.
Production of Recombinant Proteins
To produce recombinant N-terminally His6-tagged proteins, ORF fragments were subcloned into pET-15b (Novagen) using appropriate restriction enzymes. We also replaced the fragment of pET-15b encoding the His6 tag with that encoding the HA tag. This modified vector, named pHA, was used for the expression of recombinant N-terminally HA-tagged proteins. To produce synthetic mRNAs for recombinant N-terminally HA-tagged proteins, the pHA-derived expression vectors described as above were used as templates, and the fragments encompassing the T7 promoter and HA-tagged ORFs were amplified by PCR using the following primers: pET-Transcript-F (5′-CGAAATTAATACGACTCACTATAGGG-3′) and pET-Transcript-R2 (5′-TGTGCTATTTATTATCATGAACATTAACC-3′). Amplified fragments were purified and used as templates for mRNA synthesis using mMESSAGE mMACHINE T7 kit (Ambion). The concentration of each mRNA was adjusted to 1 μg/μl in H2O, and 1 μl of mRNA solution was mixed with 9 μl of CSF-arrested egg extracts. These egg extracts were incubated for 3 or 6 h in the absence or presence of Z-VAD-fmk to translate recombinant proteins. For subcellular fractionation in Fig. 4, 10 μl of egg extracts containing synthetic mRNA was incubated for 3 h in the presence of Z-VAD-fmk, diluted with 90 μl of CSF-XB (10 mm HEPES-KOH pH 7.7, 100 mm KCl, 2 mm MgCl2, 0.1 mm CaCl2, 5 mm EGTA, 50 mm sucrose), and centrifuged at 10,000 × g for 10 min. The precipitated fractions containing mitochondria were washed with 100 μl of CSF-XB and centrifuged again. Soluble fractions were pooled (∼190 μl) and precipitated with 800 μl of ice acetone. Both fractions were dissolved in SDS-PAGE buffer and subjected to Western blot. 35S-Radiolabeled recombinant N-terminally His6-tagged proteins were produced using TNT T7 Quick rabbit reticulocyte lysates (RRL; Promega). RRL were mixed with interphase extracts containing cycloheximide at 1:9 as described previously (17, 33, 36, 39).
FIGURE 4.
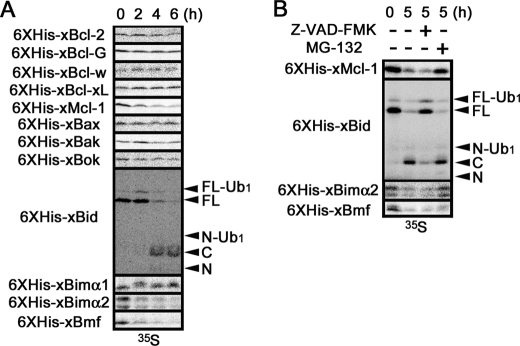
Stability of recombinant Xenopus Bcl-2 family proteins in interphase egg extracts. A, RRL containing 35S-radiolabeled, recombinant N-terminally His6-tagged proteins were mixed with interphase egg extracts containing cycloheximide. After incubation for 0, 2, 4, and 6 h, samples were separated by SDS-PAGE and analyzed by a BAS image analyzer. B, RRL containing 35S-radiolabeled, recombinant N-terminally His6-tagged xBid, xMcl-1, xBimα2, and xBmf were incubated in interphase egg extracts containing cycloheximide for 0 and 5 h in the absence (−) or presence of 100 μm Z-VAD-fmk (VAD) or 100 μm MG-132 (MG) and similarly analyzed as in A. For His6-xBid, full-length protein (FL) was cleaved into N-terminal (N) and C-terminal (C) fragments by caspases, and monoubiquitylated forms (FL-Ub1 and N-Ub1) were also observed (17).
Antibodies, Western Blot, and Immunodepletion
Recombinant His6-xMcl-1 N-terminal fragment (residues 1–174) was expressed in Escherichia coli, purified from the inclusion body fraction, refolded in PBS containing 0.1% SDS, and used for rabbit immunization by Operon Biotechnologies. The same antigen was immobilized on a HiTrap NHS-activated HP column (GE Healthcare) and used for the affinity purification of antibody. Affinity-purified anti-xBid and anti-xXIAP antibodies were prepared as described previously (17, 33). Each antibody was covalently linked to Affi-Prep Protein A beads (Bio-Rad) at 2 mg antibody/ml of beads using dimethylpimelimidate. For the immunoprecipitation-Western blot experiment in Fig. 5A, 250 μl of egg extracts was used per μl of beads. For immunodepletion experiments in Figs. 5 and 6, 10 μl of egg extracts was used per μl of beads. Monoclonal antibodies against poly(ADP-ribose) polymerase (PARP), HA tag (clone 6E2), and cytochrome c were purchased from BD Transduction, Cell Signaling Technology, and Lab Vision, respectively. Polyclonal antibody against actin was purchased from Sigma. Western blot was carried out as described previously (17, 33, 36, 39).
FIGURE 5.
Counteracting apoptosis-regulating activities of endogenous xBid and xMcl-1 in egg extracts. A, detection of endogenous xMcl-1 in egg extracts. Immunoprecipitation was carried out from CSF-arrested metaphase egg extracts using control (Immunoprecipitation: Control) or anti-xMcl-1 (Immunoprecipitation: αxMcl-1) antibody. Immunoprecipitated materials were separated by SDS-PAGE and subjected to Western blot using anti-xMcl-1 antibody (Western: αxMcl-1). B, immunodepletion of recombinant xMcl-1 from egg extracts. Recombinant HA-xMcl-1 was expressed in CSF-arrested metaphase egg extracts as in Fig. 2A, followed by immunodepletion using control (Control) or anti-xMcl-1 (αxMcl-1) antibody. Both supernatants (Sup) and immunoprecipitated materials (Ppt) were separated by SDS-PAGE and subjected to Western blot using anti-HA antibody (αHA). C, immunodepletion of endogenous xBid from egg extracts. CSF-arrested metaphase egg extracts immunodepleted with control (Control) or anti-xBid (αxBid) antibody were separated by SDS-PAGE and subjected to Western blot using anti-xBid antibody (αxBid). Both nonubiquitylated (xBid) and monoubiquitylated (xBid-Ub1) forms were depleted (17). D, counteracting apoptosis-regulating activities of xBid and xMcl-1 in egg extracts. Interphase egg extracts containing cycloheximide after control depletion, xBid depletion, xMcl-1 depletion, or xBid/xMcl-1 double depletion were incubated for up to 6 h. Samples were separated by SDS-PAGE, and apoptotic cleavage of PARP at every hour was detected by Western blot using anti-PARP antibody (αPARP). FL, full-length protein; N, N-terminal fragment.
