Abstract
CorA is a family of divalent cation transporters ubiquitously present in bacteria and archaea. Although CorA can transport both Mg2+ and Co2+ almost equally well, its main role has been suggested to be that of primary Mg2+ transporter of prokaryotes and hence the regulator of Mg2+ homeostasis. The reason is that the affinity of CorA for Co2+ is relatively low and thus considered non-physiological. Here, we show that Thermotoga maritima CorA (TmCorA) is incapable of regulating the Mg2+ homeostasis and therefore cannot be the primary Mg2+ transporter of T. maritima. Further, our in vivo experiments confirm that TmCorA is a highly selective Co2+ transporter, as it selects Co2+ over Mg2+ at >100 times lower concentrations. In addition, we present data that show TmCorA to be extremely thermostable in the presence of Co2+. Mg2+ could not stabilize the protein to the same extent, even at high concentrations. We also show that addition of Co2+, but not Mg2+, specifically induces structural changes to the protein. Altogether, these data show that TmCorA has the role of being the transporter of Co2+ but not Mg2+. The physiological relevance and requirements of Co2+ in T. maritima is discussed and highlighted. We suggest that CorA may have different roles in different organisms. Such functional diversity is presumably a reflection of minor, but important structural differences within the CorA family that regulate the gating, substrate selection, and transport.
Keywords: Bacterial Metabolism, Ion Channels, Membrane Function, Membrane Proteins, Membrane Trafficking, Metals, Transporter
Introduction
Divalent metal ions are highly essential for cellular processes in all life forms. The supply of these ions to cells and organelles at appropriate levels is therefore critical. As a result, organisms have developed transport systems for maintaining adequate concentrations of intracellular divalent cations. Distortions in these systems cause various pathological conditions in higher eukaryotes and are lethal for microorganisms. CorA is a family of divalent cation transporters that is found in most bacteria and archaea (1, 2). This family has been studied extensively at a functional level using homologues from Escherichia coli, Salmonella typhimurium, Methanococcus jannaschii, and Haemophilus influenza as model proteins (3–8). These studies have shown that CorA is able to transport Mg2+, Co2+, and Ni2+ with the binding affinities of 15–20, 20–40, and 200–400 μm, respectively (4, 8). However, because Co2+ and Ni2+ are trace elements, the required concentrations are considered non-physiological for these organisms, thus leaving Mg2+ as the main substrate of CorA. Nevertheless, the rather similar affinity of CorA for both Mg2+ and Co2+ per se indicates that it is not very selective, as each of these ions can readily inhibit the uptake of the other one via CorA.
Rather recently, crystal structures of CorA from the hyperthermophilic organism Thermotoga maritima were determined (9–11). Although some metal binding sites were identified and important structural features of the protein were revealed, the molecular mechanism for the ion transport through CorA remained unsolved. This is despite further functional characterizations of the T. maritima CorA (TmCorA)3 (12, 13).
Based on the amino acid sequence and phylogenic analyses, the CorA family can be divided into at least two rather distinct subgroups (14). In this classification, CorAs from E. coli, S. typhimurium, and H. influenza belong to one group (subgroup B), whereas TmCorA belongs to the other group (subgroup A), which is distinguished largely by CorAs originating from extremophilic bacteria. The latter opens up possibilities to find new primary roles of CorA, as the physiological conditions for many of the organisms from subgroup A are significantly different from those of E. coli and S. typhimurium, for example. Together with the fact that CorA has the ability to bind multiple substrates, one could speculate that there are differences in the physiological roles of CorA between the two subgroups. In other words, is it possible that a CorA from subgroup A has other primary substrates than Mg2+? If this would be the case, it could be a plausible explanation for why functional studies of TmCorA using Mg2+ have failed to describe how CorA transports its substrate across the membrane (12, 13). In the crystal structure of TmCorA, both Mg2+ and Co2+ were found at the metal-binding sites (9). Interestingly, in TmCorA crystals, Co2+ could outcompete Mg2+ for the metal binding sites at a four times lower concentration (9). This was the first indication that TmCorA might have a higher affinity for Co2+ over Mg2+, hence suggesting Co2+ as its primary substrate. In the present study, we have explored the substrate preference of TmCorA by performing in vitro and in vivo studies. Our data clearly show that TmCorA is specifically thermostabilized and undergoes structural changes through interactions with Co2+ but not Mg2+. Moreover, we show through in vivo competition studies that TmCorA can select Co2+ over Mg2+ for transport at >100 times lower concentrations. We also show that in contrast to E. coli CorA (EcCorA) and S. typhimurium CorA (StCorA), TmCorA is unable to regulate the Mg2+ uptake and intracellular Mg2+ concentration. Altogether, our data show that TmCorA is a highly selective Co2+ transporter and indicate that Co2+, but not Mg2+, is the primary substrate of TmCorA. Hence, this is the first study indicating functional diversity within the CorA family, which reflects important structural differences among the CorA proteins.
EXPERIMENTAL PROCEDURES
Cloning of CorA Homologues
The T. maritima corA gene was cloned into a pBAD vector as described previously (9). The E. coli corA gene was cloned into a pBAD vector (Invitrogen) according to the manufacturer's protocol. The gene was amplified by PCR using Phusion Hotstart II (Finnzyme) and the following primers: forward, 5′-AGCGCGCCTCGAGCTACCAGCCAGTTCTTGCGCTTAA-3′; and reverse, 5′-GCGCGCCATGGGTCACCATCATCATCATCA-3′. The PCR-amplified gene was inserted in the pBAD vector after 3 h of cleavage with NcoI and XhoI (New England Biolabs), followed by 16-h ligation with T4 DNA ligase (New England Biolabs). All reactions were performed at room temperature, except for the digestion, which was performed at 37 °C. Both TmCorA and EcCorA contained the MHHHHHHSSGVDLGTENLYFQSM sequence at the N terminus.
