Abstract
Tumor progression requires a crosstalk with the tumor surrounding, where the tumor matrix plays an essential role. We recently reported that only the matrix delivered by a CD44v6-competent (ASMLwt), but not that of a CD44v6-deficient (ASML-CD44vkd) rat pancreatic adenocarcinoma line supports metastasis formation. We here describe that this matrix provides an important feedback toward the tumor cell and that CD44v6 accounts for orchestrating signals received from the matrix. ASMLwt cells contain more hyaluronan synthase-3 and secrete higher amounts of >50 kDa HA than ASML-CD44vkd cells, which secrete more hyaluronidase. Only the ASMLwt-matrix supports migration and apoptosis resistance, which both can be initiated via CD44v6, c-Met, and α6β4 ligand binding and proceed via FAK, PI3K/Akt, and MAPK activation, respectively. However, c-Met- and α6β4-initiated signaling are strongly augmented by the association with CD44v6 as only very weak effects are observed in CD44v6-deficient cells. The same CD44v6-dependent convergence of motility- and apoptosis resistance-related signals also accounts for human tumor lines. Thus, CD44v6 promotes motility and apoptosis resistance via its involvement in assembling a matrix that, in turn, triggers activation of signaling cascades, which proceeds, independent of the initiating receptor-ligand interaction, in a concerted action via CD44v6.
Keywords: Extracellular Matrix, Hyaluronate, Pancreas, Signal Transduction, Tumor Metastases, CD44 Variant Isoform, Apoptosis Resistance, c-Met, Migration
Introduction
Tumor cells require a special surrounding for survival and metastatic progression (1, 2). Tumor cells support this crosstalk with the host by secreting and assembling a tumor matrix (3, 4). One of the molecules that has been amply demonstrated to contribute to the metastatic process is CD44 (5, 6), particularly the CD44 variant isoform v6 (CD44v6)2 (7, 8). In fact, this multifaceted molecule encompasses many activities facilitating the metastatic process.
First, the most important feature of CD44 in the communication with the tumor surrounding is its quality as the major hyaluronan (HA) receptor (9, 10). By HA binding, the CD44 conformation becomes altered such that its cytoplasmic tail interacts with the cytoskeleton via ankyrin (11) and ERM (Ezrin/Radixin/Moesin) proteins (12), which guide CD44 to the leading edge of migrating cells (13). The CD44-HA interaction also stimulates MMP2 and MMP9 production (14) and promotes MMP9 binding to CD44, which supports invasiveness by focalized matrix degradation (14). HA also binds to endothelial cells through CD44. Proinflammatory cytokines stimulate CD44 expression and strengthens endothelial cell HA binding (15). This, in turn facilitates tumor cell attachment via CD44, rolling and finally extravasation (16).
Second, besides binding to HA, CD44 also acts as a receptor for several cytokines and chemokines deposited in the extracellular matrix (ECM) (17–19), which trigger activation of signal transduction cascades (5, 6, 8). CD44 itself does not display kinase activity. Instead it initiates signal transduction via associated receptor tyrosine kinases (RTK) (20–24), or via interaction of the cytoplasmic tail with non-RTK and linker proteins (6, 25). Thus, CD44 co-immunoprecipitates with all ERBB RTK family members. To give an example, the CD44-HA interaction initiates ERBB2 phosphorylation and stimulation of HA production induces assembly of a lipid raft integrated complex of ERBB2, CD44, ezrin, the chaperone molecules HSP90 and CDC37 and PI3K, where activation of the PI3K/Akt pathway promotes apoptosis resistance (25). HER4 activation also proceeds via CD44, where CD44 heparan sulfate side chain-bound MMP7 cleaves heparin binding growth factor, a prerequisite for HER4 activation (26). Another important CD44 partner in apoptosis protection is c-Met. CD44 promotes c-Met phosphorylation via CD44v3- or CD44v6-bound hepatocyte growth factor (HGF). For not yet defined reasons, c-Met activation via CD44 requires the cytoplasmic tail of CD44 and the interaction with ERM proteins for activation of the Ras-MAPK pathway (27, 28). The most important CD44-associated non-RTK are members of the src family (29). As membrane-attached molecular switch, src has a central role by linking a variety of extracellular signals to crucial intracellular signaling pathways (30). Essential for their association with CD44 is the membrane subdomain location. Both CD44 and non-RTK are located in glycolipid-enriched membrane microdomains (GEM) that are prone for collecting signal transducing and linker molecules (6).
Third, there is strong evidence that CD44 is involved in the assembly of the matrix (31–33) such that the HA-CD44 association modifies the tissue matrix to support colonization (34, 35). Perturbation in matrix components can alter intracellular tension resulting in shifts in signaling events that affect gene expression (36). By its nature as a transmembrane proteoglycan CD44 allows the local concentration of glycosaminoglycan-associating proteins that frequently lowers the threshold for signal transduction (37), where binding of osteopontin, bFGF, VEGF, and HGF are of special interest for the metastatic process (38–41). We recently reported that a CD44v knockdown (kd) in a highly metastatic tumor line (ASML) (42) revealed a striking reduction in metastatic capacity, which was, at least in part, due to an altered tumor matrix. The CD44vkd, distinct to CD44v-competent cells secrete a matrix that does not support adhesion of CD44vwt or CD44vkd cells, whereas both cells readily adhere to the CD44vwt-matrix (43, 44).
Based on these latter findings, we searched for additional differences in the matrix assembled by CD44v-competent versus CD44v-deficient cells and asked, whether the feedback from the tumor matrix can account for the striking loss in metastatic capacity of ASML-v4–7kd cells. We show for human and rat pancreatic adenocarcinoma cells that only a matrix derived from CD44v6-competent cells supports tumor cell migration and apoptosis resistance. Furthermore, matrix-, as well as HA- or HGF- or laminin5 (LN5)-initiated activation of CD44v6, c-Met, and α6β4 essentially requires coordination via CD44v6.
EXPERIMENTAL PROCEDURES
Tumor Lines
ASMLwt, ASML-v4–7kd, AS, AS-v6, and AS-v4–7 clones of a BDX pancreatic adenocarcinoma (7, 42, 43, 45) were maintained in RPMI 1640/10% FCS. The level of panCD44, CD44s, and CD44v6 expression is shown in supplemental Fig. S1. c-Met-, β4-, and CD44v6-siRNA transfection followed the supplier's suggestion (Qiagen, Hildesheim, Germany). Efficiency of silencing was monitored after 48 h by Western blot (WB). Central features of these lines and of the human pancreatic adenocarcinoma lines Capan2, 8.18, and Pt45P1 are listed in supplemental Table S1.
Antibodies
Primary antibodies are listed in supplemental Table S2. Streptavidin-HRP and dye-labeled secondary antibodies were obtained commercially.
Inhibitors
LY294002 (PI3K-inhibitor) (Calbiochem, Darmstadt, Germany), SU11274 (c-Met-inhibitor), and GW5074 (raf-inhibitor) (Sigma, Munich, Germany) were used as indicated.
Cell and Conditioned Medium Fractionation
For cytosol preparation, 2.5 × 106 cells were incubated in hypotonic buffer, homogenized, and centrifuged (800 × g) to pellet nuclei. After adding Nonidet-P40 (0.5%), vortexing and centrifugation (1600 × g, 5 min), cytosolic proteins were recovered from the supernatant. Conditioned medium, derived from tumor cells cultured for 48 h in serum-free medium, was centrifuged (10 min, 1000 × g, 10 min 1500 × g, 10 min 2000 × g, 20 min 10,000 × g, 90 min 26,000 × g). This vesicle-depleted supernatant, termed “matrix,” was used throughout.
In Vitro Kinase Assay
Immune complexes were suspended in lysis buffer containing a protease inhibitor mix (Roche, Mannheim, Germany). After centrifugation, beads were resuspended in 30 μl of kinase assay buffer, 10 μCi of [γ-32P]ATP and incubated (15 min, 37 °C). The reaction was stopped by adding 10 μl of non-reducing 6× Laemmli buffer. SDS-PAGE was followed by autoradiography.
Immunoprecipitation
Cells were washed in HEPES buffer and lysed (30 min, 4 °C, HEPES buffer, 1% Lubrol, 1 mm PMSF, 1 mm NaVO4, 10 mm NaF, protease inhibitor mix). Centrifuged lysates, mixed with the antibody, were immunoprecipitated and incubated with Protein G-Sepharose. Immune complexes were washed, dissolved in Laemmli buffer, and resolved by SDS-PAGE.
SDS-PAGE and WB
SDS-PAGE resolved proteins were transferred (nitrocellulose membranes), membranes were blocked, blotted with primary and HRP-conjugated secondary antibodies and developed with the ECL kit. For MALDI-TOF analysis, proteins were separated by two-dimensional gel analysis and silver-stained.
MALDI-TOF Mass Spectrometry
Silver-stained spots were excised. Protein digestion, sample preparation, MALDI-TOF fingerprint analysis, post-source-decay fragmentation analysis, and database searches are described (46).
Flow Cytometry
Cells (2 × 105) were stained with AnnexinV-APC/PI. Samples were processed in a FACS-Calibur with the CellQuest program (BD, Heidelberg, Germany).