FIGURE 6.
Dual apoptosis-inhibiting activities of endogenous xMcl-1 and xXIAP in egg extracts. A, degradation time course of recombinant xBid, xMcl-1, and xXIAP in egg extracts. RRL containing 35S-radiolabeled, recombinant N-terminally His6-tagged xBid, xMcl-1, or xXIAP were mixed with interphase egg extracts containing cycloheximide and incubated for up to 6 h. Samples were separated by SDS-PAGE and analyzed by a BAS image analyzer. Fragments derived from His6-xBid were as described in the legend to Fig. 4. B, immunodepletion of endogenous xXIAP from egg extracts. CSF-arrested metaphase egg extracts immunodepleted with control (Control) or anti-xXIAP (αxXIAP) antibody were separated by SDS-PAGE and subjected to Western blot with anti-xXIAP antibody (αxXIAP). C, dual apoptosis inhibition by xMcl-1 and xXIAP in egg extracts. Interphase egg extracts containing cycloheximide after control depletion, xMcl-1 depletion, xXIAP depletion, or xMcl-1/xXIAP double depletion were incubated for up to 5 h. Samples were separated by SDS-PAGE, and apoptotic cleavage of PARP at every hour was detected by Western blot using anti-PARP antibody (αPARP). FL, full-length protein; N, N-terminal fragment.
RESULTS
Identification and RT-PCR Analysis of Xenopus Bcl-2 Family Proteins
Five members of the Xenopus Bcl-2 family of proteins (xBcl-w/xR1, xBcl-xL/xR11, xBax, xBid, and xBok) were functionally characterized in the previous studies (3–19). In addition, partial or complete nucleotide sequences of 10 Bcl-2 family proteins (xBcl-2, xBcl-B, xBcl-G, xBcl2L12, xBcl2L13, xMcl-1, xBak, xBim, xBmf, and xNoxa) were deposited in the Xenopus nucleotide database, but the functions of their protein products remained uncharacterized (42–44). Among these, xBcl2L13 was excluded from our study because the complete ORF of xBcl2L13 could not be determined due to limited sequence information. xBcl2L12 was also excluded because the putative ORF of xBcl2L12 showed only weak homology with chicken homolog and lacked the key signature motifs of Bcl2L12 (proline-rich region and C-terminal BH2 domain). Therefore, we aimed to characterize these 13 Xenopus Bcl-2 family proteins.
First, RT-PCR analyses were carried out to examine the expression profiles of mRNAs encoding Xenopus Bcl-2 family proteins. We used oligo(dT) primer to specifically synthesize cDNAs from poly(A)-containing mRNAs, and each primer pair was designed to amplify the fragment containing deduced ORF (supplemental material). Our RT-PCR successfully amplified the corresponding fragments of all members (Fig. 1A). Most of the members showed ubiquitous mRNA expression. However, xBcl-2 was not expressed in egg and ovary, and xBcl-B was not expressed in egg. In contrast, xBmf was not expressed in the liver and stomach. A single fragment was amplified for each of the members except xBim, suggesting the absence of splice variants. In the case of xBim, our RT-PCR amplified three fragments (Fig. 1B). The 0.5-kbp fragment encoded a 166-residue protein with a BH3 motif. The 0.25-kbp fragment encoded an 82-residue protein, which was internally deleted but still contained the BH3 motif. In contrast, the 0.4-kbp fragment encoded a frame-shifted protein of 52 residues lacking the BH3 motif. Analogous to the nomenclature by U et al. (45), we named these proteins as xBimα1, xBimα2, and xBimβ, respectively. Both xBimα1 and xBimα2 mRNAs were strongly expressed in intestine, ovary, and stomach, whereas the expression of xBimβ mRNA was high in egg and ovary (Fig. 1B).
FIGURE 1.
mRNA expression profiles and structures of Xenopus Bcl-2 family proteins. A, RT-PCR analyses (left) and schematic structures (right) of Xenopus Bcl-2 family proteins except xBim. B, RT-PCR analysis (left), and schematic structures of cDNAs (center) and proteins (right) of xBim variants. In A and B, RT-PCR was carried out without template (−) or with cDNA templates from egg (E), intestine (I), liver (L), ovary (O), or stomach (S). Xenopus ribosomal protein L8 (xRpl8) was also examined as a positive control. In schematic protein structures, BH1 (white boxes), BH2 (dotted boxes), BH3 (black boxes), BH4 (hatched boxes), and transmembrane regions (gray boxes) are indicated. The caspase-mediated cleavage site of xBid (Asp52) is indicated by an arrowhead. In the central panel of B, the lengths of the corresponding parts of cDNAs in bp and the positions of start (ATG) and stop (TGA and TAA) codons are shown.
Functional Characterization of the Recombinant Xenopus Bcl-2 Family Proteins in Egg Extracts
Next, we examined the apoptosis-regulating activities of recombinant Xenopus Bcl-2 family proteins in egg extracts. Each synthetic mRNA was added to CSF-arrested egg extracts to synthesize the corresponding recombinant N-terminally HA-tagged protein by the endogenous translation system. To confirm the correct expression of recombinant proteins, we first carried out the experiment in the presence of Z-VAD-fmk (a pancaspase inhibitor) to block apoptotic execution, followed by Western blot with the antibody against HA tag. For all members except xBimβ, recombinant proteins of the expected electrophoretic mobility were translated, but their expression levels were variable (Fig. 2, αHA). In contrast, xBimβ was not expressed in this experimental system. We then performed the same experiment in the absence of Z-VAD-fmk and analyzed the apoptosis-regulating activities of recombinant proteins by monitoring the apoptotic cleavage of endogenous PARP. In the control extract containing no synthetic mRNA, endogenous PARP remained intact after 3 h but was cleaved by caspases after 6 h (Fig. 2, αPARP). Therefore, we determined that the proteins inducing PARP cleavage by 3 h were strongly pro-apoptotic and that the proteins preventing PARP cleavage until 6 h were strongly anti-apoptotic. Our results indicated that four proteins (xBcl-B, xBcl-w/xR1, xBcl-xL/xR11, and xMcl-1) were strongly anti-apoptotic. In contrast, five proteins (xBak, xBid, xBimα1, xBimα2, and xNoxa) were strongly pro-apoptotic. Two proteins (xBcl-G and xBax) slightly accelerated PARP cleavage after 3 h and were defined as weakly pro-apoptotic. Four proteins (xBcl-2, xBok, xBimβ, and xBmf) showed no effect in our experimental system.