Overexpression and Purification of TmCorA
TmCorA was overexpressed in E. coli and purified as described previously (9), with the only exception that 1% Cymal 5 (Anatrace) was used for solubilization, and the protein was subsequently purified in the presence of 0.1% Cymal 5. The His tag was removed from the column by adding 120 μm Tobacco Etch Virus (TEV) protease. The column was incubated at room temperature overnight, and cleaved TmCorA was eluted with buffer A (20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.5 mm tris(2-carboxyethyl) phosphine (TCEP), 40 mm imidazole, and 0.1% (w/v) Cymal 5). The eluate was incubated with 5 mm EDTA to remove any divalent metals and then desalted on a PD10 column (GE Healthcare) equilibrated with desalting buffer (20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.5 mm TCEP, 0.1% Cymal 5).
Thermostability of TmCorA in the Presence of Different Metals
Protein was diluted to a concentration of about 0.5 mg/ml. The solution was divided in 50-μl aliquots, and 1 μl of stock solution of different metals was added. The samples were incubated at room temperature for about 30 min before heating in a thermocycler (Agilent Technologies). Samples were incubated for 10 min at each temperature point. To remove precipitated protein, the samples were applied on a 96-well filter-plate (0.65 μm) (Millipore). The yield of the filtered protein was analyzed by SDS-PAGE. Protein-bands were Coomassie-stained using Simply Blue Safe Stain (Invitrogen).
Thermal Aggregation Shift Assay
Purified TmCorA at 0.2 mg/ml was mixed with various concentrations of Co2+ or Mg2+ and aliquoted in a final volume of 50 μl into a clear-bottom, 384-well plate (Nunc). The mixture was covered with 45 μl of mineral oil (Sigma-Aldrich) to avoid evaporation. The mixtures were then gradually heated from 25 °C to 80 °C at 1 °C/min using StarGazer-384 (Harbinger Biotech), and the data were analyzed with Bioactive software (Harbinger Biotech) (15).
Co2+ Transport Assay
The E. coli MG1655 with knocked-out corA (ΔE. coli) (National Institute of Genetics, Japan) was transformed with corA from T. maritima in pBAD. The wild-type E. coli MG1655 and the ΔE. coli, both containing empty pBAD, were also used as positive and negative controls, respectively. Cultures were then grown overnight at 37 °C in Luria Broth (LB) medium (ForMedium, UK) supplemented with 100 μg/ml ampicillin. The overnight cultures were then diluted 50 times with LB medium supplemented with 0.02% (w/v) l-arabinose and 100 μg/ml ampicillin, followed by incubation at 37 °C for 3 h. Cells were harvested by centrifugation at 3000 × g for 5 min at room temperature. Pellets were washed twice with 10 ml N buffer (5 mm KCl, 7.5 mm (NH4)2SO4, 0.5 mm K2SO4, 1 mm KH2PO4, and 0.1 m Tris (pH 7.4)). The washed pellets were then resuspended in N buffer containing Co2+ at various concentrations to a final A600 of 0.15. The mixture was incubated 10 min at 37 °C. Three parts LB medium containing the same concentrations of Co2+ was added into each reaction and incubated at 37 °C for another 3 h. The final A600 was recorded and analyzed in comparison with the starting A600. The competition assays were done similarly by including Mg2+ or Mn2+.
Mg2+ Transport Assay
The S. typhimurium strain MM281, depleted from all Mg2+ transporters, was transformed with corA from T. maritima and E. coli, respectively. MM1278, a special strain of MM281 that encodes StCorA, as well as MM281 transformed with empty pBAD, were also used as positive and negative controls, respectively. Cultures were then grown overnight at 37 °C in LB medium supplemented with 100 μg/ml ampicillin, 50 μg/ml kanamycin, 34 μg/ml chloramphenicol, and 100 mm MgSO4. Cells were harvested by centrifugation at 3000 × g for 5 min at room temperature and then washed three times with LB medium. The washed pellets were then resuspended in LB medium supplemented with 0.02% l-arabinose and antibiotics as well as Mg2+ at various concentrations to a final A600 of 0.1. The cultures were grown at 37 °C for 10 h and the A600 was recorded every 2 h. The competition assays were done similarly by including Co2+.
Fluorescence Measurements
The protein was diluted to 6.4 μm in a desalting buffer, and the tryptophan fluorescence was measured by excitation at 283 nm and scanning the emission between 295 and 425 nm (Cary Eclipse). The tryptophan fluorescence-quenching effects of Mg2+, Ni2+, and Co2+ were determined by titration of each of these metal ions.
SDS-PAGE and Western Blot Analysis of the Whole Cells
The volume of cell cultures were adjusted to reach the same A600 for all the samples. Then, 10 μl of total cells was prepared and loaded on 4–12% gels according to the manufacturer's recommendations (Invitrogen). The protein bands were then transferred into nitro cellulose membranes using I-Blot according to the manufacturer's protocol (Invitrogen). The bands were then detected using HRP-conjugated His probe and West-Pico according to the manufacturer's protocol (Pierce).
