Successful organ homotransplantation is dependent upon the availability of satisfactory grafts. Paired organs, such as the kidney, may be obtained from living donors, whereas hepatic and cardiac grafts for human use can only be obtained post mortem. Cadaveric renal homografts have been largely unsuccessful to date.3, 11, 12, 19, 21 The major cause of early failure was probably ischemic damage. This report describes an experimental method of procuring and temporarily preserving postmortem homografts of kidney and liver, and documents the clinical use of the technique in 4 cases.
METHODS
Thirty-two dogs weighing 9 to 20 kilograms were used. After the animals were anesthetized with 30 mg. per kilogram of pentobarbital intravenously, the femoral vessels on one side were exposed. Heparin, 3 mg. per kilogram, was injected intravenously, and aortic and inferior vena caval catheters were inserted through the femoral artery and vein (Fig. 1). The animals were then sacrificed with an overdose of pentobarbital. Respiratory arrest preceded cessation of heartbeat by 5 to 15 minutes. Perfusion was subsequently begun one to 22 minutes after the disappearance of palpable pulses, when it was certain that the animal was dead.
Fig. 1.
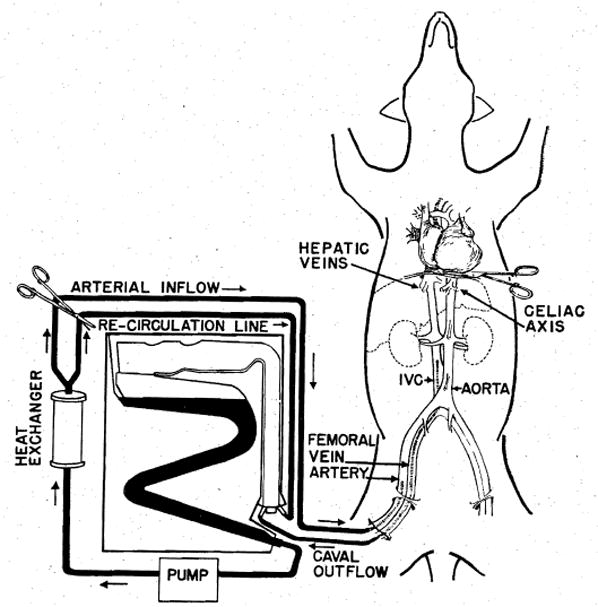
Technique of extracorporeal cadaver perfusion.
The catheters were connected to an extracorporeal perfusion system consisting of a disposable bubble oxygenator, a single DeBakey pump, and a heat exchanger (Fig. 1). Venous outflow was by gravity drainage. The oxygenator was primed with lactated Ringer’s solution in all experiments except 2 hepatic transplants in which 5 percent dextrose in water was used. The perfusate was precooled to 15° C. by recirculation through the heat exchanger. One gram of procaine chloride was added to each liter of the perfusate. In prolonged perfusions, heparin, 1.5 mg. per Kilogram, was added hourly. One third the original dose of procaine was added every 3 hours. With perfusions of 8 hours or more it was frequently necessary to add extra priming solution to the reservoir.
Pilot studies were first performed to determine suitable flow rates. The procedure was then standardized with initial flow rates of 40 to 60 ml. per kilogram per minute, and gradual reduction to 5 to 20 ml. per kilogram per minute, as the esophageal temperature fell below 20° C. Organ temperature was maintained between 12 and 15° C. thereafter by adjusting the temperature of the perfusate (Fig. 2).
Fig. 2.
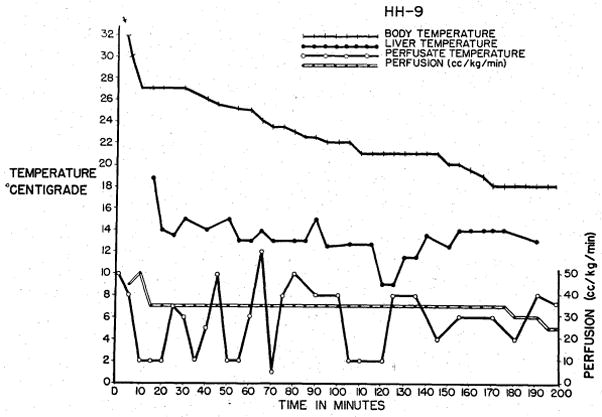
Cooling curves obtained during cadaver perfusion for a liver homograft. Note the rapid response of liver temperature to changes in perfusate temperature.