FIGURE 2.
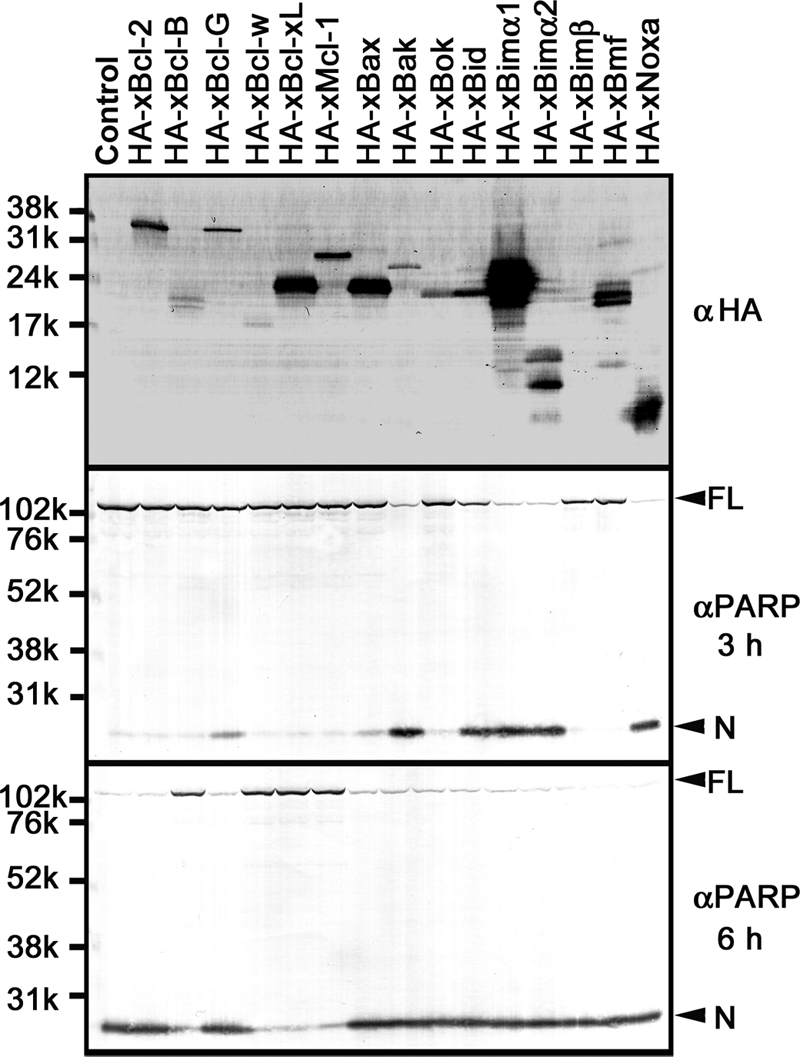
Apoptosis-regulating activities of Xenopus Bcl-2 family proteins in CSF-arrested metaphase egg extracts. Shown is expression of recombinant proteins and regulation of apoptotic PARP cleavage in CSF-arrested metaphase egg extracts. Top, recombinant N-terminally HA-tagged proteins were translated from the added synthetic mRNAs for 3 h in CSF-arrested metaphase egg extracts in the presence of Z-VAD-fmk. Samples were separated by SDS-PAGE and subjected to Western blot using anti-HA tag antibody (αHA). Middle and bottom, the same recombinant proteins as above were translated in the absence of Z-VAD-fmk, and apoptotic cleavage of endogenous PARP after incubating the extracts for 3 h (middle) and 6 h (bottom) was detected by Western blot using anti-PARP antibody (αPARP). Full-length protein (FL) and cleaved N-terminal fragment (N) of PARP are indicated by arrowheads.
Subcellular Fractionation of Recombinant Xenopus Bcl-2 Family Proteins in Egg Extracts
We next carried out subcellular fractionation analysis to examine the distribution of Xenopus Bcl-2 family proteins in egg extracts. The mitochondrial fraction is commonly separated from cytosol and microsomal membrane by low speed centrifugal precipitation (5,000–10,000 × g for 10 min). However, the density of egg extracts is so high that the mitochondrial fraction does not precipitate under this condition. Therefore, the egg extracts containing recombinant N-terminally HA-tagged proteins described as above were first diluted 10-fold with buffer to reduce the density. Diluted extracts were then centrifuged (10,000 × g for 10 min) to separate supernatant fraction containing cytosol and microsomal membrane (Fig. 3, Sup) from precipitated fraction containing mitochondria (Fig. 3, Ppt), and the fractions were analyzed by Western blot with the antibody against HA tag. Nine proteins (xBcl-2, xBcl-B, xBcl-G, xBcl-w/xR1, xBid, xBimα1, xBimα2, xBmf, and xNoxa) were detected mainly in the supernatant fraction. In contrast, three proteins (xBcl-xL/xR11, xBak, and xBok) were detected only in the precipitated fraction. Two proteins (xMcl-1 and xBax) were present in both supernatant and precipitated fractions. Actin and cytochrome c were recovered in supernatant and precipitated fractions, respectively, thereby validating our subcellular fractionation procedure. These results suggest that Xenopus Bcl-2 family proteins show different subcellular distributions in egg extracts.
FIGURE 3.