Immunofluorescence
Cells on coverslides were fixed, permeabilized, blocked, incubated with primary antibodies, fluorochrome-conjugated secondary antibodies, blocked, incubated with second, dye-labeled antibodies or with a second antibody followed by a dye-labeled secondary antibody and washed. Coverslides were mounted in Elvanol. Digitized images were generated using a 40× objective (Leica DMRBE microscope, SPOT-CCD camera, Diagnostic Instruments, Software SPOT2.1.2). Overlays of single fluorescence are shown.
ELISA
96-well plates were coated at pH 7.2 with matrix or HA. After washing, HA was determined using biotinylated HA-binding protein and streptavidin-biotin for detection. Hyaluronidase (HAase) levels were measured according to Stern and Stern (47). HAase concentrations (mU/ml) were determined by hyaluronidase dilution curves.
Apoptosis Induction
Cells (1 × 105) were grown for 48 h in RPMI/10% FCS containing serial dilutions of cisplatin (Sigma). Survival was monitored by annexinV-APC/PI staining, MTT assay, and [3H]thymidine uptake.
Migration
Cells, in the upper part of a Boyden chamber (40 μl of RPMI/0.1% BSA), were separated from the lower part, containing 30 μl of RPMI/1% FCS, 20% FCS, 5 ng/ml HGF, 10 μg/ml HA, 2 μg/ml LN5, ASMLwt-, or ASML-v4–7kd-matrix, by a 8-μm pore size polycarbonate-membrane (Neuroprobe, Gaithersburg, MD). Where indicated, the ASMLwt matrix was treated for 2 h with 0.5 mg/ml HAase type IV-S (Sigma) or the ASML-v4–7kd matrix was supplemented with HA (10 μg/ml). Migration was evaluated after 16 h by lower membrane side staining with crystal-violet. After lysis, A595 nm was measured. Migration is presented as % of input cells.
Video Microscopy
Hoechst 33342-stained cells (5 × 104) were seeded on matrix-coated migration chambers (8-well μ-slides, ibidi, Martinsried, Germany). Chambers were placed under an Olympus IX81 inverse microscope with a Hg/Xe lamp, an incubation chamber (37 °C, 5%CO2), a CCD camera (Hamamatsu), and a ScanR acquisition software (Olympus, Hamburg, Germany). Two pictures (20-fold magnification)/chamber (2ms exposure) were taken every 15 min for 12 h. Migration was quantified according to Manual_tracking plugin (F.P. Cordeliére, Centre de Recherche de l'Institute Curie) running in the open-source software Image J (NIH). Path length of 20 individual cells in each setting was calculated for every 15 min by customized programs. The mean pathway length per 1 h during the incubation period of 0–12 h and of 9–12 h is presented.
Statistics
Significance of triplicates or of 20 individual values was calculated by Student's t test. Assays were repeated three times.
RESULTS
Highly metastatic CD44v6-competent ASMLwt cells assemble a matrix, which supports metastasis formation (44). This finding suggests a feedback trigger from the matrix toward the tumor cell. The hypothesis and the involvement of CD44v6 was controlled for two most characteristic features of metastasizing tumor cells, motility, and apoptosis resistance.
CD44v6 and Matrix-supported Motility
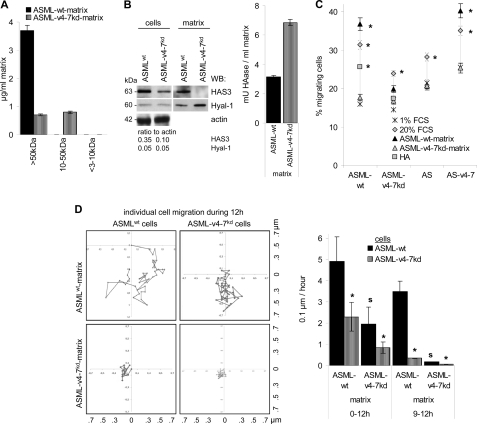
The ASMLwt-matrix contains, besides others, higher amounts of HGF, c-Met, uPA, uPAR, MMP2, and MMP9 (44, supplemental Table S3) that might support cell motility. In addition, compared with the ASML-v4–7kd-matrix, the ASMLwt-matrix is enriched in >50 kDa HA (Fig. 1A). ASMLwt cells and the matrix contain higher amounts of hyaluronan synthase-3 (HAS3). The ASML-v4–7kd-matrix, instead, contains a higher amount of HAase (Fig. 1B). These differences have significant bearing on cell motility. The ASMLwt-, but not the ASML-v4–7kd-matrix promotes transwell migration of CD44v6-expressing cells (Fig. 1C). Time lapse video microscopy for 12 h revealed migration of ASMLwt cells on the ASMLwt-, but not the ASML-v4–7kd-matrix. ASML-v4–7kd cells did not migrate on their own matrix. They showed some migratory activity on the ASMLwt-matrix in the starting hours. However, their migratory activity rapidly declined and during the last 4 h of culture ASML-v4–7kd cells no longer moved at all (Fig. 1D).
FIGURE 1.
Recovery of HA in the ASMLwt-matrix and tumor cell migration. A, ASMLwt- and the ASML-v4–7kd-matrix were fractionated by filtration through 3000, 10,000, and 50,000 MW pore size filters and titrated amounts of the size-separated matrix or HA (1–20 μg/ml) were seeded on ELISA plates and recovery of HA in the ASMLwt- and the ASML-v4–7kd-matrix was determined with biotinylated HA-binding protein and streptavidin-biotin. Mean ± S.D. of triplicates is shown. B, WB of Hyal-1 and HAS3 in ASMLwt and ASML-v4–7kd cell lysates and the matrix. Gels were loaded with 25 μg of lysate. For the quantification of HAase, titrated amounts of matrix or HAase (0–25mU/ml) were seeded on ELISA plates coated with 200 μg/ml HA. After 16 h of incubation, plates were washed and remaining HA was determined as above. HAase concentrations (mU/ml) were calculated according to standard HAase dilution curves. C, migration of AS, AS-v4–7, ASMLwt, and ASML-v4–7kd cells toward FCS, HA, the ASMLwt- and the ASML-v4–7kd-matrix (Boyden chamber). The percentage of migrating cells was determined after 18 h. Mean ± S.D. of triplicates are shown. Significant differences between ASMLwt and ASML-v4–7kd cells are indicated by *. D, migration of ASMLwt and ASML-v4–7kd cells on the ASMLwt- and the ASML-v4–7kd-matrix as revealed by video microscopy. Pictures were taken every 15 min. The movement of individual cells during 12 h and the mean movement per hour ± S.D. of 20 cells during 0–12 h and during 9–12 h after seeding are shown. Significant differences in cell motility on the ASMLwt versus the ASML-v4–7kd matrix are indicated by s; significant differences in the motility of ASMLwt versus ASML-v4–7kd cells are indicated by *. The ASMLwt matrix strongly promotes motility of ASMLwt cells, but has only a weak and transient effect on ASML-v4–7kd cell motility.
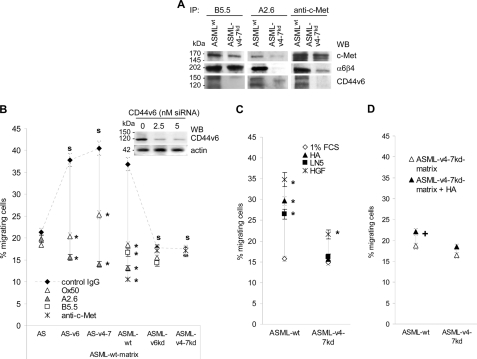
To control whether CD44v6 contributes to the response toward the ASMLwt-matrix, migration (Boyden chamber) was evaluated after pre-incubation of ASMLwt (CD44s+/CD44v+/c-Met+/α6β4+), ASML-v4–7kd (CD44s+/CD44v4–7−/c-Met+/α6β4+), ASML-v6kd (CD44s+/CD44v6−/c-Met+/α6β4+), AS (CD44s+/CD44v−/c-Met+/α6β4−), AS-v6 (CD44s+/CD44v6+/c-Met+/α6β4−), and AS-v4–7 (CD44s+/CD44v4–7+/c-Met+/α6β4−) cells with anti-panCD44 (Ox50), anti-CD44v6 (A2.6), anti-c-Met, and anti-α6β4 (B5.5). The latter two antibodies have been included, because α6β4 and c-Met co-immunoprecipitate with CD44v6 and c-Met co-immunoprecipitates with α6β4 (27, 48) (Fig. 2A). Pre-incubation with anti-panCD44, anti-CD44v6, anti-c-Met, and anti-α6β4 significantly inhibited ASMLwt cell migration toward the ASMLwt-matrix. Anti-panCD44 and anti-CD44v6 also inhibited AS-v6 and AS-v4–7, but not AS cell migration. ASML-v6kd and ASML-v4–7kd cell migration toward the ASMLwt-matrix was not significantly inhibited by anti-panCD44, anti-α6β4, or anti-c-Met (Fig. 2B). These results indicated that the ASMLwt-matrix-initiated migration essentially depends on CD44v6, but also involves c-Met- and α6β4. To control this hypothesis, HA, LN5 and HGF were used as stimulus. Surprisingly, all three molecules promoted migration of ASMLwt cells, although not as efficiently as the ASMLwt-matrix. Only HGF, albeit weakly stimulated ASML-v4–7kd cell migration (Fig. 2C). Comparable results were obtained with human pancreatic cancer lines differing in c-Met, α6β4, and CD44v6 expression. Capan2 cells (CD44v6+/c-Met+/α6β4+) migrated in response to HA, LN5 and HGF. Inhibition of migration by anti-CD44v6, anti-c-Met, and anti-β4 was stimulus-independent. Even in the absence of c-Met (8.18 cells), anti-CD44v6, and anti-β4 inhibited migration, which was not promoted by HGF and not inhibited by anti-c-Met. HA, LN5, or HGF poorly stimulated migration of Pt45P1 cells (CD44v6−/c-Met−/α6β4±), which was weakly inhibited by anti-β4 (supplemental Fig. S2).
