Abstract
Matrix metalloproteinase-9 (MMP-9) plays a critical role in tissue remodeling under both physiological and pathological conditions. Although MMP-9 expression is low in most cells and is tightly controlled, the mechanism of its regulation is poorly understood. We utilized mouse embryonic fibroblasts (MEFs) that were nullizygous for the catalytic α subunit of AMP-activated protein kinase (AMPK), which is a key regulator of energy homeostasis, to identify AMPK as a suppressor of MMP-9 expression. Total AMPKα deletion significantly elevated MMP-9 expression compared with wild-type (WT) MEFs, whereas single knock-out of the isoforms AMPKα1 and AMPKα2 caused minimal change in the level of MMP-9 expression. The suppressive role of AMPK on MMP-9 expression was mediated through both its activity and presence. The AMPK activators 5-amino-4-imidazole carboxamide riboside and A769662 suppressed MMP-9 expression in WT MEFs, and AMPK inhibition by the overexpression of dominant negative (DN) AMPKα elevated MMP-9 expression. However, in AMPKα−/− MEFs transduced with DN AMPKα, MMP-9 expression was suppressed. AMPKα−/− MEFs showed increased phosphorylation of IκBα, expression of IκBα mRNA, nuclear localization of nuclear factor-κB (NF-κB), and DNA-binding activity of NF-κB compared with WT. Consistently, selective NF-κB inhibitors BMS345541 and SM7368 decreased MMP-9 expression in AMPKα−/− MEFs. Overall, our results suggest that both AMPKα isoforms suppress MMP-9 expression and that both the activity and presence of AMPKα contribute to its function as a regulator of MMP-9 expression by inhibiting the NF-κB pathway.
Keywords: AMP Kinase, AMP-activated Kinase (AMPK), Fibroblast, Matrix Metalloproteinase, Protein Synthesis
Introduction
Matrix metalloproteinase-9 (MMP-9,2 gelatinase B) degrades denatured collagens and native collagen type IV, which is a major component of the extracellular matrix (ECM) and basement membranes (1). Under normal circumstances, the degradation of the ECM by MMP-9 is a tightly controlled process involved in physiological wound healing and embryo development (1, 2). Conversely, aberrant degradation of ECM by excess MMP-9 expression results in the pathologic destruction of connective tissue seen in cancer, arterial sclerosis, and rheumatoid arthritis (1, 3). Therefore, under physiological conditions, regulated MMP-9 expression is low (1), but the mechanisms behind this are obscure.
AMP-activated protein kinase (AMPK) is a serine/threonine kinase, which regulates energy homeostasis and metabolic stress (4). AMPK acts as a sensor of cellular energy status and maintains the balance between ATP production and consumption. In mammals, AMPK exists as a heterotrimer with α, β, and γ subunits, each of which is encoded by two or three genes (α1, α2, β1, β2, γ1, γ2, and γ3). The α subunit possesses catalytic activity, whereas the β and γ subunits are regulatory and maintain the stability of the heterotrimer complex. The importance of AMPKα is illustrated by the fact that dual deficiency of AMPKα1 and AMPKα2 is embryonic lethal (5).
Recent evidence suggests that AMPK has a much wider range of functions, including the regulation of cell growth, cell proliferation, cell polarity, and autophagy (6, 7). Because these functions are closely linked to the pathology of MMP-9-related diseases, including cancer, arterial sclerosis, and rheumatoid arthritis, we hypothesized that AMPK regulates MMP-9 expression. To address this, in the present study, we utilized AMPKα-deficient mouse embryonic fibroblasts (MEFs) to investigate the effect of the genetic deletion and activation of AMPK on MMP-9 expression.
EXPERIMENTAL PROCEDURES
Antibodies, Recombinant Proteins, and Reagents
All antibodies, except for MMP-9 (Abcam, Cambridge, MA) and AMPKα2 (Santa Cruz Biotechnology, Santa Cruz, CA), were purchased from Cell Signaling (Beverly, MA). Recombinant mouse TNF-α, MMP-9, and MMP-2 proteins were obtained from R&D Systems (Minneapolis, MN). Pharmacological activators of AMPK, 5-amino-4-imidazole carboxamide riboside (AICAR), and A769662 were purchased from Sigma-Aldrich and Tocris Bioscience (Ellisville, MO), respectively. The inhibitors for IKK (BMS345541), NF-κB (SM7368), and JNK (SP600125) were purchased from Calbiochem.
Cell Culture
The origins of primary and SV40-immortalized WT, AMPKα1 subunit single knock-out (AMPKα1−/−), ΑΜPKα2 subunit single knock-out (AMPKα2−/−), and double knock-out of AMPKα1 and AMPKα2 subunit (AMPKα−/−) MEFs have been described previously (8). MEFs were cultured in DMEM-high glucose (D6429; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). For all experiments, cells were grown at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Experiments were performed on cells below passage 10 grown to 80–90% confluence.
Protein Extraction and Subcellular Fractionation
Cells were rinsed in ice-cold Tris-buffered saline and then scraped and lysed with lysis buffer (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 5 mm CaCl2, 1% Triton X-100, 0.02% NaN3, and 0.05% Brij35) supplemented with phosphatase inhibitors (10 mm NaF, 10 mm β-glycerophosphate, and 2 mm Na3VO4) and a protease inhibitor mixture (Sigma-Aldrich). Cell suspensions were incubated on ice for 10 min and centrifuged at 17,000 × g for 10 min at 4 °C. Supernatants were collected as whole cell lysates. Subcellular fractionation was performed as described previously (7, 9).
Gelatin Zymography
Conditioned media from cultured cells were collected and subjected to gelatin zymography. After cells reached 90% confluence, they were rinsed twice, and the medium was replaced with serum-free medium with or without TNF-α (1–100 ng/ml). After 24-h incubation, the conditioned media were collected and concentrated 3-fold using an Ultrafree-MC centrifugal filter device (Millipore) with a 30,000-molecular mass cutoff. The amount of concentrated media was normalized to the amount of protein in the cell lysate, then loaded on a Zymogram 10% gel (Invitrogen). Recombinant mouse MMP-2 and MMP-9 were used as positive controls. After renaturing and developing the gels according to the manufacturer's instructions, gels were stained with Coomassie Brilliant Blue R-250 solution (Bio-Rad). The intensities of bands were quantified using ImageJ software.