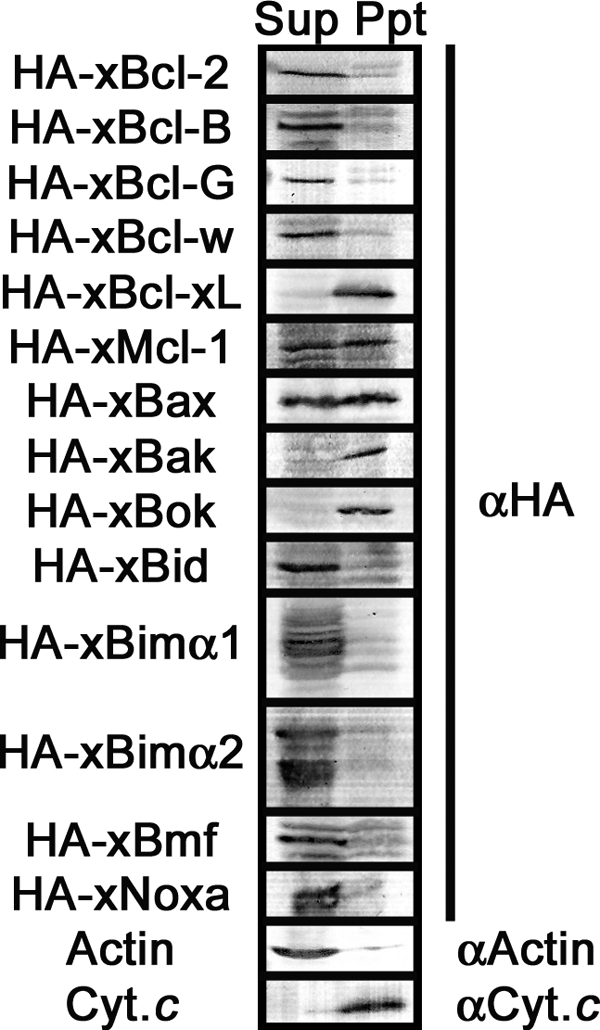
Subcellular fractionation of recombinant Xenopus Bcl-2 family proteins in CSF-arrested metaphase egg extracts. Supernatant (Sup) and precipitated membrane (Ppt) fractions were prepared from CSF-arrested metaphase egg extracts expressing recombinant N-terminally HA-tagged proteins. Samples were separated by SDS-PAGE and subjected to Western blot using anti-HA tag antibody (αHA). Samples were also probed using anti-cytochrome c (αCyt.c; mitochondria marker) and anti-actin (αActin; cytoplasm marker) to validate our fractionation procedure.
Stability Analysis of Xenopus Bcl-2 Family Proteins in Egg Extracts
Apoptosis in egg extracts is carried out without the requirement of new protein synthesis. Therefore, we hypothesized that apoptosis may be triggered by the irreversible changes of existing molecules, such as 1) activation of pro-apoptotic proteins and 2) inactivation of anti-apoptotic proteins. To examine these possibilities, we tested the behaviors of exogenously added, recombinant N-terminally His6-tagged proteins during incubation in interphase egg extracts. When translated in RRL, 12 proteins (xBcl-2, xBcl-G, xBcl-w/xR1, xBcl-xL/xR11, xMcl-1, xBax, xBak, xBok, xBid, xBimα1, xBimα2, and xBmf) were successfully 35S-radiolabeled, whereas the signals of three proteins (xBcl-B, xBimβ, and xNoxa) could not be detected due to poor expression. Each of the above 12 proteins was mixed with egg extracts and incubated for up to 6 h. As shown in Fig. 4A, four proteins (xMcl-1, xBid, xBimα2, and xBmf) of 12 showed significant changes during incubation. As we previously reported (17), recombinant xBid was gradually ubiquitylated and then cleaved by caspases after 4 h in egg extracts, and this change was prevented by Z-VAD-fmk but not by MG-132 (Fig. 4, 6XHis-xBid). In addition, the gradual decreases of recombinant xMcl-1 and xBimα2 were blocked by MG-132 but not by Z-VAD-fmk, suggesting the proteasome-mediated degradation of both proteins in egg extracts (Fig. 4, 6XHis-xMcl-1 and 6XHis-xBimα2). Recombinant xBmf was also unstable in egg extracts, but neither Z-VAD-fmk nor MG-132 blocked its degradation (Fig. 4, 6XHis-xBmf). Although MG-132 blocked the degradation of at least two Bcl-2 family proteins (xMcl-1 and xBimα2), it showed little overall effect on the timing of apoptosis execution in egg extracts (supplemental Fig. S1), as we reported previously (33).
Counteracting Apoptosis-regulating Activities of Endogenous xBid and xMcl-1 in Xenopus Egg Extracts
We already characterized endogenous xBid as a physiological accelerator of apoptosis in egg extracts (17). It is generally believed that Bid is activated by the caspase-mediated N-terminal cleavage to form tBid, which supports our first hypothesis (activation of pro-apoptotic protein). Among the remaining three unstable proteins, xMcl-1 was strongly anti-apoptotic, xBimα2 was strongly pro-apoptotic, and xBmf was inactive in our assay using recombinant proteins (Fig. 4). Therefore, only xMcl-1 fulfills our second hypothesis (inactivation of anti-apoptotic protein), and we concentrated our effort to analyze the physiological functions of endogenous xMcl-1.
Although the amount of endogenous xMcl-1 was too low to be detected by direct Western blot of egg extracts, immunoprecipitation-coupled Western blot with anti-xMcl-1 antibody clearly detected the endogenous xMcl-1 in fresh CSF-arrested metaphase egg extracts (Fig. 5A, αxMcl-1). In contrast, no xMcl-1 was detected with control antibody (Fig. 5A, Control). The concentration of endogenous immunoprecipitable xMcl-1 in normal CSF-arrested metaphase egg was less than 0.1 ng/μl. To evaluate the efficacy of immunodepletion, we tested whether our anti-xMcl-1 antibody was able to immunodeplete recombinant HA-xMcl-1 translated in CSF-arrested metaphase egg extracts. Because recombinant xMcl-1 was efficiently immunodepleted with anti-xMcl-1 antibody (Fig. 5B, HA-xMcl-1), we expected that the immunodepletion of endogenous xMcl-1 would be also efficient. Endogenous xBid was also efficiently immunodepleted with our anti-xBid antibody (Fig. 5C), as we reported previously (17). Using these antibodies, we characterized the functional relationship between endogenous xBid and xMcl-1 in egg extracts. Control-depleted interphase egg extracts executed spontaneous apoptosis after 4 h, as judged by caspase-mediated PARP cleavage (Fig. 5D, Control depletion). PARP cleavage in xBid-depleted egg extracts began after 5 h, indicating that xBid immunodepletion delayed the onset of apoptosis by ∼1 h (Fig. 5D, xBid depletion), as we reported previously (17). In contrast, PARP cleavage in xMcl-1-depleted egg extracts started after 3 h, suggesting that xMcl-1 immunodepletion accelerated the onset of apoptosis by ∼1 h (Fig. 5D, xMcl-1 depletion). Interestingly, double depletion of xBid and xMcl-1 reversed the onset of PARP cleavage to 4 h after incubation, similar to control-depleted egg extracts (Fig. 5D, Double depletion). These results indicate that the anti-apoptotic function of endogenous xMcl-1 counteracts the pro-apoptotic function of endogenous xBid in egg extracts. Our data also suggest that egg extracts can execute spontaneous apoptosis normally in the absence of endogenous xBid-xMcl-1 pair.
