Abstract
Background and methods
Perturbations in energetic metabolism and impaired atrial contractility may play an important role in the pathogenesis of atrial fibrillation (AF). Besides, atrial stretch is commonly associated with AF. However, the atrial energetics of stretch-related AF are poorly understood. Here, we measured indicators of energy metabolism during acute-stretch related AF. PCr, adenine nucleotides and derivatives concentrations as well as the activity of the F0F1-ATPase and Na,K-ATPase were obtained after one hour of stretch and/or AF in isolated rabbit hearts and compared to control hearts without stretch and AF.
Results
After one hour of stretch-related AF, the total adenine nucleotides pool was significantly lower (42.2±2.6 versus 63.7±8.3 µmol/g protein in control group, p<0.05) and the PCr/ATP ratio significantly higher (2.3±0.3 vs 1.1± 0.1 in control group p<0.05), because of ATP, ADP and AMP decrease and PCr increase. The sum of high energy phosphate compounds did not change. There were no significant differences in F0F1-ATPase nor Na,K-ATPase activity between the groups.
Conclusions
Results show that in this experimental model, acute-stretch related AF induces specific modifications of atrial myocytes energetics that may play a pivotal role in the perpetuation of the arrhythmia.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in adults (1). Classically, AF is associated with an impaired atrial contractility but the underlying molecular mechanisms contributing to these changes and more generally how this arrhythmia is maintained remain unclear (2). While ionic remodeling (3–5) as well as changes in cellular and structural architecture (6) have been described, increasing evidence for a role of perturbations in energetic metabolism exists. In fact, various studies have shown modifications in energetic metabolism in both animal models (7–11) and humans (12–14) and after both chronic or short episodes of AF (9, 10, 12–14). However, a variety of changes have been observed. Several works describe an increased demand in energy (7–9, 11) while others have suggested impaired energy production or consumption (10, 12–14). In order to clarify the atrial energetic status during AF, we investigated the consequences of acute atrial stretch on atrial myocytes energetic metabolism. This substrate is of particular interest because atrial stretch is commonly associated with AF and has been demonstrated to play a critical role in the initiation and maintenance of this arrhythmia (15–17). Previously, some changes in atrial myocardial energetic status after the onset of fibrillation have been described in models with physiological elevation of intra-atrial pressure (7, 8, 11). Nevertheless, an evaluation of the energetic status in an AF model with a controlled high level of intra-atrial pressure and stretch has not been investigated. We hypothesized that acute-stretch-related AF induces specific perturbations in energetic metabolism that are critical for AF maintenance. To this aim, we used a well-characterized stretch-related model of AF in the rabbit (17, 18) and evaluated the changes in atrial adenine nucleotides and phosphocreatine concentrations during stretch-induced AF in comparison to stretch alone and to control conditions without stretch and AF. Finally, we determined the activities of 2 ion pumps, F0F1-ATPase and Na,K-ATPase, which are known to be very sensitive to stress and tightly coupled to energetic metabolism.
Materials and methods
Experimental Model of Biatrial Dilation
Twenty one rabbits of either sex weighing 1.5 to 2 kg were used for this study. The study was approved by the ethic committee for animal investigation of the Faculty of Medicine in Marseille. The animals were sedated with an association ketamine/chlorpromazine and, after injection of 1000 IU heparin i.v., they were killed by cervical dislocation. The thorax was opened by a midsternal incision, and the heart was rapidly removed and placed in cold perfusion fluid (0–10°C). The aorta was cannulated, and the heart was perfused at a pressure of 65 mm Hg and a temperature of 37°C. The composition of the perfusion fluid was (in mM) NaCl 130, NaHCO3 24.2, KCl 4.0, CaCl2 2.2, MgCl2 0.6, Na2HPO4 1.2, and glucose 12. It was gassed with a mixture of 95% O2/5% CO2, resulting in a pH of 7.4. A model of bi-atrial dilation (19) initially described by Ravelli and Alessie (17) was used. The bi-atrial pressure was controlled as following: a Y-shaped manometer (Phymep, Paris, France) was inserted into the inferior caval vein and one of the pulmonary veins to measure biatrial pressure. Atrial pressure and degree of atrial dilatation were controlled by adjustment of the height of the outflow cannula. Intra-biatrial pressure was maintained at 15 cm H2O throughout the experiment. As it was shown in previous studies, easily inducible and sustained episodes of AF were obtained (17–19) at this level of pressure.