All animals receiving renal homografts had bilateral nephrectomies. The donor left kidney was transferred to the right pelvis and the donor right kidney to the left pelvis of the host. Each recipient received only one kidney. The renal artery was anastomosed end to end to the iliac artery of the recipient and the renal vein end to side to the iliac vein. In animals receiving hepatic homografts, the liver was transplanted orthotopically after recipient hepatectomy.17, 18 Postoperatively, azathioprine (Burroughs-Wellcome 57–322, Imuran) was administered to prevent rejection. Results were judged by function of the grafts and by subsequent histologic studies.
In clinical use, insertion of the catheters was not done until the patient’s death had been verified by the attending physician. In these instances, heparin was added to the perfusate in an amount equal to 3 mg. per kilogram of body weight of the proposed donor. Total time to make the necessary incisions, insert the catheters, and begin perfusion has not exceeded 15 minutes from death in 4 clinical applications.
RESULTS
Pressure-flow studies
Blood pressure monitoring was found to be of no value in determining flow adjustments. In order to maintain an arterial pressure of 50 to 70 mm. Hg, flow rates as great as 600 ml. per kilogram per minute were required, resulting in acute swelling or even rupture of the various organs. Flow rates were therefore arbitrarily limited to 40 to 60 ml. per kilogram per minute, and gradually reduced to 5 to 20 ml. per kilogram per minute as the temperature fell to 20° C. Under these conditions of perfusion, arterial pressures never exceeded 20 mm. Hg and were usually un-obtainable. Despite the low flow perfusion, cooling was relatively rapid (Fig 2).
Degree of cooling
Five animals were cooled to 2 to 10° C. by the low-flow technique. These temperatures were usually reached in less than 45 minutes. Renal homotransplantation was then performed. Immediately after revascularization, the organs assumed a mottled pink and blue hue with islands of cyanotic tissue interspersed among areas of normal parenchyma. During the next hour, the kidney gradually became grossly normal in appearance. Nevertheless, none of the grafts produced urine and all 5 animals died with uremia within 2 to 3 days.
When temperatures were kept at 15° C. or above, such mottling of the kidney did not occur, and urine production was prompt. In the definitive series to be described, temperatures were, therefore, not allowed to fall below 15° C.
Renal homografts
Eleven renal homografts were performed after cadaver perfusion of 1 to 14 hours (Table I). In 5 of the 11 cadavers, perfusion was carried out for 11 or more hours. Perfusion time for the entire series averaged 7 hours and 33 minutes. Revascularization in the recipient dog was usually completed within 30 minutes after removal of the kidney from the perfused cadaver.
Table I.
Renal homografts
| Dog no. | Perfusion started post mortem(min.) | Flow rates (ml./Kg.) High-low | Time to reach 15° C. (min) | Total time from death to revascularization (hr., min.) | Urine flow started | Survival post trans plantation (days) | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 40-20 | 45 (to 21° C.) | 2, 15 | Immediately | 36 | Gastrointestinal bleeding |
| 2 | 5 | 60-20 | 24 | 4, 20 | Immediately | 31 | Pneumonia |
| 3 | 4 | 19.5- 4.5 | 90 | 4, 42 | Immediately | 10 | Intussusception |
| 4 | 12 | 32-15 | 6 | 3, 31 | Second day | 10 | Pneumonia |
| P.O. | |||||||
| 5 | 8 | 30- 7 | 10 | 6, 15 | Immediately | 10 | Pneumonia |
| 6 | 8 | 30- 7 | 10 | 6, 20 | Immediately | 16 | Pneumonia |
| 7 | 5 | 40-15 | 70 | 13, 6 | Immediately | 13 | Pneumonia |
| 8 | 5 | 40-15 | 70 | 14, 33 | Immediately | 11 | Pulmonary emboli |
| 9 | 5 | 30-15 | 45 | 11, 12 | Immediately | 52 | Pneumonia |
| 10 | 5 | 30-15 | 45 | 14, 23 | Immediately | 23 | Pneumonia |
| 11 | 5 | 50-15 | 22 | 13, 27 | Immediately | 7 | Pneumonia |
Ten of the 11 animals produced urine immediately and maintained good urinary volumes until death. The eleventh animal was anuric for 2 days, with subsequent diuresis.