Western Blotting
Western blotting was carried out according to standard protocols. Densitometric analysis of bands was performed using ImageJ software.
ELISA
Analysis of accumulated MMP-9 in cell culture medium was performed using a quantitative ELISA kit (R&D Systems). After cells reached 90% confluence, they were rinsed twice, and fresh DMEM with or without reagent was added. The media were collected 12 or 24 h later, and assays were conducted according to the manufacturer's instructions. Obtained values were normalized to cell lysate protein levels.
DNA-binding Activity
The DNA-binding activity of NF-κB p50, p52, p65, and RelB was determined by the Trans AMTM NF-κB family assay kit (Active Motif, Carlsbad, CA). Nuclear extracts were prepared as described above, and 15-μg nuclear extracts were used for the detection of DNA binding following the manufacturer's protocol.
Real-time Quantitative RT-PCR (qRT-PCR)
Total RNA was harvested from cells using the RNeasy kit (Qiagen), and complementary DNA (cDNA) was generated with the First Strand cDNA synthesis kit (GE Healthcare) according to the manufacturer's instructions. Real-time PCR was carried out using the following mouse TaqMan gene expression assays (Applied Biosystems): AMPKα1 (Mm01296695_m1), AMPKα2 (Mm01264788_m1), MMP-9 (Mm00442991_m1), IκBα (Mm00477798_m1), and β-actin (Mm00607939_s1). All reactions were prepared following the manufacturer's protocol and carried out using the StepOneTM Real-time PCR System (Applied Biosystems).
Adenovirus Vector Transduction
The adenovirus vector for the dominant negative (DN) form of AMPKα2 (Ad-DN) with an inactivating mutation in the kinase domain (K45R substitution) has been described previously (10). The Ad-DN contained GFP as a marker, and the adenovirus vector 5 with GFP (Ad-GFP) (Vector BioLabs, Philadelphia, PA) was used as a control. MEFs were transduced with the adenovirus vectors at a multiplicity of infection of 300 for 48 h. The medium was then changed, and cell extracts and medium were harvested after 12 h. Under these conditions, the infection efficiency was >90%.
Statistical Analysis
All experiments were repeated a minimum of three times. All data were expressed as means ± S.E. Statistical differences between two groups were analyzed by the unpaired Student's t test. Multiple group comparison was performed by one-way analysis of variance with Scheffe's test. Differences were considered significant at p < 0.05.
RESULTS
Deletion of Both AMPKα1 and AMPKα2 Isoforms Results in Constitutive Expression of MMP-9 from MEFs
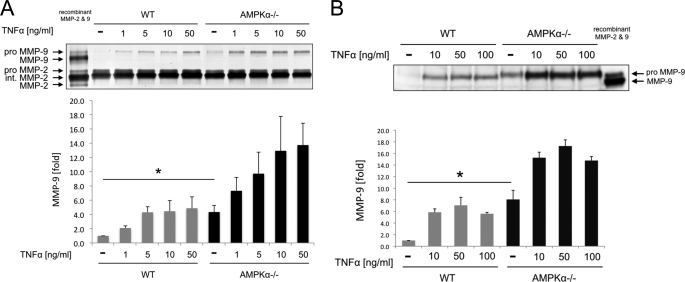
To study the role of AMPK in fibroblast expression of MMP-9, we utilized SV40-immortalized WT MEFs and MEFs that were nullizygous for both AMPKα1 and AMPKα2 subunits (AMPKα−/− MEFs). We examined the gelatinolytic activity of culture medium using gelatin zymography. As shown in Fig. 1A, the base-line gelatinolytic activity of pro-MMP-9 (under nonstimulatory conditions) of WT MEFs was barely detectable. By contrast, AMPKα−/− MEFs showed MMP-9 gelatinolytic activity that was 4.3 ± 0.9 times higher than that of WT MEFs (p < 0.05). There was no significant difference in the gelatinolytic activity of MMP-2 between the two cell types. To determine the magnitude of AMPK deletion in fibroblast MMP-9 expression, we next treated both MEFs with the major inducible factor of MMP-9, TNF-α (1). TNF-α increased the MMP-9 gelatinolytic activity of both WT and AMPKα−/− MEFs in a dose-dependent manner (Fig. 1A). Notably, the MMP-9 gelatinolytic activity of unstimulated AMPKα−/− ΜΕFs was equal to the maximum gelatinolytic activity of WT MEFs with TNF-α treatment. Furthermore, the MMP-9 gelatinolytic activity of AMPKα−/− ΜΕFs with each dose of TNF-α was two to three times higher than that of WT MEFs treated in the same way. The results of gelatin zymography were confirmed by Western blotting (Fig. 1B). Collectively, these results demonstrate that deletion of both AMPKα1 and AMPKα2 subunits leads to constitutive expression of MMP-9 and augmentation of the effect of TNF-α on MMP-9 expression.
FIGURE 1.
AMPK deletion up-regulates expression of MMP-9 but not MMP-2. A, the amounts of MMP-2 and MMP-9 in cell culture medium of WT and AMPKα−/− MEFs were examined by gelatin zymography. MEFs were stimulated with 1–50 ng/ml TNF-α for 24 h. Recombinant mouse MMP-2 and MMP-9 were used as molecular markers. B, the amounts of MMP-9 in cell culture medium of WT and AMPKα−/− MEFs were examined by Western blotting. MEFs were stimulated with 10–100 ng/ml TNF-α for 24 h. Recombinant mouse MMP-9 was used as a molecular marker. A and B, representative blots are shown. Error bars, S.E. *, p < 0.05.
