Abstract
A number of enzymes become functional by binding to zinc during their journey through the early secretory pathway. The zinc transporters (ZnTs) located there play important roles in this step. We have previously shown that two zinc transport complexes, ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers, are required for the activation of alkaline phosphatases, by converting them from the apo- to the holo-form. Here, we investigated the molecular mechanisms of this activation. ZnT1 and ZnT4 expressed in chicken DT40 cells did not contribute to the activation of tissue nonspecific alkaline phosphatase (TNAP). The reduced activity of TNAP in DT40 cells deficient in both ZnT complexes was not restored by zinc supplementation nor by exogenous expression of other ZnTs that increase the zinc content in the secretory pathway. Moreover, we showed that expression of ZnT5/ZnT6 heterodimers reconstituted with zinc transport-incompetent ZnT5 mutant failed to restore TNAP activity but could stabilize the TNAP protein as the apo-form, regardless of zinc status. These findings demonstrate that TNAP is activated not simply by passive zinc binding but by an elaborate two-step mechanism via protein stabilization followed by enzyme conversion from the apo- to the holo-form with zinc loaded by ZnT complexes in the early secretory pathway.
Keywords: Membrane Proteins, Metalloenzymes, Metals, Transport Metals, Zinc, Secretory Pathway, Zinc-requiring Enzymes, ZnT/SLC30A
Introduction
The early secretory pathway, comprising the endoplasmic reticulum (ER)2 and the Golgi apparatus, is known to play a critical role in a variety of cellular functions, including protein folding, membrane trafficking, modification, glycosylation, and cell signaling (1, 2). Approximately one-third of all proteins in eukaryotes are targeted to the ER (3), and an elaborate quality control system operates in the secretory pathway. Divalent cations are one of the important regulators of this quality control process; the functions of calcium have been extensively investigated and well elucidated (4, 5). Recently, there has been increasing interest in the function of zinc. Our group and others have shown that the heterocomplexes of zinc transporters (ZnT) 5 and 6 and their orthologs, which transport zinc into the secretory pathway, are important for homeostatic maintenance of secretory pathway function (6, 7). Vertebrate cells and yeast deficient in these transporters show exacerbation of the unfolded protein response or impairment of Golgi membrane trafficking (6, 7, 9). Several Zrt/Irt-like protein (ZIP) members are likely to function as modulators of the early secretory pathway by regulating zinc content (10–12), but the molecular evidence is far from complete.
The most important task of zinc in the secretory pathway is thought to be to bind to numerous proteins or enzymes, because there are a number of secretory, membrane-bound, or organelle-resident enzymes and proteins that are properly folded and become functional by binding to zinc in the early secretory pathway before reaching their final destination. In vertebrates, matrix metalloproteinases, angiotensin-converting enzymes, endothelin-converting enzymes, A Disintegrin and Metalloproteinase (ADAM) family proteins, alkaline phosphatases (ALPs), and secretory/membrane-bound carbonic anhydrases are well known zinc-requiring enzymes, and all play important physiological roles (13–18). There is, however, almost no information about the activation step of these enzymes in the secretory process. Using a gene disruption/re-expression system in chicken DT40 cells, and ALPs as marker proteins, we have shown that two ZnT complexes (ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers) activate zinc-requiring enzymes that have matured during the secretory process, converting them from the apo- to the holo-form (7, 8, 19, 20). We used both placenta ALP and tissue nonspecific ALP (TNAP) because their activities are wholly dependent on zinc. Of these, understanding the activation mechanism of TNAP is important because TNAP plays a crucial role promoting mineralization of the extracellular matrix by restricting the concentration of the calcification inhibitor inorganic pyrophosphate. Reduced activity of TNAP caused by missense mutations in the TNAP gene causes hypophosphatasia, a heritable form of rickets and osteomalacia (21, 22). Moreover, partial reduction of TNAP activity and impairment of osteoblast maturation is observed in osteoblasts differentiated in vitro from precursor cells prepared from Znt5−/− mice (23). TNAP activity was significantly reduced (over 95%) in DT40 cells deficient in ZnT5, ZnT6, and ZnT7 (triple knock-out cells (TKO cells)), and this reduction was restored by re-expression of the human counterparts (8, 19). Intriguingly, TKO cells express other ZnTs constitutively or transiently localized to the secretory pathway, such as ZnT1 and ZnT4. This led us to examine the possibility whether they may contribute to the activation of TNAP and to investigate the molecular basis of TNAP activation by ZnTs. In this study, we document that TNAP is activated not simply by zinc ions mobilized in the early secretory pathway but by an elaborate two-step mechanism by specific ZnTs, through protein stabilization and activation. To our knowledge, this is the first report to demonstrate directly that ZnTs operate to regulate protein stability independently of zinc transport and how ZnTs specifically regulate the activity of a zinc-requiring enzyme.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Chicken B lymphocyte-derived DT40 cells were maintained in RPMI 1640 medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% heat-inactivated fetal calf serum (FCS) (JRH Biosciences, Lenexa, KS), 1% chicken serum (Invitrogen), and 50 μm 2-mercaptoethanol (Sigma) at 39.5 °C. To generate zinc-deficient culture medium, FCS or chicken serum was treated with Chelex-100 resin as described previously (24). DNA transfection into DT40 cells was carried out as described previously (8). Other experimental strategies to generate conditional mutants are shown in the supplemental figures.
Plasmid Construction
Information about the targeting vectors of the chicken ZnT1 (cZnT1) and ZnT4 (cZnT4) genes is shown in supplemental Fig. S1. Plasmids to express N-terminally FLAG-tagged human ZnT1 (FLAG-hZnT1), C-terminally HA-tagged human ZnT2 (hZnT2-HA), C-terminally Myc-tagged human ZnT3 (hZnT3-Myc) (25), C-terminally HA-tagged human ZnT4 (hZnT4-HA) (26), or C-terminally HA-tagged mouse ZnT8 (mZnT8-HA) were constructed by inserting each cDNA into the pA-Ecogpt or pA-Puro vectors. The tagged cDNAs used in this study were constructed by two-step PCR methods. Fragments containing full-length cDNAs of hZnT1, hZnT2, or mZnT8 were amplified by RT-PCR using total RNA prepared from A549, MCF7, or MIN6 cells as a template, respectively. Amplified cDNAs were sequenced in both directions. Plasmids to express HA-hZnT6 were constructed by inserting it upstream of the internal ribosome entry site-enhanced green fluorescent protein (IRES-EGFP) sequence of the pCR3-CMVmin-loxP-MCS-IRES-EGFP-loxP vector (27), in which the HA-hZnT6 cDNA and EGFP genes are flanked by loxP sequences. Thus, the HA-hZnT6 cDNA could be excised by Cre-recombinase. Plasmids for expression of human TNAP (hTNAP) and tamoxifen-regulated chimeric Cre recombinase (pANMerCreMre) have been described previously (7, 19). All plasmids were linearized with appropriate restriction enzymes prior to electroporation.