Renal function was assessed by urine volumes and blood urea nitrogen (BUN) determinations. The animals receiving kidneys which had been perfused for 6 hours or more showed sharp postoperative rises in the BUN (Fig. 3). This subsequently returned toward normal. With perfusions of less than 6 hours, this pattern of early azotemia was not prominent (Fig. 3).
Fig. 3.
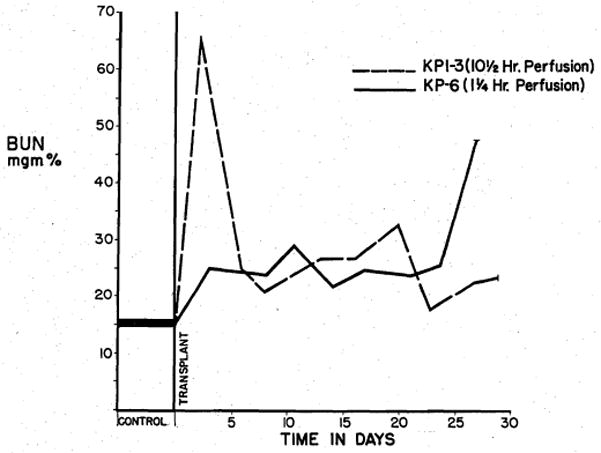
BUN level after renal homografting. Note the acute azotemia with the homograft perfused for 10½ hours. Early azotemia is minimal in the animal receiving a kidney perfused for 1¼ hours.
Survival ranged from 7 to 52 days (Table I). Mean survival time was 19.9 days. Death most commonly resulted from pneumonitis, with 8 dogs dying of this complication. Six of the animals were uremic at the time of death. Histologic sections of the transplanted kidneys removed post mortem showed varying degrees of mononuclear cell infiltrates with minimal evidence of rejection. In no instance was there evidence of histologic renal damage which could be directly attributed to the perfusion.
Hepatic homografts
Hepatic homografts were transplanted in 10 animals after perfusions ranging from 71 to 416 minutes (Table II). Revascularization after removal of the liver required an additional average time of 72 minutes. Hepatic function after transplantation was inferred from the ability of the animal to awaken from pentobarbital anesthesia and survive without supplemental glucose therapy.
Table II.
Hepatic homografts
| Dog no. | Perfusion started post mortem (min.) | Flow rates (ml./Kg.) high-low | Time to reach 15° C. (min.) | Total time from death to revascularization (hr., min.) | Survival post transplant | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 7 | 60-20 | 24 | 2, 3 | No | Hemorrhage |
| 2 | 4 | 3, 54 | No | Hemorrhage | ||
| 3 | 5 | 50-25 | 18 | 4, 22 | No | Hemorrhage |
| 4 | Immediate | 15.5- 2.5 | 17 | 3, | No | Hemorrhage |
| 5 | 7 | 32-15 | 28 | 2, 18 | 4 days | Bile peritonitis |
| 6 | 8 | 33- 3 | 22 | 3, 38 | No | Hemorrhage |
| 7 | 4 | 195-45 | 90 | 3, 42 | 5 days | Intussusception |
| 8 | Immediate | 29-12 | 19 | 8, 18 | 2 days | Hemorrhage |
| 9 | 5 | 40-15 | 8 | 4, 55 | 3 days | Hemorrhage |
| 10 | 22 | 27-12 | 10 | 4, 47 | 24 hr. | Hemorrhage |
Five animals survived the immediate postoperative period. Severe hepatic damage was observed in all, as evidenced by sharp rises in serum glutamic oxalacetic acid transaminase (SGOT) and bilirubin levels (Fig. 4). Three of these animals later died of hemorrhage at 24 hours, 2 days, and 3 days postoperatively.
Fig. 4.
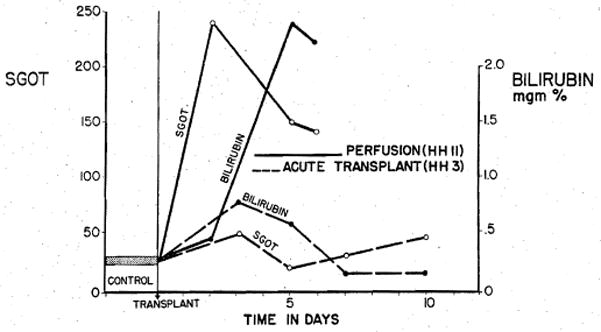
Patterns of injury in dogs observed following transplantation of a perfused hepatic homograft, and of a liver obtained after sacrifice of a living donor. Total time from death to reimplantation for the perfused organ was 140 minutes as compared to 55 minutes for the graft transplanted immediately after sacrifice (acute transplant).