Electrophysiological measurements
Epicardial electrograms were recorded from the left mid-atrial free wall by a bipolar electrode. To measure atrial effective refractory periods (ERPs) an electrical stimulation was delivered with a bipolar epicardial hook electrode attached to the right atrial appendage. Stimuli of 1-ms pulse duration and 2-fold diastolic pacing threshold were delivered with a stimulator (Medtronic, Boulogne-Billancourt, France). Electrograms were registered using a data acquisition system (PowerLab, Phymep, Paris, France) for conversion and analysis of signals. The electrograms were filtered (bandwidth, 1 to 500 Hz) and amplified (gain 1000) with the aortic cannula used as an indifferent electrode. ERPs were measured at a cycle length of 250 ms with single premature stimuli interpolated every 8th basic interval. The shortest coupling interval that resulted in a propagated atrial response was considered as the ERP value.
Experimental protocol
Atrial ERPs were measured at 0 cm H2O and after elevating the pressure at 15 cm H2O. Then stretch was maintained during 1 hour (stretch, n=7) or AF was induced after delivering a burst of stimulation (20 Hz, 10 seconds) or else during the ERPs measurements after the premature stimulus was delivered (AF, n=7) and sustained thereafter during 1 hour. A control group (control, n=7) was composed by hearts in which pressure was maintained at 0 cmH2O during an equal time. As described previously AF episodes are very sustained in this model when intra-atrial pressure is set above 10 cm H2O (17, 19). After one hour of stretch or sustained AF or control the two atria were freeze-clamped with a Wollenberg clamp that was pre-cooled in liquid nitrogen and kept at − 80° C.
Atrial metabolism measurements
Determination of adenine nucleotides and derivatives, PCr in freeze-clamped atria by ion-exchange high performance liquid chromatography
After perchloric acid extraction of a portion of freeze-clamped atria (left and right) (50mg), separation of PCr, adenine nucleotides and derivatives (nucleosides and nucleobases) and NAD was performed by the ion-pairing reverse phase technique of Lazzarino et al. (20). Results were expressed in µmoles per g of protein as previously described (21). Total adenine nucleotides (TAN) were calculated from the sum ATP+ADP+AMP. The sum of high energy phosphates was the sum PCr+ATP+ADP+AMP. Atrial energetic charge (EC) was calculated from the formula (ATP + 0.5ADP)/(ATP+ADP+AMP).
Preparation of atrial mitochondrial and microsomal membranes
Small pieces (100 to 200mg) of frozen right or left atria were homogenized with a polytron PT2100 (Polytron Kinematica.A.G., Luzern, Switzerland) in a buffer (pH 7.4) with the following composition (mM) : Na pyrophosphate 20, KCL 80, sucrose 250, Tris 20, PMSF 0.1 and EDTA 0.1. Mitochondrial membranes were obtained from two successive centrifugations (Sorvall, Evolution RC and SA 300 rotor, Kendro Scientific). After a first centrifugation at 600g for 10 minutes to eliminate the cells, membrane sediments at 6800g for 15 minutes were harvested as a source of mitochondrial membranes. The supernatant was centrifuged for 60 minutes at 62300 g and then membranes were harvested as a source of microsomal membranes. The 2 pellets were resuspended in a mix of tris 20mM and sucrose 250mM.
Measurement of F0F1-ATPase activity
F0F1-ATPase activity was measured in triplicate in mitochondrial membranes preparations using an Ultraspec 2000 spectrophotometer with a Swift software (Pharmacia, Biotech) at 37°C. Ten µg of the suspension of mitochondrial membranes were added to the test tube containing the following (mM) : Tris acetate 33, sucrose 83, MgCl2 10, EDTA 1, ATP 2, phosphoenolpyruvate 1.5, NADH 0.17, pyruvate kinase 3.5 IU, lactate dehydrogenase 10IU at pH 7.2. The decrease in NADH absorbance at 340nm (in triplicate experiments) was observed for 10 minutes with or without NaN3 to inhibit F0F1-ATPase. The activity was measured from the difference between the two slopes and was expressed in micromoles of hydrolyzed ATP per min per mg of mitochondrial proteins.
Measurement of Na,K-ATPase activity
Na,K-ATPase activity was defined at 37°C in triplicate in microsomal membranes preparations using an ultraspec spectrophotometer as previously described (22). Ten µg of the suspension of microsomal membranes were added to the test tube containing the following (mM) : NaCl 100, KCl 10, MgCl2 4, ATP 4, phosphoenolpyruvate (trisodium salt) 2, Imidazole 30, pyruvate kinase 3.5 IU, lactate dehydrogenase 5 IU, NADH 0.4 mM at pH 7,4. The decrease in NADH absorbance at 340 nm was observed with or without ouabain to inhibit Na,K-ATPase activity. Maximal Na,K-ATPase activity was defined as activity inhibited by 50µM ouabain.