Northern Blotting
RNA (20 μg) extracted from DT40 cells using Sepasol I (Nacalai Tesque) was electrophoresed on an agarose gel and then transferred to a nitrocellulose membrane filter in 20× SSC. The membrane was hybridized to appropriately radiolabeled cDNA probes. Radioimages were obtained as described previously (8).
Measurement of TNAP Activity
Total cellular protein extracts were prepared from cells lysed in ALP lysis buffer, and 2 or 3 μg of protein was used to measure TNAP activity as described previously (8). Shrimp ALP (Roche Applied Science) was used as the standard. For the zinc supplementation experiment, total cellular protein was prepared from cells incubated in the presence of 100 μm ZnSO4 or 5 μm zinc pyrithione for 30 min, followed by two washes with culture medium and incubation in medium for another 8 h at 39.5 °C.
Immunoblotting and Immunoprecipitation
Immunoblotting and immunoprecipitation were performed as described previously (7, 19). The blotted membrane was blocked with blocking solution (5% skim milk and 0.1% Tween 20 in phosphate-buffered saline) and then incubated with anti-HA HA-11 (1:1000 dilution; Covance, Emeryville, CA), anti-FLAG M2 (1:1000; Sigma), anti-Myc 9E10 (1:1000; Sigma), anti-calnexin (1:1000; StressGen, Ann Arbor, MI), anti-hTNAP (1:2000), anti-hZnT5 (1:1000), or anti-hZnT6 (1:1000; Proteintech Group Inc., Chicago) antibodies in blocking solution. Immobilon Western chemiluminescent HRP substrates (Millipore, Billerica, MA) were used for detection. The fluorimager was obtained using a LAS1000 Plus image analyzer (Fujifilm, Tokyo, Japan).
Immunofluorescence
Immunostaining for FLAG-hZnT1, hZnT2-HA, hZnT3-Myc, hZnT4-HA, or mZnT8-HA expressed in TKO cells was performed as described previously (20). Briefly, the cells were stained with anti-HA antibody (1:50; Sigma) for hZnT4-HA, with anti-HA tag antibody (1:100; MBL, Nagoya, Japan) for hZnT2-HA and mZnT8-HA, with anti-Myc tag antibody (1:100; MBL) for hZnT3-Myc, or with anti-FLAG tag antibody (anti-DDDDK; 1:100; MBL) for FLAG-hZnT1, followed by Alexa 488-conjugated goat anti-rabbit IgG (1:250; Molecular Probes, Eugene, OR), or with anti-GM130 antibody (1:100; BD Transduction Laboratories), followed by Alexa 594-conjugated goat anti-mouse IgG (1:250; Molecular Probes). The stained cells were observed using an Olympus IX70 microscope equipped with an Olympus digital camera (Olympus, Tokyo) and Metamorph software (Molecular Devices Inc., Downingtown, PA) for image acquisition. Images were analyzed using Adobe Photoshop CS (Adobe Systems, Inc., San Jose, CA).
Zinc-specific Fluorescence Staining
Cells were fixed on coverslips coated with poly-l-lysine and incubated in the presence of 100 μm ZnSO4 or 5 μm zinc pyrithione for 30 min. After the treatment, cells were washed twice with culture medium and subsequently incubated in medium for another 8 h at 39.5 °C. For imaging zinc, cells were loaded with the cell-permeable, zinc-specific dye FluoZin-3 (5 μm; Invitrogen) for 1 h before fixation.
RESULTS
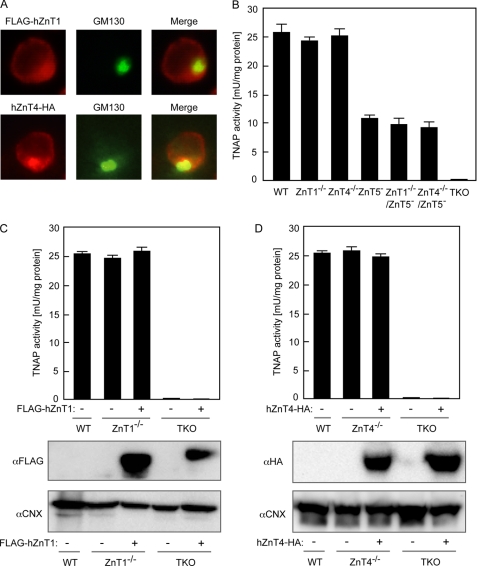
It has been shown previously that TNAP activity is significantly reduced (over 95%) in DT40 cells deficient in ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers (ZnT5− ZnT6−/−ZnT7−/−; TKO cells) (19) but that TKO cells express other ZnT mRNAs, such as cZnT1 and cZnT4 (8). Here, we found that expression of cZnT1 mRNA, but not cZnT4, was increased in response to high zinc concentrations in DT40 cells in the same manner as in mammalian cells, and there were no significant differences in their expression levels and patterns between wild-type (WT) and TKO cells (Fig. 1). Chicken metallothionein mRNA also dramatically increased in response to high zinc concentrations in both cells in the same manner, indicating that cytoplasmic zinc homeostasis is not significantly disturbed in TKO cells, in contrast to marked reduction of TNAP activity. Exogenously expressed hZnT1 and hZnT4 were partially localized to the early secretory pathway, including the Golgi apparatus, in DT40 cells (Fig. 2A) as shown previously (28–30), although that of hZnT1 was most likely transient. This led us to investigate the possibility that these ZnTs may be involved in the activation of TNAP. We generated cZnT1 disruptant (ZnT1−/−) cells and cZnT4 disruptant (ZnT4−/−) DT40 cells (supplemental Fig. S1). Both cell types proliferated and demonstrated TNAP activity at a rate comparable with that of WT cells (Fig. 2B). To examine this in more detail, we generated cZnT1/cZnT5 and cZnT4/cZnT5 double disruptant (ZnT1−/−ZnT5− cells and ZnT4−/−ZnT5− cells, respectively) and measured the TNAP activity. Again, the TNAP activity was not significantly changed compared with that of ZnT5− cells (Fig. 2B). Furthermore, overexpression of hZnT1 or hZnT4 caused no increase in TNAP activity in either disruptant or TKO cells (Fig. 2, C and D), indicating that ZnT1 and ZnT4 do not contribute significantly to the activation of TNAP even though they localize to the early secretory pathway.
FIGURE 1.