FIGURE 2.
The contribution of CD44v6, c-Met, and α6β4 to ASMLwt cell migration. A, ASMLwt and ASML-v4–7kd lysates from 107 cells were immunoprecipitated with B5.5 (anti-α6β4), anti-c-Met, and A2.6 (anti-CD44v6). Immunoprecipitates were separated by SDS-PAGE and after transfer blotted with anti-c-Met, B5.5, and A2.6. CD44v6, c-Met and α6β4 co-immunoprecipitate. B, ASMLwt, ASML-v4–7kd, ASML-v6kd, AS, AS-v4–7, and AS-v6 cells were pre-incubated with Ox50 (anti-panCD44), A2.6, B5.5, and anti-c-Met. Migration toward the ASMLwt matrix was evaluated. C, migration of ASMLwt and ASML-v4–7kd cells toward HA, LN5 and HGF was evaluated. D, migration of ASMLwt and ASML-v4–7kd cells toward the ASML-v4–7kd-matrix with/without addition of 10 μg/ml HA was evaluated. B–D, the percentage of migrating cells (Boyden chamber) was determined after 18 h. Mean ± S.D. of triplicates are shown. Significant inhibition by antibody pre-incubation is indicated by *. Significant differences in dependence on CD44v6 expression is indicated by s. Significant differences by HA addition is indicated by +. The ASMLwt-matrix, but also HA, LN5 and HFG promote ASMLwt cell migration and anti-CD44v6, -c-Met, or -α6β4 inhibit ASMLwt cell migration. Thus, CD44v6, c-Met, and α6β4 jointly promote ASMLwt cell migration. However, anti-CD44v6 only inhibits migration of AS-v4–7, AS-v6, and ASMLwt cells. On the contrary, anti-panCD44 does not inhibit migration of CD44v6-deficient AS, ASML-v4–7kd, and ASML-v6kd cells, which implies that migration-promoting activity of CD44, c-Met, and α6β4 is coordinated by CD44v6.
Having demonstrated that rat and human tumor lines migrate toward HGF and LN5 only as far as they express the corresponding receptors plus CD44v6, we finally asked, whether the failure of ASMLwt cells to migrate toward the ASML-v4–7kd-matrix relies exclusively on the low level of higher molecular weight HA. Addition of HA to the ASML-v4–7kd-matrix hardly strengthened ASMLwt and not at all ASML-v4–7kd cell migration (Fig. 2D). This indicates that the ASML-v4–7kd matrix, by assembly or composition, actively inhibits tumor cell migration.
Thus, CD44v6-competent tumor cells can assemble a matrix that promotes migration, whereas in the absence of CD44v6, at least in ASML cells, a matrix is assembled that actively inhibits migration. Though less efficiently, migration can also be initiated by HA, HGF, and LN5. However, irrespective of the initiating stimulus, CD44v6 expression is essentially required as CD44v6-deficient cells do not or poorly respond to the migration-promoting stimuli.
CD44v6-dependent Motility Is Accompanied by c-Met, Ezrin, and Src Activation
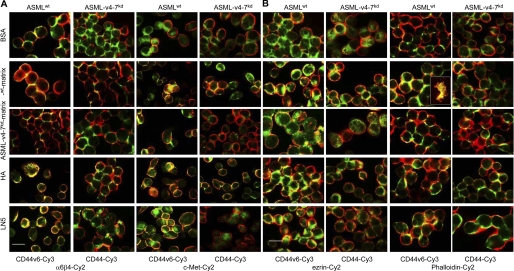
Immunofluorescence of ASMLwt cells revealed pronounced co-localization of CD44v6 with α6β4 and c-Met, when seeded on HA-, LN5-, ASMLwt-, but not ASML-v4–7kd-matrix-coated slides. Instead, the CD44 standard molecule poorly co-localized with α6β4 and c-Met in ASML-v4–7kd cells (Fig. 3A). As CD44, upon activation, also associates via ezrin with the actin cytoskeleton, we evaluated CD44 co-localization with ezrin and F-actin, which was strong in ASMLwt cells, when seeded on HA-, LN5-, or ASMLwt-matrix-, but not ASML-v4–7kd-matrix-coated slides. Co-localization was weak in ASML-v4–7kd cells (Fig. 3B). FAK, which can become attracted via CD44-associated src, also preferentially co-localized with CD44v6 when ASMLwt cells were seeded on the ASMLwt-matrix or on LN5 (data not shown). The same observations accounted for CD44v6 co-localization with β4, ezrin, FAK, c-Met, and actin bundles in Capan2 cells. HA- and LN5 also stimulated, albeit less pronounced, co-localization of CD44v6 and β4 with actin bundles in 8.18 cells (supplemental Fig. S3).
FIGURE 3.
The ASMLwt matrix supports co-localization of CD44v6 with α6β4, c-Met, ezrin, and F-actin. ASMLwt and ASML-v4–7kd cells were seeded on HA, LN5, the ASMLwt-, or ASML-v4–7kd-matrix and were stained with A2.6 or Ox50 plus (A) B5.5 and anti-c-Met or (B) anti-ezrin and phalloidin. Inset, membrane fragments, left behind (migrating) cells, have only been seen, when ASMLwt cells were seeded on the ASMLwt-matrix. Overlays of single fluorescence microscopy are shown (bar size: 5 μm). The ASMLwt-matrix, HA, and LN5, but not the ASML-v4–7kd-matrix strengthen co-localization of CD44v6 with α6β4, c-Met, ezrin, and F-actin.
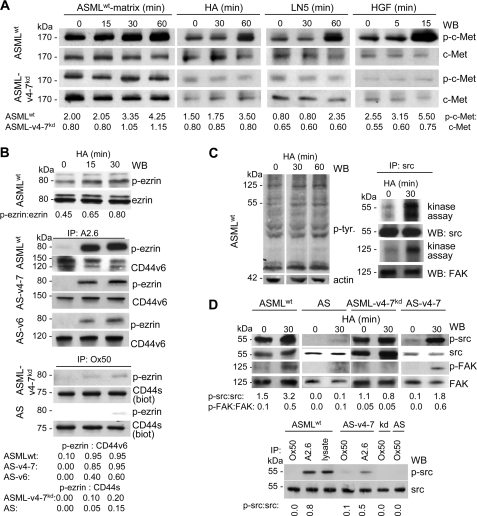
Co-localization of c-Met with CD44v6 was accompanied by strong c-Met phosphorylation, when cells were stimulated by the ASMLwt-matrix, HA, LN5, or HGF. Instead, only weak c-Met phosphorylation was observed, when ASML-v4–7kd cells were stimulated by the ASMLwt-matrix, or HGF, but not when stimulated with HA or LN5 (Fig. 4A). The finding not only confirms the impact of the ASMLwt-matrix on c-Met activation, but also strengthens the notion that irrespective of the initiating stimulus, c-Met activation is greatly facilitated by CD44v6. As demonstrated for HA cross-linking, ezrin phosphorylation is also strongly promoted by CD44v6 expression. In lysates of ASMLwt, AS-v6, or AS-v4–7 cells cultured on HA-coated plates, an increased amount of phosphorylated ezrin co-immunoprecipitated with CD44v6. However, HA-binding of CD44s in ASML-v4–7kd or AS cells hardly strengthened co-immunoprecipitation of phosphorylated ezrin with CD44s (Fig. 4B).
FIGURE 4.
CD44v6 dependence of c-Met, ezrin, src, and FAK activation. A, ASMLwt and ASML-v4–7kd cells were stimulated with ASMLwt-matrix, HA (10 μg/ml), LN5 (2 μg/ml), and HGF (5 ng/ml). Lysates (25 μg) were separated by SDS-PAGE and after protein transfer blotted with anti-p-c-Met and anti-c-Met. B, ASMLwt cells were stimulated with HA (10 μg/ml). Lysates (25 μg) were separated by SDS-PAGE and after protein transfer blotted with anti-p-ezrin or anti-ezrin or lysates of ASMLwt, AS-v6, AS-v4–7 cells were immunoprecipitated with A2.6 or lysates of biotinylated ASML-v4–7kd and AS cells were immunoprecipitated with Ox50. After SDS-PAGE and protein transfer membranes were blotted with anti-p-ezrin and A2.6, respectively, streptavidin. C, untreated and HA-treated (10 μg/ml) ASMLwt cells were lysed separated by SDS-PAGE and after protein transfer blotted with anti-p-tyrosine and anti-actin or were immunoprecipitated with anti-src. Precipitates were labeled with [γ-32P]ATP. Autoradiography and blotting after SDS-PAGE separation and transfer with anti-src and anti-FAK are shown. D, HA-treated ASMLwt, AS-v4–7, ASML-v4–7kd, and AS cells were lysed and were blotted, after SDS-PAGE separation and transfer, with anti-p-src, -src, -p-FAK, and -FAK. Alternatively, lysates were immunoprecipitated with A2.6 or Ox50 and SDS-PAGE separated immunoprecipitates were blotted with anti-p-src and -src. A, B, and D, ratios of non-phosphorylated to phosphorylated protein or of precipitated to co-precipitated protein are included. The ASMLwt-matrix, HA, LN5, and HGF strengthen the CD44v6, c-Met, and α6β4 association, which is accompanied by ezrin, src, and FAK association/phosphorylation only in CD44v6-competent cells.