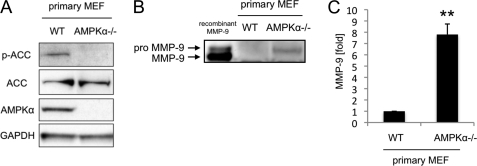
SV40 immortalization is known to inactivate the function of the cancer suppressor genes Rb and p53 (11, 12). To exclude the effects of SV40 immortalization on MMP-9 expression, we cultured primary MEFs of WT and AMPKα−/− and analyzed the level of MMP-9 protein in their culture media by Western blotting and ELISA. Fig. 2A shows a representative result of confirmatory Western blotting for the deletion of AMPKα in AMPKα−/− MEFs. The ability of antibody to detect phosphorylated acetylcoenzyme A carboxylase (ACC), which is a downstream target of AMPK, was taken as an indicator of AMPK activation. In accordance with the results for SV40-immortalized MEFs, both Western blotting and the ELISA showed that MMP-9 expression from primary AMPKα−/− MEFs was significantly higher than that of primary WT MEFs (7.8 ± 0.9 times higher in the ELISA, p < 0.01, Fig. 2, B and C). These results show that SV40 immortalization does not affect differences in MMP-9 expression between WT and AMPKα−/− MEFs. Hereafter, all experiments were performed with SV40-immortalized MEFs unless otherwise noted.
FIGURE 2.
AMPK deletion up-regulates expression of MMP-9 from primary MEFs. A, the AMPK deletion in primary AMPKα−/− MEFs was confirmed by Western blotting of whole cell lysates. B, the amounts of MMP-9 in cell culture medium of primary WT and AMPKα−/− MEFs were examined by Western blotting. Recombinant mouse MMP-9 was used as a molecular marker. Representative blots are shown. C, the MMP-9 concentrations in cell culture medium of primary WT and AMPKα−/− MEFs were measured by ELISA. Error bars, S.E. **, p < 0.01.
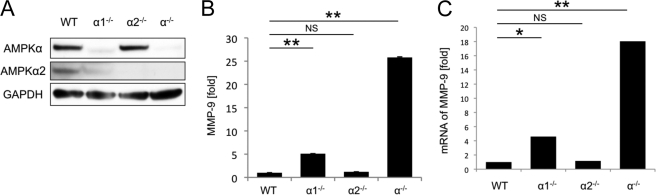
The AMPKα catalytic subunit has two isoforms, AMPKα1 and AMPKα2, which show differential tissue-specific expression (4). To determine the role of both isoforms in the expression of MMP-9, we utilized MEFs nullizygous for AMPKα1 (AMPKα1−/− MEFs) and AMPKα2 (AMPKα2−/− MEFs). As shown in Fig. 3A, the protein amount of pan-AMPKα subunit (AMPKα1 + α2) decreased considerably in AMPKα1−/− MEFs, but not in AMPKα2−/− MEFs. This indicates that the majority of AMPKα subunit in MEF is AMPKα1 (8). AMPKα1−/− MEFs showed a significant up-regulation of MMP-9 expression (5.1 ± 0.02 times higher than WT MEFs, p < 0.01, Fig. 3B), but the level of MMP-9 up-regulation was less than that seen in AMPKα−/− MEFs. By contrast, MMP-9 expression from AMPKα2−/− MEFs was similar to that seen in WT MEFs, consistent with the minimal change in the pan-AMPKα amount. These results were confirmed at the mRNA level by qRT-PCR (Fig. 3C), suggesting that complete deletion of both AMPKα1 and AMPKα2 isoforms is required to cause maximum overexpression of MMP-9 in MEFs.
FIGURE 3.
Complete deletion of both AMPKα1 and AMPKα2 isoforms is required to cause maximum over expression of MMP-9. A, the amounts of pan-AMPKα (AMPKα1 + α2) and AMPKα2 in WT, AMPKα1−/−, AMPKα2−/−, and AMPKα−/− MEFs were examined by Western blotting of whole cell lysates. Representative blots are shown. B, the MMP-9 concentrations in cell culture medium of WT, AMPKα1−/−, AMPKα2−/−, and AMPKα−/− MEFs were examined by ELISA. C, the expression of MMP-9 mRNA in WT, AMPKα1−/−, AMPKα2−/−, and AMPKα−/− MEFs was examined by qRT-PCR. *, p < 0.05; **, p < 0.01; NS, not significant. Error bars, S.E.
AMPK Activation Inhibits MMP-9 Expression from MEFs
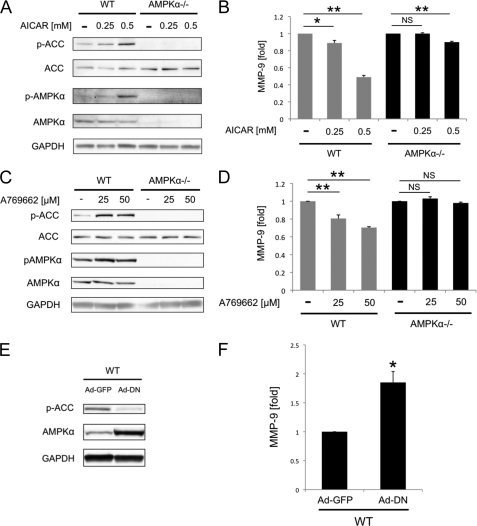
The finding that AMPKα deletion leads to the constitutive expression of MMP-9 prompted us to examine whether the kinase activity of AMPK is involved in the regulation of MMP-9 expression. To this end, we first treated WT and AMPKα−/− MEFs with a pharmacological activator of AMPK, AICAR (13, 14). AICAR activated AMPK signaling in WT MEFs in a dose-dependent manner, but not in AMPKα−/− MEFs (Fig. 4A). MMP-9 expression from WT MEFs decreased dose-dependently by 11 and 52% following 0.25 mm and 0.5 mm AICAR treatment, respectively (Fig. 4B). By contrast, 0.25 mm AICAR treatment of AMPKα−/− MEFs did not alter MMP-9 expression. With 0.5 mm AICAR treatment, MMP-9 expression in AMPKα−/− MEFs decreased minimally (by 10%, p < 0.01, Fig. 4B).
FIGURE 4.