The above result suggested that xBid and xMcl-1 might be physically associated in egg extracts. We initially examined this possibility by the reciprocal co-immunoprecipitation assays between endogenous xBid and endogenous xMcl-1 but failed to detect their interaction in egg extracts (data not shown). However, this might be due to the low endogenous concentration and proteasome-mediated degradation of endogenous xMcl-1. To solve this problem, we established a pull-down assay system using recombinant xMcl-1. When maltose-binding protein (MBP) was fused to the N terminus of xMcl-1, this fusion protein was stable in egg extracts in contrast with His6-xMcl-1 and showed a strong anti-apoptotic activity. We fixed MBP-xMcl-1 or control MBP on amylose beads and used them to pull down endogenous xBid from healthy or apoptotic egg extracts. Again, we could not pull down the full length, N terminus, or C terminus of endogenous xBid (supplemental Fig. S2). We also tried to pull down recombinant Bcl-2 family proteins using this experimental system. Although xBim specifically bound to xMcl-1, we did not observe the specific interaction between xBid-FL or -C and xMcl-1 (supplemental Fig. S3). Altogether, these additional experiments still did not allow us to observe the physical interaction between xBid and xMcl-1.
Dual Apoptosis-inhibiting Activities of Endogenous xMcl-1 and xXIAP in Xenopus Egg Extracts
We previously reported that xXIAP, a Xenopus homolog of mammalian X-linked inhibitor of apoptosis protein that blocks the activity of caspases, was also a physiological apoptosis inhibitor in egg extracts (33). To study the functional relationships among xBid, xMcl-1, and xXIAP, we first compared the stabilities of exogenously added, recombinant N-terminally His6-tagged proteins during incubation in interphase egg extracts. Caspase activation resulted in the cleavage of recombinant xBid after 4 h (Fig. 6A, 6XHis-xBid), whereas recombinant xMcl-1 was rapidly degraded before caspase activation (Fig. 6A, 6XHis-xMcl-1). In contrast, recombinant xXIAP was stable before caspase activation but suddenly disappeared after 4 h (Fig. 6A, 6XHis-xXIAP). We therefore speculated that the loss of endogenous xMcl-1 might be compensated by the presence of endogenous xXIAP. To examine this possibility, we tried to deplete both endogenous xMcl-1 and xXIAP from egg extracts. Our anti-xXIAP antibody, further purified since our first report (33), enabled us to detect and deplete endogenous xXIAP from egg extracts (Fig. 6B). As shown in Fig. 6C, apoptotic PARP cleavage began earlier in xXIAP-depleted extracts (after 2 h; xXIAP depletion) than control-depleted extracts (after 4 h; Control depletion) and xMcl-1-depleted extracts (after 3 h; xMcl-1 depletion), suggesting that xXIAP was a stronger apoptosis inhibitor than xMcl-1. Double depletion of xXIAP and xMcl-1 showed a similar effect to single depletion of xXIAP (after 2 h; Double depletion). These results suggest that both xMcl-1 and xXIAP are physiological apoptosis inhibitors in egg extracts.
Identification of the Destabilizing Region in the N Terminus of xMcl-1
Human Mcl-1 is also reported to be an unstable protein, and the regulatory mechanism of proteasome-mediated degradation of Mcl-1 has been well documented. PEST region, MULE-mediated ubiquitylation, Jun N-terminal kinase- and glycogen synthase kinase 3-mediated phosphorylation, and phosphodependent recognition by β-TrCP are reported to be important for proteasome-mediated degradation of human Mcl-1. In addition, caspase-mediated cleavage converts the C-terminal fragment into the pro-apoptotic molecule (reviewed in Refs. 46 and 47). However, the N terminus of xMcl-1 does not show significant amino acid sequence homology with that of mammalian Mcl-1 or any other protein in the database, and the sequence motifs of human Mcl-1 described above are not conserved in xMcl-1. Moreover, we did not observe the caspase-mediated cleavage of recombinant xMcl-1 in apoptotic egg extracts (Fig. 4). Therefore, the regulation of xMcl-1 stability may be different from that of human Mcl-1. We first examined the relative contribution of RRL and egg extracts to the instability of His6-xMcl-1 and confirmed that the components in egg extracts, rather than those in RRL, were important for the rapid degradation of His6-xMcl-1 (supplemental Fig. S4). Next, to examine whether the stability of xMcl-1 is regulated by phosphorylation, we compared the degradation rate of exogenously added recombinant His6-xMcl-1 in four egg extracts of different phosphorylation conditions. CSF-arrested metaphase egg extracts naturally arrest at the second meiotic metaphase, and mitotic kinases, including Cdc2-cyclin B and the components of the Mos-MEK-p42MAPK-p90Rsk kinase cascade known as CSF, are active (Fig. 7A, CSF). In contrast, mitotic kinases and CSF are both inactive in interphase egg extracts (Fig. 7A, INT). The addition of 10 μm okadaic acid to interphase egg extracts inhibited PP1 and PP2A to induce a hyperphosphorylated condition (Fig. 7A, +OA). Staurosporine at 10 μm is expected to inhibit various Ser/Thr kinases in interphase egg extracts (Fig. 7A, +STS). Recombinant xMcl-1 was rapidly degraded in all four types of egg extracts, suggesting that the stability of xMcl-1 was not significantly affected by phosphorylation status (Fig. 7A, 6XHis-xMcl-1). To elucidate how the stability of xMcl-1 is controlled, we next constructed several N-terminal deletion mutants of xMcl-1 (Fig. 7B). Only FL, Δ(1–30), and Δ(1–79) were successfully translated in RRL, whereas Δ(1–39) and Δ(1–60) were not. In contrast, all five proteins were expressed in E. coli extracts (supplemental Fig. S5), but further examination suggested that E. coli-synthesized recombinant proteins were insoluble and unsuitable for stability analysis. We therefore examined the stability of RRL-synthesized FL, Δ(1–30), and Δ(1–79) in interphase egg extracts. Both FL and Δ(1–30) were rapidly degraded in the absence of MG-132 but stabilized in the presence of MG-132 (Fig. 7C, FL and Δ(1–30)). In contrast, Δ(1–79) remained stable even in the absence of MG-132 (Fig. 7C, Δ(1–79)), indicating that residues 31–79 were required for the proteasome-mediated degradation of xMcl-1 in egg extracts. When RRL containing His6-xMcl-1 FL were added to egg extracts, apoptosis inhibition was not observed due to rapid degradation of recombinant xMcl-1 (Fig. 7D, compare +Control RRL and +FL RRL). In contrast, the addition of RRL containing stable His6-xMcl-1 Δ(1–79) delayed the onset of PARP cleavage by ∼1 h (Fig. 7D, +Δ(1–79) RRL), suggesting that stabilization of xMcl-1 increased its anti-apoptotic activity.
