Abstract
Background
Impulsive physical aggression is a common and problematic feature of many personality disorders. The serotonergic system is known to be involved in the pathophysiology of aggression, and multiple lines of evidence have implicated the 5-HT2A receptor (5-HT2AR). We sought to examine the role of the 5-HT2AR in impulsive aggression specifically in the orbitofrontal cortex (OFC), given that our own studies and an extensive literature indicate that serotonergic disturbances in the OFC are linked to aggression. We have previously hypothesized that increased 5-HT2AR function in the OFC is a state phenomenon which promotes impulsive aggression.
Methods
5-HT2AR availability was measured with positron emission tomography and the selective 5-HT2AR antagonist radioligand [11C]MDL100907 in two groups of impulsively aggressive personality disordered patients --14 with current physical aggression, and 15 without current physical aggression --and 25 healthy controls. Clinical ratings of various symptom dimensions were also obtained.
Results
Orbitofrontal 5-HT2AR availability was greater in patients with current physical aggression compared to patients without current physical aggression and healthy controls; no differences in OFC 5-HT2AR availability were observed between patients without current physical aggression and healthy controls. No significant differences in 5-HT2AR availability were observed in other brain regions examined. Among both groups of impulsively aggressive personality disordered patients combined, OFC 5-HT2AR availability was correlated, specifically, with a state measure of impulsive aggression.
Conclusions
These findings are consistent with our previously described model in which impulsive aggression is related to dynamic changes in 5-HT2AR function in the OFC.
Keywords: Aggression, Personality Disorder, Intermittent Explosive Disorder, Serotonin, Positron Emission Tomography, Orbitofrontal Cortex
INTRODUCTION
Impulsive physical aggression is a common and problematic feature of severe personality disorders, of which borderline personality disorder (BPD) is a prototype. Integrated research criteria have been developed for the diagnosis of intermittent explosive disorder (IED-IR), and the confirmation of its discriminant and convergent validity has previously been described (1). In addition to its clinical importance, IED-IR serves as a useful paradigm to study impulsive aggression in humans. A particular advantage of the IED-IR criteria is the ability to qualify aggression in terms of whether it is expressed in a physical and/or verbal form; as there are specific temporal parameters, IED-IR can also be designated as either being current or past. Moreover, IED-IR criteria were purposely developed to not exclude for comorbid personality disorders that characteristically manifest aggressive behavior, e.g., BPD and antisocial personality disorder (ASPD). These characteristics of IED-IR offer the capacity to reliably stratify subgroups of highly aggressive personality disordered patients, and examine more precisely specific hypotheses about the neurobiology of aggression.
Altered serotonergic function has consistently been implicated in the pathophysiology of aggression (2); the 5-HT2AR, in particular, appears to be involved. In animal models, antagonists and agonists to the 5-HT2AR attenuate and augment, respectively, aggressive and impulsive behaviors (3–5). Platelet 5-HT2AR binding is associated with aggression in personality disordered patients but not in healthy controls (6). In a post-mortem study, 5-HT2AR expression in prefrontal cortical regions correlated positively with lifetime aggression in subjects who committed suicide, but not in those who died by other causes (7). Specific genetic polymorphisms of the 5-HT2AR are associated with aggression (8) and impulsivity (9,10).
The orbitofrontal cortex (OFC) is our a priori region of interest to examine the means by which the 5-HT2AR may modulate aggression. Methyl-L-tryptophan trapping --a putative index of serotonin synthesis --is lower in the ventromedial PFC in suicide attempters (11) and impulsive patients (12). Patients with BPD exhibit decreased activation in orbitofrontal regions in response to fenfluramine, a presynaptic 5-HT secretagogue (13). Blunted medial prefrontal and orbitofrontal activation in response to fenfluramine (14) and meta-chlorophenylpiperazine (m-CPP) (15), a 5-HT2CR agonist, has been demonstrated in impulsive-aggressive patients. Treatment of impulsive-aggressive patients with fluoxetine, a selective serotonin reuptake inhibitor (SSRI), improved symptoms of aggression and irritability, and enhanced prefrontal glucose metabolism (16).
We have previously described a model in which attenuated presynaptic cortico-limbic 5-HT represents a vulnerability trait, predisposing an individual to impulsive aggression; dynamic functional changes of the 5-HT2AR in orbitofrontal regions, however, determines the degree of impulsive aggressive behavior, in a state dependent manner. To further assess this model, we examined 5-HT2AR availability with positron emission tomography (PET) and the selective 5-HT2AR antagonist radioligand [11C]MDL100907 in personality disordered patients with IED-IR. In order to determine whether changes in 5-HT2AR availability would reflect a state as opposed to trait phenomenon, IED-IR patients were stratified into two groups: those with current physical IED-IR and those without. We selected physical as opposed to verbal IED-IR, as the former is the more severe form, manifesting in terms of physical assault against others and destruction of valuable property. Consistent with our model, we predicted: 1) greater [11C]MDL100907 binding potential in the OFC of the IED-IR group with current physical aggression compared to the IED-IR group without current physical aggression and healthy controls; and 2) an association between OFC [11C]MDL100907 binding potential and state aggression.
METHODS AND MATERIALS
Human Subjects
The study was approved by the Institutional Review Boards of the New York State Psychiatric Institute, Columbia University Medical Center, Mount Sinai Hospital, and the Bronx Veterans Affairs Medical Center. Written informed consent was obtained from each research participant after explanation of study procedures. Participants were recruited through advertisements in local newspapers and the internet. All participants underwent a medical clearance, consisting of a medical history, physical exam, basic blood and urine tests, and electrocardiogram. All participants were free of significant medical/neurological problems, and were not pregnant or nursing. None of the [11C]MDL100907 binding potential data of any of the subjects in this study have been previously published. Fourteen participants (10 males, 4 females, mean age = 36.75 [SD = 10.31, range = 21–54]) met criteria for at least one DSM-IV personality disorder and current IED-IR with ‘current physical aggression’ (1); current physical aggression was designated if patients met IED-IR criterion A2: three episodes of physical assault against other people or destruction of property over the past year. It is important to note that unlike DSM-IV IED criteria (17), IED-IR criteria (1) were deliberately designed to not exclude for a comorbid personality disorder that characteristically manifests aggressive behavior. Fifteen participants (12 males, 3 females, mean age = 36.40 [SD = 9.25, range = 20–51]) met criteria for at least one personality disorder and IED-IR (either current or past) without current physical aggression (i.e., either past physical, and/or current or past verbal aggression). Verbal aggression was operationalized in terms of IED-IR criterion A1: verbal aggression towards others occurring twice weekly on average for one month; these episodes may have been accompanied by incidents of minor physical aggression (e.g., brief pushing/shoving, or slamming a door shut) that did not le to physical ad assault against others or destruction of valuable property. The Structured Clinical Interview for DSM-IV Axis I Disorder (18) was used for Axis I diagnoses. The Structured Interview for DSM-IV Personality Disorders (SIDP-IV) (19) was used for Axis II diagnoses.