In addition, FAK and src co-immunoprecipitation and phosphorylation became strengthened by CD44-crosslinking via HA (Fig. 4C). Src and FAK also became phosphorylated in HA-treated AS-v4–7, but not in ASML-v4–7kd and AS cells. Furthermore, although src co-immunoprecipitated with CD44s and CD44v, p-src was only recovered in anti-CD44 precipitates of CD44v6-competent cells (Fig. 4D).
Taken together, migratory activity of CD44v6-competent tumor cells is accompanied by c-Met phosphorylation, recruitment, and phosphorylation of ezrin and pronounced phosphorylation of FAK via CD44-associated src. None of these observations are discerned for CD44v6-deficient cells. This confirms that transition toward the migratory phenotype, initiated via c-Met, α6β4, or CD44v6 ligand binding, essentially requires CD44v6 for signal transduction downstream of c-Met, ezrin, and src. This central role of CD44v6 in signal transduction has also been seen in matrix-supported drug resistance.
Matrix-assisted Drug Resistance
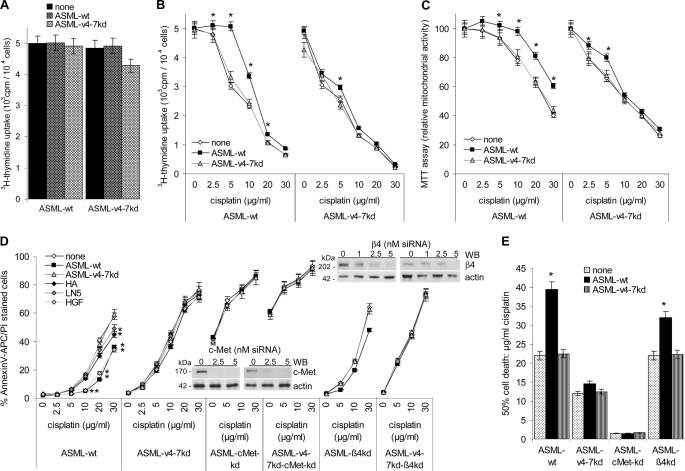
ASML cells grow anchorage-independent with a soft agar cloning efficacy of close to 100%, which is not affected by the CD44v4–7kd (43). They have a slow cell cycle, where the proliferative activity is not altered in ASML-v4–7kd cells (43) and neither the ASMLwt- nor the ASML-v4–7kd-matrix influence cell cycle progression of ASMLwt and ASML-v4–7kd cells (Fig. 5A). Instead, proliferation and mitochondrial respiratory activity of ASML-v4–7kd, but not of ASMLwt cells is impaired after 48 h of culture in the presence of 2.5 μg/ml cisplatin. As in the absence of cisplatin, proliferative activity of ASMLwt, and ASML-v4–7kd cells did not differ, this indicates that the high drug resistance of ASMLwt cells (43) may be affected by the CD44v4–7kd. Notably, the ASMLwt-matrix obviously supports drug resistance of ASMLwt, but hardly of ASML-v4–7kd cells. In the presence of the ASMLwt-matrix, proliferative and mitochondrial respiratory activity of ASMLwt cells was unimpaired by 5 μg/ml cisplatin, while in the absence of the ASMLwt-matrix proliferative activity was reduced by roughly 30%.The ASML-v4–7kd-matrix hardly exerted any protective effect on ASMLwt cells and none on ASML-v4–7kd cells (Fig. 5 B and C). To control that the ASMLwt-matrix indeed protects from apoptosis, the apoptosis rate of ASMLwt and ASML-v4–7kd cells cultured for 48 h in the presence of increasing doses of cisplatin was evaluated by AnnexinV/PI staining. High apoptosis resistance of ASMLwt cells was further increased in the presence of the ASMLwt-matrix. The ASML-v4–7kd-matrix exerted no effect. ASML-v4–7kd cells were not protected from apoptosis by their own and only weakly by the ASMLwt-matrix. HGF also promoted apoptosis resistance. Protection by HA and LN5 was weaker, but reached a significant level in ASMLwt cells at high cisplatin concentrations (Fig. 5D).
FIGURE 5.
The ASMLwt-matrix supports apoptosis resistance. A, ASMLwt and ASML-v4–7kd cells were seeded on BSA-, ASMLwt-matrix, or ASML-v4–7kd-matrix and cultured for 48 h in the presence of RPMI/5%FCS containing [3H]thymidine (10 μCi/ml). Mean + S.D. (triplicates) of [3H]thymidine incorporation are shown. B and C, ASMLwt and ASML-v4–7kd cells were seeded on BSA or ASMLwt-matrix- or ASML-v4–7kd-matrix-coated plates and were cultured for 48 h in the presence of titrated amounts of cisplatin. Mean ± S.D. of triplicates of (B) proliferative activity ([3H]thymidine incorporation) and (C) the relative mitochondrial respiratory activity (MTT assay) are shown. D, ASMLwt and ASML-v4–7kd cells were seeded on BSA- or ASMLwt-matrix-, ASML-v4–7kd-matrix-, HA-, LN5-, or HGF-coated plates; ASMLwt-cMetkd, ASML-v4–7kd-cMetkd, ASMLwt-β4kd, and ASML-v4–7kd-β4kd cells were seeded on BSA or ASMLwt-matrix- or ASML-v4–7kd-matrix-coated plates. Cells were cultured for 48 h in the presence of titrated amounts of cisplatin. Mean ± S.D. of triplicates of the percentage of dead cells (AnnexinV-APC/PI staining) is shown. Insets, c-Met, β4, and, as control, actin WB after c-Met siRNA and β4-siRNA treatment. Mean ± S.D. of triplicates of the percentage of dead cells (AnnexinV-APC/PI staining) are shown. E, amount of cisplatin that is required to kill 50% of the indicated cells on uncoated or matrix coated plates is shown. A–E, significant differences between uncoated and coated plates are indicated by *. Proliferative activity of ASML cells is independent of CD44v6 and not supported by the ASMLwt-matrix. Instead, apoptosis resistance of ASMLwt and ASML-v4–7kd cells essentially depends on c-Met expression and is supported by α6β4. The ASMLwt-matrix supports apoptosis resistance only in CD44v6-compentent cells.
These findings indicated that CD44v6 plays a central role in apoptosis protection and that particularly c-Met might support CD44v6-mediated apoptosis resistance. The latter assumption was controlled by a transient c-Met and β4 knockdown. C-Met siRNA strongly affected ASMLwt and ASML-v4–7kd viability even in the absence of cisplatin and the ASMLwt matrix did not exert a protective effect. Viability of ASML cells was not affected by the β4 knockdown. Nonetheless, the protective effect of the ASMLwt-matrix was weaker in ASML-β4kd cells and was not seen in ASML-v4–7kd-β4kd cells (Fig. 5E). The contribution of the tumor matrix and the CD44v6-c-Met-α6β4 complex to apoptosis resistance was confirmed in Capan2, 8.18, and Pt45P1 cells. In the presence of the Capan2-matrix, proliferation, mitochondrial respiratory activity, and apoptosis were not affected by 7.5 μg/ml cisplatin. The Capan2-matrix also protected 8.18 cells (CD44v6+, but c-Met−), but hardly Pt45P1 cells (CD44v6−, c-Met−, α6β4±). The Pt45P1-matrix was not protective, and the 8.18-matrix showed only minor protection on Capan2 and 8.18 cells (supplemental Fig. S4).
The impact of the matrix and of the CD44v6-c-Met-α6β4 complex on apoptosis resistance is summarized in Fig. 5F, which shows the dose of cisplatin that is required to kill 50% of the tumor cells. From there it becomes obvious that only the ASMLwt-, but not the ASML-v4–7kd-matrix exerts a protective effect, when cells express, at least, CD44v6 and c-Met. Furthermore, c-Met exerts the strongest and α6β4 the weakest effect on apoptosis resistance. Finally, the partial rescue of apoptosis resistance by the ASMLwt-matrix in ASMLwt-β4kd, but not in ASML-v4–7kd-β4kd cells is compatible with the interpretation that CD44v6 coordinates apoptosis-protecting signals delivered by c-Met and α6β4 (Fig. 5F).
CD44v6 Coordinates Activation of Anti-apoptotic Molecules
Activation of apoptosis-related signal transduction was evaluated in ASMLwt and ASML-v4–7kd cells after stimulation with HA, HGF, and LN5.
Caspase-8 is the downstream caspase of death receptors. Caspase-8 activity increased in cisplatin-treated ASMLwt as well as ASML-v4–7kd cells. This indicates that receptor-mediated apoptosis of ASML cells is not affected by the CD44v4–7kd and that receptor-mediated apoptosis does not proceed through CD44v6, c-Met, or α6β4.