Activation of AMPK inhibits MMP-9 expression from MEFs. A, WT and AMPKα−/− MEFs were treated with 0.25 and 0.5 mmol/liter AICAR. The phosphorylation of AMPKα (p-AMPKα) and ACC (p-ACC) after AICAR treatment was examined by Western blotting. B, after 12-h AICAR treatment, the MMP-9 concentrations in cell culture medium of WT and AMPKα−/− MEFs were measured by ELISA. C, WT and AMPKα−/− MEFs were treated with 25 and 50 μmol/liter A769662. The phosphorylation of AMPKα and ACC after A769662 treatment was examined by Western blotting. D, after 12-h A769662 treatment, the MMP-9 concentrations in cell culture medium of WT and AMPKα−/− MEFs were measured by ELISA. E, WT MEFs were transduced with adenovirus vectors expressing GFP (Ad-GFP) or the DN form of AMPKα (Ad-DN). Whole cell lysates of transduced MEFs were examined by Western blotting to confirm gene transduction. F, MMP-9 concentrations in cell culture medium of transduced WT MEFs were measured by ELISA. A, C, and E, representative blots are shown. *, p < 0.05; **, p < 0.01; NS, not significant. Error bars, S.E.
Although AICAR is used extensively as an AMPK activator, it has been shown to regulate other AMP-sensitive enzymes (13, 14). Furthermore, AICAR has been reported to inhibit cellular respiration by an AMPK-independent mechanism and decrease intracellular ATP (15–18). These could account for the suppressive effects seen on MMP-9 expression in AMPKα−/− MEFs at the higher levels of AICAR (0.5 mm). In addition, we tested the more specific AMPK activator, A769662 (13, 14). A769662 activated AMPK signaling in WT MEFs in a dose-dependent manner, but not in AMPKα−/− MEFs (Fig. 4C). In accordance with the results from AICAR-treated MEFs, 25 and 50 μm A769662 decreased MMP-9 expression from WT MEFs dose-dependently by 20 and 30%, respectively (Fig. 4D). By contrast, A769662 did not alter expression of MMP-9 from AMPKα−/− MEFs in either concentration used (Fig. 4D).
Next, we investigated the effects of AMPK inhibition on MMP-9 expression by overexpressing the DN form of AMPKα in WT MEFs. Western blotting of Ad-DN-transduced WT MEF cell lysates showed a decrease in ACC phosphorylation, indicating inhibition of AMPK activity (Fig. 4E). The MMP-9 level of Ad-DN-transduced WT MEFs was 1.85 ± 0.19 times higher than that of Ad-GFP-transduced WT MEFs (Fig. 4F). Taken together, these results indicate the importance of AMPK kinase activity for the inhibition of MMP-9 expression from MEFs.
Presence of AMPK Inhibits MMP-9 Expression from MEFs
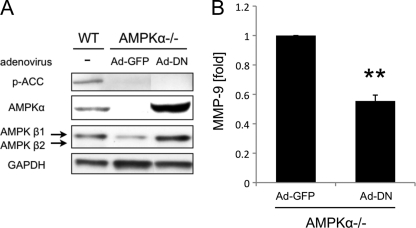
It has been reported that protein kinase including AMPK can bind to different protein and regulates signal transduction independently of its kinase catalytic activity (19, 20). This suggests that not only the kinase activity but also the presence of AMPK may play an important role in the regulation of MMP-9 expression. To address this, we transduced AMPKα−/− MEFs with the Ad-DN. Transduction was confirmed by Western blotting (Fig. 5A). Western blotting showed inhibition of ACC phosphorylation in Ad-GFP- and Ad-DN-transduced AMPKα−/− MEFs. It also revealed the up-regulation of AMPKβ1 in Ad-DN-transduced AMPKα−/− MEFs. Interestingly, ELISA revealed that the MMP-9 protein level of Ad-DN-transduced AMPKα−/− MEFs was significantly lower than that of Ad-GFP-transduced AMPKα−/− MEFs (p < 0.01, Fig. 5B). These results, in addition to the previous experiments mentioned above, indicate the importance of both the activity and the presence of AMPK in inhibiting MMP-9 expression in MEFs.
FIGURE 5.
Presence of AMPKα inhibits MMP-9 expression from AMPKα−/− MEFs. A, AMPKα−/− MEFs were transduced with Ad-GFP and Ad-DN vectors. Whole cell lysates of WT MEFs and transduced AMPKα−/− MEFs were examined by blotting to confirm gene transduction. Representative blots are shown. B, MMP-9 concentrations in cell culture medium of transduced AMPKα−/− MEFs were measured by ELISA. **, p < 0.01. Error bar, S.E.
Constitutive Activation of the NF-κB Pathway Is Involved in Up-regulating MMP-9 Expression by AMPKα−/− MEFs
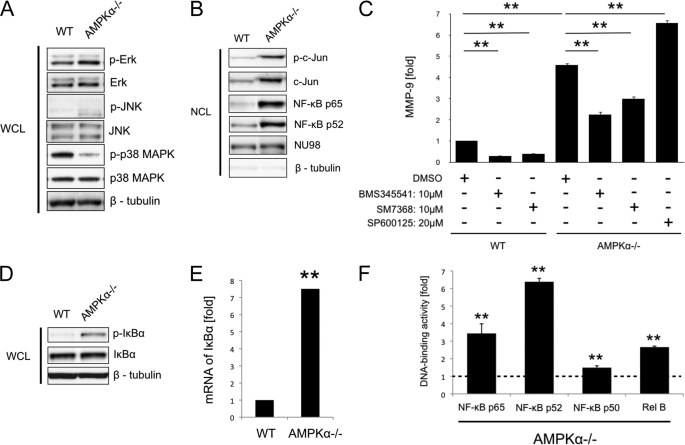
MMP-9 expression is largely controlled by transcription of the gene, although mRNA stability and translational efficiency also play a role in regulating protein levels (1). Regulation of transcription is achieved via a regulatory sequence containing binding sites for activator protein 1 (AP-1), NF-κB, Sp1, and PEA3/Ets (21–23). To investigate which of these transcription factors are involved in the up-regulation of MMP-9 expression in AMPKα−/− MEFs, we used Western blotting to examine the effects of AMPKα deletion on the phosphorylation of c-Jun, major subunits of AP-1, and its upstream MAPKs (i.e. ERK, JNK, and p38 MAPK), and the nuclear translocation of c-Jun and NF-κB. As shown in Fig. 6A, the phosphorylation of JNK was increased in AMPKα−/− ΜEFs, whereas there was no change in the phosphorylation of ERK. The phosphorylation of p38 MAPK was down-regulated in AMPKα−/− ΜΕFs. Nuclear extracts from WT and AMPKα−/− ΜΕFs showed an increase in the phosphorylation and the nuclear translocation of c-Jun, indicating that it is activated through the activation of JNK (Fig. 6B). Western blotting of the nuclear extract also revealed nuclear translocation of NF-κΒ p65 and p52 in AMPKα−/− ΜΕFs, indicating that both canonical and noncanonical NF-κB pathways are activated in AMPKα−/− ΜEFs (Fig. 6B).