FIGURE 7.
Identification of destabilizing region in the N terminus of xMcl-1. A, stability of recombinant xMcl-1 in four egg extracts of different phosphorylation conditions. RRL containing 35S-radiolabeled recombinant His6-xMcl-1 were mixed with CSF-arrested metaphase egg extracts (CSF), interphase egg extracts (INT), interphase egg extracts with 10 μm okadaic acid (+OA), or interphase egg extracts with 10 μm staurosporine (+STS), all containing cycloheximide. After incubation for 0, 2, and 4 h, samples were separated by SDS-PAGE and analyzed by a BAS image analyzer. B, schematic structures of N-terminally deleted xMcl-1 mutants used in this study. Human Mcl-1 (HsMcl-1) is also presented for comparison. Arrowheads, caspase-cleaved sites in HsMcl-1 (Asp127 and Asp157). C, stability of recombinant xMcl-1 deletion mutants in interphase egg extracts. RRL containing 35S-radiolabeled FL, Δ(1–30), or Δ(1–79) were mixed with interphase egg extracts containing cycloheximide in the absence (top, −MG) or presence (bottom, +MG) of 100 μm MG-132. After incubation for 0, 2, and 4 h, samples were separated by SDS-PAGE and analyzed by a BAS image analyzer. D, increased apoptosis inhibition by the stabilized xMcl-1 mutant. Control RRL (+Control RRL), RRL containing FL (+FL RRL), or RRL containing Δ(1–79) (+Δ(1–79) RRL) were mixed with interphase egg extracts as in C. After incubation for up to 6 h, samples were separated by SDS-PAGE, and apoptotic cleavage of PARP at every hour was detected by Western blot using anti-PARP antibody (αPARP). FL, full-length protein; N, N-terminal fragment.
DISCUSSION
Functional Characterization of Xenopus Bcl-2 Family Proteins in Egg Extracts
In this study, we comprehensively characterized the apoptosis-regulating activities of Xenopus Bcl-2 family proteins in egg extracts. xBcl-2, xBcl-B, xBcl-G, xMcl-1, xBak, xBim isoforms, xBmf, and xNoxa were functionally characterized for the first time. However, the complete genomic sequence of X. laevis is still not available, and we do not exclude the possibility that other members, such as Xenopus Bad or Puma, may be encoded in the genome but are not represented in the current nucleotide database. The molecular mechanisms of apoptosis regulation by Bcl-2 family proteins appear to be evolutionarily conserved between Xenopus and mammals, but several differences are also observed as described below.
Multidomain Anti-apoptotic Proteins
Our RT-PCR suggested that xBcl-w/xR1, xBcl-xL/xR11, and xMcl-1 were expressed at the mRNA level in egg, whereas xBcl-2 and xBcl-B were not (Fig. 1). In mice and humans, however, Bcl-B is expressed in oocytes and early embryos (48, 49). Thus, Bcl-B may be a physiological apoptosis inhibitor of egg in mammals but not in Xenopus. Our functional assay revealed that recombinant xBcl-B, xBcl-w/xR1, xBcl-xL/xR11, and xMcl-1 were strongly anti-apoptotic (Fig. 2). The initial characterization of xBcl-w/xR1 was incomplete due to poor expression of recombinant protein (3), but we optimized the expression system and clearly showed the anti-apoptotic activity of xBcl-w/xR1. In contrast, recombinant xBcl-2 was inactive in our experiment (Fig. 2), whereas previous reports indicated the anti-apoptotic role of mammalian Bcl-2 expressed in Xenopus egg extracts (22–24, 26, 27). This discrepancy may be due to the relatively low level expression of recombinant xBcl-2 in our study. Otherwise, the molecular function of xBcl-2 may not be identical with that of mammalian Bcl-2.
Multidomain Pro-apoptotic Proteins
Our results indicated that xBcl-G, xBax, xBak, and xBok were all expressed at mRNA level in egg (Fig. 1A). When exogenously overexpressed, xBak was strongly pro-apoptotic, xBcl-G and xBax were weakly pro-apoptotic, and xBok was inactive (Fig. 2). In mammals, Bax and Bak are believed to be functionally redundant, but our results using Xenopus egg extracts showed a clear functional difference between xBax and xBak. Weak pro-apoptotic activity of overexpressed xBax by itself suggests that additional activation steps, such as mitochondria membrane translocation and conformational change, may be required to exert its pro-apoptotic activity in egg extracts. In contrast, overexpression may be sufficient for xBak to become fully active. Alternatively, egg extracts may have a capacity to selectively neutralize xBax but not xBak. A number of studies have suggested the pro-apoptotic function of xBax during Xenopus early embryonic development (4, 7–9, 16, 19), and the apoptosis-inducing activity of xBax may be tissue- or developmental stage-specific. Similarly, overexpressed xBok was inactive in egg extracts (this study) but accelerated apoptosis in oocytes (18). This may be due to the difference of experimental systems or expression levels of recombinant xBok.
BH3-only Pro-apoptotic Proteins
We previously reported that endogenous xBid was a physiological accelerator of apoptosis in egg extracts (17). In addition, RT-PCR analysis detected the mRNA expressions of xBimβ, xBmf, and xNoxa in egg, whereas xBimα1 and xBimα2 mRNAs were poorly expressed in egg (Fig. 1). Overexpression analysis showed that xBimα1, xBimα2, and xNoxa were strongly pro-apoptotic, whereas xBimβ and xBmf were inactive in egg extracts (Fig. 2). Our results are different from those of another study by Uren et al. (37), in which Noxa BH3 peptide alone did not release cytochrome c from Xenopus egg mitochondria. This discrepancy may be due to the experimental differences between nearly physiological levels of full-length Xenopus proteins against unfractionated egg extracts (this study) and high concentrations of mammalian synthetic BH3 peptides against isolated mitochondria (37). In addition, our subcellular fractionation analysis indicated that only five proteins (xBcl-xL/xR11, xMcl-1, xBax, xBak, and xBok) were present in the mitochondrial fraction after centrifugation, whereas the remaining nine proteins (xBcl-2, Bcl-B, xBcl-G, xBcl-w/xR1, xBid, xBimα1, xBimα2, xBmf, and xNoxa) were not (Fig. 3). Therefore, the composition and functional contribution of endogenous Xenopus Bcl-2 family proteins may be different between unfractionated egg extracts and purified mitochondrial fraction.