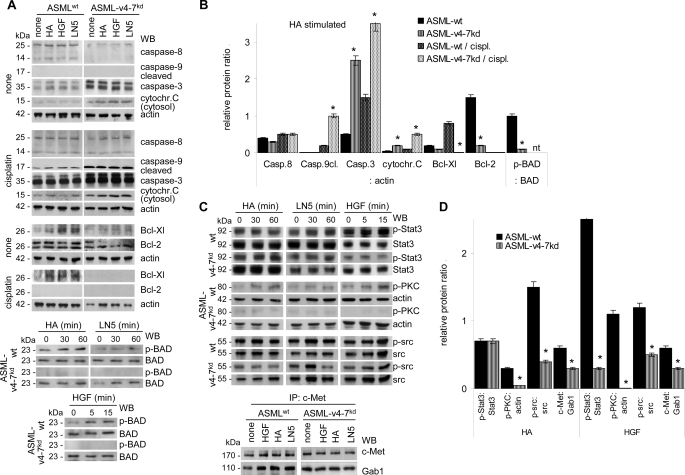
On the contrary, caspase-9 cleavage and, less pronounced, caspase-3 expression was stronger in cisplatin-treated ASML-v4–7kd than ASMLwt cells and higher amounts of cytochrome c were recovered in the cytosol. Thus, ASMLwt cells display more efficient protection toward the mitochondrial pathway of apoptosis than ASML-v4–7kd cells, even though a protective effect of HA, LN5, or HGF was not observed after the prolonged culture period in the presence of cisplatin.
Reduced cytochrome c release and reduced caspases 9 and 3 activation in cisplatin-treated ASMLwt cells could be due to up-regulation of anti-apoptotic proteins. Indeed, Bcl-Xl and weakly Bcl-2 expression was increased in HA-, HGF-, and LN5-treated ASMLwt, but not ASML-v4–7kd cells. Furthermore, Bcl-Xl expression remained up-regulated under cisplatin-treatment only in ASMLwt cells. Finally, in ASMLwt, but not in ASML-v4–7kd cells, HA, LN5, and HGF supported phosphorylation of pro-apoptotic BAD, which allows for the release/functional activity of Bcl-2 and Bcl-Xl (Fig. 6A). Calculating the relative increase/decrease of these pro-and anti-apoptotic proteins in HA-stimulated untreated or cisplatin-treated ASMLwt versus ASML-v4–7kd lysates revealed comparable activity of caspase-8 in both lines. This further supports that receptor-mediated apoptosis is CD44v6-independent. The relative increase of caspase-3, cleaved caspase-9 and cytosolic cytochrome c in cisplatin-treated ASML-v4–7kd compared with ASMLwt cells confirms the higher apoptosis susceptibility of ASML-v4–7kd cells to be due to the absence of CD44v6. This also accounts for the reduced recovery of the anti-apoptotic proteins Bcl-Xl and Bcl-2 as well as the reduced phosphorylation (inactivation) of BAD in HA-stimulated ASML-v4–7kd compared with ASMLwt cells (Fig. 6B).
FIGURE 6.
The ASMLwt-matrix, HA, HGF, and LN5 promote up-regulation of anti-apoptotic proteins. A–D, ASMLwt and ASML-v4–7kd cells were stimulated with HA, LN5, and HGF as described above. A, where indicated, cells were cultured for 48 h in the presence or absence of 30 μg/ml cisplatin. Cells were lysed and, where indicated, the cytosolic fraction was isolated. WB anti-caspase-8, -caspase-9 (cleaved), -caspase-3, -cytochrome c (cytosolic fraction), -Bcl-Xl, -Bcl-2, -actin, BAD, and p-BAD; B, mean ± S.D. (3 experiments) of the band intensity ratio compared with actin or phosphorylated to non-phosphorylated protein is shown. Significant differences are indicated by *. C, WB anti-p-Stat3, -Stat3, -p-PKC, -actin, -p-src, -src, and WB with anti-c-Met and anti-Gab1 after IP with anti-c-Met. D, mean ± S.D. (three experiments) of the band intensity ratio of non-phosphorylated to phosphorylated protein or to actin is shown. Significant differences are indicated by *. Stimulation with HA, HGF, and LN5 is accompanied by reduced caspase-9 and caspase-3 activation, stabilization of mitochondrial membrane integrity, promotion of BAD phosphorylation, Bcl-2, and Bcl-Xl expression, strengthening of Stat3, PKC, and src phosphorylation and the association of c-Met with Gab1 indicating that CD44v6, c-Met, and α6β4 jointly support apoptosis protection.
To further strengthen our hypothesis that in CD44v6-competent cells activation of signaling molecules via CD44 or c-Met or α6β4 predominantly proceeds via CD44v6, we stimulated ASMLwt and ASML-v4–7kd cells with HA, LN5, or HGF and evaluated activation of src, STAT3, and PKC, which are directly associated with c-Met. Stat3 phosphorylation was most pronounced after HGF-cross-linking, but only in ASMLwt cells. PKC and src phosphorylation were equally well promoted by HA, LN5, and HGF in ASMLwt, but not ASML-v4–7kd cells. Furthermore, HGF, HA, and LN5 promoted co-immunoprecipitation of c-Met with Gab1 more efficiently in ASMLwt than ASML-v4–7kd cells (Fig. 6C). Calculating the ratio of the phosphorylated to non-phosphorylated proteins in ASMLwt and ASML-v4–7kd cells confirmed that HGF and HA equally well supported PKC, src, and Gab1 phosphorylation, however, only in CD44v6-competent cells. STAT phosphorylation has been the only exception in as much as it was more strongly stimulated by HGF than HA. Nonetheless, the effect was not seen in CD44v-deficient cells (Fig. 6D). These findings imply that activation of anti-apoptotic proteins can be initiated via HGF or HA. But, activation of downstream signaling cascades is greatly facilitated by CD44v6.
Apoptosis Resistance Proceeds via the PI3K/Akt and MAPK Pathways
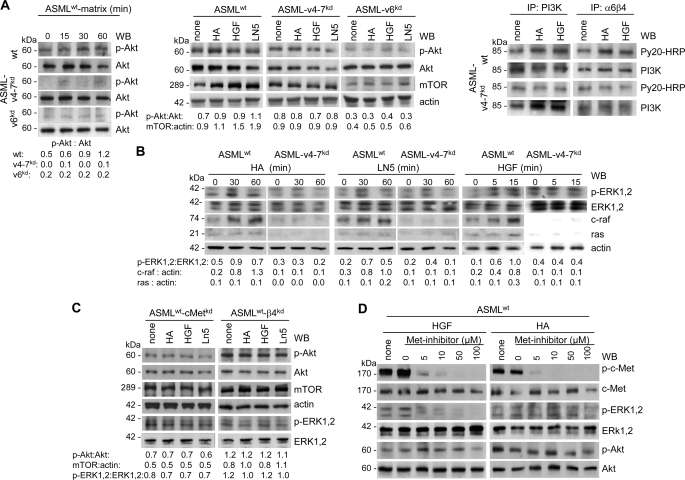
Apoptosis protection can be initiated by activation of the PI3K/Akt or the ras-raf-MAPK pathway. Akt phosphorylation and mTOR expression were up-regulated in ASMLwt-matrix, HA-, LN5-, and HGF-stimulated ASMLwt, but not ASML-v4–7kd or ASML-v6kd cells. Furthermore, a higher amount of phosphorylated PI3K was recovered in HA- or HGF-stimulated ASMLwt than ASML-v4–7kd cells and p-PI3K co-immunoprecipitated with α6β4 (Fig. 7A). Ligand binding of CD44v6, c-Met, and α6β4 also sufficed for ras, c-raf, and ERK1,2 activation. Activation was not observed in the absence of CD44v6 (Fig. 7B).
FIGURE 7.
The ASMLwt-matrix, HA, HGF, and LN5 initiate activation of the PI3K/Akt and the MAPK pathway. A and B, ASMLwt, ASML-v4–7kd, and ASML-v6kd cells and (C) ASMLwt-cMetkd and ASMLwt-β4kd cells were stimulated with the ASMLwt-matrix, HA, LN5, and HGF as described above. Cells were lysed, and lysates were separated by SDS-PAGE and after transfer blotted with the indicated antibodies or (A, right) lysates were immunoprecipitated with anti-PI3K or B5.5 and immunoprecipitates, after SDS-PAGE and transfer, were blotted with anti-p-tyrosine and anti-PI3K. A–C, ratio of non-phosphorylated to phosphorylated protein or to actin is included. D, ASMLwt cells were stimulated with HGF or HA in the presence of the Met inhibitor SU11274. After lysis, SDS-PAGE and transfer, blots were incubated with the indicated antibodies. The ASMLwt-matrix, HA, HGF, and LN5 support activation of both the PI3K/Akt and the ras/raf/MAPK pathway. Selective inhibition of the MAPK pathway suggests, at least, a partly independent activation of the PI3K and the MAPK pathway.