FIGURE 6.
Constitutive activation of the NF-κB pathway is involved in the up-regulation of MMP-9 expression from AMPKα−/− MEFs. A, whole cell lysates (WCL) of WT and AMPKα−/− MEFs were examined by Western blotting to determine activation of ERK, JNK, and p38 MAPK pathways. β-Tubulin antibody was used as a control. B, nuclear cell lysates (NCL) of WT and AMPKα−/− MEFs were examined by Western blotting to determine activation of c-Jun and NF-κB pathways. NU98 and β-tubulin antibodies were used to confirm equal protein loading and to assess the relative purity of the nuclear cell lysates. C, amounts of MMP-9 in cell culture media from WT and AMPKα−/− MEFs after treatment with IKK inhibitor (BMS345541, 10 μm), NF-κB inhibitor (SM7368, 10 μm), and JNK inhibitor (SP600125, 20 μm) were examined by ELISA. MEFs were treated with each inhibitor for 12 h. Dimethyl sulfoxide (DMSO) was used as a control. D, whole cell lysates of WT and AMPKα−/− MEFs were examined by Western blotting to determine IκBα phosphorylation. E, expression of IκBα mRNA in WT, AMPKα−/− MEFs was examined by qRT-PCR. F, DNA-binding activities of NF-κB p65, p52, p50, and RelB in nuclear cell lysates of WT and AMPKα−/− MEFs were measured by ELISA-based assay. The data are shown as -fold changes relative to WT MEFs, which is set as 1. A, B, and D, representative blots are shown. **, p < 0.01. Error bars, S.E.
To determine whether the activation of c-Jun and NF-κB pathways is responsible for the up-regulation of MMP-9 expression in AMPKα−/− MEFs, we treated them with IKK inhibitor (BMS345541), NF-κB inhibitor (SM7368), or JNK inhibitor (SP600125) and evaluated MMP-9 protein levels by ELISA. Treatment with both BMS345541 and SM7368 significantly suppressed MMP-9 expression by 51 and 34%, respectively (both p < 0.01), whereas application of SP600125 did not. This indicates that the NF-κB pathway is partially responsible for the up-regulation of MMP-9 expression in AMPKα−/− MEFs (Fig. 6C). AMPKα deletion (comparing WT versus AMPKα−/− MEFs) increases MMP-9 expression by about 5-fold (Fig. 6C), but as shown previously, activation of endogenous AMPKα only suppresses MMP-9 in WT MEFs by about 50% (Fig. 4, B and D). This would suggest that endogenous AMPKα activity is sufficient to suppress MMP-9 expression tonically. Indeed, when NF-κB inhibitors BMS345541 and/or SM7368 were given in WT MEFs, they markedly suppressed endogenous MMP-9 expression by 71 and 61%, respectively (both p < 0.01, Fig. 6C). Together, these results highlight the importance of the AMPKα/NF-κB pathway in MMP-9 expression.
To investigate whether the AMPKα deletion causes the constitutive activation of the NF-κΒ pathway in MEFs, we next investigated the phosphorylation and mRNA expression of IκBα (p-IκBα). Western blotting showed that IκBα phosphorylation was markedly higher in AMPKα−/− MEFs than in WT MEFs (Fig. 6D). The expression level of IκBα mRNA in AMPKα−/− MEFs was also significantly higher than in WT MEFs (p < 0.01, Fig. 6E). As IκBα is degraded by the ubiquitin process after phosphorylation (24–26), these findings suggest that IκBα might undergo chronic degradation and resynthesis in AMPKα−/− ΜΕFs and that the AMPKα deletion causes constitutive activation of the NF-κΒ pathway in MEFs (27).
To confirm activation of the NF-κB pathways further, we next evaluated the binding activity of nuclear extracts to NF-κΒ subunits of p65, p52, p50, and RelB by ELISA. We found that the binding activities of all NF-κB subunits were up-regulated in AMPKα−/− MEFs (p < 0.01, Fig. 6F), indicating that both canonical and noncanonical NF-κB pathways are activated. Collectively, these results suggest that the AMPK deletion leads to the constitutive activation of the NF-κB pathway, which is at least partly responsible for the up-regulation of MMP-9 expression.
DISCUSSION
MMP-9 plays a critical role in tissue remodeling under both physiological and pathological conditions. Its expression is low in most cells and is tightly controlled. Although many factors have been identified as stimulators of MMP-9 expression, only integrin α1 and transgelin have been shown to inhibit MMP-9 expression in normal cells under physiological conditions (28, 29). Here, we utilized MEFs that were nullizygous for the catalytic α subunit of AMPK to identify AMPK as a negative regulator of MMP-9 expression, thus adding it to the short list of known MMP-9 repressors.
Using the gene knock-out system, Pozzi et al. (28) and Nair et al. (29) reported that integrin α1 and transgelin are negative regulators of MMP-9 expression by demonstrating over-expression of MMP-9 in normal lung endothelial cells and uterine epithelial cells from knock-out mice. Other groups have shown that proteins such as kisspeptin-1 (KiSS-1), PKCs, heme oxygenase 1, reversion-inducing cysteine-rich protein with kazal motifs (RECK), and caveolin-1 down-regulate MMP-9 expression (30–35). However, the latter studies used cell lines such as HT-1080, prostate cancer cells, pancreatic cancer cells, and NMuMG which, in contrast to normal cells and MEFs, express MMP-9 constitutively at high levels. Constitutive up-regulation of MMP-9 in these cells is considered to be due to constitutive activation of MAPKs by oncogenic transformation of Ras (36–38). Even if these proteins can decrease MMP-9 expression in Ras-transformed cell lines, it does not follow that they are responsible for the basal suppression of MMP-9 expression in non-Ras-transformed cells.