RESULTS
Thermostability of TmCorA Is Improved with the Substrate
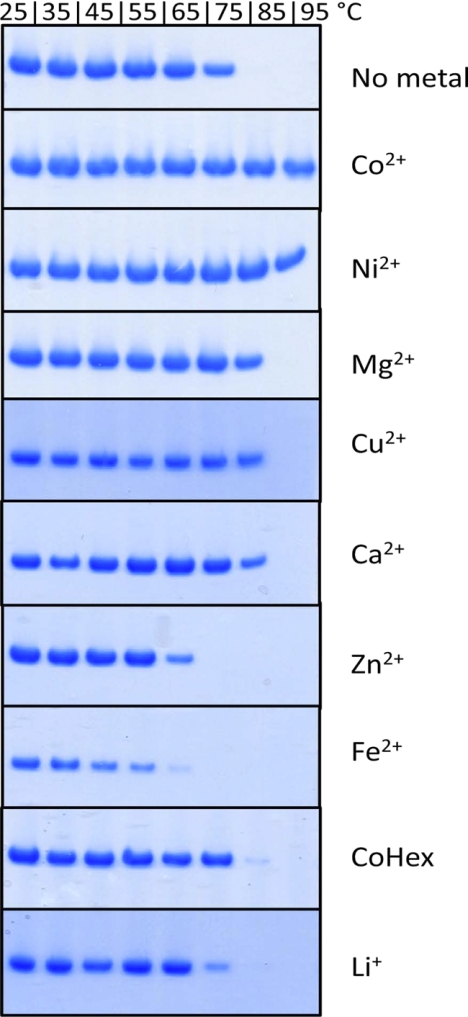
To test the thermostability of TmCorA, the isolated protein was gradually heated from 25 °C to 95 °C, and samples were taken for analysis at every 10 °C increment. The precipitated (i.e. destabilized) protein was removed by filtration. The stability of the protein at each temperature was monitored through analyzing the level of retained protein with SDS-PAGE. In the absence of divalent metals, TmCorA was totally stable up to 65 °C (Fig. 1). At 75 °C, almost 50% of the protein had precipitated, and complete denaturation was observed at 85 °C. When 1 mm MgCl2 was added to the buffer, TmCorA thermostability was improved, and the protein was almost totally stable at 85 °C. However, in the presence of 1 mm CoCl2 or NiCl2, TmCorA was almost fully stable at 95 °C. These results indicate that Co2+ and Ni2+ are more competent than Mg2+ to keep TmCorA stable at temperatures that are physiologically relevant. In the presence of cobalt hexamine, the traditional inhibitor of CorA, no significant changes in the protein stability in comparison with the control were observed (Fig. 1).
FIGURE 1.
Thermostability of TmCorA in the presence of 1 mm divalent cations as well as cobalt-hexamine (CoHex) and lithium (Li+) at different temperatures. The control (C) shows the thermostability of the protein without any additives.
To verify whether the stabilizing effect of the metal ions were relevant and specific, we also tested thermostability of CorA in the presence of non-substrate metal ions. Ca2+ showed the same stabilizing effect as Mg2+, whereas Fe2+ and Zn2+ showed a negative effect on the stability of TmCorA, already causing protein destabilization at 65 °C or even lower temperatures. Thus, the thermostability of CorA was presumably specifically improved with the metal substrates and not generally by any divalent metal ions.
Co2+ and Ni2+ Specifically Stabilize TmCorA at Low Concentrations
A metal ion concentration of 1 mm is a relatively high substrate concentration and may not reflect the physiologically relevant concentrations for T. maritima. Therefore, the stabilizing effects obtained by the metals at this concentration could be consequences of nonspecific binding to the protein. To explore how specific each substrate thermostabilizes the protein, a titration of metal ions from 10 to 1000 μm was performed at 85 °C because at this temperature, all three known substrates (Mg2+, Co2+, and Ni2+) could stabilize the protein. Our analysis showed that below 1 mm Mg2+ (same as the non-substrate ion Ca2+) was no longer able to keep the protein stable at 85 °C (Fig. 2). On the other hand, thermostabilization could be detected already in the presence of 80 μm of Co2+ or Ni2+, and a major fraction of the protein remained stable in the presence of ≥200 μm CoCl2 or NiCl2 (Fig. 2). To further investigate the specificity of the stabilizing effect of Co2+ and Ni2+ on TmCorA, we performed a competition study in which titration of Co2+ and Ni2+ was done in the presence of 1 mm MgCl2. Interestingly, Mg2+ showed no negative effect, even at 10 times the concentration of Co2+ or Ni2+ (supplemental Fig. S1). These data indicate that in an environment containing a mixture of Mg2+, Ni2+, and Co2+, either of the last two can compete out Mg2+ for binding to TmCorA, even at much lower concentrations.
FIGURE 2.
Stability of TmCorA at 85 °C in the presence of divalent cations at different concentrations. The control (C) is the unheated protein and shows the amount of starting material (100%) of each experiment.