Sixteen patients were naïve to psychotropic medications and the remaining 13 patients were free of psychotropic medications for a minimum of 6 months prior to initial screening. Patients were excluded if they met criteria for a current major depressive episode, a history of schizophrenia or other psychotic disorder, bipolar-I, or current/recent (within the past 6 months) alcohol or substance abuse/dependence. Patients were also excluded for histories of serious past alcohol/substance abuse/dependence which may have led to long-standing neurochemical sequelae, namely: delirium tremens or medically complicated alcohol withdrawal, significant methylene-dioxymethamphetamine (MDMA) use, intravenous drug use, or chronic/persistent cocaine dependence.
All IED-IR patients were assessed with the Overt Aggression Scale-Modified (OAS-M) by an experienced clinical psychologist. The initial development and psychometric characterization of the OAS-M has previously been described (20). In brief, the OAS-M exhibits excellent interrater reliability, significant one week test-retest stability, and internal consistency. OAS-M ratings were performed twice, once at the initial screen and then again approximately 2–4 weeks later, and the means of these two scores were used. The OAS-M is composed of three subscales: Assaultiveness, Global Irritability, and Suicidality. The former two were used as measures of state aggression. Numerous clinical studies have validated the utility of the Assaultiveness and Global Irritability subscales as state measures sensitive to changes in impulsive aggression in response to various pharmacological treatments (21–26). The score of the Assaultiveness subscale is a sum of incidents of verbal and physical aggression, weighted according to severity, which occurred over the preceding week; technically there is no maximum score for this subscale. The Global Irritability subscale score is based on the rating of the patient’s level of reported subjective irritability and overt/expressed irritability over the preceding two weeks; both subjective and overt/expressed irritability are rated on a scale of 0–5 resulting in a maximum score of 10.
All patients completed self-report measures of trait aggression (Buss-Durkee Hostility Inventory and/or Buss-Perry Aggression Questionnaire) (27,28), trait impulsivity (Barratt Impulsiveness Scale version-11) (29), affective lability (Affective Lability Scale) (30), and state depressive symptoms (Beck Depression Inventory) (31). The Buss-Perry Aggression Questionnaire (BPAQ) is an updated version of the Buss-Durkee Hostility Inventory (BDHI). In the IED-IR group with current physical aggression six completed the BDHI, six did the BPAQ, and two did both. In the group without current physical aggression, nine completed the BDHI, five did the BPAQ, and one completed both. In order to derive one trait aggression score that was comparable across patients, T scores were derived for each scale and the mean of these scores was calculated for each patient.
Healthy comparison participants (n = 25, 15 males, 10 females, mean age = 32.86 [SD = 10.52, range = 19–55]) had no current or past DSM-IV Axis I or II psychiatric disorder or first-degree relative with a history of schizophrenia, bipolar disorder, major depression, or Axis II disorder.
PET Acquisition and Reconstruction
Following a 15-minute transmission scan for attenuation correction, [11C]MDL100907 was injected intravenously as a single bolus over 30 seconds. Emission data were acquired for 90 minutes (see Supplement 1). Primary outcome measures were binding potential relative to the nondisplaceable radioligand concentration in the brain (BPND), and binding potential relative to the unmetabolized radioligand concentration in the plasma (BPP). See Supplement 1 and Innis et al. (32) for definitions of these outcome measures, and Supplement 1 for additional information regarding PET scan acquisition and estimation procedures.
Data analysis and statistics
One-way ANOVAs were used to examine potential group differences in scan parameters (i.e., parameters that do not reflect receptor availability). Using the General Linear Model framework, group differences in BPND and BPP for the hypothesized ROI (OFC; Figure S1 in Supplement 1) were tested with 3-group ANOVAs, controlling for covariates as indicated (see below). Group differences for non-normally distributed variables were examined with non-parametric tests (i.e., Kruskal-Wallis H for 3-group and Mann-Whitney U for 2-group comparisons). Associations between clinical variables and binding potential measures (patient groups only) were examined with Pearson product-moment correlations, or Spearman rank coefficients for non-normally distributed variables. Tests for exploratory analyses were corrected for multiple comparisons using the false discovery rate procedure (FDR) (33).
RESULTS
Demographic and Clinical Characteristics
There were no significant group differences on any of the demographic parameters examined (Table 1). Axis I and II diagnoses, and IED-IR designations of patients are presented in Table S1 in Supplement 1. The great majority of IED-IR patients met criteria for BPD.
Table 1.