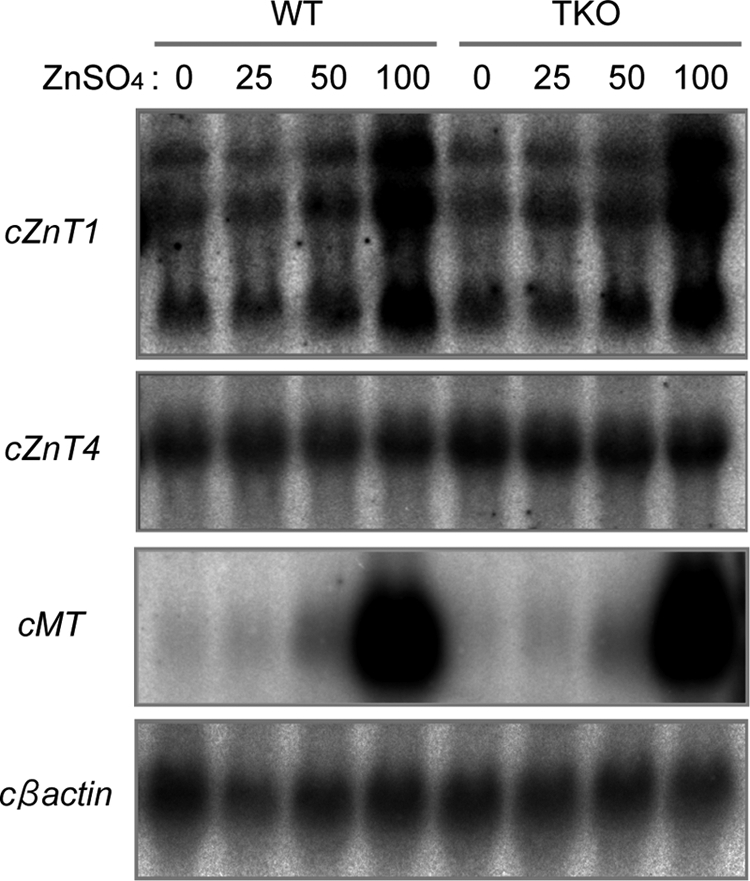
cZnT1 and cZnT4 are highly expressed in DT40 cells. Total RNA was prepared from WT and TKO cells cultured in the presence of the indicated concentrations of ZnSO4 (0–100 μm) for 6 h. Note that cZnT1 mRNA expression (detected as three transcripts) is clearly dependent on the zinc content of the culture medium in parallel with chicken metallothionein (cMT) induction, and the expression pattern of cZnT1 and cZnT4 mRNA was almost identical between WT and TKO cells. Levels of cβ-actin are shown as a control.
FIGURE 2.
ZnT1 and ZnT4 do not contribute significantly to the activation of TNAP. A, subcellular localization of hZnT1 or hZnT4 expressed in TKO cells. Stably expressed FLAG-hZnT1 (upper left-hand panel) or hZnT4-HA (lower left-hand panel) was double stained with a marker for the Golgi apparatus, GM130 (middle panels). The merged images are shown in the right-hand panels. Indirect immunofluorescence was performed using anti-FLAG or anti-HA antibodies and anti-GM130 antibodies followed by Alexa 594- or 488-conjugated secondary antibodies. B, ZnT1 and ZnT4 are unlikely to be involved in the activation of TNAP. TNAP activity was not changed in ZnT1- or ZnT4-deficient DT40 cells compared with that in WT cells, or in DT40 cells deficient in both ZnT1 and ZnT5 or ZnT4 and ZnT5, compared with that in ZnT5-deficient DT40 cells. C, TNAP activity was not increased by overexpression of hZnT1 in either ZnT1-deficient or TKO cells. Expression of FLAG-hZnT1 was confirmed by immunoblotting (lower panels). D, TNAP activity was not increased by overexpression of hZnT4 in either ZnT4-deficient or TKO cells. Expression of hZnT4-HA was confirmed by immunoblotting (lower panels). B–D, TNAP activity of the total cellular protein prepared from the indicated cells was assayed and is the mean ± S.D. of triplicate experiments. C and D, calnexin (CNX) is shown as a loading control.
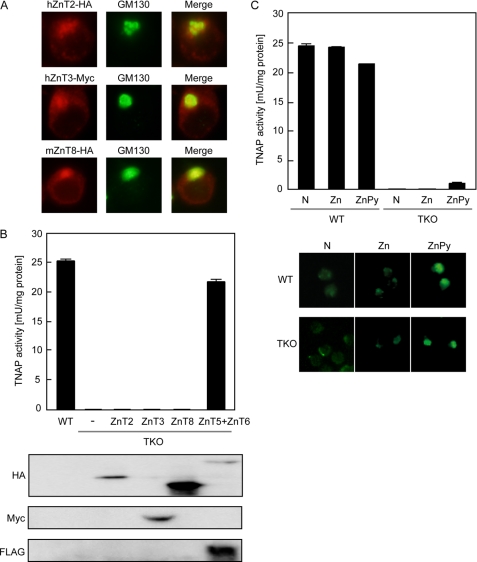
As TNAP activity was not activated by endogenously expressed ZnT4 nor ZnT1, we next examined whether other ZnTs, which have been shown to localize to and mobilize zinc into the secretory pathway but are not expressed in DT40 cells, increase TNAP activity when exogenously expressed in TKO cells. We generated TKO cells stably expressing hZnT2, hZnT3, or mZnT8, where all of them were partially localized to the Golgi apparatus (Fig. 3A), and measured TNAP activity in these cells. Here, we used ZnT2 as the exogenously expressed ZnT because the expression level of cZnT2 mRNA was very low in DT40 cells, and the partial fragment was detected only when nested PCR was performed (data not shown) (8). TNAP activity in TKO cells showed almost no increase in the presence of these exogenously expressed ZnTs, in contrast to simultaneous expression of hZnT5 and hZnT6 (Fig. 3B). Thus, in addition to ZnT1 and ZnT4, ZnT2, -3, and -8 failed to increase TNAP activity in TKO cells. To investigate whether zinc mobilization into the early secretory pathway increases TNAP activity more directly, we added zinc sulfate into the medium, but this did not increase TNAP activity in TKO cells (Fig. 3C). To penetrate more zinc ions into the pathway, we treated the cells with zinc pyrithione, a zinc ionophore, for 30 min followed by another 8-h incubation, and performed the same experiments. When zinc was added as zinc pyrithione, FluoZin-3 staining revealed more zinc ions could be mobilized into the early secretory pathway in TKO cells, similar to WT cells, despite lacking two ZnT complexes (Fig. 3C, lower panel). TNAP activity was increased by this treatment, but the effect was relatively small compared with that caused by simultaneous expression of ZnT5 and ZnT6, and it was never completely restored to that level (Figs. 3C and 4B). These results, taken together with our previous results, suggest that metalation and activation of TNAP are not simply passive binding with zinc after its mobilization into the early secretory pathway but are specific regulatory steps that require ZnT5/ZnT6 heterodimers or ZnT7 homo-oligomers.
FIGURE 3.