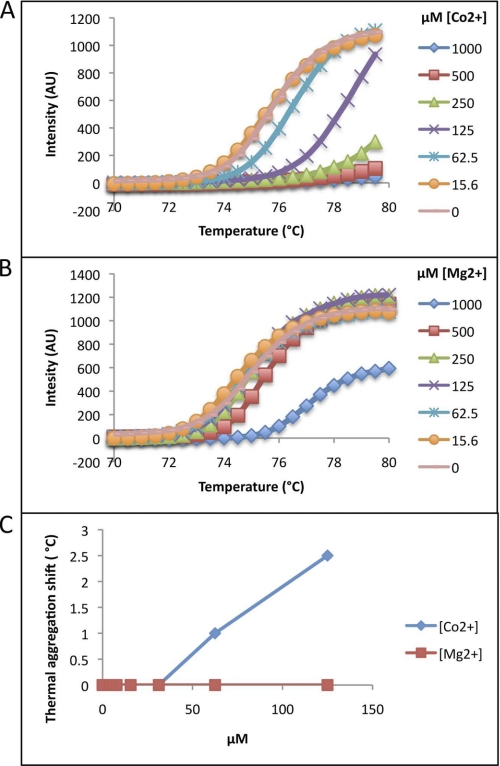
The thermostability effect of Co2+ as well as Mg2+ on TmCorA was further explored by thermal aggregation shift assay. This assay is more sensitive and can detect protein destabilization and aggregation in real time. However, the upper temperature limit for this assay is 80 °C, which makes it less suitable for such a hyperthermostable protein. Nevertheless, at temperatures below 80 °C, this assay clearly shows that at ∼60 μm Co2+, already the aggregation temperature (Tagg) is shifted by +1 °C and that the Tagg continues to increase dramatically by increasing Co2+ concentration until complete stabilization is reached (Fig. 3). On the other hand, Mg2+ could only moderately cause a shift in Tagg without reaching complete stabilization. The results obtained with the differential thermal shift assay were thus in full agreement with the other results shown in Figs. 1 and 2.
FIGURE 3.
Thermal aggregation shift scanning of TmCorA in the presence of different concentrations of Co2+ (A) and Mg2+ (B). The increase in thermostability obtained by various concentrations of the added divalent cations per degrees Celsius is presented in C. TmCorA in the presence of >125 μm Co2+ was so stable that the shift could not be detected and analyzed. The measurements were carried out at 25 °C, and the represented curves are the average of three different measurements with 2–5% deviations.
TmCorA Undergoes Structural Changes upon Specific Binding of Co2+
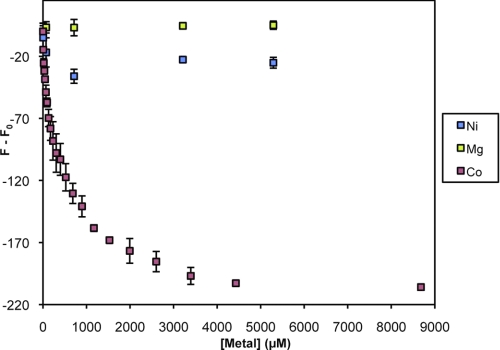
At this point, our results suggest dominant and specific binding of Co2+ and Ni2+ to TmCorA, whereas Mg2+ appeared to bind much weaker. Nevertheless, these experiments do not provide any information on whether the three substrates bind to and stabilize the protein in a similar manner. Therefore, we also performed a ligand dose tryptophan fluorescence quenching experiment (16). Surprisingly, Mg2+ did not induce any quenching of the fluorescence signal from TmCorA, even at a concentration of 5 mm, whereas Co2+ quenched the signal already at ∼10 μm (Fig. 4), indicating structural changes in the protein induced upon Co2+ binding. The titration with Ni2+shows that this metal ion has some effect on the structure of TmCorA, but this effect is much smaller than for Co2+. Thus, the ligand dose tryptophan fluorescence quenching clearly indicated that Co2+ could specifically interact with TmCorA, resulting in structural changes already at low concentrations.
FIGURE 4.
Ligand dose-induced tryptophan quenching (Fq) of TmCorA by magnesium (Mg), cobalt (Co), and nickel (Ni) ions at various concentrations. A TmCorA solution with a protein concentration of 6.4 μm in desalting buffer was used. The fluorescence decrease because of dilution was measured and has been subtracted from the data. The measurements were carried out at 25 °C, and each represented read is an average of three different measurements.
TmCorA Is a Highly Potent Co2+ Transporter
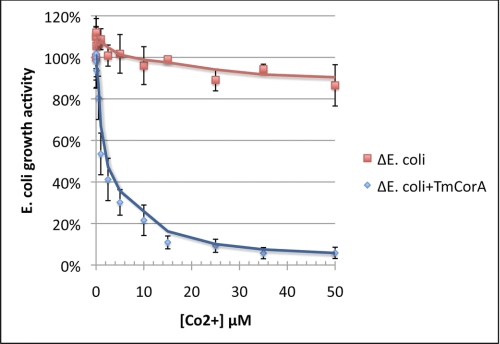
All the results discussed above have shown specific and stabilizing binding of Co2+ to TmCorA. To investigate whether this specific binding is related to the transport activity, the ability of TmCorA to transport Co2+ was examined. For these experiments, we used a corA-less mutant of the E. coli MG1655 strain (ΔE. coli), which is highly resistant to Co2+. Thus, ΔE. coli was incubated with Co2+ at various concentrations for 10 min, followed by 3 h of growth. Using this assay, we could monitor the Co2+ uptake of the strain as a direct measure of the growth activity, i.e. the more Co2+ uptake, the lower the growth activity. In the absence of any corA, ΔE. coli remained resistant toward Co2+ and retained ∼80% of its growth activity at concentrations up to 500 μm (data not shown). Once the ΔE. coli was transformed with the corA from T. maritima, it became highly sensitive toward Co2+ and already lost ∼50% of its growth activity in the presence of 1 μm Co2+ (Fig. 5). Thus, TmCorA could readily transport Co2+ at submicromolar concentrations and thereby reduce the ΔE. coli growth activity. The growth activity was completely abolished at 100 μm Co2+, indicating total toxification of ΔE. coli cells (data not shown).