We observed that deletion of both AMPKα1 and α2 isoforms in primary or immortalized MEFs led to a significant up-regulation of MMP-9 expression (Figs. 1, A and B, and 2, B and C). However, single deletion of AMPKα1 or α2 did not cause overexpression of MMP-9 to the levels seen in MEFs that are nullizygous for both AMPKα1 and α2 (Fig. 3, B and C). Notably, the MMP-9 expression level of AMPKα1−/− MEFs, which is lacking a majority of AMPKα (Fig. 3A), was only 20% of that of AMPKα−/− MEFs. These results suggest that AMPKα1 and AMPKα2 both function to inhibit MMP-9 expression, and that even a small amount of AMPKα can potently inhibit MMP-9 expression.
Previous experimental inflammation animal models and cancer cell lines have been used to show that the broad and nonspecific AMPK activators metformin and AICAR decrease MMP-9 expression; however, base-line MMP-9 expression in these artificial models was already up-regulated, and metformin and AICAR can have many off-target effects (39, 40). Similarly, here we found that the activity of AMPK was partially responsible for the regulation of basal MMP-9 levels. However, the magnitude of the effect of AMPK activity manipulation on MMP-9 expression was not as large as the effect seen by deletion of both AMPKα isoforms (compare Fig. 3B and Fig. 4, B, D, and F). This led us to investigate whether not only the activity but also the presence of AMPKα is important for inhibition of basal MMP-9 expression. Bronner et al. reported that AMPK binds to PPARα and co-activates PPARα-mediated transcription, independently of its catalytic activity (19). Indeed, transduction with kinase-dead AMPKα (DN AMPKα) suppressed MMP-9 expression in AMPKα−/− MEFs (Fig. 5B). Although the reduction rate in this experiment (45%, Fig. 5B) was not as drastic as we expected, we think that one possible reason for this can be the limited infection efficiency of Ad-DN AMPK. Interestingly, AMPKα−/− MEFs transduced with Ad-DN showed a greater increase in AMPKβ1 protein levels than Ad-GFP-transduced AMPKα−/− MEFs (Fig. 5A). This can be explained by the importance of heterotrimeric complex in the stabilization of individual AMPK subunit. AMPK is stable as a heterotrimer complex, whereas each of its subunits is subject to an increased turnover rate and is depleted from the cell when not associated with others (41–43). Therefore, the observed higher expression of AMPKβ1 in AMPKα−/− MEFs indicates that the transduction of the Ad-DN resulted in an increase in the AMPK heterotrimer complex in the cell. Recently, it was reported that the AMPKβ subunit is not only a scaffold that assembles α and γ subunits, but also determines the subcellular localization and substrate specificity of the AMPK heterotrimer complex (44, 45). Thus, understanding the function of the AMPK heterotrimer complex in the regulation of MMP-9 is important, and further study is required to achieve this.
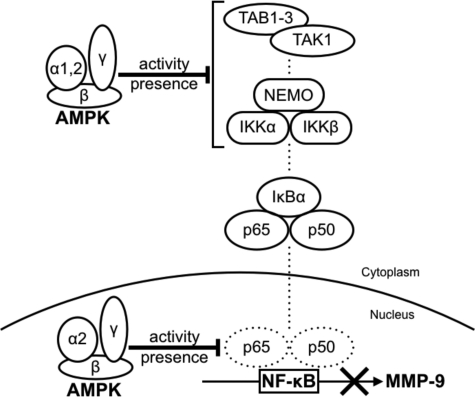
We found that AMPK suppresses MMP-9 expression by inhibiting the NF-κB pathway in MEFs. The gelatin zymography result showed that the AMPKα deletion did not affect MMP-2 expression in MEFs (Fig. 1A). These findings are consistent with the fact that the regulatory sequence of MMP-2 does not contain a NF-κB-binding site (46). Furthermore, the nuclear localization and NF-κB DNA-binding activity results suggest that activation of both the canonical and the noncanonical NF-κB pathways is responsive to overexpression of MMP-9 in AMPKα−/− MEFs (Fig. 6, B, C, and F). Wang et al. reported that deletion of AMPKα2 results in the constitutive activation of NF-κB in mouse aortic endothelial cells (47). In addition, many studies have reported that the activation of AMPK inhibits the NF-κB pathway (48–51). Although the mechanism of AMPK inhibition of the NF-κB pathway is not fully elucidated, there are at least two main possibilities (Fig. 7).
FIGURE 7.
Proposed model for the mechanism by which AMPK suppresses MMP-9 expression. AMPK negatively regulates MMP-9 expression by inhibiting the NF-κB pathway. Our results suggest at least two possibilities for the mechanism: 1) AMPK inhibits IKK activity or upstream proteins of IKK, such as TAK1, TAB1–3, and NEMO; and 2) AMPK directly inhibits the DNA-binding activity of NF-κB.
The first possibility is that AMPK inhibits IKK-dependent IκBα phosphorylation. Phosphorylation of IκBα by the upstream kinase IKK is essential for NF-κB nuclear translocation. Our results demonstrate that the AMPKα deletion led to the phosphorylation of IκBα and nuclear translocation of NF-κB, indicating that AMPK targets IKK activity or upstream proteins of IKK (Fig. 6, B and D). These include transforming growth factor β-activated kinase 1 (TAK1), TAK1-binding proteins 1–3 (TAB1–3), NF-κB essential modulator (NEMO), and deubiquitinating enzymes such as A20 and cylindromatosis tumor suppressor protein (CYLD) (27, 52–56). Among these, Li et al. reported that AMPKα2 associates with TAB1 and activates p38 MAPK in mouse heart (57). Furthermore, TAK1 is a major upstream activating kinase of AMPK (45). Although we tried to demonstrate an interaction between these proteins and AMPK, we were unable to clarify their associations in MEFs.
The second possibility is that AMPK directly inhibits the DNA-binding activity of NF-κB. It has been reported that the AMPKα1 isoform is primarily cytoplasmic, whereas AMPKα2 is predominantly nuclear and plays a role in transcriptional regulation (58, 59). Indeed, Katerelos et al. reported the possibility that AMPK reduces the NF-κB DNA-binding activity in bovine aortic endothelial cells (51). Further study is required to determine the target of AMPK for interference with the NF-κB pathway.