The membrane orientation has not been characterized here since in our previous experiments this standardized membrane isolation did not produce inside out vesicles but leaky right side out vesicles in which ouabain and ATP have free access to their site of action. Similar results in Na,K-ATPase activity have been obtained for this preparation with native membrane or with detergent (23). Furthermore to avoid the alteration of membrane protein environment, the enzymatic activity was carried out with native membranes (without detergent treatment) (24).
Statistical analysis
Results are expressed as means ± SEM. Comparison between average values of ERPs before and after stretch was performed with a paired t-test. One way ANOVA with Tukey post-hoc test was used for comparison of metabolic measurements between the 3 groups. A value of p < 0.05 was considered as significant.
Results
ERPs and AF inducibility
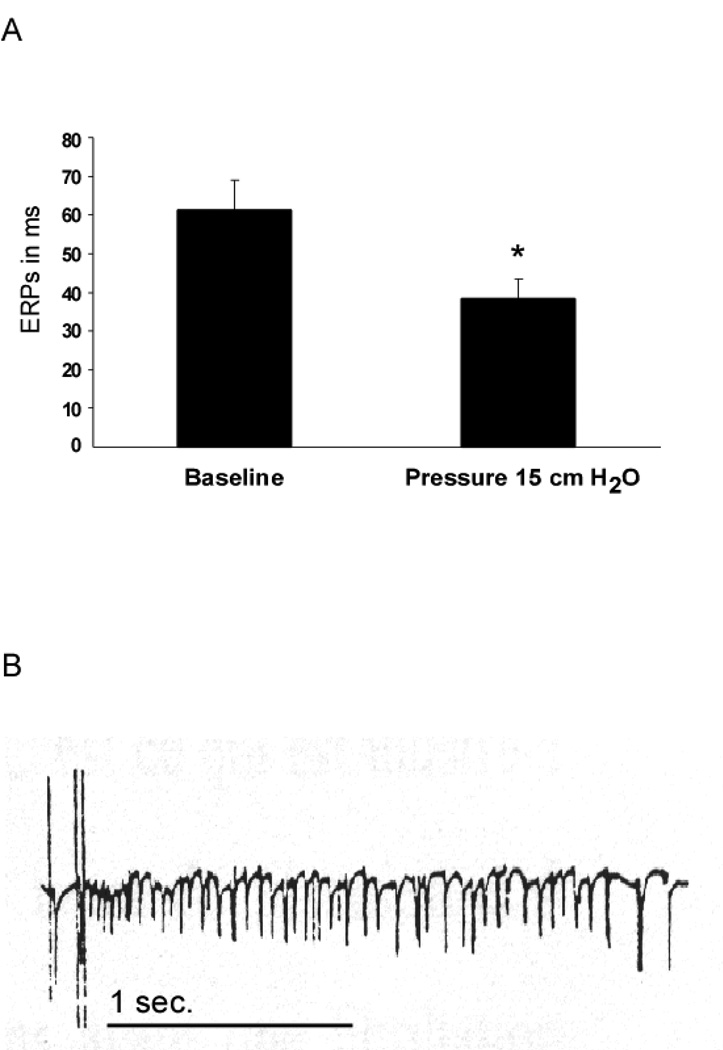
As expected in this model (17), after elevating intra-atrial pressure, right atrial ERPs are significantly reduced from 61.2±7.8 to 38.3±4.9 ms (p= 0.03), see Figure 1A. Consistently, sustained episodes of AF (more than one hour) were obtained after elevating the intra-atrial pressure at 15 cm H2O. In Figure 1B a representative bipolar recording after initiation of AF is presented. In previous experiments with a similar protocol (19) we recorded the spontaneous heart rate in 30 isolated rabbit hearts before and more than 3 minutes after having elevated the left intra-atrial pressure to 15 cm H2O. We observed that the sinus rate was raised from 215±6 to 252±3 beats/min corresponding to a rate increase of 17±1%. This increase can be explained by the activation of sinus node stretch-activated channels which are known to be responsible for an elevation of the resting membrane potential and a reduction in maximum diastolic potential as shown by Cooper et al. (25).
Figure 1.
(A) Right atrial ERPs before and after elevation of intra-atrial pressure, means ± SEM, * p=0.03. (B) Representative example of a bipolar recording at the RA appendage of an initiation of AF after delivery of a burst of stimulation.