Consistent clotting defects were encountered in all animals. Increased fibrmolysin, decreased plasma fibrinogen contents, and increased thrombin times were uniformly observed. The significance of the clotting defects is reflected in the high death rate from hemorrhage (Table II). Five animals died from this cause either on the operating table or immediately postoperatively; another 3 which survived the procedure had extensive hemoperitoneum at autopsy. The incidence of fatal hemorrhage increased with increased length of perfusion of the donor liver. Six of the 8 animals that died from this cause had received hepatic homografts from donors that had been perfused in excess of 2 hours (Table II).
Histologic sections taken post mortem correlated well with the clinical observations. All livers showed moderate to marked centrilobular congestion and hepatic cell necrosis (Fig. 5) without evidence of rejection.
Fig. 5.
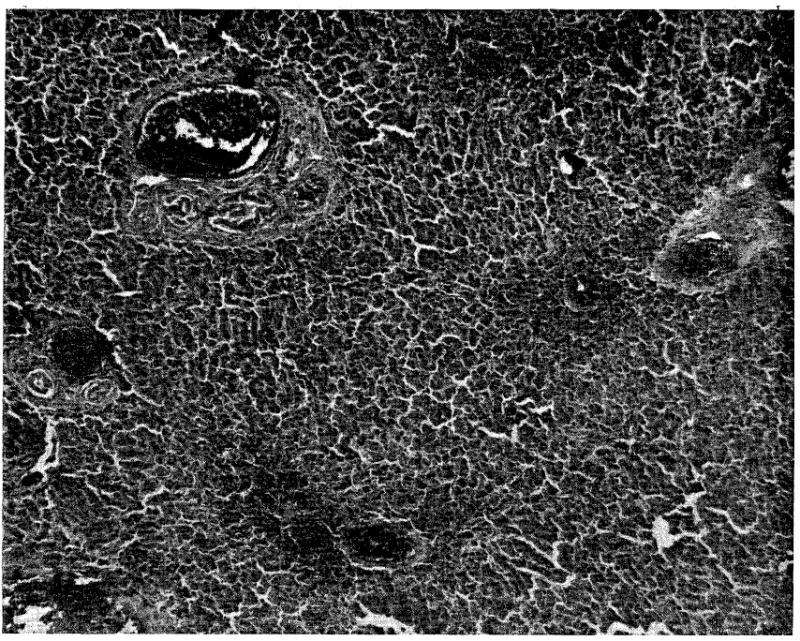
Typical injury observed in livers perfused for more than 2 hours. Note the marked centrilobular congestion and parenchymal necrosis. (×32.)
Clinical use of cadaveric renal homografts
The first homograft was obtained from a patient who was thought to have a brain tumor, but who was subsequently shown at autopsy to have died of actue bacterial endocarditis. His blood pressure was 60 mm. Hg or less for 4 hours prior to death. Cadaver perfusion was not satisfactory, the maximal flow rates being 15 to 20 ml. per kilogram per minutes. Revascularization of the kidney in the recipient bed was accomplished 106 minutes after death. The homograft functioned for 2 weeks postoperatively, although at reduced efficiency. The BUN fell from 176 to 119 mg. percent, and then rose again after 12 days. Death of the host from sepsis occurred on the twenty-fourth day after transplantation. Histologic sections of the transplanted kidney showed severe interstitial hemorrhage and the presence of proteinaceous material in the tubules. There was minimal mononuclear infiltrate (Fig. 6). Sections obtained from the opposite donor kidney after perfusion revealed normal architecture (Fig. 6). There was no evidence that septic emboli in the transplanted tissue contributed to the unfavorable outcome.
Fig. 6.