TNAP activity is not restored by supplementation with zinc into the early secretory pathway in TKO cells. A, subcellular localization of hZnT2, hZnT3, or mZnT8 expressed in TKO cells. Stably expressed hZnT2-HA (upper left-hand panel), hZnT3-Myc (middle left-hand panel), or mZnT8-HA (lower left-hand panel) was double stained with a marker for the Golgi apparatus, GM130 (middle panels). The merged images are shown in the right-hand panels. Indirect immunofluorescence was performed using anti-HA or anti-Myc antibodies and anti-GM130 antibodies followed by Alexa 594- or 488-conjugated secondary antibodies. B, exogenously expressed ZnTs failed to restore TNAP activity in TKO cells. The TNAP activity of the total cellular protein prepared from TKO cells expressing hZnT2, hZnT3, or mZnT8 was compared with the levels in WT and TKO cells expressing both hZnT5 and hZnT6. Expression of each ZnT was verified by immunoblotting using an antibody detecting the tag fused to each ZnT (HA, Myc, or FLAG). C, zinc supplementation into the early secretory pathway by a zinc ionophore did not significantly increase TNAP activity in TKO cells. TKO cells were cultured in normal medium (N) or normal medium supplemented with 100 μm ZnSO4 (Zn), or were treated with 5 μm zinc pyrithione (ZnPy) for 30 min and then washed twice and cultured for 8 h in normal medium. TNAP activity was measured in the total cellular protein prepared from the indicated cells and is the mean ± S.D. of triplicate experiments (upper panels). Zinc ions were detected using FluoZin-3 as described under “Experimental Procedures” (lower panels).
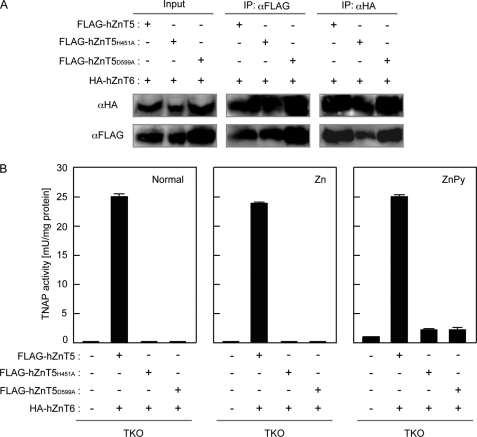
FIGURE 4.
Zinc transport activity of ZnT5/ZnT6 heterodimers is required for the activation of TNAP. A, mutants of hZnT5 interact with hZnT6, as does wild-type hZnT5. Whole cell lysates were prepared from TKO cells expressing HA-hZnT6 and the indicated wild-type or mutant FLAG-hZnT5. Tagged-hZnT5 or -hZnT6 was immunoprecipitated (IP) with antibodies against either the FLAG or HA epitopes. The immunoprecipitates were analyzed by immunoblotting using antibodies against the FLAG or HA tags. The specificity of the interaction between hZnT5 and hZnT6 has been previously verified (19, 20). To estimate the amount of FLAG-hZnT5 and HA-hZnT6 in the whole cell lysates, 10% of each aliquot was subjected to immunoblotting (Input panels). B, TNAP activity was measured using total cellular protein prepared from the indicated cells and is the mean ± S.D. of triplicate experiments. TKO cells expressing the indicated FLAG-hZnT5 and HA-hZnT6 were cultured in the same conditions as described for Fig. 3C.
To test this speculation, we used a re-expression system using hZnT5 and hZnT6, because they activate TNAP robustly, and useful mutants of hZnT5 have been reported. Replacement of essential hZnT5 hydrophilic residues such as histidine or aspartic acid with alanines, in transmembrane domains XI and XIV (D599A and H451A), abolishes the zinc transport activity of hZnT5/hZnT6 heterodimers (31). We generated TKO cells expressing transport-incompetent mutants of hZnT5 (FLAG-hZnT5D599A and FLAG-hZnT5H451A) with HA-hZnT6 and measured their TNAP activity. Physical interaction between both mutants of hZnT5 and HA-hZnT6 was detected by immunoprecipitation as in the case of wild-type hZnT5 (Fig. 4A), but TNAP activity was not restored in either cell type expressing mutant hZnT5 (Fig. 4B, left panel). Zinc mobilization into the early secretory pathway of these cells using zinc pyrithione increased TNAP activity, but the effects were small (less than 10% of that of the cells expressing both hZnT5 and hZnT6), and expression of the transport-incompetent mutants of hZnT5 with hZnT6 had almost no effect (Fig. 4B, right panel). These results indicate that the zinc transport activity of ZnT5/ZnT6 heterodimers is essential for the activation of TNAP. Moreover, ZnT5/ZnT6 heterodimers likely use unknown mechanisms to activate TNAP, in addition to supplying it with zinc.
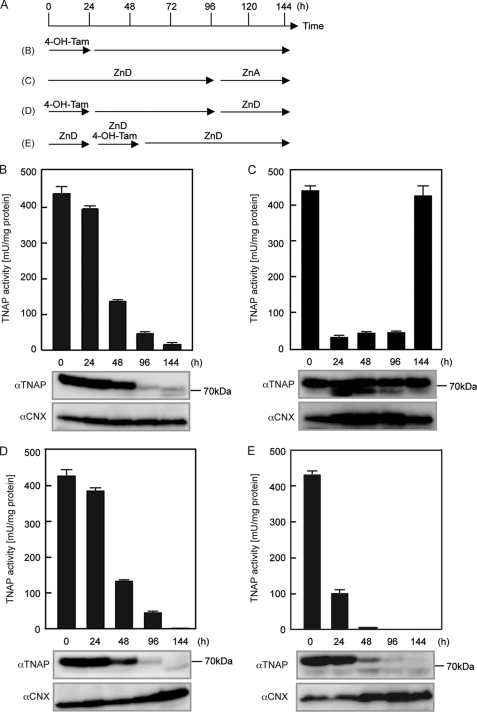
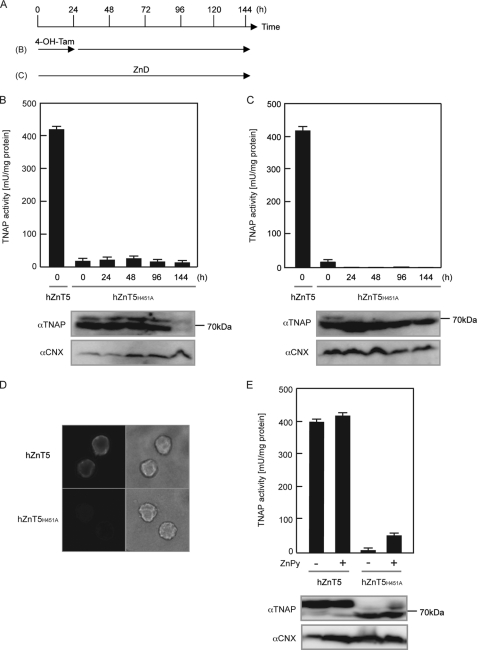
We previously reported that hTNAP exogenously expressed in DT40 cells was detected as two bands, a complex carbohydrate form (Endo-H-resistant form) and an N-linked high mannose form (Endo-H-sensitive form) (7). In DT40 cells lacking ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers, hTNAP protein was mainly detected as the Endo-H-sensitive form and was readily degraded by proteasome-mediated degradation during the secretory process (7). We next investigated in more depth the relationships between degradation (or stabilization) of hTNAP and expression of ZnT5/ZnT6 heterodimers. We generated conditional TKO cells expressing hZnT5, hTNAP, and HA-hZnT6, in which the HA-hZnT6 gene could be excised using a tamoxifen-regulated chimeric Cre-loxP system after 4-hydroxytamoxifen (4-OH-Tam) treatment. In the conditional TKO cells, hZnT5 and hTNAP are constitutively expressed at high levels because their expression is driven by the strong chicken β-actin promoter, whereas expression of HA-hZnT6 is driven by the weak CMV minimal promoter, which keeps expression of HA-hZnT6 at a very low level (∼100-fold less than that driven by the chicken β-actin promoter; supplemental Fig. S2). Thereby, expression of HA-hZnT6 was almost undetectable in the conditional TKO cells at the 48-h time point following 4-OH-Tam treatment, which enabled us to perform a direct comparison using the same cells without clonal variation in the regulation of TNAP.