High energy phosphates and related compounds
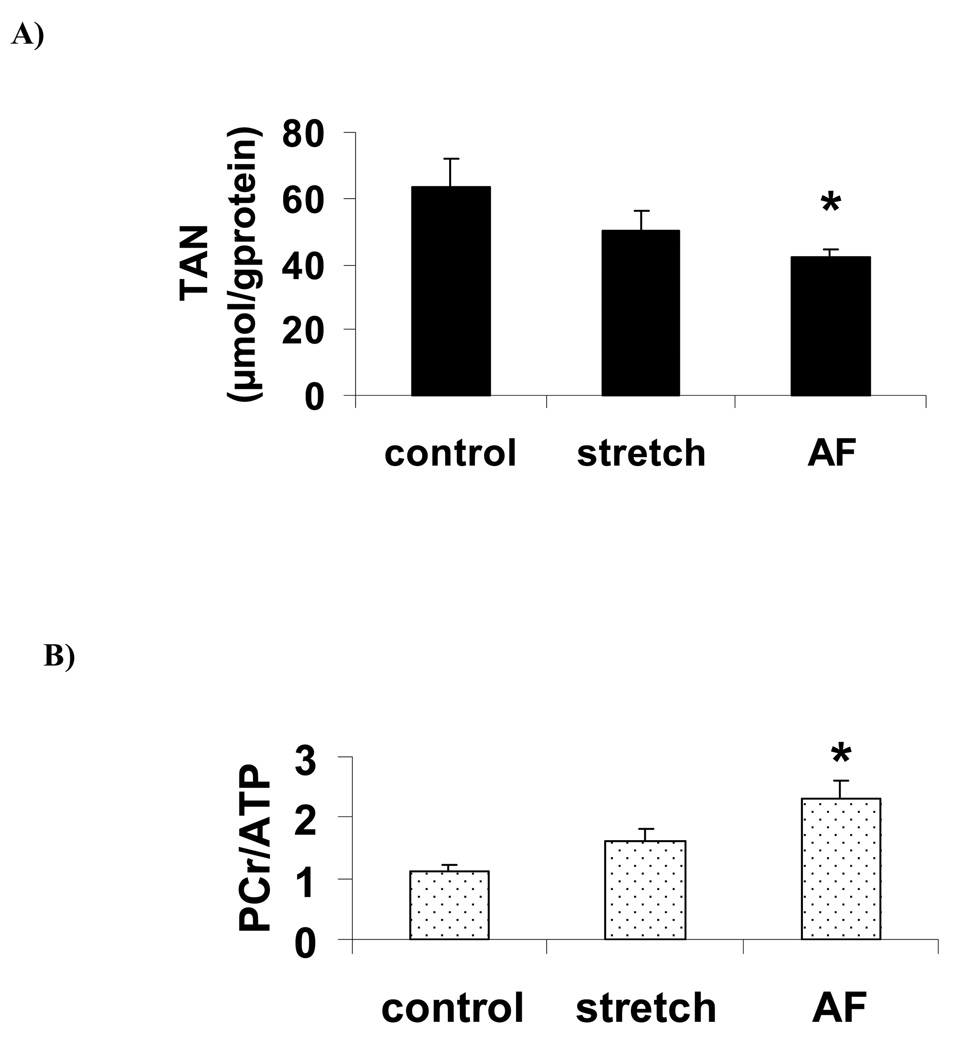
Significant statistical differences among the three groups were revealed for the total adenine nucleotides pool (TAN) (ANOVA, p= 0.045) and for the ratio PCr/ATP (ANOVA, p=0.0025). Post-hoc tests show that TAN pool was significantly lower (figure 2A) and PCr/ATP ratio was significantly higher during AF (figure 2B) compared to control, resulting both from PCr increase as well as ATP, ADP and AMP decrease although individual changes were not significantly different (table I). The same trend was observed in the stretch group but did not reach the level of significance. The sum of high energy phosphate compounds (ΣHEP), energetic charge and NAD were constant among the 3 groups. The concentration of the degradation products of adenine nucleotides in atrial tissues (Σnucleosides, nucleobases = adenosine, inosine, hypoxanthine and xanthine) was very low and was not significantly different between the 3 groups (table I).
Figure 2.
Total adenine nucleotides (TAN) (A) and PCr/ATP ratio (B) expressed as means ± SEM. *: p<0.05 vs control group.
Table 1.