Demographic and Scan Parameters by Diagnostic Group
| Characteristic | Current Physical IED-IR (n=14)1 | Without Current Physical IED-IR (n=15)2 | Controls (n=25) | Group comparisons3 |
|---|---|---|---|---|
| Age (M (SD)) | 36.75 (10.31) | 36.40 (9.25) | 32.86 (10.52) | NS |
|
| ||||
| Sex (% male) | 71.4% | 80.0% | 60.0% | NS |
|
| ||||
| Ethnicity (n (%)) | NS4 | |||
| Caucasian | 50.0% | 53.3% | 48.0% | |
| Black | 21.4% | 13.3% | 28.0% | |
| Hispanic | 21.4% | 33.3% | 16.0% | |
| Asian | 7.1% | --- | 8.0% | |
|
| ||||
|
Scan parameter (M (SD))
| ||||
| Injected dose (mCi) | 14.28 (3.65) | 13.42 (3.46) | 15.05 (2.63) | NS |
|
| ||||
| Injected mass (μg) | 3.74 (1.79) | 3.83 (2.58) | 5.42 (2.63) | Ctrl > Current Physical IED-IR (p = .043) Ctrl > Without Current Physical IED-IR (p = .050) |
|
| ||||
| Specific activity (Ci/mmol) | 1701.37 (832.02) | 1651.30 (772.73) | 1266.38 (595.91) | NS |
|
| ||||
| Plasma free fraction (fp) | .31 (.06) | .31 (.03) | .30 (.04) | NS |
|
| ||||
| Clearance (liters/hour) | 181.11 (67.64) | 220.62 (56.91) | 172.23 (57.13) | Without Current Physical IED-IR > Ctrl (p = .017)5 |
|
| ||||
| VND (ml/g) | 21.57 (4.03) | 22.59 (3.10) | 20.57 (2.91) | NS |
Note. Ctrl = control group; IED-IR = intermittent explosive disorder-integrated research criteria; M = mean; NS = nonsignificant; SD = standard deviation; VND = nondisplaceable distribution volume
Personality-disorder patients who met IED-IR with current physical aggression
Personality-disorder patients who met IED-IR without current physical aggression
3-group chi-square for dichotomous variables and 3-group ANOVA for continuous variables (with least-significance difference post-hoc tests when overall F significant).
Groups were compared on dichotomized ethnicity variable (Caucasian v. ethnic minority).
IED-IR patients without current physical aggression were increased at the trend level compared to IED-IR patients with current physical aggression (p = .08).
Scores of the Assaultiveness subscale of the OAS-M were significantly increased among the IED-IR group with current physical aggression compared to those without (Table 2). When excluding 2 outlying scores from the group with current physical aggression, a significant group difference was still observed (p = .020). Scores on the OAS-M Global Irritability subscale were significantly greater among the patients with current physical aggression compared to those without (Table 2). The two IED-IR groups did not differ significantly on the other clinical measures employed (Table 2). Therefore, of the various clinical measures employed, the two IED-IR groups differed significantly only with respect to our two measures of state aggression.
Table 2.
Patient Group Comparisons on Clinical Measures
| Clinical Measure | Current Physical IED-IR (n=14)1 | Without Current Physical IED-IR (n=15)2 | Group comparisons3 |
|---|---|---|---|
| M (SD) | M (SD) | ||
| State aggression: Assaultiveness4 | 50.71 (84.98) | 12.10 (15.08) | p = .0065 |
| State aggression: Global Irritability6 | 5.96 (1.61) | 4.33 (2.11) | p = .0287 |
| Trait aggression8 | 59.74 (7.07) | 55.21 (7.73) | p = .112 |
| Trait impulsivity9 | 54.56 (7.20) | 52.52 (7.50) | p = .461 |
| Trait affective lability10 | 1.71 (.40) | 1.42 (0.54) | p = .111 |
| State depressive symptoms11 | 15.79 (8.35) | 12.79 (9.35) | p = .378 |
Note. IED-IR = intermittent explosive disorder-integrated research criteria; M = mean; SD = standard deviation
Personality-disorder patients who met IED-IR with current physical aggression
Personality-disorder patients who met IED-IR without current physical aggression
For Assaultiveness, Mann-Whitney test performed due to the non-normal distribution of scores (positive skew); for other measures, t-tests performed.
ssaultiveness subscale of the Overt Aggression Scale-Modified (OAS-M)
Mann-Whitney U = 42.5
Global Irritability subscale of the OAS-M
t (27) = 2.33
Patients were administered the Buss-Durkee Hostility Inventory and/or Buss-Perry Aggression Questionnaire, and a T score was derived for each measure; for patients who completed both questionnaires, the mean of the two T scores was used; see text for additional information.
Barratt Impulsiveness Scale, Version-11
Affective Lability Scale
Beck Depression Inventory
Scan Parameters
There were no significant group differences in injected dose of [11C]MDL100907 or in specific activity at time of injection (Table 1). Injected mass was significantly greater in the control group compared to both IED-IR groups (Supplement 1). Injected mass was not related to OFC [11C]MDL100907 BPND or BPP across the total sample, or within any of the 3 diagnostic groups (Supplement 1). Nevertheless, group differences in OFC BPND and BPP were examined with and without injected mass as a covariate. Peripheral clearance of [11C]MDL100907 was significantly faster in the IED-IR group without current physical aggression compared to the control group, and increased at the trend level compared to the group with current physical aggression (Supplement 1). Note, however, that binding potential measures are independent of peripheral clearance (32). Neither the plasma free fraction of [11C]MDL100907 nor the non-displaceable distribution volume (VND, measured as cerebellum distribution volume) differed among the 3 groups (Table 1).
Regional Volumes
The mean ROI volumes by group are presented in Table S2 in Supplement 1. There were no group differences in the OFC or other ROI volumes examined with the exception of the parietal cortex, which was significantly smaller among patients with current physical aggression compared to controls (Table S2, Supplement 1 for Results and Discussion). Across the total sample, age was significantly negatively related to the volumes of the DLPFC and MPFC; and, amygdala volumes were significantly greater in males compared to females (Results and Discussion in Supplement 1).
Associations of Demographic Features with OFC [11C]MDL100907 BPND and BPP
Neither sex nor ethnicity (dichotomized) was related to [11C]MDL100907 OFC BPND or BPP. There was a significant negative association between age and OFC BPND (r = − .31, p = .022), but not with BPP (r = − .17, p = .218). Further examination of this relation revealed a trend for an age-by-group interaction [F(2,48) = 2.73, p = .076] such that age was negatively associated with OFC BPND in patients with current physical aggression (r = − .65, p = .013) but not in patients without (r = − .12, p = .68) or healthy controls (r = − .26, p = .22). Therefore, analyses were performed with age and the age-by-group interaction as predictor terms in the 3-group ANOVAs of OFC BPND and BPP, and age and age-by-group were controlled for when examining associations of OFC BPND and BPP with clinical measures.