To confirm a joint contribution of CD44v6, c-Met, and α6β4, the experiment was repeated with c-Met- and β4-siRNA-treated ASML cells. HA-, HGF-, and LN5-initiated ERK1,2 and Akt phosphorylation and mTOR up-regulation were strongly reduced after c-Met silencing. The β4-knockdown exerted a similar, though less pronounced effect (Fig. 7C). Finally, when ASMLwt cells were stimulated with HGF in the presence of the Met-inhibitor SU11274, c-Met, and ERK1,2 phosphorylation of ASMLwt cells was strongly reduced at 5 μm SU11274. However, 10 μm SU11274 did not affect Akt phosphorylation and a partial reduction was only seen with 100 μm SU11274. A similar inhibition profile was observed upon HA stimulation: SU11274 strongly inhibited c-Met and ERK1,2, but only partially Akt phosphorylation (Fig. 7D).
We interpret these findings to indicate that (i) both PI3K and MAPK pathway activation contribute to ASMLwt-matrix, HA-, LN5-, and HGF-initiated apoptosis resistance and (ii) anti-apoptotic signal transduction converges downstream of CD44v6. However, (iii) the weak effect of c-Met inhibition on Akt phosphorylation pointed toward independent activation of the MAPK and PI3K/Akt pathway.
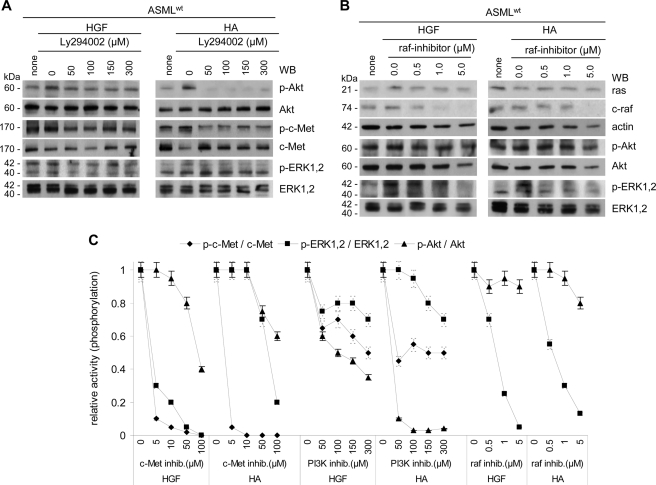
To control for independent PI3K and MAPK pathway activation, ASMLwt cells were stimulated by HGF and HA in the presence of the PI3K-inhibitor Ly294002 or the raf-inhibitor GW5074. Akt inhibition was more pronounced in HA- than HGF-stimulated cells. Nonetheless, already 50 μm Ly294002 inhibited Akt phosphorylation in HA-stimulated ASMLwt cells. On the contrary, c-Met and ERK1,2 phosphorylation was less severely impaired (Fig. 8A). The raf-inhibitor (1 μm) strongly affected c-raf expression and ERK1,2 phosphorylation. Akt phosphorylation remained unaltered (Fig. 8B).
FIGURE 8.
Preferential activation of the MAPK pathway by HGF and of the PI3K/Akt pathway by HA. A and B, ASMLwt cells were stimulated with HGF or HA in the presence of (A) the PI3K inhibitor Ly294002 or (B) the raf inhibitor GW5074. After lysis, SDS-PAGE and transfer, blots were incubated with the indicated antibodies. C, relative ratios of p-Akt:Akt, p-c-Met:c-Met, and p-ERK1,2:ERK1,2 (mean ± S.D. of three assays) depending on the stimulus and the inhibitor are shown. The ratio in the absence of an inhibitor has arbitrarily been taken as 1.0. Inhibition of c-Met and raf hardly affects Akt activation. Instead, activation of the MAPK pathway becomes only partially inhibited by a blockade of PI3K. These findings imply an independent activation of both pathways, despite the essential requirement of CD44v6 in activation of both.
Thus, PI3K/Akt and ras-raf-MAPK pathway activation contribute to apoptosis resistance. Though activation of both pathways involves CD44v6 due to its association with c-Met and α6β4, PI3K/Akt and MAPK pathway activation are not linked (Fig. 8C).
Taken together, ASMLwt cells secrete a matrix, whose composition is strongly influenced by CD44v6. The matrix, prone for storage of matrix-degrading enzymes and growth factors, facilitates migration via the association of ezrin and src/FAK with CD44, most pronounced CD44v6 and promotes apoptosis resistance via MAPK and PI3K/Akt pathway activation. Motility and apoptosis resistance can become initiated via CD44v6-HA, c-Met-HGF, and α6β4-LN5 binding. Nonetheless, increased motility and apoptosis resistance in response to HGF, HA, and LN5 are only seen in CD44v6-competent cells (Fig. 9). Thus, activation of motility-promoting and apoptosis-protecting signals via c-Met and α6β4 ligand binding are strongly supported by CD44v6.
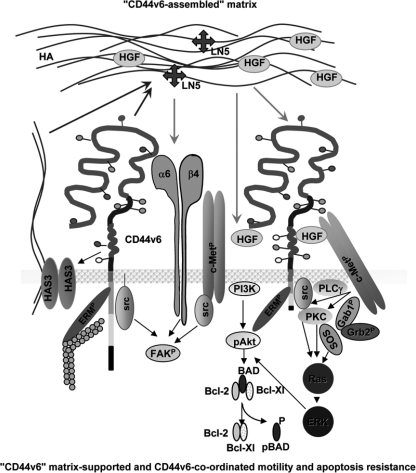
FIGURE 9.
Coordinating activity of CD44v6 in tumor matrix assembly and matrix assisted tumor cell motility and apoptosis resistance. CD44v6 promotes by not yet defined mechanisms up-regulation of HAS3 with more abundant secretion of HMW HA. Besides others, this matrix is enriched in LN5 and HGF. By receptor binding HA, LN5, and HGF stimulate motility and apoptosis resistance. Both activities, motility and apoptosis resistance become significantly strengthened in cell expressing CD44v6. This is due to CD44v6 supporting c-Met activation by presenting HGF (27, 28) as well as by CD44v6 coordinating signals downstream of α6β4 and c-Met as outlined in the scheme.
DISCUSSION
Metastasizing tumor cells rely on a crosstalk with the environment, which at least in part, is supported by the tumor matrix (49). Furthermore, we recently reported that assembly of a metastasis-promoting matrix (44) was strikingly CD44v6-dependent and provided evidence for a feedback loop, such that the matrix strengthened tumor cell adhesiveness and drug resistance (43). We here explored the molecular mechanisms underlying this feedback cross-talk, which unexpectedly revealed that CD44v6 ligation/activation besides being an initial trigger, most efficiently coordinates signals initiated by c-Met and α6β4 ligand binding.
CD44v6 and Matrix Assembly
In line with published evidence, HA-bound CD44 can contribute to c-Met, uPAR, and HAS3 transcription (50–52), expression of the three molecules being strongly reduced in ASML-v4–7kd cells and the matrix (44). The increased level of HAS3, known to favor the malignant phenotype (53), in ASMLwt cells corresponds to the higher amount and higher MW of HA in the ASMLwt- than the ASML-v4–7kd-matrix. However, we do not yet know at what level CD44v6 expression regulates HAS3. Recovery of lower MW HA in the ASML-v4–7kd-matrix is in line with the higher recovery of HAase, where we do not yet know the linkage between CD44v deficiency and HAase transcription or stabilization. However, it is known that HA functions vary considerably with length (54). Thus, pronounced HA degradation in the ASML-v4–7kd-matrix might well contribute to its inefficacy compared with the ASMLwt-matrix. Nonetheless, it is not merely the reduction in high MW HA, which hampers functional activity of the ASML-v4–7kd-matrix, as addition of HA to the ASML-v4–7kd-matrix provides only a weak migratory stimulus for ASMLwt and none for ASML-v4–7kd cells. Taking this into account and the notion that HAase can also act as a tumor promoter (55), it becomes unlikely that only the higher level of HAase in the ASML-v4–7kd matrix suffices to explain its inhibitory effect.
In fact, additional molecule recovery is strongly reduced in the ASML-v4–7kd-matrix, although expression is largely unaltered in ASML-v4–7kd cells. These include α6β4, the proteases MMP2, MMP9, uPA, CD13, and 2 hrta serine peptidases, chaperons involved in removing improperly folded molecules (56). Reduced protease recovery in the ASML-v4–7kd-matrix could well influence matrix assembly. Hepatoma-derived growth factor (HDGF), too, is more abundantly recovered in the ASMLwt- than the ASML-v4–7kd-matrix. High level HDGF expression protects from apoptosis and is associated with poor survival of cancer patients (57). Vimentin, enolase-1, clusterin, ECM protein-1, and DJ-1 are also strongly enriched in the ASMLwt-matrix. Soluble clusterin, a prosurvival chaperon-like molecule, influences chemokine secretion and supports intercellular communication (58). ECM-1, which interacts with perlecan, fibulin-1C/D and MMP-9, interferes with angiogenesis (59). High level ECM-1 in the ASMLwt-matrix might explain the poor vascularization of ASML tumors (42). Potential activities of DJ-1, a negative regulator of PTEN (60) within the matrix are unknown. Loss of the anaphylatoxin C3a and the CD11b ligand C3b likely affect host cell recruitment (61).
Taken together, these multiple effects of a CD44vkd on protein delivery were unexpected and require further exploration. This implies that, at the present state of knowledge, we cannot speculate on a possible negative impact of the ASML-v4–7kd matrix on either the tumor cell or the surrounding. However, the higher recovery of e.g. HA, HGF, MMPs, uPA, LN5, and others in the ASMLwt-matrix could well contribute to the crosstalk between the matrix and the tumor cells and allowed us to proceed toward unraveling the particular contribution of CD44v6.