In conclusion, we identified AMPK as a novel negative regulator of MMP-9 expression in MEFs under physiological conditions. Recently, ample evidence indicates the importance of AMPK in the pathogenesis of cancer and arterial sclerosis (6, 7, 60–62). Because MMP-9 plays an important role in these diseases, in the invasion and metastasis of cancer cells and the rupture of atheromatous plaques, our findings might provide fundamental insights not only into the regulatory mechanism of MMP-9 expression and the function of AMPK, but also into the pathogenesis of these diseases.
Acknowledgments
We thank Drs. Shintaro Nakao (Kyushu University, Japan), Yasuhiro Ito (Kumamoto University, Japan), and Jun Kosaka and Tomoko Yonezawa (Okayama University, Japan) for technical assistance and insightful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant EY014104 through the NEI. This work was also supported by the Bacardi Fund (to D. G. V.), the Research to Prevent Blindness Foundation (to D. G. V.), the Lions Eye Research Fund (to D. G. V.), the Onassis Foundation (to D. G. V.), a Fight for Sight Grant in Aid (to D. G. V.), the Harvard Ophthalmology Department (to D. G. V.), and a Bausch and Lomb Japan Vitreoretinal fellowship (to Y. M. and M. K.).
- MMP
- matrix metalloproteinase
- ACC
- acetylcoenzyme A carboxylase
- Ad
- adenovirus
- AICAR
- 5-amino-4-imidazole carboxamide riboside
- AMPK
- AMP-activated protein kinase
- DN
- dominant negative
- ECM
- extracellular matrix
- MEF
- mouse embryonic fibroblast
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Van den Steen P. E., Dubois B., Nelissen I., Rudd P. M., Dwek R. A., Opdenakker G. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 375–536 [DOI] [PubMed] [Google Scholar]
- 2. Sternlicht M. D., Werb Z. (2001) Annu. Rev. Cell Dev. Biol. 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. (2003) Mol. Cell. Biochem. 253, 269–285 [DOI] [PubMed] [Google Scholar]
- 4. Hardie D. G., Hawley S. A. (2001) Bioessays 23, 1112–1119 [DOI] [PubMed] [Google Scholar]
- 5. Viollet B., Athea Y., Mounier R., Guigas B., Zarrinpashneh E., Horman S., Lantier L., Hebrard S., Devin-Leclerc J., Beauloye C., Foretz M., Andreelli F., Ventura-Clapier R., Bertrand L. (2009) Front. Biosci. 14, 19–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang W., Guan K. L. (2009) Acta Physiol. 196, 55–63 [DOI] [PubMed] [Google Scholar]
- 7. Theodoropoulou S., Kolovou P. E., Morizane Y., Kayama M., Nicolaou F., Miller J. W., Gragoudas E., Ksander B. R., Vavvas D. G. (2010) FASEB J. 24, 2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laderoute K. R., Amin K., Calaoagan J. M., Knapp M., Le T., Orduna J., Foretz M., Viollet B. (2006) Mol. Cell. Biol. 26, 5336–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh S., Saunders G. F. (1998) Anal. Biochem. 265, 185–187 [DOI] [PubMed] [Google Scholar]
- 10. Aguilar V., Alliouachene S., Sotiropoulos A., Sobering A., Athea Y., Djouadi F., Miraux S., Thiaudière E., Foretz M., Viollet B., Diolez P., Bastin J., Benit P., Rustin P., Carling D., Sandri M., Ventura-Clapier R., Pende M. (2007) Cell Metab. 5, 476–487 [DOI] [PubMed] [Google Scholar]
- 11. Stahl H., Knippers R. (1987) Biochim. Biophys. Acta 910, 1–10 [DOI] [PubMed] [Google Scholar]
- 12. Moens U., Seternes O. M., Johansen B., Rekvig O. P. (1997) Virus Genes 15, 135–154 [DOI] [PubMed] [Google Scholar]
- 13. Göransson O., McBride A., Hawley S. A., Ross F. A., Shpiro N., Foretz M., Viollet B., Hardie D. G., Sakamoto K. (2007) J. Biol. Chem. 282, 32549–32560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guigas B., Sakamoto K., Taleux N., Reyna S. M., Musi N., Viollet B., Hue L. (2009) IUBMB Life 61, 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guigas B., Bertrand L., Taleux N., Foretz M., Wiernsperger N., Vertommen D., Andreelli F., Viollet B., Hue L. (2006) Diabetes 55, 865–874 [DOI] [PubMed] [Google Scholar]
- 16. Mukhtar M. H., Payne V. A., Arden C., Harbottle A., Khan S., Lange A. J., Agius L. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R766–774 [DOI] [PubMed] [Google Scholar]
- 17. Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. (2010) J. Clin. Invest. 120, 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guigas B., Taleux N., Foretz M., Detaille D., Andreelli F., Viollet B., Hue L. (2007) Biochem. J. 404, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bronner M., Hertz R., Bar-Tana J. (2004) Biochem. J. 384, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S., Kim S. F., Maag D., Maxwell M. J., Resnick A. C., Juluri K. R., Chakraborty A., Koldobskiy M. A., Cha S. H., Barrow R., Snowman A. M., Snyder S. H. (2011) Cell Metab. 13, 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gum R., Lengyel E., Juarez J., Chen J. H., Sato H., Seiki M., Boyd D. (1996) J. Biol. Chem. 271, 10672–10680 [DOI] [PubMed] [Google Scholar]
- 22. Himelstein B. P., Lee E. J., Sato H., Seiki M., Muschel R. J. (1997) Oncogene 14, 1995–1998 [DOI] [PubMed] [Google Scholar]
- 23. Robert I., Aussems M., Keutgens A., Zhang X., Hennuy B., Viatour P., Vanstraelen G., Merville M. P., Chapelle J. P., de Leval L., Lambert F., Dejardin E., Gothot A., Chariot A. (2009) Oncogene 28, 1626–1638 [DOI] [PubMed] [Google Scholar]
- 24. Karin M., Ben-Neriah Y. (2000) Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 25. Gilmore T. D. (2006) Oncogene 25, 6680–6684 [DOI] [PubMed] [Google Scholar]