PCr, adenine nucleotides, derivatives (Σnucleosides, nucleobases =adenosine, hypoxanthine, inosine, xanthine), ΣHEP (PCr+ATP+ADP+AMP), NAD (µmol/g of protein) and energetic charge (EC) in each of the 3 groups. Results are expressed as means ± SEM.
| PCr | AMP | ADP | ATP | ΣHEP | Σnucleosides, nucleobases |
NAD | EC | |
|---|---|---|---|---|---|---|---|---|
| control | 49.8±8.2 | 4.5±1.2 | 14.2±2.7 | 45.1±5.3 | 113.5±13.8 | 1.8±0.9 | 6.4±1.2 | 0.82±0.02 |
| stretch | 54.0±4.5 | 2.8±0.5 | 11.2±1.4 | 36.2±4.4 | 104.2±7.9 | 2.5±0.9 | 5.7±0.5 | 0.83±0.01 |
| AF | 63.8±6.2 | 2.1±0.6 | 9.2±0.7 | 30.8±2.1 | 106.0±18.7 | 0.5±0.2 | 5.7±0.5 | 0.84±0.01 |
| P(ANOVA) | 0.31 | 0.15 | 0.18 | 0.08 | 0.79 | 0.17 | 0.79 | 0.73 |
F0F1-ATPase activity (figure 3A) and Na,K-ATPase activity (figure 3B)
Figure 3.
Mitochondrial F0F1-ATPase activity (A) and sarcolemmal Na,K-ATPase activity (B) from atrial membranes in control, stretch and AF groups. Results are expressed as means ± SEM.
There were no significant differences in F0F1-ATPase activity (ANOVA, p=0,17) nor Na,K-ATPase activity between control, stretch and AF (ANOVA, p=0.32) although a trend to higher value in the stretch group was observed for Na,K-ATPase.
Discussion
Main findings
After one hour of acute stretch-related AF significant changes in energetic metabolism were observed; PCr/ATP ratio was increased due to both PCr increase and ATP (and the pool of total adenine nucleotides) decrease. Besides, the sum of high energy phosphates, F0F1-ATPase and Na,K-ATPase activity were not significantly altered. The same trend was observed in the stretch group but did not reach the level of significance.
AF energetics
Understanding the molecular mechanisms of energetic metabolism modulation during AF is crucial to elucidate the pathogenesis of this arrhythmia and its propensity to self-perpetuate. However, the nature of these changes remains controversial. AF has been described either as a high energy demanding state or as an ischemic state in both animals and humans. An ischemic state has been suggested by (i) White CW et al. (8) demonstrating, that after 15 minutes of AF in a dog open chest model, a significant increase in oxygen consumption resulted in the reduction of atrial flow reserve; (ii) Ausma et al (26) who documented a cellular structural remodeling during chronic AF similar to that occurring during chronic hibernation; (iii) Tsuboi et al (14) who found a reduction of adenine nucleotides (associated with mitochondrial deletion) in human atria; (iv) Seppet et al (12) who quantified an alteration of oxidative phosphorylation and (v) Cha et al (10) who demonstrated, in a dog model of tachypacing-induced heart failure and chronic atrial dilatation, a depletion of high energy phosphates (ATP and PCr), reduced activities of phosphotransfer enzymes and a decrease in the cellular energy potential. Conversely, in favor of a status of high energy demand, Leistad et al. (11) have shown in a pig open chest model that after 15 and 25 minutes there was no increase in lactate levels and, subsequently, Barbey et al (7) confirmed that after 100 minutes of AF, a high demand metabolic state translated into an increased F0F1-ATPase activity. Further, Ausma et al (9) reported that AF-induced atrial remodeling in the goat, was associated with transient lowering of phosphocreatine (with no changes in adenine nucleotides or in the mitochondrial enzymes), this study being consistent with a high demand energy state.