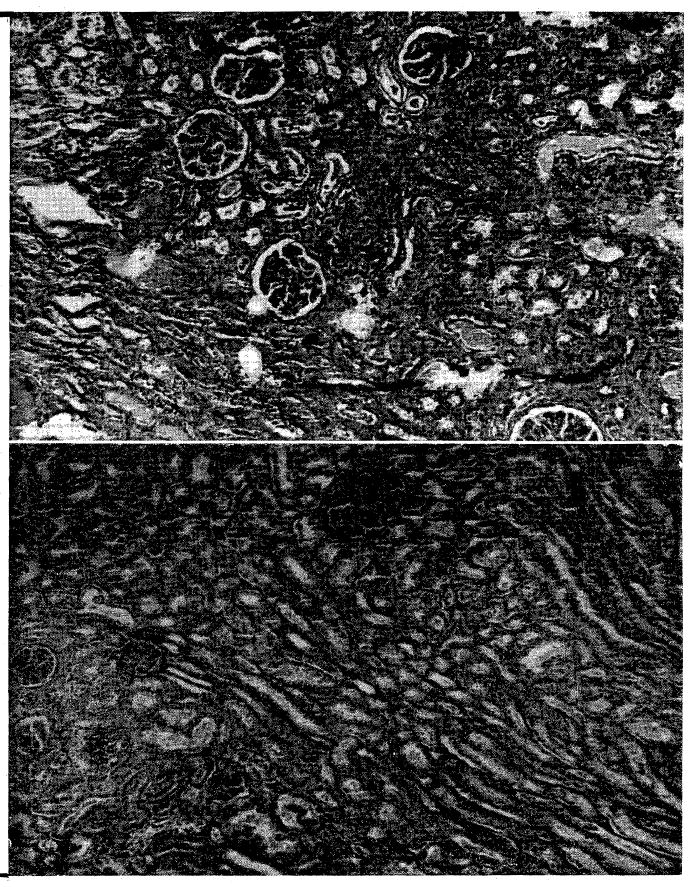
A, Appearance of homografted cadaveric left kidney 24 days after transplantation. B, Cadaveric contralateral kidney immediately after cessation of perfusion. (×32.)
Postmortem hypothermic perfusion in the second case failed within a few minutes after its inception. The patient, who died of subarachnoid hemorrhage, was severely dehydrated terminally, and probably had a low blood volume. Adequate venous return could not be obtained. The kidney was revascularized in the recipient bed 124 minutes after death. Renal function did not return. Twelve days later, the graft was removed, after it had ruptured following relatively minor trauma to the recipient patient in a fall from bed. Histologic sections showed typical rejection (Fig. 7).
Fig. 7.
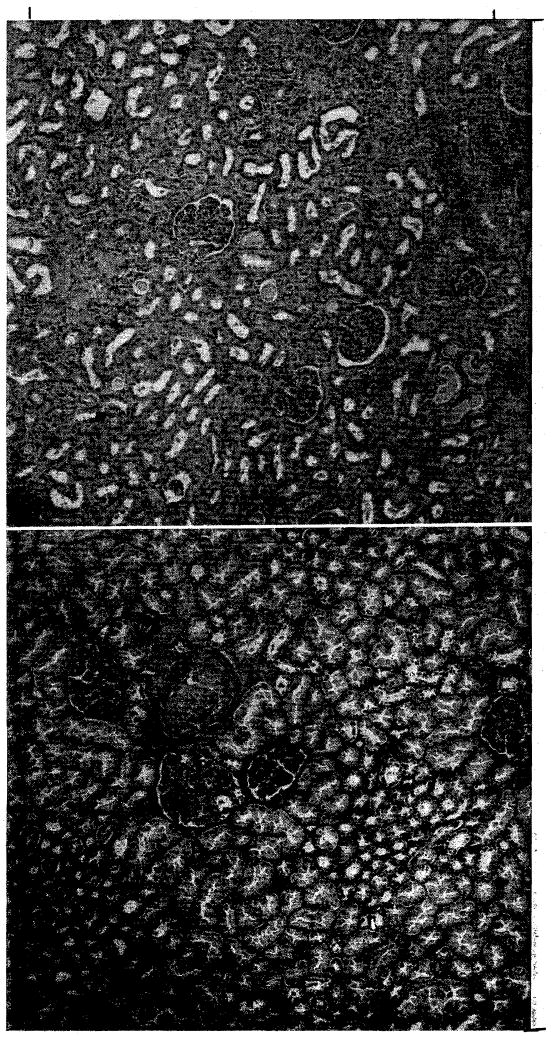
A, Homografted cadaveric right kidney removed 12 days after transplantation. Note rejection. B, Contralateral donor kidney obtained at conclusion of perfusion. (×32.)