FIGURE 5.
Assaying Co2+ transport activity of TmCorA (ΔE. coli + TmCorA) by monitoring the loss of ΔE. coli growth activity caused by Co2+ toxicity. ΔE. coli lacking any functional CorA shows resistance toward Co2+ by retaining its growth activity. The presented data points are the average of three different measurements as described under “Experimental Procedures.”
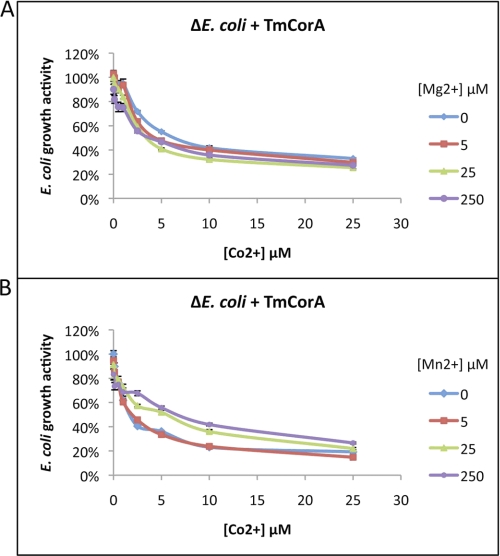
EcCorA and StCorA can transport both Mg2+ and Co2+, and each of these ions can inhibit the uptake of the other one via CorA. We attempted to reproduce this inhibitory effect on the EcCorA using our Co2+ transport assay, where the wild-type E. coli strain MG1655 with the endogenous CorA was used. As expected, the wild-type E. coli showed Co2+ sensitivity through its endogenous CorA by showing reduction in growth activity. However, the growth activity was restored by the addition of Mg2+ at concentrations similar to Co2+ or slightly higher (supplemental Fig. S2). In fact, Mg2+ was able to completely restore the E. coli growth activity already at concentrations three to four times higher than that of Co2+ (data now shown). This result was in good agreement with the results from a previous study (8). We then examined the ability of TmCorA to select Co2+ in the presence of Mg2+ or Mn2+ in ΔE. coli. The data show that TmCorA was extremely selective in Co2+ uptake because neither Mg2+ nor Mn2+ could restore the ΔE. coli growth activity, even at concentrations >100 times higher than those of Co2+ (Fig. 6). It is noteworthy that Mn2+ was able to show some inhibitory effect, whereas Mg2+ was almost completely incapable of outcompeting Co2+. In a previous study, Mn2+ showed to be more competent than Co2+ to inhibit the Mg2+ uptake by StCorA (8).
FIGURE 6.
Assaying the substrate selectivity of TmCorA by monitoring the loss of ΔE. coli growth activity caused by Co2+ toxicity and the recovery of growth activity caused by addition of Mg2+ (A) or Mn2+ (B). The presented data points are the average of three different measurements as described under “Experimental Procedures.”
Mg2+ Is a Poor Substrate for TmCorA
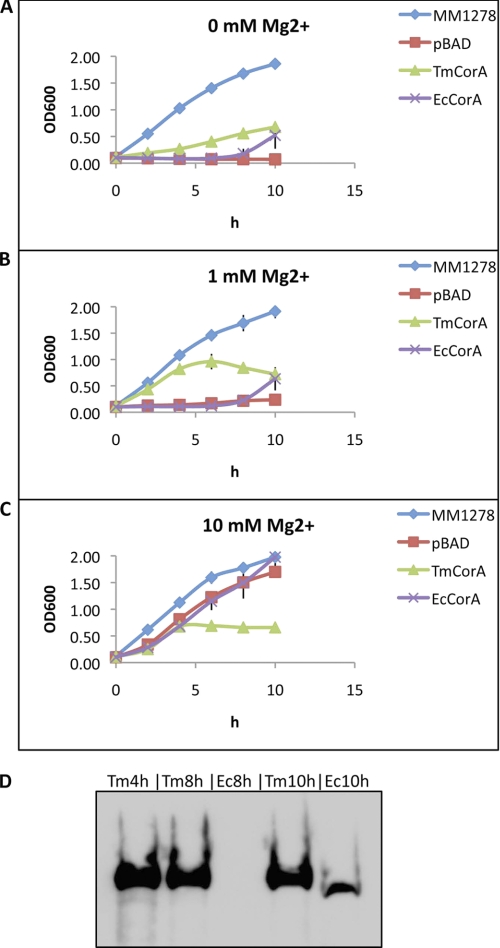
Finally, we performed a Mg2+ transport assay using the S. typhimurium MM281 strain, which is depleted of all Mg2+ transporter genes. This strain can grow only in the presence of ≥10 mm Mg2+, i.e. the Mg2+ content of the LB medium is not sufficient for the growth. MM281, when expressing TmCorA (MM281TmCorA), could slowly grow in LB medium without supplementary Mg2+ and seemed to reach a growth steady state (at A600 of ∼0.6) after 10 h (Fig. 7A). Addition of 1 mm Mg2+ to LB medium increased the growth activity of MM281TmCorA; however, after 6 h the cell density declined and reached an A600 of ∼0.6 after 10 h (Fig. 7B). Interestingly, when 10 mm Mg2+ was added to the LB medium, the MM281TmCorA reached an A600 of ∼0.6 after only 4 h, and no further growth was observed after 4 h (Fig. 7C). These results indicate a slow transport of Mg2+ through TmCorA, as increasing supplementary Mg2+ increased the growth of MM281. However, the Mg2+ uptake by TmCorA seems to be uncontrolled and not regulated because it could stop the growth and even cause cell death, indicating toxicity to the cell. On the other hand, MM281 expressing EcCorA (MM281EcCorA), although showing a slow start in growth, had a very stable growth rate regardless of Mg2+ concentration in the medium (Fig. 7, A and B). Even with an additional 10 mm Mg2+ in the medium, MM281EcCorA continued a stable growth, indicating a tight regulation of Mg2+ uptake through EcCorA as expected (Fig. 7C). Notably, the initial slow growth rate of MM281EcCorA was significantly elevated after 8 h. An expression analysis of both CorA proteins showed a strong expression of TmCorA, already reaching saturation after 4 h, whereas the expression of EcCorA could first be detected after 10 h (Fig. 7D). The analysis of the expression levels could clearly show that EcCorA is significantly more competent than TmCorA in transporting Mg2+ and, especially, in regulating the Mg2+ import and homeostasis. Hence, these results underscore that TmCorA, unlike EcCorA, has a very slow Mg2+ uptake activity and is incapable of controlling the intracellular Mg2+ concentration.