In an attempt to clarify the energetic status during AF, we chose to investigate specifically the consequences of acute atrial stretch that is well-known to predispose to AF (27). To the best of our knowledge, acute atrial stretch energetics during AF have not been investigated previously. Myocardial stretch has been shown to lead to increased oxygen consumption and increased ATP synthesis in the intact, non contracting heart (28). In the present study stretch group shows a trend to lower nucleotides levels together with higher PCr/ATP levels while the sum of high energy phosphate compounds was maintained constant and these changes became statistically significant when atrial fibrillation was superimposed on stretch. Interestingly, lower adenine nucleotide levels have been associated with permanent atrial fibrillation in the case of mitochondrial DNA deletion in atria of patients (14). We may thus hypothesize that loss of adenine nucleotides in this model of acute stretch-induced AF participates in perpetuating AF. We also observed a loss of ATP and adenine nucleotides compounds without increase in adenosine, inosine, hypoxanthine and xanthine in the tissue. The nucleotides ATP, ADP, AMP do not cross the cell membrane but the nucleosides and nucleobases adenosine, inosine and hypoxanthine diffuse across the cell wall down their concentration gradients and this phenomenon is particularly relevant in the presence of flow and a release of these compounds has been observed in various situations of ATP degradation with a flow allowing the release of these metabolites (hypoxia, post-ischemic reperfusion (29)). Interestingly release of adenine nucleotide metabolites has been observed at toxic concentrations of glycosides and also when fibrillation was elicited electrically in normal hearts (30) and the decrease in myocardial ATP observed in glycoside-intoxicated heart preparation has been suggested to be partly due to the loss of nucleotide precursors. Similarly the changes observed here could be partly due to the loss of nucleotide precursors. Concomitantly to ATP decrease, phosphocreatine levels were increased. This may be explained by alteration of the PCr/Cr ratio towards increased PCr (31) due to elevated Pi associated to the loss of adenine nucleotides (31, 32) as was also observed during post-ischemic reperfusion (31). Alternatively, as reported by Mihm et al. in atria of patients with AF (13), increased phosphocreatine and lower adenine nucleotide levels could be caused by altered energetics at the site of utilization of ATP i.e at the myofibrillar level where a selective impairment of myofibrillar creatine kinase activity can occur. An increased activity of adenylate kinase could also explain at least partly these data. However the measurement of adenylate kinase and creatine kinase activities was not performed thus not allowing final conclusions. Changes in creatine content could also play a role. Creatine content was not measured in the present experiments however as PCr was increasing we do not expect a decrease in total creatine.
F0F1-ATPase and Na,K-ATPase activity
F0F1-ATPase and Na,K-ATPase are likely to be modulated by AF as they are tightly related to energetic metabolism and ionic gradients. Besides, direct and indirect evidence suggest that the activity of these two pumps might have been modified in our model. First, Barbey et al (12) have shown an increased activity of F0F1-ATPase but in absence of any change in Na,K-ATPase activity (7). On the other hand, the work by Songu-Mize et al (33) has shown increased Na pump activity by short term cyclic stretch in aortic smooth muscle cells. A tendency towards increased Na,K-ATPase activity was observed in the stretch group but did not reach the level of significance, however we cannot exclude a lack of power of our experiment. Conversely we did not observe any significant modification in the activity in the AF group. Similarly to the results of the present study for sarcolemmal and mitochondrial ATPases, the sarcoplasmic Ca2+-pump was found unaltered (34); AF sequence did not change the protein and mRNA levels of this membrane function although an impaired Ca2+ homeostasis is supposed to contribute to initiation and perpetuation of AF. However, El Armouche et al (35) recently provide convincing evidence that atrial fibrillation is associated to activation of cellular regulatory proteins (ie kinases and phosphatases activity with increased PP1 and PP2A) resulting in impaired phosphorylation of MyBP-C but hyperphosphorylation of phospholamban. This phenomenon resulted in myosin as well as sarcolemmal and sarcoplasmic membrane regulations that could be associated to the modifications of energy metabolism shown in the present study. Similarly Na,K-ATPase and mitochondrial ATPase could be modulated by these phosphatase and kinase regulations as well as other signal transduction during AF (36, 37).
Conclusions
In this experimental model of acute-stretch related AF, data show modifications in energetic metabolites different from findings previously reported in the literature with AF models in which no controlled elevation of intra-atrial pressure was achieved. These results provide a new insight for the understanding of initiation and maintenance of AF when intra-atrial pressure is acutely increased like during acute pulmonary embolism (38). Lower adenine nucleotides levels together with the absence of a decrease in PCr and in F0F1-ATPase activity exclude the occurrence of ischemia in this experimental model and rather documents a state of impaired energy utilization. Such metabolic changes may play a pivotal role in the perpetuation of stretch-related AF as they are tightly related to calcium handling which is known to play a key-role in AF perpetuation (39, 40); calcium handling is ATP-dependent and alterations in calcium homeostasis may lead to impaired ATP synthesis (41). Putatively, an alternative mechanisms related to changes in gene expression (42) consequent to atrial mechanical stretch and yielding variations in the level of mitochondrial enzymes could have impaired ATP synthesis in our model. In this regard, it has been shown that mitochondrial DNA deletion in human atria is associated with AF (14). However, testing this hypothesis, as well as the detailed molecular mechanisms of the energetic changes presented here, and also the extent to which this energetic status would be similar after chronic stretch, will require different experimental models and further studies. Importantly, our observations only relate to our specific experimental model and may not be extrapolated to AF in patients.