TNAP activity was expressed in the conditional TKO cells (hereafter referred to as conditional TKO(WT-hZnT5) cells), and hTNAP protein was mainly detected as the Endo-H-resistant form, as in the previous study (7). After 4-OH-Tam treatment, the Endo-H-resistant form of hTNAP protein gradually decreased, almost paralleling the decreases in activity, and was significantly decreased after 96 h (Fig. 5B). At the 144-h time point, both the Endo-H-resistant and -sensitive forms of hTNAP were detected, although its overall expression level was significantly decreased (Fig. 5B). In contrast, TNAP activity in the conditional TKO(WT-hZnT5) cells rapidly decreased to basal levels when cultured in medium containing zinc-deficient FCS (zinc-deficient conditions) for 24 h, but hTNAP protein was not degraded and was detected in both the Endo-H-resistant and -sensitive forms during this time course. The addition of zinc into the zinc-deficient culture medium rapidly restored both the activity and the proportion of the Endo-H-resistant form, even after 96 h of culture in zinc-deficient conditions (Fig. 5C). Degradation of hTNAP was not zinc-dependent because hTNAP protein was still absent in the conditional TKO(WT-hZnT5) cells in zinc-deficient culture after excision of the HA-hZnT6 gene (Fig. 5D, last 48 h) or in conditional TKO(WT-hZnT5) cells in which the HA-hZnT6 gene was excised during the zinc-deficient conditions (Fig. 5E). These results indicate that the cellular zinc level does not trigger degradation of hTNAP, but a lack of ZnT5/ZnT6 heterodimers does. The Endo-H-resistant form of hTNAP also decreased in parallel with decreases in activity in conditional TKO(hZnT7) cells that express hTNAP and FLAG-hZnT7, in which the FLAG-hZnT7 gene can be excised using the same Cre-loxP system (supplemental Fig. S3). Collectively, ZnT complexes regulate the stability of hTNAP during the secretory process.
FIGURE 5.
ZnT5/ZnT6 heterodimers are important for the stability of TNAP, independent of zinc status. A, schematic representation of the time course used in the following studies using conditional TKO(WT-hZnT5) cells. Treatment with 4-OH-Tam excises the HA-hZnT6 gene, which leads to a lack of hZnT5/hZnT6 heterodimers in the conditional TKO(WT-hZnT5) cells (see supplemental Fig. S2). ZnD, cultured in medium containing 10% zinc-deficient FCS; ZnA, ZnD plus 20 μm ZnSO4. B, stabilization of hTNAP requires hZnT5/hZnT6 heterodimers. Conditional TKO(WT-hZnT5) cells were cultured in medium containing normal FCS for 144 h (with 4-OH-Tam for the first 24 h). C, inactive hTNAP, when cultured in zinc-deficient conditions, was not degraded in conditional TKO(WT-hZnT5) cells. Conditional TKO(WT-hZnT5) cells were cultured in medium containing 10% zinc-deficient FCS for 96 h and then cultured for a further 48 h after supplementation with 20 μm ZnSO4. D, degradation of hTNAP triggered by the lack of hZnT5/hZnT6 heterodimers was not inhibited by zinc deficiency. The conditional TKO(WT-hZnT5) cells were cultured in the same conditions as in B for up to 96 h and then cultured in zinc-deficient conditions for the last 48 h. E, stabilization of hTNAP by hZnT5/hZnT6 heterodimers can operate in zinc-deficient conditions. TKO(WT-hZnT5) cells were cultured in zinc-deficient conditions for 24 h, treated with 4-OH-Tam for 24 h, and then cultured in zinc-deficient conditions for 96 h. B–E, TNAP activity at the indicated time points (upper graphs) is expressed as described above. The expression level of hTNAP protein at each time point is shown in the lower panels; 20 μg of total cellular protein was loaded in each lane. hTNAP was detected by anti-hTNAP antibody as two bands of ∼60 kDa (Endo-H-sensitive form) and ∼80 kDa (Endo-H-resistant form). Calnexin (CNX) is shown as a loading control. Calnexin expression is moderately increased in D and E because the unfolded protein response (UPR) was exacerbated by the lack of zinc transport complexes in the zinc-deficient conditions, as described previously (7). Equal loading was confirmed by Coomassie Brilliant Blue staining (data not shown).
To clarify whether zinc transport activity is essential for protein stabilization, we examined the expression level of hTNAP using the same Cre-loxP system but with transport-incompetent mutants of hZnT5 (hZnT5H451A; hereafter referred to as conditional TKO(mut-hZnT5) cells). In the conditional TKO(mut-hZnT5) cells, hTNAP was constitutively expressed mainly as the Endo-H-sensitive form (Fig. 6, B and C); thus, it was barely trafficked to the plasma membrane (Fig. 6D). Consistent with Fig. 4, the TNAP activity of the conditional TKO(mut-hZnT5) cells was significantly reduced compared with that of the conditional TKO(WT-hZnT5) cells, although residual activity (less than 5% of that in the conditional TKO(WT-hZnT5) cells) was detected. This residual activity is attributed to minor pathway(s) that are independent of ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers. Although TNAP activity was not changed after treatment with 4-OH-Tam, constitutively expressed hTNAP was degraded in the conditional TKO(mut-hZnT5) cells, as it was in the conditional TKO(WT-hZnT5) cells (144 h in Fig. 6B). Expression of hTNAP was not significantly changed in the conditional TKO(mut-hZnT5) cells cultured in zinc-deficient conditions, although its activity was moderately decreased (Fig. 6C). We confirmed that zinc supplementation using zinc pyrithione failed to significantly increase either TNAP activity or the level of hTNAP protein in the conditional TKO(mut-hZnT5) cells before 4-OH-Tam treatment, indicating that hTNAP is sufficiently stabilized by heterodimers of hZnT5H451A and hZnT6 in our experimental conditions (Fig. 6E). Taken together, ZnT5/ZnT6 heterodimers are required for the stabilization of TNAP in the early secretory process, which are independent of zinc transport activity.