FIGURE 7.
Assaying Mg2+ transport activity of CorA homologues (A–C). The strain MM1278, a derivate of MM281 that expresses StCorA and can completely restore the growth activity of the strain, was used as positive control. pBAD indicates MM281 strains transformed with the empty pBAD vector. TmCorA and EcCorA are MM281 expressing TmCorA and EcCorA, respectively. The presented data points are the average of three independent experiments. The concentration of supplementary Mg2+ in the LB medium is indicated. D, the expression levels of TmCorA and EcCorA at different time points were analyzed by Western blotting, e.g. Tm4h indicates the expression level of TmCorA after 4 h.
DISCUSSION
Despite the ability of various CorA homologues to transport Co2+, CorA has generally been considered as the primary Mg2+ transporter of all organisms in which it is encoded. This is a result of extrapolating the functional characterization of a few organisms, such as S. typhimurium and E. coli. The reason for this generalization is that the micromolar concentration of Co2+ that is required for a transport to occur is not physiologically relevant for these organisms, which led to excluding Co2+ as the physiological substrate of CorA. Nevertheless, when performing functional analyses of CorA from T. maritima using Mg2+ as a substrate, the results were not easily explainable and are rather inconclusive. For example, the function of TmCorA was investigated by focusing on its Mg2+ transport activity using numerous mutants of the protein that were designed based on the crystal structures (13, 17). In addition, unexpected behavior such as gain of function was observed (13), which means that some mutants were more capable in transporting Mg2+ than the wild-type TmCorA and became as active as the wild-type EcCorA. In a recent in vivo study, when monitoring the Mg2+-transport activity of TmCorA, the host strain showed sensitivity to Mg2+ as if the metal ion was toxic (17). Thus, despite the available crystal structures as well as well performed functional studies, it is yet unclear how TmCorA can transport its substrate across the membranes and how its function is regulated. The reason could simply be that the right substrate was not used in these studies.
The capacity of Co2+ to replace Mg2+ at the metal-binding sites at four times lower concentration (9) was the first indication of TmCorA to be more selective for Co2+ rather than for Mg2+. However, this conclusion could have been biased because Ca2+, which is not a substrate of CorA, could also bind to the same site (11). Also, the actual role of these metal-binding sites is not yet known. Nevertheless, our further exploration of this possibility has clearly shown that the TmCorA-Co2+ interactions are highly specific. Among the many divalent cations tested, Co2+ and Ni2+ were the only ions that could increase the thermostability of TmCorA at relatively low concentrations (Fig. 1–3), but only Co2+ showed specific interactions with TmCorA (Fig. 4). Various studies have already shown that thermostability is a good measure for assaying the substrate or ligand binding (18–20). Our thermostability data here were complementary to the in vivo transport studies, which showed that not only is TmCorA capable of transporting Co2+ at submicromolar concentrations, but it also can efficiently select Co2+ over other divalent ions such as Mg2+ (Fig. 5 and 6). The poor ability of Mg2+ to stabilize TmCorA was reflected by the poor capability of TmCorA to take up Mg2+ and its inability to regulate the uptake (Fig. 7), which can explain the unusual toxicity observed in (17). Altogether, it means that in an environment with mixed metal concentrations, Co2+ is the preferred substrate of TmCorA as it is taken up much faster and at much lower concentrations than Mg2+. This leads to the question regarding the physiological relevance of Co2+ uptake and the need for Co2+.