- 26. Scheidereit C. (2006) Oncogene 25, 6685–6705 [DOI] [PubMed] [Google Scholar]
- 27. Reiley W. W., Jin W., Lee A. J., Wright A., Wu X., Tewalt E. F., Leonard T. O., Norbury C. C., Fitzpatrick L., Zhang M., Sun S. C. (2007) J. Exp. Med. 204, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pozzi A., Moberg P. E., Miles L. A., Wagner S., Soloway P., Gardner H. A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2202–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nair R. R., Solway J., Boyd D. D. (2006) J. Biol. Chem. 281, 26424–26436 [DOI] [PubMed] [Google Scholar]
- 30. Yan C., Wang H., Boyd D. D. (2001) J. Biol. Chem. 276, 1164–1172 [DOI] [PubMed] [Google Scholar]
- 31. Urtreger A. J., Grossoni V. C., Falbo K. B., Kazanietz M. G., Bal de Kier Joffé E. D. (2005) Mol. Carcinog. 42, 29–39 [DOI] [PubMed] [Google Scholar]
- 32. Grossoni V. C., Falbo K. B., Mauro L. V., Krasnapolski M. A., Kazanietz M. G., Bal De Kier Joffé E. D., Urtreger A. J. (2007) Clin. Exp. Metastasis 24, 513–520 [DOI] [PubMed] [Google Scholar]
- 33. Gueron G., De Siervi A., Ferrando M., Salierno M., De Luca P., Elguero B., Meiss R., Navone N., Vazquez E. S. (2009) Mol. Cancer Res. 7, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 34. Takagi S., Simizu S., Osada H. (2009) Cancer Res. 69, 1502–1508 [DOI] [PubMed] [Google Scholar]
- 35. Han F., Zhu H. G. (2010) J. Surg. Res. 159, 443–450 [DOI] [PubMed] [Google Scholar]
- 36. Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. (1985) Mol. Cell. Biol. 5, 259–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. (1987) Cancer Res. 47, 1523–1528 [PubMed] [Google Scholar]
- 38. Gum R., Wang H., Lengyel E., Juarez J., Boyd D. (1997) Oncogene 14, 1481–1493 [DOI] [PubMed] [Google Scholar]
- 39. Nath N., Khan M., Paintlia M. K., Singh I., Hoda M. N., Giri S. (2009) J. Immunol. 182, 8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hwang Y. P., Jeong H. G. (2010) Br. J. Pharmacol. 160, 1195–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woods A., Azzout-Marniche D., Foretz M., Stein S. C., Lemarchand P., Ferré P., Foufelle F., Carling D. (2000) Mol. Cell. Biol. 20, 6704–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foretz M., Hébrard S., Guihard S., Leclerc J., Do Cruzeiro M., Hamard G., Niedergang F., Gaudry M., Viollet B. (2011) FASEB J. 25, 337–347 [DOI] [PubMed] [Google Scholar]
- 43. Steinberg G. R., O'Neill H. M., Dzamko N. L., Galic S., Naim T., Koopman R., Jørgensen S. B., Honeyman J., Hewitt K., Chen Z. P., Schertzer J. D., Scott J. W., Koentgen F., Lynch G. S., Watt M. J., van Denderen B. J., Campbell D. J., Kemp B. E. (2010) J. Biol. Chem. 285, 37198–37209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warden S. M., Richardson C., O'Donnell J., Jr., Stapleton D., Kemp B. E., Witters L. A. (2001) Biochem. J. 354, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sanz P. (2008) Curr. Protein Pept. Sci. 9, 478–492 [DOI] [PubMed] [Google Scholar]
- 46. Yan C., Boyd D. D. (2007) J. Cell. Physiol. 211, 19–26 [DOI] [PubMed] [Google Scholar]
- 47. Wang S., Zhang M., Liang B., Xu J., Xie Z., Liu C., Viollet B., Yan D., Zou M. H. (2010) Circ. Res. 106, 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giri S., Nath N., Smith B., Viollet B., Singh A. K., Singh I. (2004) J. Neurosci. 24, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cacicedo J. M., Yagihashi N., Keaney J. F., Jr., Ruderman N. B., Ido Y. (2004) Biochem. Biophys. Res. Commun. 324, 1204–1209 [DOI] [PubMed] [Google Scholar]
- 50. Hattori Y., Suzuki K., Hattori S., Kasai K. (2006) Hypertension 47, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 51. Katerelos M., Mudge S. J., Stapleton D., Auwardt R. B., Fraser S. A., Chen C. G., Kemp B. E., Power D. A. (2010) Immunol. Cell Biol. 88, 754–760 [DOI] [PubMed] [Google Scholar]
- 52. Qian Y., Commane M., Ninomiya-Tsuji J., Matsumoto K., Li X. (2001) J. Biol. Chem. 276, 41661–41667 [DOI] [PubMed] [Google Scholar]
- 53. Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. (1999) Nature 398, 252–256 [DOI] [PubMed] [Google Scholar]
- 54. Skaug B., Jiang X., Chen Z. J. (2009) Annu. Rev. Biochem. 78, 769–796 [DOI] [PubMed] [Google Scholar]
- 55. Adhikari A., Xu M., Chen Z. J. (2007) Oncogene 26, 3214–3226 [DOI] [PubMed] [Google Scholar]
- 56. Takaesu G., Kishida S., Hiyama A., Yamaguchi K., Shibuya H., Irie K., Ninomiya-Tsuji J., Matsumoto K. (2000) Mol. Cell 5, 649–658 [DOI] [PubMed] [Google Scholar]
- 57. Li J., Miller E. J., Ninomiya-Tsuji J., Russell R. R., 3rd, Young L. H. (2005) Circ. Res. 97, 872–879 [DOI] [PubMed] [Google Scholar]
- 58. Salt I., Celler J. W., Hawley S. A., Prescott A., Woods A., Carling D., Hardie D. G. (1998) Biochem. J. 334, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Turnley A. M., Stapleton D., Mann R. J., Witters L. A., Kemp B. E., Bartlett P. F. (1999) J. Neurochem. 72, 1707–1716 [DOI] [PubMed] [Google Scholar]
- 60. Luo Z., Saha A. K., Xiang X., Ruderman N. B. (2005) Trends Pharmacol. Sci. 26, 69–76 [DOI] [PubMed] [Google Scholar]
- 61. Steinberg G. R., Kemp B. E. (2009) Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 62. Motoshima H., Goldstein B. J., Igata M., Araki E. (2006) J. Physiol. 574, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]