Group Differences in [11C]MDL100907 BPND and BPP
Orbitofrontal Cortex
A 3-group ANOVA (controlling for age and age-by-group) of OFC [11C]MDL100907 BPND indicated significant group differences (Table 3; Figure 1). Specifically, BPND in the OFC was significantly higher among patients with current physical aggression compared to those without current physical aggression and healthy controls. Patients without current physical aggression did not differ significantly from healthy controls.
Table 3.
[11C]MDL 100907 BPND by Diagnostic Group
| Region | Current Physical IED- IR (n=14)1 | Without Current Physical IED-IR (n=15)2 | Controls (n=25) | Group comparisons3: Overall F (df=2,48) | p |
|---|---|---|---|---|---|
| OFC | 2.44 (.79) | 1.98 (.48) | 2.07 (.52) | 4.41 |
.017 Current Physical > Ctrl: .010 Current Physical > Without Current Physical: .013 Without Current Physical v. Ctrl: .677 |
| Overall χ2 (df=2) | p | ||||
| GEN | 2.39 (.53) | 2.20 (.70) | 2.57 (.87) | 1.83 | .400 |
| MPFC | 2.49 (.63) | 2.37 (.31) | 2.41 (.55) | 1.10 | .577 |
| ACC | 2.46 (.68) | 2.36 (.48) | 2.39 (.55) | 1.02 | .602 |
| TC | 2.48 (.79) | 2.26 (.34) | 2.29 (.60) | 1.48 | .477 |
| DLPFC | 2.21 (.52) | 2.09 (.30) | 2.17 (.50) | 0.77 | .681 |
| OC | 2.23 (.59) | 2.17 (.34) | 2.13 (.51) | 1.04 | .595 |
| INS | 2.07 (.55) | 1.97 (.27) | 2.00 (.38) | 0.50 | .780 |
| PC | 2.14 (.58) | 1.93 (.35) | 1.96 (.45) | 3.26 | .196 |
| ENT | 1.18 (.46) | 1.00 (.28) | 1.09 (.24) | 1.08 | .582 |
| UNC | .97 (.39) | 1.03 (.39) | 1.09 (.40) | 0.39 | .825 |
| PHG4 | .86 (.26) | .91 (.18) | .97 (.22) | 1.31 | .520 |
| AMYG | .69 (.20) | .52 (.13) | .62 (.17) | 6.68 | .0355 |
Note. Mean (standard deviation).
ACC = anterior cingulate cortex; AMYG = amygdala; Ctrl = control group; DLPFC = dorsolateral prefrontal cortex; ENT = entorhinal cortex; GEN = genu; IED-IR = intermittent explosive disorder-integrated research criteria; INS = insula; MPFC = medial prefrontal cortex; OC = occipital cortex; OFC = orbitofrontal cortex; PC = parietal cortex; PHG = parahippocampal gyrus; TC = temporal cortex; UNC = uncus
Personality-disorder patients who met IED-IR with current physical aggression
Personality-disorder patients who met IED-IR without current physical aggression
For OFC, 3-group ANOVA, controlling for age and ageXgroup, conducted. For other regions, several of which were non-normally distributed, age-adjusted BPND values were assessed with 3-group Kruskal-Wallis H tests.
AgeXgroup term was significant for PHG; thus age adjustments of BPND values were performed using group-specific parameter estimates.
This p value was not considered statistically significant when adjusting for multiple comparisons with the false discovery rate procedure.
Figure 1.
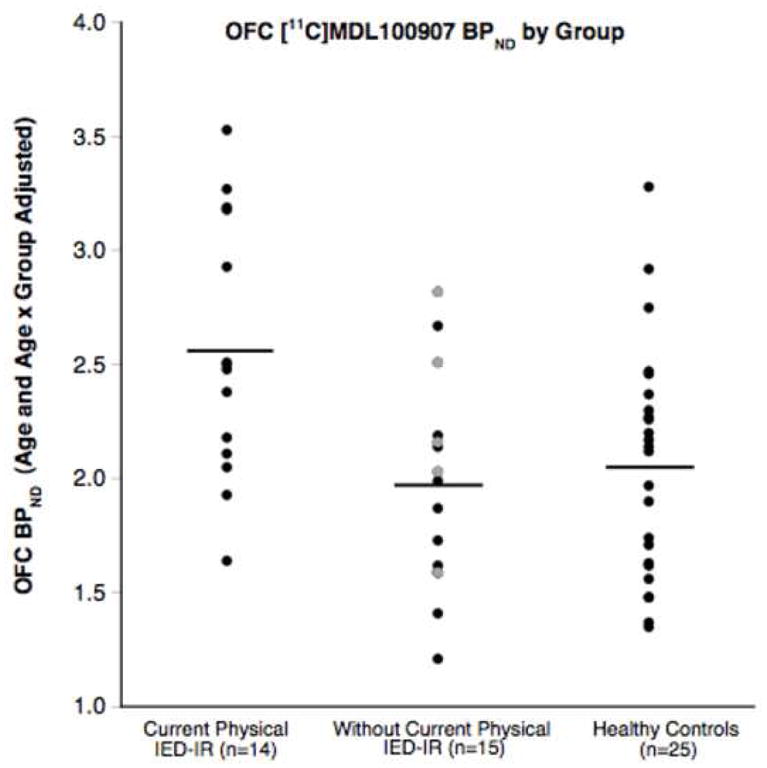
[11C]MDL100907 BPND (adjusted for age and age x group) in the orbitofrontal cortex (OFC) of intermittent explosive disorder-integrated research criteria (IED-IR) groups with and without current physical aggression and healthy controls. The mean BPND for each group is depicted by the horizontal line. BPND was significantly greater in IED-IR patients with current physical aggression compared to those without current physical aggression and healthy controls. IED-IR patients without current physical aggression did not differ significantly from healthy controls. Gray circles represent the 5 patients without current physical aggression who only met criteria for verbal, but never physical, IED-IR.