FIGURE 6.
Presence of ZnT5/ZnT6 heterodimers regulates the stability of TNAP independently of their zinc transport activities. A, schematic representation of the time course used in the following studies using conditional TKO(mut-hZnT5) cells. ZnD, cultured in medium containing 10% zinc-deficient FCS. B, expression of the zinc transport-incompetent hZnT5 mutant and hZnT6 stabilizes the hTNAP protein. C, inactive hTNAP protein in the conditional TKO(WT-hZnT5) cells was not degraded even when cultured in zinc-deficient conditions. D, hTNAP was barely trafficked to the plasma membrane in the conditional TKO(mut-hZnT5) cells. Conditional TKO(WT-hZnT5) cells and TKO(mut-hZnT5) cells were fixed with formaldehyde, and indirect immunofluorescence was performed without permeabilization. E, expression of hTNAP was not significantly changed in the conditional TKO(mut-hZnT5) cells by zinc supplementation. Conditional TKO(mut-hZnT5) cells were cultured in the absence (−) or presence (+) of zinc pyrithione (ZnPy) for 30 min and then cultured for 8 h before harvesting the cells. B, C, and E, TNAP activity (upper graphs) and the expression level of TNAP protein (lower panels) are shown in the same way as for Fig. 5, B–E. CNX, calnexin.
DISCUSSION
A number of ZnTs have been identified in the secretory pathway, but detailed information on their cellular functions is far from complete (32–35). Fortunately, DT40 cells moderately express almost all ZnTs that do not show cell type-specific expression, such as ZnT3 or ZnT8, although the expression of cZnT2 mRNA is quite low (8). This enabled us to perform detailed analysis of the functions of ZnTs in the supply of zinc to zinc-requiring enzymes such as TNAP. Our findings revealed that over 95% of TNAP protein is activated by ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers and that the other ZnTs barely contribute, despite having zinc transport activity in the secretory pathway when exogenously expressed (25, 36, 37). Treatment with zinc pyrithione increased the zinc content in the early secretory pathway and, consistently, increased TNAP activity via other minor pathways, but this effect was not comparable in magnitude with that of ZnT5/ZnT6 heterodimers and/or ZnT7 homo-oligomers. Here, we elucidated the reason why both complexes specifically activate TNAP, mainly by means of re-expression of ZnT5/ZnT6 heterodimers.
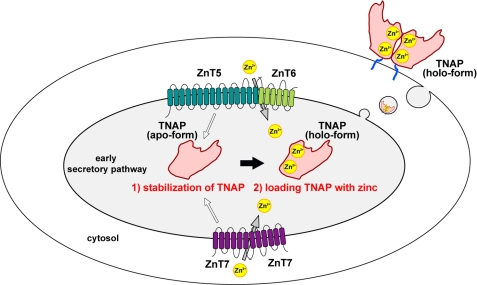
TNAP activity decreased significantly in TKO cells, which highlights the requirement for the zinc transport activity of both complexes (ZnT5-ZnT6 and ZnT7) to supply zinc to hTNAP. In this study, we demonstrated this by using a combination of methods, including disruption/re-expression of ZnT1 and ZnT4, exogenous expression of ZnT2, ZnT3, or ZnT8, enforced zinc addition using zinc pyrithione, and the re-expression of zinc transport-incompetent mutants of hZnT5 with hZnT6 in TKO cells. However, inconsistent with our previous results that hTNAP protein was degraded when expressed in DT40 cells deficient in both ZnT complexes (7), hTNAP was constitutively expressed and not readily degraded in TKO cells expressing zinc transport-incompetent mutants of hZnT5 with hZnT6 (Fig. 6, B and C). This discrepancy excludes the possibility that decreases in zinc content in the early secretory pathway result in the degradation of hTNAP. A simple comparison using different clones expressing various combinations of ZnT mutants would be possible, but the results would be difficult to verify because of clonal variation. Therefore, we employed a tamoxifen-regulated chimeric Cre-loxP system to express hZnT5/hZnT6 heterodimers, including mutant hZnT5, which enabled us to show conclusively that ZnT5/ZnT6 heterodimers regulate the stability of hTNAP. Considering that hTNAP was degraded in ZnT5−ZnT7−/− cells expressing endogenous cZnT6 (7), and in the conditional TKO(WT-hZnT5) cells after excision of the HA-hZnT6 gene where hZnT5 was still being expressed, the expression of each monomer (ZnT5 or ZnT6) was insufficient for stabilization of hTNAP. Therefore, formation of the complexes is likely critical, and this would also be true of ZnT7. The unique feature of heterodimer formation of ZnT5/ZnT6 clarifies this point, which is one of the reasons why we predominantly used the hZnT5/hZnT6 re-expression system in this study. Thus, ZnT5/ZnT6 heterodimers (and ZnT7 homo-oligomers) activate TNAP with a two-step mechanism: regulating the stability of TNAP independently of its zinc transport activity and supplying zinc to TNAP in the early secretory pathway (Fig. 7).
FIGURE 7.
Model for the two-step mechanism for the activation of TNAP by ZnTs. ZnT5/ZnT6 heterodimers and ZnT7 homo-oligomers activate TNAP with two steps as follows: first, regulating the protein stability of apo-form of TNAP independently of their zinc transport activity (white arrow); second, enzyme conversion from the apo- to the holo-form of TNAP by loading zinc for expressing the activity in the early secretory pathway. The holo-form of TNAP is trafficked and localized to the plasma membrane as a homodimer via a glycophosphatidylinositol anchor, which possesses two zinc ions in the active site of each monomer.