Anti-apoptotic Activity and Proteasome-mediated Degradation of xMcl-1 in Egg Extracts
We demonstrated in this study that endogenous xMcl-1 physiologically inhibited apoptosis by counteracting the pro-apoptotic activity of endogenous xBid in egg extracts (Fig. 5D). However, we were not able to detect the physical association of xBid and xMcl-1 in egg extracts (supplemental Figs. S2 and S3). Therefore, these two proteins may not exist as a tightly bound complex in normal egg extracts. Recombinant xMcl-1 (and probably also endogenous xMcl-1) was constitutively degraded by proteasome in healthy egg extracts before apoptotic execution (Figs. 4B and 7A). This instability of xMcl-1 may contribute to the initiation of apoptosis in egg extracts. The degradation of recombinant xMcl-1 required the presence of the N-terminal 31–79 residues, but this region does not contain the key regulatory residues identified in the N terminus of mammalian Mcl-1 (reviewed in Refs. 46 and 47). We currently hypothesize that intrinsic instability, rather than signal-dependent modifications, may drive the constitutive proteasome-mediated degradation of xMcl-1 in egg extracts. This proteolytic decay keeps the concentration of endogenous xMcl-1 relatively low, and endogenous xMcl-1 will disappear when protein translation is inhibited by cycloheximide or slowed down by long incubation. We also showed that xXIAP, a downstream backup of apoptosis inhibitor in egg extracts, was abruptly degraded at the onset of apoptosis (Fig. 6), as we reported previously (33). From the current knowledge, we speculate that the mechanism of two-tier apoptosis inhibition by xMcl-1 and xXIAP may be as follows. When protein synthesis is blocked or slowed down, exhaustion of endogenous xMcl-1 by constitutive proteasome-mediated degradation changes the functional balance between pro- and anti-apoptotic activities of Bcl-2 family proteins. This eventually leads to cytoplasmic release of cytochrome c, formation of apoptosome, and activation of caspases. However, endogenous xXIAP immediately binds to and inhibits activated caspases. In turn, the xXIAP-caspase complex is degraded as shown in Fig. 6A, probably by the self-ubiquitylation activity of the C-terminal RING finger domain of xXIAP (reviewed in Ref. 50). This mechanism removes activated caspases from egg extracts during the initial phase of apoptosis, but inhibition of protein synthesis leads to the gradual loss of endogenous xXIAP. Our preliminary analysis suggests that the endogenous amounts of effector caspases are larger than that of xXIAP,3 and apoptosis is finally executed after the exhaustion of endogenous xXIAP. This hypothesis also explains the dominant anti-apoptotic role of xXIAP over xMcl-1. However, double depletion of endogenous xMcl-1 and xXIAP still required ∼2 h of incubation to execute apoptosis in egg extracts (Fig. 6C). Therefore, other upstream or parallel mechanisms may regulate cytochrome c release from mitochondria. They might be either activation of pro-apoptotic proteins, such as xBax, inactivation of anti-apoptotic proteins other than xMcl-1, or activation of hitherto unidentified pathway(s). Further characterization of other Xenopus Bcl-2 family proteins at endogenous levels, if present, will be necessary for a complete understanding of apoptosis regulation in egg extracts.
Supplementary Material
Acknowledgments
We thank the members of our laboratory for discussion.
This work was supported by Toho University School of Medicine Project Research Grant 20-19 and in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s)AB427203 and AB427204.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Y. Tsuchiya and S. Yamashita, unpublished data.
- CSF
- cytostatic factor
- MBP
- maltose-binding protein
- PARP
- poly(ADP-ribose) polymerase
- RRL
- rabbit reticulocyte lysates
- FL
- full length
- ORF
- open reading frame
- Z
- benzyloxycarbonyl
- fmk
- fluoromethylketone.
REFERENCES
- 1. Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 2. Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz-Reyes J., Tata J. R. (1995) Gene 158, 171–179 [DOI] [PubMed] [Google Scholar]
- 4. Finkielstein C. V., Lewellyn A. L., Maller J. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coen L., du Pasquier D., Le Mevel S., Brown S., Tata J., Mazabraud A., Demeneix B. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7869–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakajima K., Yaoita Y. (2003) Dev. Dyn. 227, 246–255 [DOI] [PubMed] [Google Scholar]
- 7. Tríbulo C., Aybar M. J., Sánchez S. S., Mayor R. (2004) Dev. Biol. 275, 325–342 [DOI] [PubMed] [Google Scholar]
- 8. Sachs L. M., Le Mevel S., Demeneix B. A. (2004) Dev. Dyn. 231, 671–682 [DOI] [PubMed] [Google Scholar]
- 9. Rowe I., Le Blay K., Du Pasquier D., Palmier K., Levi G., Demeneix B., Coen L. (2005) Dev. Dyn. 233, 76–87 [DOI] [PubMed] [Google Scholar]
- 10. Johnston J., Chan R., Calderon-Segura M., McFarlane S., Browder L. W. (2005) BMC Dev. Biol. 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Pasquier D., Rincheval V., Sinzelle L., Chesneau A., Ballagny C., Sachs L. M., Demeneix B., Mazabraud A. (2006) Dev. Dyn. 235, 2083–2094 [DOI] [PubMed] [Google Scholar]
- 12. Kominami K., Takagi C., Kurata T., Kitayama A., Nozaki M., Sawasaki T., Kuida K., Endo Y., Manabe N., Ueno N., Sakamaki K. (2006) Genes Cells 11, 701–717 [DOI] [PubMed] [Google Scholar]