Clinical use of two cadaveric hepatic homografts
The first organ was obtained from a 3-year-old child who died in the operating room during resection of a medulloblastoma. The recipient was another 3-year-old child with terminal biliary cirrhosis secondary to congenital atresia of the bile ducts. The time from death to the institution of extracorporeal perfusion was 15 minutes. For the preceding 30 minutes, open cardiac massage was performed. Initial perfusion flow rates were 30 to 50 ml. per kilogram per minute and cooling proceeded rapidly. Perfusion was then reduced to 10 to 20 ml. per kilogram per minute. After 2 hours, venous return began to decline and during the next 110 minutes perfusion ceased altogether. The temperature of the liver at the time of perfusion failure was 15° C. After an additional 45 minutes, the liver was removed. Orthotopic revascularization in the recipient took 85 minutes.
Following restoration of blood supply to the donor liver, a bleeding diathesis developed with hypofibrinogenemia and fibrinolysis. Despite the administration of fresh whole blood, fibrinogen, and epsilon-aminocaproic acid, the hemorrhage could not be controlled. Histologic sections of the transplanted liver showed well-advanced, nonspecific autolysis which could not be attributed to ordinary postmortem changes (Fig. 8).
Fig. 8.

Photomicrograph of first clinical hepatic homograft. The patient died on the operating table. Note advanced hepatic cell necrosis. (×32.)
The second liver homograft was obtained from a 55-year-old man who died from a cerebral glioma. The recipient was a 47-year-old man in whom a primary hepatoma had been found at operation one week earlier. Several measures were taken to shorten the necessary time for and to improve the quality of perfusion. The recipient was prepared for the homograft by a preliminary operation, 24 hours before definitive transplantation, at which time his own liver was freed of all attachments except the bile ducts and vessels. Intravenous infusions of glucose were given to the prospective donor in order to maintain hepatic glycogen. Immediately after death had been certified, acute expansion of the intravascular volume was achieved by forced transfusion of whole blood and plasma. Finally, the thoracic aorta was occluded just above the diaphragm (Fig. 1).
Perfusion at 40 ml. per kilogram per minute was begun 5 minutes after death, and gradually reduced to 20 ml. per kilogram per minute during the next 45 minutes, during which time the body temperature fell to 15° C. Dissection and extirpation of the donor organ required 94 minutes. After removal, 500 c.c. of cooled lactated Ringer’s solution was used to wash out residual blood. The liver was then carried to the recipient operating room and revascularized in the ensuing 63 minutes. Bile flow through the transected donor common duct was noted shortly thereafter. Epsilon-aminocaproic acid, fibrinogen, and fresh blood were administered during transplantation. Bleeding was not a major problem.
In spite of the success of perfusion and the short time from death of the donor to revascularization of the liver in the host, evidence of hepatic damage was observed in the first few postoperative days with rises of SGOT to as high as 1,150 units and an early rise of bilirubin to 12 mg. percent. Liver function tests gradually returned toward normal until death occurred on the twenty-second postoperative day as a result of multiple pulmonary emboli. Histologic sections of the liver showed preservation of architecture (Fig. 9). There was some increased fibrosis and bile stasis. No damage due to perfusion was seen, nor was there unequivocal microscopic evidence of rejection.
Fig. 9.
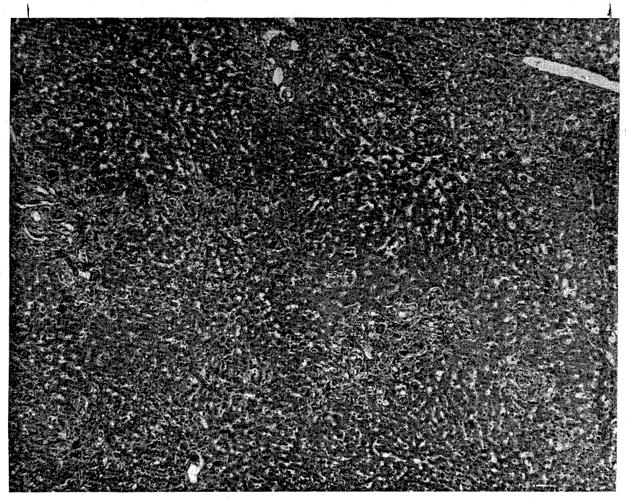
Photomicrograph of liver from the second clinical hepatic transplant, obtained at autopsy 22 days after transplantation. Note preservation of hepatic architecture. Moderate periportal fibrosis and bile stasis are present. (×32.)