The hyperthermophilic T. maritima is a rod-shaped, Gram-negative bacterium found in geothermal heated marine sediment, and it can live at temperatures between 55–90 °C, with an optimum growth activity at 80 °C (21). In the natural habitat of T. maritima, the Mg2+ concentration is relatively low, and it decreases with increasing temperatures (22). In contrast, in the same environment the level of Co2+ is increased to even micromolar concentrations (22). Thus, T. maritima has a very different habitat than e.g. E. coli, even based on the concentration and availability of metal ions. In addition, there are reports of cytosolic enzymes from this organism that prefer Co2+ as the cofactor, rather than Mg2+ or Zn2+, which are the most common divalent cations in E. coli, for example (23–26). Co2+ has also been shown to thermostabilize proteins from T. maritima (Refs. 23, 24 and Figs. 1–3). Therefore, T. maritima most likely has a higher intracellular Co2+ concentration than E. coli, for example, as it has a higher requirement for Co2+. The intracellular Co2+ concentration of T. maritima is not known. However, it must have a very tightly regulated Co2+-uptake system and/or high intracellular Co2+ content because in laboratories it is usually grown in a medium containing nearly 1 mm Co2+ (27, 28), and it can also tolerate >1 mm Co2+ (29). Hence, the Co2+ requirement for T. maritima and its ability to grow in environments containing high Co2+ concentrations points to the necessity of a selective and tightly regulated uptake system for Co2+. Whether or not CorA is the main system responsible for regulating the Co2+ uptake in T. maritima cannot be established at this moment.
It is also unknown how well the Co2+ uptake is regulated by TmCorA. In our in vivo studies, the ΔE. coli already becomes sensitive toward Co2+ at very low concentrations (Fig. 5). It would be reasonable to ask why TmCorA does not regulate the Co2+ transport by closing and thus rescuing the cell. However, this could be explained by the differences between E. coli and T. maritima in Co2+ sensitivity/tolerance. The above-mentioned necessity of Co2+ for the stability and activity of the T. maritima cellular enzymes would most certainly result in a higher intracellular Co2+ concentration compared with E. coli. For example, the intracellular concentration of Cu2+ in T. maritima has been measured to be 15 μm (29), which is unusually high and for E. coli is considered toxic. Another important parameter to consider when studying the activity of proteins from thermophilic organisms is the temperature. Isolated proteins from thermophilic organisms typically display optimal activities at elevated temperatures (30–33). Therefore, to better understand the biological role of TmCorA, one should perform the studies using corA-deleted T. maritima strains or alternatively create assays mimicking conditions similar to the environment of T. maritima.
The high selectivity of TmCorA for Co2+ with its poor ability to transport and regulate Mg2+ uptake is in contrast to the function of EcCorA as well as StCorA. This is most likely a direct reflection of the structural differences between TmCorA and the other two CorAs, and perhaps more generally also between subgroups A and B (14).
Although the results from two previous studies have indicated EcCorA to be a tetramer (34, 35), a pentameric structure for all CorAs is most probable, considering that even the crystal structure of a distant homologue of CorA, ZntB, was determined as a pentamer (36). However, only a minor difference, such as the nature or position of the side chains in the active or substrate-binding site, can make a major difference, as has been reported in other cases (37, 38). Therefore, it is important to avoid unreasonable extrapolations and excessive generalization of the CorA function before more functional as well as structural data from various subgroups is obtained. Understanding the functional differences between various CorA proteins at the molecular level is important for understanding ion selectivity, gating, and transport mechanisms of the different CorA subgroups. This information should also be valuable for understanding the evolutionary aspects in the differences between the CorA proteins.
This study is the first report of a CorA protein that is highly selective for Co2+ and incapable of Mg2+ transport and regulation, suggesting new roles for this transporter family.
Supplementary Material
Acknowledgment
We thank Prof. Michael Maguire (Case Western Reserve University, Cleveland, OH) for providing us with the MM281 and MM1278 strains.
This work was supported by grants from Nanyang Technological University, the National Research Foundation (NRF) and Biomedical Research Council (BMRC) of Singapore, the Swedish Research Council, the Center for Biomembrane Research at Stockholm University, and the Knut and Alice Wallenberg Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- TmCorA
- Thermotoga maritima CorA
- EcCorA
- Escherichia coli CorA
- StCorA
- Salmonella typhimurium CorA
- LB
- Luria broth.