A 3-group ANOVA (controlling for age and age-by-group) of OFC [11C]MDL100907 BPP indicated group differences consistent with BPND (Table 4). Although the overall F reached the level of a statistical trend, pairwise comparisons indicated that BPP in the group with current physical aggression was significantly higher compared to healthy controls, and increased at the trend level compared to patients without current physical aggression. No significant differences in BPP were observed between patients without current physical aggression and healthy controls. Including injected mass as a covariate in the above 3-group ANOVAs for OFC BPND and BPP led to results essentially identical to those when injected mass was not a covariate.
Table 4.
[11C]MDL 100907 BPP by Diagnostic Group
| Region | Current Physical IED- IR (n=14)1 | Without Current Physical IED-IR (n=15)2 | Controls (n=25) | Group comparisons3: Overall F (df=2,48) | p |
|---|---|---|---|---|---|
| OFC | 51.60 (16.52) | 44.94 (13.22) | 42.70 (12.72) | 2.54 |
.089 Current Physical > Ctrl: .035 Current Physical > Without Current Physical: .086 Without Current Physical v. Ctrl: .971 |
| Overall χ2 (df=2) | p | ||||
| GEN | 51.40 (14.25) | 50.0 (17.90) | 52.64 (17.91) | 0.23 | .891 |
| MPFC | 52.19 (10.63) | 53.53 (10.34) | 49.46 (12.24) | 3.06 | .217 |
| ACC | 51.51 (11.64) | 53.26 (13.53) | 48.65 (11.11) | 1.83 | .400 |
| TC | 51.54 (12.27) | 51.41 (11.99) | 46.95 (13.14) | 2.53 | .283 |
| DLPFC | 46.25 (8.38) | 47.11 (9.58) | 44.46 (10.78) | 0.74 | .692 |
| OC | 46.62 (10.10) | 49.05 (9.77) | 43.65 (11.45) | 3.26 | .196 |
| INS | 43.38 (8.99) | 44.79 (10.20) | 40.89 (8.65) | 2.69 | .260 |
| PC | 44.63 (9.55) | 43.54 (9.36) | 39.96 (9.12) | 3.49 | .174 |
| ENT | 25.01 (9.76) | 23.06 (8.52) | 22.31 (5.73) | 0.23 | .893 |
| UNC | 20.61 (8.43) | 23.31 (10.29) | 22.40 (8.18) | 0.31 | .857 |
| PHG4 | 18.32 (5.92) | 20.60 (5.82) | 19.84 (5.07) | .95 | .621 |
| AMYG | 14.80 (5.51) | 12.00 (4.31) | 12.77 (3.84) | 2.11 | .349 |
Note. Mean (standard deviation).
ACC = anterior cingulate cortex; AMYG = amygdala; Ctrl = control group; DLPFC = dorsolateral prefrontal cortex; ENT = entorhinal cortex; GEN = genu; IED-IR = intermittent explosive disorder-integrated research criteria; INS = insula; MPFC = medial prefrontal cortex; OC = occipital cortex; OFC = orbitofrontal cortex; PC = parietal cortex; PHG = parahippocampal gyrus; TC = temporal cortex; UNC = uncus
Personality-disorder patients who met IED-IR with current physical aggression
Personality-disorder patients who met IED-IR without current physical aggression
For OFC, 3-group ANOVA, controlling for age and ageXgroup, conducted. For other regions, several of which were non-normally distributed, age-adjusted BPP values were assessed with 3-group Kruskal-Wallis H tests.
AgeXgroup term was significant for PHG; thus age adjustments of BPP values were performed using group-specific parameter estimates.
As one-third of patients without current physical aggression had no history of prior physical IED-IR, an exploratory analysis was performed in which only the 10 patients with past physical IED-IR were included in the group without current physical aggression. A 3-group ANOVA (controlling for age and age-by-group) showed significant group differences for both OFC BPND [F(2,43) = 5.61, p = .007] and BPP [F(2,43) = 3.94, p = .027]. Patients with current physical aggression had significantly greater OFC BPND and BPP compared to this subset of patients with past physical aggression (p = .004 for BPND and p = .012 for BPP) and healthy controls (p = .010 for BPND and p = .034 for BPP). Patients with past physical aggression did not differ significantly from healthy controls.
Exploratory ROIs
Exploratory analyses were performed across the other brain regions for which reliable measures of [11C]MDL100907 binding potential could be obtained (Tables 3 and 4). The only 3-group comparison for which p was below .05 was the amygdala BPND; this result was not considered statistically significant after correcting for multiple comparisons using the FDR procedure.
Effect of Past History of Alcohol/Substance Abuse/Dependence
As can be appreciated from Table S1 (see Supplement 1), the IED-IR group with and the group without current physical aggression each consisted of approximately 50% of patients with a past history of alcohol/substance abuse/dependence. There were no significant differences between the IED-IR group with and the group without a past history of alcohol/substance abuse/dependence in OFC [11C]MDL100907 binding potential or with respect to our two measures of state aggression (Table S3 in Supplement 1).
Associations of Clinical Measures with OFC [11C]MDL100907 BPND and BPP
Scores of the OAS-M Assaultiveness subscale were positively skewed; thus Spearman correlation coefficients (controlling for age and age-by-group) were used to examine associations between Assaultiveness and OFC BPND and BPP among the IED-IR patients (both groups combined . No significant associations were observed, whether including or excluding the two patients with outlying Assaultiveness subscale scores from the group with current physical aggression (Table 5).
Table 5.
Associations of Clinical Measures with OFC [11C]MDL 100907 BPND, controlling for age and age-by-group, across the two IED-IR patient groups (n = 29).
| Clinical Measure | rs or r1 | p |
|---|---|---|
| State aggression: Assaultiveness2 | .22 | .263 |
| State aggression: Global Irritability3 | .48 | .009 |
| Trait aggression4 | −.01 | .955 |
| Trait impulsivity5 | −.04 | .843 |
| Trait affective lability6 | −.01 | .947 |
| State depressive symptoms7 | −.15 | .436 |
Note. The two state aggression clinical measures (above the double line) were our pre-selected measures of interest; the measures below the double line were used as exploratory measures; IED-IR = intermittent explosive disorder-integrated research criteria; OFC = orbitofrontal cortex
For Assaultiveness, Spearman correlation coefficient calculated due to non-normal distribution of scores (positive skew); for other measures, Pearson correlation coefficients calculated.