Our finding here is somewhat unexpected, but it is in accordance with increasing evidence that metal transporters possess functions other than simply transporting their substrate metals. For example, the Xenopus copper transporter CTR1 is thought to be a critical component of the membrane-associated FGF signaling complex, transducing FGF signals independently of copper (38). ZnT1 has been shown to regulate Raf1 biological activity, down-regulate transcription factors stimulated by MTF1, c-Jun, and Elk, or inhibit L-type calcium channels by decreasing the trafficking of the L-type calcium channel α1-subunit (28, 39, 40). Our findings here are novel in that ZnTs are involved in the regulation of protein stability in addition to supplying zinc. What is the mechanism to explain this novel ZnT function? Given that the above ZnT1 functions are mediated via interaction with Raf1, EVERs, or the L-type calcium channel β-subunit, the most plausible explanation is that ZnT5/ZnT6 heterodimers interact directly with TNAP to stabilize it. A recent report by Qin et al. (41) that ATP7A interacts directly with extracellular superoxide dismutase to supply copper in a copper-dependent manner in the early secretory pathway potentiates this idea. However, we have not yet obtained data to support this by immunoprecipitation using hZnT5/hZnT6 and hTNAP with or without cross-linkers. In our immunoprecipitation conditions, we detected an interaction of hZnT6 with calnexin only when cultured in zinc-deficient conditions,3 which may suggest that interaction between ZnT5/ZnT6 heterodimers and TNAP would be very weak and transient, if it even existed. In general, ZnTs have relatively long N- and C-terminal portions and a long cytoplasmic His-rich loop in the cytosol, but very short loops between the transmembrane domains that face the lumen. This may make it difficult to detect the interaction of hZnT5/hZnT6 heterodimers with hTNAP and requires further investigation.
In the conditional TKO(mut-hZnT5) cells, constitutively expressed hTNAP was mainly detected as the Endo-H-sensitive form, and it seems also to be in the apo-form because its activity was significantly reduced (see Fig. 6, B and C). The Endo-H-sensitive form of hTNAP increased accompanied by a reduction in its activity in the conditional TKO(WT-hZnT5) cells cultured in zinc-deficient conditions (see Fig. 5C, 24–96 h). These results indicate that the apo-form of hTNAP is not preferentially degraded and is not predominantly trafficked to the medial Golgi apparatus, when ZnT5/ZnT6 heterodimers are expressed. In contrast, when hTNAP degradation was blocked by the proteasome inhibitor MG132 and/or the lysosome inhibitor bafilomycin in the conditional TKO(WT-hZnT5) cells after 4-OH-Tam treatment, the protein seemed to be in the apo-form because of the reduction in its activity (supplemental Fig. S4). This is despite the fact that it was detected as the Endo-H-resistant form (and thus trafficked to the medial Golgi apparatus) (supplemental Fig. S4). Together, this suggests that hTNAP in the apo-form is promptly degraded if ZnT5/ZnT6 heterodimers are not present and that ZnT5/ZnT6 heterodimers are involved in the regulation of TNAP folding before it reaches the medial Golgi. Some missense mutations in the TNAP gene that cause hypophosphatasia have been shown to result in degradation of the TNAP protein due to misfolding (42, 43). Thus, the stabilization of TNAP by ZnT complexes is extremely important in conferring its enzymatic activity.
In contrast to the present results, heat-resistant placenta ALP is constitutively expressed as the apo-form and is trafficked to the plasma membrane in ZnT5−ZnT7−/− cells (8). Recently, Qiao et al. (44) showed a similar regulation of the yeast ALP Pho8. Pho8 is not activated by Msc2/Zrg17 hetero-oligomers, which are the yeast counterpart of ZnT5/ZnT6 heterodimers (6, 45), but is activated by vacuolar ZnTs, Cot1 or Zrc1, which are orthologs of ZnT4. The apo-form of Pho8 was present in vacuoles in mutant yeast deficient in both Cot1 and Zrc1 but was degraded when cultured in zinc-deficient conditions. Moreover, its activity was restored by zinc supplementation using zinc pyrithione and was partially restored by exogenous expression of mZnT4 (44). Thus, even ALP proteins activated by ZnTs demonstrate totally different activation events, suggesting that there is a variety of activation processes for zinc-requiring enzymes in the secretory pathway.
Our findings raise the question as to where ZnT5/ZnT6 heterodimers regulate TNAP stability. As the Golgi lumen is more acidic (pH 6.0–6.7) than the ER (pH 7.0–7.4) (46), ZnT5/ZnT6 heterodimers that function as Zn2+/H+ exchangers would become more active in the Golgi apparatus (31). As described above, the degradation of TNAP seems to occur in the Endo-H-sensitive form, which suggests the possibility that ZnT5/ZnT6 heterodimers stabilize TNAP in the ER but supply it with zinc in the Golgi apparatus. That the zinc transport activity of the ZnT complexes is required for the activation of TNAP indicates that these steps may operate sequentially. The elucidation of how, when, and where ZnT5/ZnT6 heterodimers (or ZnT7 homo-oligomers) regulate the stability of TNAP and supply it with zinc will provide insight into the activation mechanisms used by other zinc-requiring enzymes in the secretory pathway.
Supplementary Material
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to A. F. and T. K.), grants-in-aid for scientific research from the Ministry of Health, Labor, and Welfare of Japan (to S. E.), Nestle Nutrition Council, Japan, 2010 research grant from Danone Institute of Japan, Central Miso Research Institute, Kieikai Research Foundation, Yamada Bee Farm grant for Honeybee Research, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Fuji Foundation for Protein Research (to T. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and an additional reference.
A. Fukunaka and T. Kambe, unpublished data.
- ER
- endoplasmic reticulum
- ZnT
- Zn transporter
- ALP
- alkaline phosphatase
- TNAP
- tissue nonspecific ALP
- TKO
- triple knockout
- 4-OH-Tam
- 4-hydroxy tamoxifen
- h
- human
- Endo-H
- endo-β-N-acetylglucosaminidase H
- c
- chicken
- m
- mouse.