The ASMLwt-matrix Supports Migration via CD44v6
A subfraction of the ASMLwt-matrix promotes β1-integrin-dependent, but CD44v6-independent tumor cell adhesion such that ASML-CD44v4–7kd cells readily adhere to the ASMLwt-matrix (43). Instead, tumor cell migration toward the ASMLwt-matrix strikingly depends on CD44v6, as neither AS nor ASML-v4–7kd nor ASML-v6kd cells receive a migratory stimulus from the ASMLwt-matrix. Nonetheless, ASMLwt cell migration is equally well inhibited by anti-CD44v6, anti-α6β4 and anti-c-Met. Conversely, HA, LN5, and HGF support ASMLwt, but not ASML-v4–7kd cell migration. This has been a first hint that ligand interaction of CD44v, c-Met, and α6β4 contributes to migration, but transition to the migratory phenotype is strongly promoted by CD44v6, where signaling converges due to the association of the three molecules (20, 28). Indeed, signal transduction can proceed, independent of the initiating stimulus, downstream of CD44v6 toward ERM binding and phosphorylation (28). In addition, CD44-associated src becomes phosphorylated, which is accompanied by FAK recruitment toward CD44v6 and FAK phosphorylation. Phosphorylation of the β4 chain can contribute by binding of the PTB domain of shc, which is required for shc phosphorylation and recruitment of Grb/SOS (62). C-Met also binds shc and src, which leads to FAK activation and by Gab1 binding many signaling cascades, including the MAPK pathway, become activated (63).
Taken together, though not excluding a direct contribution by c-Met and α6β4 to matrix-initiated migration, our data convincingly demonstrate an essential contribution of CD44v6, as only CD44v6-competent, but not CD44v6-deficient human and rat pancreatic adenocarcinoma cells respond to HA, LN5, HGF and, importantly, the tumor cell natural surrounding, as far as the matrix is delivered by a CD44v6-competent cell. The contribution of CD44v6 relies on its association with c-Met and α6β4 and, beyond this, the coordination of signal transduction initiated through this complex.
Matrix-supported Drug Resistance and CD44v6-dependent MAPK and PI3K/Akt Pathway Activation
The ASMLwt-matrix protects only CD44v6-expressing cells from cisplatin-induced apoptosis. However, protection is abolished in ASMLwt-cMetkd and weakened in ASMLwt-β4kd cells. Receptor-mediated apoptosis is not involved, as caspase-8 activation does not differ in ASMLwt versus ASML-v4–7kd cells. Instead, caspase-9 activation and release of cytochrome c are stronger in cisplatin-treated ASML-v4–7kd than ASMLwt cells. In line with these findings, BAD phosphorylation and Bcl-Xl expression become strengthened by CD44v6, c-Met, and α6β4 activation in ASMLwt cells. Only in ASMLwt cells, phosphorylation of directly c-Met-associated Stat3 and src (64), which both also associate with CD44 (11), was particularly pronounced after c-Met triggering. PKC phosphorylation and the c-Met-Gab1 association was stimulated by HA, LN5, and HGF in ASMLwt cells. Similar to matrix-assisted migration, drug-resistance depended on CD44v6-coordinated signal transduction as demonstrated for the PI3K/Akt and MAPK pathways.
Though CD44v6 contributed to both PI3K/Akt and MAPK activation, Akt phosphorylation was only slightly impaired, when cells were stimulated with HA or HGF in the presence of a c-Met or a raf inhibitor. On the other hand, ERK1,2 phosphorylation became only partly blocked by a PI3K-inhibitor, which efficiently interfered with Akt phosphorylation. Thus, the MAPK and PI3K/Akt pathways contribute independently to apoptosis resistance initiated by CD44v6 or c-Met ligand binding. Nonetheless, in line with the CD44v6-c-Met-α6β4 association (20, 27, 28, 65), pronounced activation of both pathways essentially requires CD44v6 and c-Met, deletion of either molecule being accompanied by impaired apoptosis resistance. The point of convergence of signals initiated via c-Met, α6β4, and CD44v6 remains to be defined. We expect and are currently exploring, whether joint signal transduction depends on complex assembly in tetraspanin-enriched membrane microdomains, known as docking sites for kinases and adaptor proteins (66). Our hypothesis is supported by the findings that tetraspanins associate with c-Met, CD44v, and α6β4 (67–69).
In the latter context, it also should be mentioned that induction of a migratory phenotype can be accompanied by increased apoptosis resistance (reviewed in Ref. 70). This notion is well in line with activation-induced recruitment of c-Met, CD44v, and α6β4 into tetraspanin-enriched membrane microdomains as well as the convergence of signal transduction downstream of CD44v6. Nonetheless, α6β4-LN5 binding more efficiently promoted migration than drug resistance. Furthermore, activation of anti-apoptotic proteins and inactivation of pro-apoptotic proteins independently proceeded through the MAPK and the PI3K/Akt pathway, though activation of both pathways is coordinated by CD44v6. Therefore, we consider it likely that transition toward a migratory phenotype and apoptosis resistance can, but must not essentially be linked, which may depend on cell furnishings with signal transduction molecules.
CD44v6 can essentially contribute to tumor progression. This, at least in part, is due to CD44v6 taking an essential role in assembling a tumor matrix that, beside others, is enriched in several proteases, high MW HA, HGF, and LN5. This matrix supports motility and apoptosis resistance, which is accompanied by ERM protein, src, FAK, c-Met, MAPK, and PI3K/Akt pathway activation via CD44v6 and CD44v6-associated c-Met and α6β4. These findings not only confirm the contribution of CD44v6 to c-Met and α6β4 activation, when stimulated with the separate ligands, but extends these findings to the natural surrounding of CD44v6-competent tumor cells. Beyond this, CD44v6 coordinates signals initiated via FAK, MAPK, and PI3K/Akt independent of whether or not CD44v6 has been directly engaged in the initial trigger. Thus, a soluble tumor matrix, whose assembly depends on CD44v6 forces the cross-talk with host tissue allowing for premetastatic niche preparation and triggers via CD44v6 metastasizing tumor cell migration and apoptosis resistance.
Supplementary Material
Acknowledgments
We thank Dr. C. Claas, Bioquant, University Heidelberg, for help with video microscopy, Dr. T. Kempf, Proteomic-Core-Facility, German Cancer Research Center, Heidelberg, for MALDI-TOF/PSDF analysis and Dr. T. S. Johnson for language corrections.
This work was supported by the DFG/SPP1190 and NCT (to M. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Figures S1–S4.
- CD44v
- CD44 variant isoforms
- AS
- BSp73AS
- ASML
- BSp73ASML
- ECM
- extracellular matrix
- ERM
- ezrin, radixin, moesin
- GEM
- glycolipid-enriched membrane microdomain
- HA
- hyaluronan
- HAase
- hyaluronidase
- HAS3
- hyaluronan synthase-3
- HGF
- hepatocyte growth factor
- kd
- knockdown
- LN5
- laminin 5
- RTK
- receptor tyrosine kinase
- WB
- Western blot.