Assaultiveness subscale of the Overt Aggression Scale-Modified (OAS-M)
Global Irritability subscale of the OAS-M
Patients were administered the Buss-Durkee Hostility Inventory and/or Buss-Perry Aggression Questionnaire, and a T score was derived for each measure; for patients who completed both questionnaires, the mean of the two T scores was used; see text for additional information.
Barratt Impulsiveness Scale, Version-11
Affective Lability Scale
Beck Depression Inventory (BDI); one patient was missing BDI data, thus n=28.
Using Pearson correlation coefficients (controlling for age and age-by-group), the Global Irritability subscale was significantly associated with OFC BPND (Table 5; Figure 2) and BPP (r = .42, p = .023). These associations were similar when adjusting only for age (Supplement 1). The other clinical measures employed, however, were not associated with BPND or BPP (Table 5). Therefore, [11C]MDL100907 binding potential in the OFC correlated specifically with a measure of state aggression.
Figure 2.
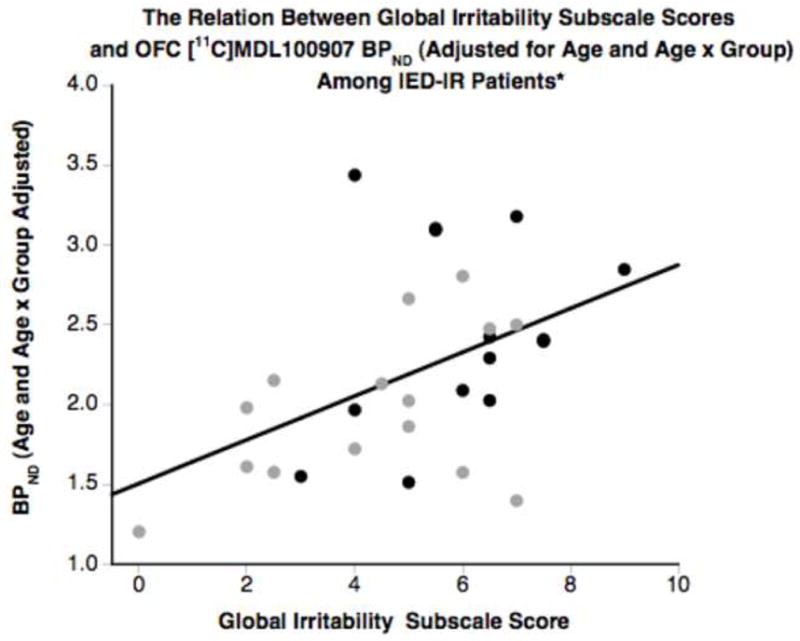
The relation between [11C]MDL100907 BPND (adjusted for age and age x group) in the orbitofrontal cortex (OFC)of both intermittent explosive disorder-integrated research criteria (IED-IR) groups combined and the Global Irritability subscale of the Overt Aggression Scale-Modified (OAS-M; a measure of state aggression). A positive association (r = .48, p = .009) was found between BPND and this state measure of aggression. Black circles = IED-IR patients with current physical aggression; gray circles = IED-IR patients without current physical aggression.
DISCUSSION
This is the first study of its kind to examine 5-HT2AR availability in two groups of impulsively aggressive personality disordered patients that differed namely in terms of their state levels of aggression --i.e., one with and one without current physical IED-IR --as well a healthy control group. We found greater OFC 5-HT2AR availability, specifically in the IED-IR group with current physical aggression compared to the other two groups. Moreover, OFC 5-HT2AR availability correlated specifically with a measure of state aggression among the IED-IR patients (both groups combined). Patients in our study were medication-free, and without a current major depressive episode or active alcohol/substance abuse. Additionally, in contrast to similar studies (34,35), we excluded for histories of serious past alcohol/substance abuse which could potentially have had long-lasting neurochemical sequelae.
Results for BPP were slightly less significant though qualitatively similar to BPND; the slightly smaller effect size for BPP may owe to added variance from the measurement of unmetabolized [11C]MDL100907 in arterial plasma, which can influence the calculation of BPP more than that of BPND. The modest effect size of our primary finding of increased [11C]MDL100907 binding potential in the OFC of IED-IR patients with current physical aggression may owe to the variability (in terms of both measurement error and biological variability) associated with clinically assessing complex and episodic human behavioral constructs, such as state aggression, as well as examining group differences in receptor availability. However, despite the modest effect size, the significance of greater OFC [11C]MDL100907 binding potential in the IED-IR group with current physical aggression compared to the IED-IR group without --i.e., that it reflects greater levels of state aggression --is supported by the correlation between OFC [11C]MDL100907 binding potential and the OAS-M Global Irritability subscale.
The lack of a correlation between the Assaultiveness subscale and [11C]MDL100907 binding potential in the OFC was unexpected; however, there are important differences between the Assaultiveness and Global Irritability subscales, which may account for why a correlation was found only with the latter. The Assaultiveness subscale score is based on events that cross the threshold for overt verbally or physically assaultive events. Generally speaking, assaultive events --in particular, high-severity ones --occur with relatively low frequency and consistency; this quality leads to greater ‘temporal variability’ or what Coccaro et al. (21,22) refer to as greater intra-individual variability. The Global Irritability subscale, on the other hand, is sensitive to both overt assaultive acts, as well as milder events that occur on a more regular and consistent basis and reflect an individual’s propensity for assaultive behavior. Therefore, Global Irritability may afford greater statistical precision, compared to Assaultiveness; thus, we speculate that with additional assessments around the time of scanning, we may have observed a significant correlation between Assaultiveness scores and OFC 5-HT2AR availability. Similarly, Coccaro et al. (21,22) and Mattes (26) have described how these qualities of the Assaultiveness subscale contribute to it being less sensitive than the Global Irritability subscale at detecting drug-placebo differences in impulsive aggression.