Acknowledgements
CNRS (UMR 6612). JK: Grants ADEREM Marseille, NIH RO1 087055-01 and ACCF/GE Healthcare Career Development Award
References
- 1.Alpert JS, Petersen P, Godtfredsen J. Atrial fibrillation: natural history, complications, and management. Annu Rev Med. 1988;39:41–52. doi: 10.1146/annurev.me.39.020188.000353. [DOI] [PubMed] [Google Scholar]
- 2.Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 3.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 4.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol. 1998;9:1378–1393. doi: 10.1111/j.1540-8167.1998.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S. Atrial electrophysiological remodeling caused by rapid atrial activation: underlying mechanisms and clinical relevance to atrial fibrillation. Cardiovasc Res. 1999;42:298–308. doi: 10.1016/s0008-6363(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 6.Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol. 2000;9:17–28. doi: 10.1016/s1054-8807(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 7.Barbey O, Pierre S, Duran MJ, Sennoune S, Levy S, Maixent JM. Specific up-regulation of mitochondrial F0F1-ATPase activity after short episodes of atrial fibrillation in sheep. J Cardiovasc Electrophysiol. 2000;11:432–438. doi: 10.1111/j.1540-8167.2000.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 8.White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res. 1982;51:205–215. doi: 10.1161/01.res.51.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Ausma J, Coumans WA, Duimel H, Van der Vusse GJ, Allessie MA, Borgers M. Atrial high energy phosphate content and mitochondrial enzyme activity during chronic atrial fibrillation. Cardiovasc Res. 2000;47:788–796. doi: 10.1016/s0008-6363(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 10.Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol. 2003;284:H1313–H1320. doi: 10.1152/ajpheart.00337.2002. [DOI] [PubMed] [Google Scholar]
- 11.Leistad E, Aksnes G, Verburg E, Christensen G. Atrial contractile dysfunction after short-term atrial fibrillation is reduced by verapamil but increased by BAY K8644. Circulation. 1996;93:1747–1754. doi: 10.1161/01.cir.93.9.1747. [DOI] [PubMed] [Google Scholar]
- 12.Seppet E, Eimre M, Peet N, Paju K, Orlova E, Ress M, Kovask S, Piirsoo A, Saks VA, Gellerich FN, Zierz S, Seppet EK. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Mol Cell Biochem. 2005;270:49–61. doi: 10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 13.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 14.Tsuboi M, Hisatome I, Morisaki T, Tanaka M, Tomikura Y, Takeda S, Shimoyama M, Ohtahara A, Ogino K, Igawa O, Shigemasa C, Ohgi S, Nanba E. Mitochondrial DNA deletion associated with the reduction of adenine nucleotides in human atrium and atrial fibrillation. Eur J Clin Invest. 2001;31:489–496. doi: 10.1046/j.1365-2362.2001.00844.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 16.Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000;101:2200–2205. doi: 10.1161/01.cir.101.18.2200. [DOI] [PubMed] [Google Scholar]
- 17.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 18.Zarse M, Deharo JC, Mast F, Allessie MA. Importance of right and left atrial dilation and linear ablation for perpetuation of sustained atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:164–171. doi: 10.1046/j.1540-8167.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalifa J, Bernard M, Gout B, Bril A, Cozma D, Laurent P, Chalvidan T, Deharo JC, Djiane P, Cozzone P, Maixent JM. Anti-arrhythmic effects of I (Na), I (Kr), and combined I (Kr)-I (CaL) blockade in an experimental model of acute stretch-related atrial fibrillation. Cardiovasc Drugs Ther. 2007;21:47–53. doi: 10.1007/s10557-007-6001-y. [DOI] [PubMed] [Google Scholar]
- 20.Lazzarino G, Di Pierro D, Tavazzi B, Cerroni L, Giardina B. Simultaneous separation of malondialdehyde, ascorbic acid, and adenine nucleotide derivatives from biological samples by ion-pairing high-performance liquid chromatography. Anal Biochem. 1991;197:191–196. doi: 10.1016/0003-2697(91)90378-7. [DOI] [PubMed] [Google Scholar]
- 21.Desrois M, Sciaky M, Lan C, Cozzone PJ, Bernard M. Preservation of amino acids during long term ischemia and subsequent reflow with supplementation of L-arginine, the nitric oxide precursor, in the rat heart. Amino Acids. 2003;24:141–148. doi: 10.1007/s00726-002-0321-9. [DOI] [PubMed] [Google Scholar]