REFERENCES
- 1. Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
- 2. Trombetta E. S., Parodi A. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 649–676 [DOI] [PubMed] [Google Scholar]
- 3. Vembar S. S., Brodsky J. L. (2008) Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szigeti R., Kellermayer R. (2006) J. Invest. Dermatol. 126, 2370–2376 [DOI] [PubMed] [Google Scholar]
- 5. Brini M., Carafoli E. (2009) Physiol. Rev. 89, 1341–1378 [DOI] [PubMed] [Google Scholar]
- 6. Ellis C. D., Wang F., MacDiarmid C. W., Clark S., Lyons T., Eide D. J. (2004) J. Cell. Biol. 166, 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishihara K., Yamazaki T., Ishida Y., Suzuki T., Oda K., Nagao M., Yamaguchi-Iwai Y., Kambe T. (2006) J. Biol. Chem. 281, 17743–17750 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T., Ishihara K., Migaki H., Matsuura W., Kohda A., Okumura K., Nagao M., Yamaguchi-Iwai Y., Kambe T. (2005) J. Biol. Chem. 280, 637–643 [DOI] [PubMed] [Google Scholar]
- 9. Fang Y., Sugiura R., Ma Y., Yada-Matsushima T., Umeno H., Kuno T. (2008) Mol. Biol. Cell 19, 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukada T., Civic N., Furuichi T., Shimoda S., Mishima K., Higashiyama H., Idaira Y., Asada Y., Kitamura H., Yamasaki S., Hojyo S., Nakayama M., Ohara O., Koseki H., Dos Santos H. G., Bonafe L., Ha-Vinh R., Zankl A., Unger S., Kraenzlin M. E., Beckmann J. S., Saito I., Rivolta C., Ikegawa S., Superti-Furga A., Hirano T. (2008) PloS ONE 3, e3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor K. M., Vichova P., Jordan N., Hiscox S., Hendley R., Nicholson R. I. (2008) Endocrinology 149, 4912–4920 [DOI] [PubMed] [Google Scholar]
- 12. Matsuura W., Yamazaki T., Yamaguchi-Iwai Y., Masuda S., Nagao M., Andrews G. K., Kambe T. (2009) Biosci. Biotechnol. Biochem. 73, 1142–1148 [DOI] [PubMed] [Google Scholar]
- 13. Overall C. M., López-Otín C. (2002) Nat. Rev. Cancer 2, 657–672 [DOI] [PubMed] [Google Scholar]
- 14. Natesh R., Schwager S. L., Sturrock E. D., Acharya K. R. (2003) Nature 421, 551–554 [DOI] [PubMed] [Google Scholar]
- 15. Turner A. J., Tanzawa K. (1997) FASEB J. 11, 355–364 [DOI] [PubMed] [Google Scholar]
- 16. Seals D. F., Courtneidge S. A. (2003) Genes Dev. 17, 7–30 [DOI] [PubMed] [Google Scholar]
- 17. Millán J. L., Fishman W. H. (1995) Crit. Rev. Clin. Lab. Sci. 32, 1–39 [DOI] [PubMed] [Google Scholar]
- 18. Purkerson J. M., Schwartz G. J. (2007) Kidney Int. 71, 103–115 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki T., Ishihara K., Migaki H., Ishihara K., Nagao M., Yamaguchi-Iwai Y., Kambe T. (2005) J. Biol. Chem. 280, 30956–30962 [DOI] [PubMed] [Google Scholar]
- 20. Fukunaka A., Suzuki T., Kurokawa Y., Yamazaki T., Fujiwara N., Ishihara K., Migaki H., Okumura K., Masuda S., Yamaguchi-Iwai Y., Nagao M., Kambe T. (2009) J. Biol. Chem. 284, 30798–30806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whyte M. P. (2010) Ann. N.Y. Acad. Sci. 1192, 190–200 [DOI] [PubMed] [Google Scholar]
- 22. Fukushi-Irié M., Ito M., Amaya Y., Amizuka N., Ozawa H., Omura S., Ikehara Y., Oda K. (2000) Biochem. J. 348, 633–642 [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue K., Matsuda K., Itoh M., Kawaguchi H., Tomoike H., Aoyagi T., Nagai R., Hori M., Nakamura Y., Tanaka T. (2002) Hum. Mol. Genet. 11, 1775–1784 [DOI] [PubMed] [Google Scholar]
- 24. Kambe T., Andrews G. K. (2009) Mol. Cell. Biol. 29, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salazar G., Falcon-Perez J. M., Harrison R., Faundez V. (2009) PLoS One 4, e5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michalczyk A. A., Allen J., Blomeley R. C., Ackland M. L. (2002) Biochem. J. 364, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujimori A., Tachiiri S., Sonoda E., Thompson L. H., Dhar P. K., Hiraoka M., Takeda S., Zhang Y., Reth M., Takata M. (2001) EMBO J. 20, 5513–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lazarczyk M., Pons C., Mendoza J. A., Cassonnet P., Jacob Y., Favre M. (2008) J. Exp. Med. 205, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang L., Kirschke C. P., Gitschier J. (2002) J. Biol. Chem. 277, 26389–26395 [DOI] [PubMed] [Google Scholar]
- 30. Ranaldi G., Perozzi G., Truong-Tran A., Zalewski P., Murgia C. (2002) Am. J. Physiol. Renal Physiol. 283, F1365–F1375 [DOI] [PubMed] [Google Scholar]
- 31. Ohana E., Hoch E., Keasar C., Kambe T., Yifrach O., Hershfinkel M., Sekler I. (2009) J. Biol. Chem. 284, 17677–17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmiter R. D., Huang L. (2004) Pflugers Arch. 447, 744–751 [DOI] [PubMed] [Google Scholar]
- 33. Kambe T., Yamaguchi-Iwai Y., Sasaki R., Nagao M. (2004) Cell. Mol. Life Sci. 61, 49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kambe T., Weaver B. P., Andrews G. K. (2008) Genesis 46, 214–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eide D. J. (2006) Biochim. Biophys. Acta 1763, 711–722 [DOI] [PubMed] [Google Scholar]
- 36. Palmiter R. D., Cole T. B., Findley S. D. (1996) EMBO J. 15, 1784–1791 [PMC free article] [PubMed] [Google Scholar]
- 37. Chimienti F., Devergnas S., Pattou F., Schuit F., Garcia-Cuenca R., Vandewalle B., Kerr-Conte J., Van Lommel L., Grunwald D., Favier A., Seve M. (2006) J. Cell Sci. 119, 4199–4206 [DOI] [PubMed] [Google Scholar]
- 38. Haremaki T., Fraser S. T., Kuo Y. M., Baron M. H., Weinstein D. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12029–12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy S., Beharier O., Etzion Y., Mor M., Buzaglo L., Shaltiel L., Gheber L. A., Kahn J., Muslin A. J., Katz A., Gitler D., Moran A. (2009) J. Biol. Chem. 284, 32434–32443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jirakulaporn T., Muslin A. J. (2004) J. Biol. Chem. 279, 27807–27815 [DOI] [PubMed] [Google Scholar]
- 41. Qin Z., Itoh S., Jeney V., Ushio-Fukai M., Fukai T. (2006) FASEB J. 20, 334–336 [DOI] [PubMed] [Google Scholar]
- 42. Fukushi M., Amizuka N., Hoshi K., Ozawa H., Kumagai H., Omura S., Misumi Y., Ikehara Y., Oda K. (1998) Biochem. Biophys. Res. Commun. 246, 613–618 [DOI] [PubMed] [Google Scholar]
- 43. Ishida Y., Komaru K., Ito M., Amaya Y., Kohno S., Oda K. (2003) J. Biochem. 134, 63–70 [DOI] [PubMed] [Google Scholar]
- 44. Qiao W., Ellis C., Steffen J., Wu C. Y., Eide D. J. (2009) Mol. Microbiol. 72, 320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ellis C. D., Macdiarmid C. W., Eide D. J. (2005) J. Biol. Chem. 280, 28811–28818 [DOI] [PubMed] [Google Scholar]
- 46. Hassinen A., Rivinoja A., Kauppila A., Kellokumpu S. (2010) J. Biol. Chem. 285, 17771–17777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.