- 13. Zhang C., Carl T. F., Trudeau E. D., Simmet T., Klymkowsky M. W. (2006) PLoS ONE 1, e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coen L., Le Blay K., Rowe I., Demeneix B. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 8502–8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du Pasquier D., Chesneau A., Ymlahi-Ouazzani Q., Boistel R., Pollet N., Ballagny C., Sachs L. M., Demeneix B., Mazabraud A. (2007) Genesis 45, 1–10 [DOI] [PubMed] [Google Scholar]
- 16. Kloc M., Shirato Y., Bilinski S., Browder L. W., Johnston J. (2007) Genesis 45, 523–531 [DOI] [PubMed] [Google Scholar]
- 17. Saitoh T., Tsuchiya Y., Kinoshita T., Itoh M., Yamashita S. (2009) Biochem. Biophys. Res. Commun. 384, 491–494 [DOI] [PubMed] [Google Scholar]
- 18. Johnson C. E., Freel C. D., Kornbluth S. (2010) Cell Death Differ. 17, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Marco N., Iannone L., Carotenuto R., Biffo S., Vitale A., Campanella C. (2010) Cell Death Differ. 17, 360–372 [DOI] [PubMed] [Google Scholar]
- 20. von Ahsen O., Newmeyer D. D. (2000) Methods Enzymol. 322, 183–198 [DOI] [PubMed] [Google Scholar]
- 21. Deming P., Kornbluth S. (2006) Methods Mol. Biol. 322, 379–393 [DOI] [PubMed] [Google Scholar]
- 22. Newmeyer D. D., Farschon D. M., Reed J. C. (1994) Cell 79, 353–364 [DOI] [PubMed] [Google Scholar]
- 23. Cosulich S. C., Green S., Clarke P. R. (1996) Curr. Biol. 6, 997–1005 [DOI] [PubMed] [Google Scholar]
- 24. Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. (1997) Science 275, 1132–1136 [DOI] [PubMed] [Google Scholar]
- 25. Cosulich S. C., Worrall V., Hedge P. J., Green S., Clarke P. R. (1997) Curr. Biol. 7, 913–920 [DOI] [PubMed] [Google Scholar]
- 26. Cosulich S. C., Savory P. J., Clarke P. R. (1999) Curr. Biol. 9, 147–150 [DOI] [PubMed] [Google Scholar]
- 27. Kluck R. M., Esposti M. D., Perkins G., Renken C., Kuwana T., Bossy-Wetzel E., Goldberg M., Allen T., Barber M. J., Green D. R., Newmeyer D. D. (1999) J. Cell Biol. 147, 809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newmeyer D. D., Bossy-Wetzel E., Kluck R. M., Wolf B. B., Beere H. M., Green D. R. (2000) Cell Death Differ. 7, 402–407 [DOI] [PubMed] [Google Scholar]
- 29. von Ahsen O., Renken C., Perkins G., Kluck R. M., Bossy-Wetzel E., Newmeyer D. D. (2000) J. Cell Biol. 150, 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moradi-Améli M., Lorca T., Ficheux D., di Pietro A., Gillet G. (2002) Biochemistry 41, 8540–8550 [DOI] [PubMed] [Google Scholar]
- 31. Kuwana T., Mackey M. R., Perkins G., Ellisman M. H., Latterich M., Schneiter R., Green D. R., Newmeyer D. D. (2002) Cell 111, 331–342 [DOI] [PubMed] [Google Scholar]
- 32. Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. (2005) Mol. Cell 17, 525–535 [DOI] [PubMed] [Google Scholar]
- 33. Tsuchiya Y., Murai S., Yamashita S. (2005) FEBS J. 272, 2237–2250 [DOI] [PubMed] [Google Scholar]
- 34. Nutt L. K., Margolis S. S., Jensen M., Herman C. E., Dunphy W. G., Rathmell J. C., Kornbluth S. (2005) Cell 123, 89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonzon C., Bouchier-Hayes L., Pagliari L. J., Green D. R., Newmeyer D. D. (2006) Mol. Biol. Cell 17, 2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsuchiya Y., Yamashita S. (2007) BMC Biochem. 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uren R. T., Dewson G., Chen L., Coyne S. C., Huang D. C., Adams J. M., Kluck R. M. (2007) J. Cell Biol. 177, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freel C. D., Richardson D. A., Thomenius M. J., Gan E. C., Horn S. R., Olson M. R., Kornbluth S. (2008) J. Biol. Chem. 283, 367–379 [DOI] [PubMed] [Google Scholar]
- 39. Saitoh T., Tsuchiya Y., Kinoshita T., Itoh M., Yamashita S. (2009) Reprod. Med. Biol. 8, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schafer B., Quispe J., Choudhary V., Chipuk J. E., Ajero T. G., Du H., Schneiter R., Kuwana T. (2009) Mol. Biol. Cell 20, 2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nutt L. K., Buchakjian M. R., Gan E., Darbandi R., Yoon S. Y., Wu J. Q., Miyamoto Y. J., Gibbon J. A., Andersen J. L., Freel C. D., Tang W., He C., Kurokawa M., Wang Y., Margolis S. S., Fissore R. A., Kornbluth S. (2009) Dev. Cell 16, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coultas L., Huang D. C., Adams J. M., Strasser A. (2002) Cell Death Differ. 9, 1163–1166 [DOI] [PubMed] [Google Scholar]
- 43. Aouacheria A., Brunet F., Gouy M. (2005) Mol. Biol. Evol. 22, 2395–2416 [DOI] [PubMed] [Google Scholar]
- 44. Blaineau S. V., Aouacheria A. (2009) Apoptosis 14, 923–925 [DOI] [PubMed] [Google Scholar]
- 45. U M., Miyashita T., Shikama Y., Tadokoro K., Yamada M. (2001) FEBS Lett. 509, 135–141 [DOI] [PubMed] [Google Scholar]
- 46. Akgul C. (2009) Cell Mol. Life Sci. 66, 1326–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas L. W., Lam C., Edwards S. W. (2010) FEBS Lett. 584, 2981–2989 [DOI] [PubMed] [Google Scholar]
- 48. Guillemin Y., Lalle P., Gillet G., Guerin J. F., Hamamah S., Aouacheria A. (2009) J. Mol. Med. 87, 923–940 [DOI] [PubMed] [Google Scholar]
- 49. Yoon S. J., Kim E. Y., Kim Y. S., Lee H. S., Kim K. H., Bae J., Lee K. A. (2009) Biol. Reprod. 81, 497–506 [DOI] [PubMed] [Google Scholar]
- 50. Galbán S., Duckett C. S. (2010) Cell Death Differ. 17, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.