- 22.Bernard M, Robert K, Caus T, Desrois M, Paganelli F, Cozzone PJ, Maixent JM. Protective effect of a low K+ cardioplegic solution on myocardial Na,K-ATPase activity. Cell Mol Biol (Noisy-le-grand) 2004;50:841–844. [PubMed] [Google Scholar]
- 23.Gerbi A, Barbey O, Raccah D, Coste T, Jamme I, Nouvelot A, Ouafik L, Levy S, Vague P, Maixent JM. Alteration of Na,K-ATPase isoenzymes in diabetic cardiomyopathy: effect of dietary supplementation with fish oil (n-3 fatty acids) in rats. Diabetologia. 1997;40:496–505. doi: 10.1007/s001250050707. [DOI] [PubMed] [Google Scholar]
- 24.Foussard-Guilbert F, Ermias A, Laget P, Tanguy G, Girault M, Jallet P. Detergent effects of kinetic properties of (Na+ +K+)-ATPase from kidney membranes. Biochim Biophys Acta. 1982;692:296–304. doi: 10.1016/0005-2736(82)90534-x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J Appl Physiol. 2000;89:2099–2104. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- 26.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 27.Power JM, Beacom GA, Alferness CA, Raman J, Farish SJ, Tonkin AM. Effects of left atrial dilatation on the endocardial atrial defibrillation threshold: a study in an ovine model of pacing induced dilated cardiomyopathy. Pacing Clin Electrophysiol. 1998;21:1595–1600. doi: 10.1111/j.1540-8159.1998.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 28.Bittl JA, Ingwall JS. The energetics of myocardial stretch. Creatine kinase flux and oxygen consumption in the noncontracting rat heart. Circ Res. 1986;58:378–383. doi: 10.1161/01.res.58.3.378. [DOI] [PubMed] [Google Scholar]
- 29.Bak MI, Ingwall JS. Acidosis during ischemia promotes adenosine triphosphate resynthesis in postischemic rat heart. In vivo regulation of 5'-nucleotidase. J Clin Invest. 1994;93:40–49. doi: 10.1172/JCI116974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernauer W. Release of adenine nucleotide metabolites by toxic concentrations of cardiac glycosides. Basic Res Cardiol. 1994;89:308–321. doi: 10.1007/BF00795200. [DOI] [PubMed] [Google Scholar]
- 31.Straeter-Knowlen IM, Butterworth EJ, Buchthal SD, Hollander JA, Caulfield JB, Jennings RB, Evanochko WT. PCr overshoot': a study of the duration in canine myocardium. NMR Biomed. 2002;15:52–59. doi: 10.1002/nbm.757. [DOI] [PubMed] [Google Scholar]
- 32.Kalil-Filho R, Gerstenblith G, Hansford RG, Chacko VP, Vandegaer K, Weiss RG. Regulation of myocardial glycogenolysis during post-ischemic reperfusion. J Mol Cell Cardiol. 1991;23:1467–1479. doi: 10.1016/0022-2828(91)90192-o. [DOI] [PubMed] [Google Scholar]
- 33.Songu-Mize E, Sevieux N, Liu X, Jacobs M. Effect of short-term cyclic stretch on sodium pump activity in aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;281:H2072–H2078. doi: 10.1152/ajpheart.2001.281.5.H2072. [DOI] [PubMed] [Google Scholar]
- 34.Ohkusa T, Ueyama T, Yamada J, Yano M, Fujumura Y, Esato K, Matsuzaki M. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol. 1999;34:255–263. doi: 10.1016/s0735-1097(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 35.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 36.Xie Z. Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell Mol Biol (Noisy-le-grand) 2001;47:383–390. [PubMed] [Google Scholar]
- 37.Liu L, Askari A. On the importance and mechanism of amplification of digitalis signal through Na+/K+-ATPase. Cell Mol Biol (Noisy-le-grand) 2006;52:28–30. [PubMed] [Google Scholar]
- 38.Klein HO, Bakst A, Kaplinsky E. Pulmonary embolism and atrial fibrillation. Am J Cardiol. 1988;61:498–499. doi: 10.1016/0002-9149(88)90326-8. [DOI] [PubMed] [Google Scholar]
- 39.Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, Huang SK, Tseng YZ, Lien WP. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 40.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 41.Ban K, Handa S, Chapman RA. On the mechanism of the failure of mitochondrial function in isolated guinea-pig myocytes subjected to a Ca2+ overload. Cardiovasc Res. 1999;44:556–567. doi: 10.1016/s0008-6363(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 42.Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]