DISCUSSION
The beneficial effects of hypothermia in preventing ischemic damage to tissue have been documented for several organs, including kidney,5, 6, 8, 10, 15 liver,1, 4, 13, 16, 17 lung,2 and heart.9 Perfusion of isolated organs with various solutions has also been shown to prolong function. The technique described here incorporates the advantages of both hypothermia and perfusion. The practicality of the method is considerably increased by the use of the electrolyte- or glucose-primed disposable bubble oxygenator, thereby eliminating the need for blood typing. The resultant hemodilution reduces viscosity14 and promotes increased renal blood flow.7 Moreover, oxygen is available to the tissues in quantities equal to those obtained from whole blood.20 The favorable effect of procaine on renal perfusion at low temperatures has been demonstrated by Kiser and his colleagues.8
Extracorporeal perfusion in cadavers differs from that in the living patient. Serious organ damage resulted from overperfusion when it was attempted to maintain arterial pressures which would be clinically acceptable. Apparently there is a rapid and complete loss of vascular tone shortly after death, making the maintenance of significant arterial pressure impossible except with exorbitant flow rates. The use of low flows in combination with hypothermia provides some protection against hydrostatic damage to the organs from the pump system. While low-flow perfusion has metabolic disadvantages, it has appeared to be satisfactory for short-term preservation.
Although others have been able to obtain satisfactory renal function after storage of kidneys at low temperatures (1 to 4° C.) for as long as 8 hours,8, 15 we were unable to do so with continuous perfusion at these temperatures. Renal homografts cooled below 10° C. failed to function. When temperature was controlled at 15° C. all renal homografts were functional (Table I). The use of extremely low temperatures with continuous perfusion appears to be harmful. The mechanism of injury is unknown, but the gross appearance of the kidneys was strongly reminiscent of frostbitten extremities.
Although satisfactory cadaveric renal homografts have been consistently obtained with the use of the low-flow perfusion at temperatures of 15° C. for as long as 14 hours after death, there are limitations to the method. Kidneys perfused for longer than 6 hours showed temporarily depressed renal function. The liver has been shown to be even more susceptible to postmortem injury. This is evidenced by the high failure rate in the animals perfused over 2 hours (Table II). Even with perfusion for less than 2 hours, serious hepatic damage occurs, as shown by elevation in SGOT and bilirubin levels, and by the development of coagulation defects.
Comparing the animal results with the clinical material, it is apparent that success depends as much on the terminal course of the donor as it does on the perfusion. Renal homografts obtained from animals sacrificed with normal cardiovascular dynamics have been uniformly successful. Cadaveric perfusion in such dogs requires no special precautions. In contrast, kidneys and livers obtained from patients who have had a protracted terminal course are variably damaged before death, resulting in unpredictable function of the homografts. In addition, difficulties with perfusion have been encountered, apparently due to a reduced blood volume, inasmuch as transfusion of the cadaver partially corrects this problem. If flows are still inadequate, selective perfusion of the lower half of the corpse has been obtained by clamping the lower thoracic aorta. Despite these adverse factors, it is encouraging to note the fact that functional hepatic and renal homografts have, nevertheless, been obtained for clinical use.
Other organs, including lung and heart, have been successfully transplanted in animals, from living donors. Homografts of heart and lung, to be clinically useful, must be obtainable from other sources. The technique described should be applicable with some modifications. It will be necessary to decompress the pulmonary circuit with left atrial or ventricular vents to prevent damage to the lung or overdistention of the left ventricle.
SUMMARY
Functional renal homografts have been obtained as long as 14 hours post mortem by a technique of extracorporeal cadaver perfusion. The method has also given satisfactory liver homografts after perfusion up to 2 hours. Details of the technique and experimental and clinical applications are described. Analysis of the data indicates that optimal perfusion times for renal homografts should not exceed 6 hours. For the liver, 2 hours appears to be the maximum tolerable period of perfusion.
For clinical application, certain adjuvant measures are important. Perfusion failure has been encountered in human cadavers, presumably as a result of the presence of a reduced blood volume. This can be prevented by acute postmortem expansion of the intravascular volume with compatible whole blood or plasma. Proper donor selection is as important a factor as the method used for preservation. Indirect evidence is cited that premortem tissue injury in protracted terminal disease may be a greater deterent to success than the parenchymal damage which occurs during postmortem perfusion.
The extension of this technique for the procurement of other organs such as heart and lung is discussed.