REFERENCES
- 1. Kehres D. G., Lawyer C. H., Maguire M. E. (1998) Microb. Comp. Genomics 3, 151–169 [DOI] [PubMed] [Google Scholar]
- 2. Kehres D. G., Maguire M. E. (2002) Biometals 15, 261–270 [DOI] [PubMed] [Google Scholar]
- 3. Hmiel S. P., Snavely M. D., Florer J. B., Maguire M. E., Miller C. G. (1989) J. Bacteriol. 171, 4742–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hmiel S. P., Snavely M. D., Miller C. G., Maguire M. E. (1986) J. Bacteriol. 168, 1444–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith R. L., Gottlieb E., Kucharski L. M., Maguire M. E. (1998) J. Bacteriol. 180, 2788–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith R. L., Kaczmarek M. T., Kucharski L. M., Maguire M. E. (1998) Microbiology 144, 1835–1843 [DOI] [PubMed] [Google Scholar]
- 7. Smith R. L., Maguire M. E. (1995) J. Bacteriol. 177, 1638–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. (1989) J. Bacteriol. 171, 4761–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eshaghi S., Niegowski D., Kohl A., Martinez Molina D., Lesley S. A., Nordlund P. (2006) Science 313, 354–357 [DOI] [PubMed] [Google Scholar]
- 10. Lunin V. V., Dobrovetsky E., Khutoreskaya G., Zhang R., Joachimiak A., Doyle D. A., Bochkarev A., Maguire M. E., Edwards A. M., Koth C. M. (2006) Nature 440, 833–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Payandeh J., Pai E. F. (2006) EMBO J. 25, 3762–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakrabarti N., Neale C., Payandeh J., Pai E. F., Pomès R. (2010) Biophys. J. 98, 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Payandeh J., Li C., Ramjeesingh M., Poduch E., Bear C. E., Pai E. F. (2008) J. Biol. Chem. 283, 11721–11733 [DOI] [PubMed] [Google Scholar]
- 14. Niegowski D., Eshaghi S. (2007) Cell. Mol. Life Sci. 64, 2564–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vedadi M., Niesen F. H., Allali-Hassani A., Fedorov O. Y., Finerty P. J., Jr., Wasney G. A., Yeung R., Arrowsmith C., Ball L. J., Berglund H., Hui R., Marsden B. D., Nordlund P., Sundstrom M., Weigelt J., Edwards A. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15835–15840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tetin S. Y., Hazlett T. L. (2000) Methods 20, 341–361 [DOI] [PubMed] [Google Scholar]
- 17. Svidova S., Sponder G., Schweyen R. J., Djinovic-Carugo K. (2010) Biochim. Biophys. Acta, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexandrov A. I., Mileni M., Chien E. Y., Hanson M. A., Stevens R. C. (2008) Structure 16, 351–359 [DOI] [PubMed] [Google Scholar]
- 19. Ericsson U. B., Hallberg B. M., Detitta G. T., Dekker N., Nordlund P. (2006) Anal. Biochem. 357, 289–298 [DOI] [PubMed] [Google Scholar]
- 20. Majava V., Löytynoja N., Chen W. Q., Lubec G., Kursula P. (2008) FEBS J. 275, 4583–4596 [DOI] [PubMed] [Google Scholar]
- 21. Huber R., Langworthy T. A., König H., Thomm M., Woese C. R., Sleytr U. B., Stetter K. O. (1986) Arch. Microbiol. 144, 324–333 [Google Scholar]
- 22. Holden J. F., Adams M. W. (2003) Curr. Opin. Chem. Biol. 7, 160–165 [DOI] [PubMed] [Google Scholar]
- 23. Nakajima M., Imamura H., Shoun H., Wakagi T. (2003) Arch. Biochem. Biophys. 415, 87–93 [DOI] [PubMed] [Google Scholar]
- 24. Wang J., Stieglitz K. A., Kantrowitz E. R. (2005) Biochemistry 44, 8378–8386 [DOI] [PubMed] [Google Scholar]
- 25. Epting K. L., Vieille C., Zeikus J. G., Kelly R. M. (2005) FEBS J. 272, 1454–1464 [DOI] [PubMed] [Google Scholar]
- 26. Wojciechowski C. L., Cardia J. P., Kantrowitz E. R. (2002) Protein Sci. 11, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. (1979) Microbiol. Rev. 43, 260–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schumann J., Wrba A., Jaenicke R., Stetter K. O. (1991) FEBS Lett. 282, 122–126 [DOI] [PubMed] [Google Scholar]
- 29. Llanos J., Capasso C., Parisi E., Prieur D., Jeanthon C. (2000) Curr. Microbiol. 41, 201–205 [DOI] [PubMed] [Google Scholar]
- 30. Cannio R., Di Prizito N., Rossi M., Morana A. (2004) Extremophiles 8, 117–124 [DOI] [PubMed] [Google Scholar]
- 31. Jorge C. D., Sampaio M. M., Hreggvidsson G. O., Kristjánson J. K., Santos H. (2007) Extremophiles 11, 115–122 [DOI] [PubMed] [Google Scholar]
- 32. Sterner R., Kleemann G. R., Szadkowski H., Lustig A., Hennig M., Kirschner K. (1996) Protein Sci. 5, 2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song S. H., Ahluwalia N., Leduc Y., Delbaere L. T., Vieille C. (2008) Appl. Microbiol. Biotechnol. 81, 485–495 [DOI] [PubMed] [Google Scholar]
- 34. Wang S. Z., Chen Y., Sun Z. H., Zhou Q., Sui S. F. (2006) J. Biol. Chem. 281, 26813–26820 [DOI] [PubMed] [Google Scholar]
- 35. Warren M. A., Kucharski L. M., Veenstra A., Shi L., Grulich P. F., Maguire M. E. (2004) J. Bacteriol. 186, 4605–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan K., Sather A., Robertson J. L., Moy S., Roux B., Joachimiak A. (2009) Protein Sci. 18, 2043–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Högbom M., Stenmark P., Voevodskaya N., McClarty G., Gräslund A., Nordlund P. (2004) Science 305, 245–248 [DOI] [PubMed] [Google Scholar]
- 38. Spraggon G., Schwarzenbacher R., Kreusch A., Lee C. C., Abdubek P., Ambing E., Biorac T., Brinen L. S., Canaves J. M., Cambell J., Chiu H. J., Dai X., Deacon A. M., DiDonato M., Elsliger M. A., Eshagi S., Floyd R., Godzik A., Grittini C., Grzechnik S. K., Hampton E., Jaroszewski L., Karlak C., Klock H. E., Koesema E., Kovarik J. S., Kuhn P., Levin I., McMullan D., McPhillips T. M., Miller M. D., Morse A., Moy K., Ouyang J., Page R., Quijano K., Robb A., Stevens R. C., van den Bedem H., Velasquez J., Vincent J., von Delft F., Wang X., West B., Wolf G., Xu Q., Hodgson K. O., Wooley J., Lesley S. A., Wilson I. A. (2004) Proteins 55, 1078–1081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.