An additional limitation of this study is the lack of a second urine toxicology screen performed the day of scanning. However, IED-IR patients who may have had the greatest propensity for substance use around the time of scanning --i.e., those with a history of past alcohol/substance abuse/dependence --did not differ from IED-IR patients without such a history in terms of OFC 5-HT2AR availability nor on measures of state aggression.
Comparison with Related Imaging Studies
In a study using PET with [18F]altanserin, increased hippocampal 5-HT2AR availability was demonstrated in a cohort of female patients with BPD selected for high impulsivity compared to healthy controls (34). Hippocampal 5-HT2AR availability was not correlated with impulsivity or other clinical measures.
The results of this comparison study and ours converge, as they both demonstrate a relationship between behavioral dysregulation in personality disorders and increased 5-HT2AR availability in cortico-limbic regions. We were unable to assess the hippocampus, as [11C]MDL100907 binding potential values were unreliable for this region. The absence of findings in the OFC in this comparison study may owe to: their selection for high impulsivity as opposed to aggression (IED-IR), the presence of current major depression and active alcohol/substance abuse in a significant portion of patients in their study, and differences from our study in average age and gender ratio, and radioligand used.
In another study, using PET with [18F]setoperone, altered 5-HT2AR availability in prefrontal cortical regions was found in a highly violent/assaultive sample meeting criteria for ASPD (35). In comparison to healthy controls, this study showed decreased 5-HT2AR availability among subjects 19–24, and no differences in subjects 25–33 years of age; however, similar to our study, in which the average age was 36, this study showed an increase in subjects 34–39 years of age. Unlike our study, in which findings were specific to the OFC, altered 5-HT2AR availability in this study was observed in multiple prefrontal regions, as well as the temporal cortex. All patients in this comparison study were diagnosed with ASPD, were recruited from a clinical setting, and aggression was not operationalized using a specific diagnostic construct; in contrast, patients in our study were predominantly diagnosed with BPD, recruited from a non-clinical setting, and aggression was operationalized as IED-IR.
One possible explanation for these disparities is that two separate pathophysiologic processes may be involved that both manifest impulsive aggression. While ASPD and BPD both share impulsive aggression as a common symptom dimension, they characteristically differ in terms of their interpersonal/affective dimensions. More specifically, ASPD is associated with the interpersonal/affective deficits of psychopathy (36), e.g., deceitfulness/conning and lack of remorse. On the other hand, BPD is noted for significant interpersonal sensitivity (e.g., frantic efforts to avoid real or perceived abandonment) and affective instability. Accordingly, psychopathic individuals exhibit attenuated responses to emotional stimuli (37,38), whereas patients with BPD exhibit amplified ones (39,40). Further, aggression in ASPD/psychopathy has been characterized in terms of a ‘hypoarousal’ pathophysiologic process (41), consistent with the decreased influence that emotion has on cognitive processes in patients with psychopathic traits (42). In contrast, a ‘hyperarousal’ (43) form of aggression, in which there is an excessive influence of emotional processes, likely characterizes IED-IR and BPD. Moreover, the serotonergic system is differentially involved in hypo-compared to hyperarousal aggression (44,45).
Functional Implications of Increased 5-HT2AR Availability in the OFC
Increases in 5-HT2AR expression may be mediated in part by psychosocial stress (46–49). Specific genetic polymorphisms of the 5-HT2AR(8–10), differential regulation of BDNF in the mature CNS (50), and possibly, epigenetic effects due to developmental psychosocial adversity (51), also likely influence 5-HT2AR function, and thus modify an individual’s risk for aggression.
Multiple lines of evidence are inconsistent with the notion that elevated 5-HT2AR expression simply reflects a compensatory upregulation in response to low presynaptic serotonin (52–55). Studies have demonstrated that, in a non-classical manner, the 5-HT2AR does not upregulate in response to serotonergic or adrenergic dennervation. Another atypical feature is that treatment with either agonists or antagonists of the 5-HT2AR leads to its downregulation. Our preliminary findings from an ongoing PET study using [11C]DASB in a partially overlapping cohort of IED-IR patients concurrently imaged with [11C]MDL100907 indicate that 5-HTT and 5-HT2AR availability in the anterior cingulate are not significantly associated (Siever et al., unpublished results).
Owing to its dual distribution in cortical regions (56,57) --i.e., the apical dendrites of cortical pyramidal cells and a sub-population of inhibitory interneurons known as basket cells --the 5-HT2AR is believed to regulate the signal-to-noise ratio among cortical columns (57). Therefore, changes in 5-HT2AR availability may lead to a deviation from the optimal signal-to-noise ratio, impairing the OFC’s ability to assess threatening stimuli.
Conclusion
In summary, these findings are consistent with our previously described model in which we propose that heightened 5-HT2AR function in the OFC is a state condition associated with increased impulsive aggression in a population with a vulnerability trait (namely, attenuated presynaptic serotonin in cortico-limbic regions) that predisposes an individual to impulsive aggression.