Acknowledgments
Supported by United States Public Health Service Grants A 6283, A 6344, and HE 07735-01.
References
- 1.Bernhard W, Cahill G, Curtis G. The rationale of surgery under hypothermia in patients with severe hepatocellular disease. Ann Surg. 1957;145:289. doi: 10.1097/00000658-195703000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenstock DA, Hechtman HB, Collins JA. Preservation of the canine lung. J Thoracic Surg. 1962;44:771. [PubMed] [Google Scholar]
- 3.Goodwin WE, Kaufman JJ, Mims MM, Turner RD, Glassock R, Goldman R, Maxwell MM. Human renal transplantation. I. Clinical experiences with 6 cases of renal transplantation. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Carter E, McDermott W. Differential hypothermia in experimental hepatic surgery. A M A Arch Surg. 1957;74:327. doi: 10.1001/archsurg.1957.01280090025005. [DOI] [PubMed] [Google Scholar]
- 5.Humphries AL, Jr, Russell R, Ostofin J, Goodrich SM, Moretz WH. Successful reimplantation of dog kidney after 24 hour storage. S Forum. 1962;13:380. [PubMed] [Google Scholar]
- 6.Jones WR, Politano VA. Acute renal ischemia and regional renal hypothermia. S Forum. 1962;13:497. [PubMed] [Google Scholar]
- 7.Kinter WB, Pappenheimer JR. Role of red blood corpuscles in regulation of renal blood flow and glomerular filtration rate. Am J Physiol. 1956;185:399. doi: 10.1152/ajplegacy.1956.185.2.399. [DOI] [PubMed] [Google Scholar]
- 8.Riser JC, Telander RL, Peterson TA, Coe JI, Hitchcock CR. Canine renal autografts: studies of reversible histopathologic changes following prolonged extracorporeal refrigeration. A M A Arch Surg. 1961;83:502. doi: 10.1001/archsurg.1961.01300160014003. [DOI] [PubMed] [Google Scholar]
- 9.Lower RR, Stofer RC, Hurley EJ, Dong E, Jr, Cohn RB, Shumway NE. Successful homotransplantation of the canine heart after anoxic preservation for seven hours. Am J Surg. 1962;104:302. doi: 10.1016/0002-9610(62)90332-x. [DOI] [PubMed] [Google Scholar]
- 10.Macksood AJ, Szilagyi DE, Smith RF. The maintenance by cold perfusion of the viability of the devascularized canine kidney. S Forum. 1961;12:190. [PubMed] [Google Scholar]
- 11.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human kidney homografts by immunosuppressive drug therapy. New England J Med. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 12.Parsons FM, Markland C, Raper FP, Fox M. Cadaveric renal transplantation. Brit M J. 1963;1:930. [Google Scholar]
- 13.Raffucci RL, Lewis FJ, Wangensteen OH. Hypothermia in experimental hepatic surgery. Proc Soc Exper Biol & Med. 1953;83:639. doi: 10.3181/00379727-83-20443. [DOI] [PubMed] [Google Scholar]
- 14.Reemtsma K, Creech O. Viscosity studies of blood, plasma, and plasma substitute. J Thoracic Surg. 1962;44:674. [PubMed] [Google Scholar]
- 15.Schloerb PR, Waldorf RD, Welsh JS. The protective effect of kidney hypothermia on total renal ischemia. Surg Gynec & Obst. 1959;109:561. [PubMed] [Google Scholar]
- 16.Sicular A, Moore FD. The postmortem survival of tissues. 2 The effect of time and temperature on the survival of liver as measured by glucose oxidation rate. J S Res. 1961;1:16. [Google Scholar]
- 17.Starzl TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RU. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynec & Obst. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Kaupp HA, Brock DR, Linman J, Moss WT. Studies on the rejection of the transplanted homologous dog liver. Surg Gynec & Obst. 1961;112:135. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Marchioro TL, Holmes J, Brittain RS, Hermann G, Knight CS, Talmage DW, Waddell WR. Clinical problems in renal homotransplantation. J A M A. In press. [Google Scholar]
- 20.Tanaka T, Inoue T, Paton BC. Oxygen availability during hypothermic perfusions using diluted blood. S Forum. 1962;13:138. [PubMed] [Google Scholar]
- 21.Woodruff MFA. The transplantation of tissues and organs. Springfield, Ill: Charles C Thomas, Publisher; 1960. pp. 521–525. [Google Scholar]