REFERENCES
- 1. Kopfstein L., Christofori G. (2006) Cell Mot. Life Sci. 63, 449–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nie D. (2010) Frontier Biosciences 2, 184–193 [Google Scholar]
- 3. Stern R. (2008) Semin. Cancer Biol. 18, 238–243 [DOI] [PubMed] [Google Scholar]
- 4. Tsuji T., Ibaragi S., Hu G. F. (2009) Cancer Res. 69, 7135–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naor D., Wallach-Dayan S. B., Zahalka M. A., Sionov R. V. (2008) Semin. Cancer Biol. 18, 260–267 [DOI] [PubMed] [Google Scholar]
- 6. Marhaba R., Zöller M. (2004) J. Mol. Histol. 35, 211–231 [DOI] [PubMed] [Google Scholar]
- 7. Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. (1991) Cell 65, 13–24 [DOI] [PubMed] [Google Scholar]
- 8. Ponta H., Sherman L., Herrlich P. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 33–45 [DOI] [PubMed] [Google Scholar]
- 9. Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. (1990) Cell 61, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 10. Toole B. P. (2004) Nat Rev Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 11. Bourguignon L. Y., Zhu H., Shao L., Chen Y. W. (2001) J. Biol. Chem. 276, 7327–7336 [DOI] [PubMed] [Google Scholar]
- 12. Tsukita S., Yonemura S., Tsukita S. (1997) Trends Biochem. Sci 22, 53–58 [DOI] [PubMed] [Google Scholar]
- 13. Lamontagne C. A., Grandbois M. (2008) Exp. Cell Res. 314, 227–236 [DOI] [PubMed] [Google Scholar]
- 14. Yu Q., Stamenkovic I. (2004) Clin Exp Metastasis 21, 235–242 [DOI] [PubMed] [Google Scholar]
- 15. Lesley J., English N. M., Gál I., Mikecz K., Day A. J., Hyman R. (2002) J. Biol. Chem. 277, 26600–26608 [DOI] [PubMed] [Google Scholar]
- 16. Mohamadzadeh M., DeGrendele H., Arizpe H., Estess P., Siegelman M. (1998) J. Clin. Invest. 101, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corso S., Comoglio P. M., Giordano S. (2005) Trends Mol Med 11, 284–292 [DOI] [PubMed] [Google Scholar]
- 18. Fjeldstad K., Kolset S. O. (2005) Curr. Drug Targets 6, 665–682 [DOI] [PubMed] [Google Scholar]
- 19. Taylor K. R., Gallo R. L. (2006) FASEB J. 20, 9–22 [DOI] [PubMed] [Google Scholar]
- 20. Bertotti A., Comoglio P. M. (2003) Trends Biochem. Sci 28, 527–533 [DOI] [PubMed] [Google Scholar]
- 21. Boccaccio C., Andò M., Tamagnone L., Bardelli A., Michieli P., Battistini C., Comoglio P. M. (1998) Nature 391, 285–288 [DOI] [PubMed] [Google Scholar]
- 22. Bourguignon L. Y., Gilad E., Peyrollier K. (2007) J. Biol. Chem. 282, 19426–19441 [DOI] [PubMed] [Google Scholar]
- 23. Sherman L., Wainwright D., Ponta H., Herrlich P. (1998) Genes Dev. 12, 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghatak S., Misra S., Toole B. P. (2005) J. Biol. Chem. 280, 8875–8883 [DOI] [PubMed] [Google Scholar]
- 25. Bourguignon L. Y. (2001) J. Mammary Gland Biol. Neoplasia 6, 287–297 [DOI] [PubMed] [Google Scholar]
- 26. Lynch C. C., Vargo-Gogola T., Martin M. D., Fingleton B., Crawford H. C., Matrisian L. M. (2007) Cancer Res. 67, 6760–6767 [DOI] [PubMed] [Google Scholar]
- 27. Orian-Rousseau V., Chen L., Sleeman J. P., Herrlich P., Ponta H. (2002) Genes Dev. 16, 3074–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orian-Rousseau V., Morrison H., Matzke A., Kastilan T., Pace G., Herrlich P., Ponta H. (2007) Mol. Biol. Cell 18, 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Föger N., Marhaba R., Zöller M. (2000) Eur. J. Immunol. 30, 2888–2899 [DOI] [PubMed] [Google Scholar]
- 30. Ingley E. (2008) Biochim. Biophys. Acta 1784, 56–65 [DOI] [PubMed] [Google Scholar]
- 31. Girish K. S., Kemparaju K. (2007) Life Sci. 80, 1921–1943 [DOI] [PubMed] [Google Scholar]
- 32. Turley E. A., Noble P. W., Bourguignon L. Y. (2002) J. Biol. Chem. 277, 4589–4592 [DOI] [PubMed] [Google Scholar]
- 33. Knudson C. B., Knudson W. (2004) Clin. Orthop. Relat. Res. 427, (suppl.), S152–S162 [PubMed] [Google Scholar]
- 34. Hill A., McFarlane S., Johnston P. G., Waugh D. J. (2006) Cancer Lett. 237, 1–9 [DOI] [PubMed] [Google Scholar]
- 35. Kuhn N. Z., Tuan R. S. (2010) J. Cell. Physiol. 222, 268–277 [DOI] [PubMed] [Google Scholar]
- 36. Wang N., Tytell J. D., Ingber D. E. (2009) Nat. Rev. Mol. Cell Biol. 10, 75–82 [DOI] [PubMed] [Google Scholar]
- 37. Couchman J. R. (2010) Annu. Rev. Cell Dev. Biol. 26, 89–114 [DOI] [PubMed] [Google Scholar]
- 38. Bennett K. L., Jackson D. G., Simon J. C., Tanczos E., Peach R., Modrell B., Stamenkovic I., Plowman G., Aruffo A. (1995) J. Cell Biol. 128, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wai P. Y., Kuo P. C. (2008) Cancer Metastasis Rev. 27, 103–118 [DOI] [PubMed] [Google Scholar]
- 40. Berdiaki A., Nikitovic D., Tsatsakis A., Katonis P., Karamanos N. K., Tzanakakis G. N. (2009) Biochim. Biophys. Acta 1790, 1258–1265 [DOI] [PubMed] [Google Scholar]
- 41. van der Voort R., Taher T. E., Wielenga V. J., Spaargaren M., Prevo R., Smit L., David G., Hartmann G., Gherardi E., Pals S. T. (1999) J. Biol. Chem. 274, 6499–6506 [DOI] [PubMed] [Google Scholar]
- 42. Matzku S., Komitowski D., Mildenberger M., Zöller M. (1983) Invasion Metastasis 3, 109–123 [PubMed] [Google Scholar]
- 43. Klingbeil P., Marhaba R., Jung T., Kirmse R., Ludwig T., Zöller M. (2009) Mol. Cancer Res. 7, 168–179 [DOI] [PubMed] [Google Scholar]
- 44. Jung T., Castellana D., Klingbeil P., Cuesta Hernández I., Vitacolonna M., Orlicky D. J., Roffler S. R., Brodt P., Zöller M. (2009) Neoplasia 11, 1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudy W., Hofmann M., Schwartz-Albiez R., Zöller M., Heider K. H., Ponta H., Herrlich P. (1993) Cancer Res. 53, 1262–1268 [PubMed] [Google Scholar]
- 46. Gobom J., Schuerenberg M., Mueller M., Theiss D., Lehrach H., Nordhoff E. (2001) Anal. Chem. 73, 434–438 [DOI] [PubMed] [Google Scholar]
- 47. Stern M., Stern R. (1992) Matrix 12, 397–403 [DOI] [PubMed] [Google Scholar]
- 48. Trusolino L., Bertotti A., Comoglio P. M. (2001) Cell 107, 643–654 [DOI] [PubMed] [Google Scholar]
- 49. De Wever O., Mareel M. (2003) J. Pathol. 200, 429–447 [DOI] [PubMed] [Google Scholar]
- 50. Kobayashi H., Suzuki M., Kanayama N., Nishida T., Takigawa M., Terao T. (2002) Int. J. Cancer 102, 379–389 [DOI] [PubMed] [Google Scholar]
- 51. Comoglio P. M., Giordano S., Trusolino L. (2008) Nat. Rev. Drug Discov. 7, 504–516 [DOI] [PubMed] [Google Scholar]
- 52. Lee K. H., Choi E. Y., Hyun M. S., Jang B. I., Kim T. N., Lee H. J., Eun J. Y., Kim H. G., Yoon S. S., Lee D. S., Kim J. H., Kim J. R. (2008) Clin. Exp. Metastasis 25, 89–96 [DOI] [PubMed] [Google Scholar]
- 53. Adamia S., Maxwell C. A., Pilarski L. M. (2005) Curr. Drug Targets Cardiovasc. Haematol. Disord. 5, 3–14 [DOI] [PubMed] [Google Scholar]
- 54. Stern R., Asari A. A., Sugahara K. N. (2006) Eur J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 55. Stern R., Jedrzejas M. J. (2006) Chem. Rev. 106, 818–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clausen T., Southan C., Ehrmann M. (2002) Mol. Cell 10, 443–455 [DOI] [PubMed] [Google Scholar]
- 57. Tsang T. Y., Tang W. Y., Tsang W. P., Co N. N., Kong S. K., Kwok T. T. (2008) Apoptosis 13, 1135–1147 [DOI] [PubMed] [Google Scholar]
- 58. Pucci S., Mazzarelli P., Nucci C., Ricci F., Spagnoli L. G. (2009) Adv. Cancer Res. 105, 93–113 [DOI] [PubMed] [Google Scholar]
- 59. Sercu S., Zhang L., Merregaert J. (2008) Cancer Invest. 26, 375–384 [DOI] [PubMed] [Google Scholar]
- 60. da Costa C. A. (2007) Curr. Mol. Med. 7, 650–657 [DOI] [PubMed] [Google Scholar]
- 61. Erler J. T., Bennewith K. L., Cox T. R., Lang G., Bird D., Koong A., Le Q. T., Giaccia A. J. (2009) Cancer Cell 15, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dans M., Gagnoux-Palacios L., Blaikie P., Klein S., Mariotti A., Giancotti F. G. (2001) J. Biol. Chem. 276, 1494–1502 [DOI] [PubMed] [Google Scholar]
- 63. Benvenuti S., Comoglio P. M. (2007) J. Cell. Physiol. 213, 316–325 [DOI] [PubMed] [Google Scholar]
- 64. Boccaccio C., Comoglio P. M. (2006) Nat Rev Cancer 6, 637–645 [DOI] [PubMed] [Google Scholar]
- 65. Klosek S. K., Nakashiro K., Hara S., Goda H., Hasegawa H., Hamakawa H. (2009) Biochem. Biophys. Res. Commun. 379, 1097–1100 [DOI] [PubMed] [Google Scholar]
- 66. Lingwood D., Simons K. (2010) Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 67. Hemler M. E. (2005) Nat. Rev. Mol. Cell Biol. 610, 801–811 [DOI] [PubMed] [Google Scholar]
- 68. Yáñez-Mó M., Barreiro O., Gordon-Alonso M., Sala-Valdés M., Sánchez-Madrid F. (2009) Trends Cell Biol. 19, 434–446 [DOI] [PubMed] [Google Scholar]
- 69. Zöller M. (2009) Nat. Rev. Cancer 9, 40–55 [DOI] [PubMed] [Google Scholar]
- 70. Lefranc F., Brotchi J., Kiss R. (2005) J. Clin. Oncol. 23, 2411–2422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.