Supplementary Material
Acknowledgments
This research was supported by a Grant MH063875 from the National Institute of Mental Health and by a Veterans Affairs Merit Review Grant (7609-028) to Larry J. Siever; and by the Veterans Affairs VISN 3 Mental Illness Research, Education & Clinical Center. Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. This publication was made possible by Grant Number MO1-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
FINANCIAL DISCLOSURES
Dr. Slifstein reports the following: Grant Support: Intracellular Therapies, Inc.; Consulting: Glaxo-SmithKline, Inc., Amgen Inc. Dr. Frankle reports the following: Consulting: Ono Pharma USA, Inc., Sepracor, Inc.; Speaking Fees: Bristol-Meyers Squibb, Inc.; Research Support: Glaxo-SmithKline, Inc., Sepracor, Inc. Dr. Laruelle is a full time employee of Glaxo-SmithKline, Inc. Dr. Abi-Dargham reports the following: Grant support: Glaxo-SmithKline, Inc.; compensation received from BMS-Otsuka for consulting and speaking engagements. The other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCloskey MS, Berman ME, Noblett KL, Coccaro EF. Intermittent explosive disorder-integrated research diagnostic criteria: convergent and discriminant validity. J Psychiatr Res. 2006;40:231–242. doi: 10.1016/j.jpsychires.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:449–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaue M, Ago Y, Sowa C, Sakamoto Y, Nishihara B, Koyama Y, et al. Modulation by 5-HT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol. 2002;89:89–92. doi: 10.1254/jjp.89.89. [DOI] [PubMed] [Google Scholar]
- 4.Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology. 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- 5.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 6.Coccaro EF, Kavoussi RJ, Sheline YI, Berman ME, Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5-HT2A receptor binding. Neuropsychopharmacology. 1991;16:211–216. doi: 10.1016/S0893-133X(96)00194-7. [DOI] [PubMed] [Google Scholar]
- 7.Oquendo MA, Russo SA, Underwood MD, Kassir SA, Ellis SP, Mann JJ, et al. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Giegling I, Hartmann AM, Möller HJ, Rujescu D. Anger-and aggression-related traits are associated with polymorphisms in the 5-HT-2A gene. J Affect Disord. 2006;96:75–81. doi: 10.1016/j.jad.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Nomura M, Kusumi I, Kaneko M, Masui T, Daiguji M, Ueno T, et al. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacology. 2006;187:30–35. doi: 10.1007/s00213-006-0398-z. [DOI] [PubMed] [Google Scholar]
- 10.Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL. Serotonin 2A receptor T102C polymorphism and impaired impulse control. Am J Med Genet. 2002;114:336–339. doi: 10.1002/ajmg.10206. [DOI] [PubMed] [Google Scholar]
- 11.Leyton M, Paquette V, Gravel P, Rosa-Neto P, Weston F, Diksic M, et al. alpha-[11C]Methyl-L-tryptophan trapping in the orbital and ventral medial prefrontal cortex of suicide attempters. Eur Neuropsychopharmacol. 2006;16:220–223. doi: 10.1016/j.euroneuro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, et al. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158:775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- 13.Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 14.Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, et al. d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 15.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, et al. Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- 16.New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, et al. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 19.Pfohl B, Blum N, Zimmerman M. Structured Interview for DSMIV Personality: SIDP-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 20.Coccaro EF, Harvey PD, Kupsaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3:S44–51. [PubMed] [Google Scholar]
- 21.Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 22.Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70:653–662. doi: 10.4088/JCP.08m04150. [DOI] [PubMed] [Google Scholar]
- 23.Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry. 1998;59:676–680. doi: 10.4088/jcp.v59n1206. [DOI] [PubMed] [Google Scholar]
- 24.Hollander E, Tracy KA, Swann AC, Coccaro EF, McElroy SL, Wozniak P. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28:1186–1197. doi: 10.1038/sj.npp.1300153. [DOI] [PubMed] [Google Scholar]
- 25.Zanarini MC, Frankenburg FR, Parachini EA. A preliminary, randomized trial of fluoxetine, olanzapine, and the olanzapine-fluoxetine combination in women with borderline personality disorder. J Clin Psychiatry. 2004;65:903–907. doi: 10.4088/jcp.v65n0704. [DOI] [PubMed] [Google Scholar]
- 26.Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25:575–579. doi: 10.1097/01.jcp.0000186739.22395.6b. [DOI] [PubMed] [Google Scholar]
- 27.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 28.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 29.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate – A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 34.Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biol Psychiatry. 2007;62:580–587. doi: 10.1016/j.biopsych.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Meyer JH, Wilson AA, Rusjan P, Clark M, Houle S, Woodside S, et al. Serotonin2A receptor binding potential in people with aggressive and violent behaviour. J Psychiatry Neurosci. 2008;33:499–508. [PMC free article] [PubMed] [Google Scholar]
- 36.Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. J Abnorm Psychol. 1991;100:391–398. doi: 10.1037//0021-843x.100.3.391. [DOI] [PubMed] [Google Scholar]
- 37.Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Arch Gen Psychiatry. 2001;58:737–745. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- 38.Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 39.Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 40.Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 41.Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell DG, Richell RA, Leonard A, Blair RJ, et al. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. J Abnorm Psychol. 2006;115:559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- 43.Haller J, Mikics E, Halász J, Tóth M. Mechanisms differentiating normal from abnormal aggression: glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Haller J, Horváth Z, Bakos N. The effect of buspirone on normal and hypoarousal-driven abnormal aggression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:27–31. doi: 10.1016/j.pnpbp.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Haller J, Tóth M, Halász J. The activation of raphe serotonergic neurons in normal and hypoarousal-driven aggression: a double labeling study in rats. Behav Brain Res. 2005;161:88–94. doi: 10.1016/j.bbr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Schiller L, Jähkel M, Kretzschmar M, Brust P, Oehler J. Autoradiographic analyses of 5-HT1A and 5-HT2A receptors after social isolation in mice. Brain Res. 2003;980:169–178. doi: 10.1016/s0006-8993(03)02832-4. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Kramer GL, Kram M, Steciuk M, Crawford IL, Petty F. Serotonin and learned helplessness: a regional study of 5-HT1A, 5-HT2A receptors and the serotonin transport site in rat brain. J Psychiatr Res. 1999;33:17–22. doi: 10.1016/s0022-3956(98)00041-7. [DOI] [PubMed] [Google Scholar]
- 48.Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–214. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Günther L, Liebscher S, Jähkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group-and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Rios M, Lambe EK, Liu R, Teillon S, Liu J, Akbarian S, et al. Severe deficits in 5-HT2A-mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- 51.Sumner BE, D’Eath RB, Farnworth MJ, Robson S, Russell JA, Lawrence AB, et al. Early weaning results in less active behaviour, accompanied by lower 5-HT1A and higher 5-HT2A receptor mRNA expression in specific brain regions of female pigs. Psychoneuroendocrinology. 2008;33:1077–1092. doi: 10.1016/j.psyneuen.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 53.Eison AS, Mullins UL. Regulation of central 5-HT2A receptors: a review of in vivo studies. Behav Brain Res. 1996;73:177–181. doi: 10.1016/0166-4328(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 54.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 55.Stockmeier CA, Kellar KJ. In vivo regulation of the serotonin-2 receptor in rat brain. Life Sci. 1986;38:117–127. doi: 10.1016/0024-3205(86)90003-2. [DOI] [PubMed] [Google Scholar]
- 56.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


